Abstract
In this study, we evaluated a new biopesticide containing different combinations of Photorhabdus luminescens (ATCC 29,999; Pl) and Bacillus thuringiensis subsp. aizawai (Bt) to leverage their insecticidal activity against Plutella xylostella. Mixtures containing proteins of various sizes were assayed to determine which combination of the two bacteria would yield the maximum insecticidal activity. A histopathologic slide revealed vacuole formations and rifts near the apical membrane (a symptom of Bt) and severe thinning of the intestinal wall (a symptom of Pl). When the two bacteria were cultured separately and then mixed, the insecticidal activity of the treatment reached 83.33% ± 8.82%. The insecticidal activity was elevated and significantly accelerated when Bt was mixed with both the Pl supernatant and the isolated protein with a molecular mass \(\ge\) 100 kDa of Pl. These results highlight the potential of Pl as a potent bioinsecticide to economically and sustainably control Pl. xylostella and other lepidopteran pests.
Key points
• Growth inhibition by Bacillus thuringiensis exerted a significant effect on insecticidal activity.
• Large Photorhabdus luminescens proteins can accelerate the synergistic insecticidal effect on Plutella xylostella.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photorhabdus luminescens (ATCC 29,999, Pl) is a gram-negative species of bacterium belonging to the Enterobacteriaceae family. In addition to its toxicity to a range of pests, it is also an intestine symbiont of the infective juvenile entomopathogenic nematodes of the Heterorhabditidiae family (Zhu et al. 2011). Pl is pathogenic to a wide range of insects, and its genome comprises more toxin genes than does any other bacterial genome sequenced to date (Duchaud et al. 2003). Additionally, numerous adhesions, proteases, and lipases, which may be expressed during the pathogenic phase of its complex lifecycle, have been discovered from its complete genome sequence. Numerous toxin complex (tc) loci were observed on the chromosomes of Pl strains (ffrench-Constant et al. 2007), all of which code for different high molecular–weight insecticidal toxins. Some of the tc proteins may function to destroy the insect midgut, similar to Bacillus thuringiensis (Bt) delta-endotoxins. Pl can also kill insect hosts by using chromosomal insertion through the expression of a single gene called makes caterpillars floppy (mcf) (Zalucki et al. 2012). Pl is often naturally delivered into the target insect hemocoel, where Pl reproduces rapidly after inducing immunosuppression in its host. The bacteria inhibit eicosanoid synthesis and multiply, resulting in septicemia and death of the infected host (Park and Kim 2000; ffrench-Constant et al. 2007). Bt exerts a potentiating effect on the orally ingested entomopathogenic bacterium Xenorhabdus nematophila ATCC19061 (Thomas and Poinar 1979), and Bt that infects the midgut epithelium allows X. nematophila to move from gut lumen to the hemocoel (Jung and Kim 2007).
Through microaggregation, spreading behavior, and phagocytosis against invasive bacteria, eicosanoids mediate both cellular and humoral immune responses to bacterium pathogenicity in insect hosts (Shrestha and Kim 2008; 2009). Some metabolites—derivatives of the bacterial family Enterobacteriaceae—can suppress cellular immune responses and considerably enhance the pathogenicity of Bt against the second instar larvae of Plutella xylostella (Lepidoptera: Plutellidae) (Shrestha et al. 2010).
Bt, a gram-positive spore-forming bacterium that synthesizes delta-endotoxins (toxic crystal [Cry] proteins), also exhibits entomopathogenic activity against different insect orders with high specificity (Bravo et al. 2011). These Cry proteins are packaged into crystals and activated through the solubilization of the crystals in the insect midgut and proteolytic processing of protoxins by midgut proteases. The activated toxins then bind to specific receptors on the midgut brush border membrane surface, which causes pore formation and cell lysis, thereby leading to insect death (Bravo et al. 2007). In addition to Cry proteins, Bt isolates can also synthesize other insecticidal proteins, including cytotoxic proteins and proteins synthesized during the vegetative growth phase (vegetative and secreted insecticidal proteins) (Estruch et al. 1996; Donovan et al. 2006).
The diamondback moth, Pl. xylostella, is a worldwide pest that causes destructive damage to Brassicaceae crops (Furlong et al. 2013). Indiscriminate use of various insecticides to control Pl. xylostella has resulted in a high level of resistance or multiple resistance of the species to every insecticide used extensively against it (Mohan et al. 2009). Mixtures of active agents of biopesticides may be used as a pesticide resistance management strategy and improve pest control efficiency (Gao et al. 2012; Mantzoukas et al. 2013). Several studies have evaluated the interactions between and potentiating effects of Bt and the entomopathogenic bacteria X. nematophila and Pl as well as the distinct effects of toxins, bacterial cells, and supernatant mixtures with different ratios, most of which enhance the effects of their respective combination treatments, resulting in greater pest mortality (Jung and Kim 2007; Sayed and Behle 2017).
Jung and Kim (2007) reported that when a mixed treatment of X. nematophila and Bt was used to target late instars of Pl. xylostella, the moths exhibited significantly higher mortality than when only X. nematophila was used, and the X. nematophila cells and their pathogenies were recovered from the hemocoel. However, no cells were recovered when only X. nematophila was used. This observation of synergism is consistent with that of BenFarhat et al. (2013) who explored the high affinity of Bt delta-endotoxins Cry1Ac and Cry2Aa for membrane receptors on larval Ephestia kurstaki, which leads to extensive pore formation, allowing the transfer of numerous Xenorhabdus cells. A histopathological study of other entomopathogenic Pl reported the rapid invasion of the hemocoel Agrotis segetum by Pl cells (Jallouli et al. 2018). Despite these generally encouraging results, few studies have attempted to combine and optimize these pathogens into integrated protocols stipulating the optimal ratios of mixed treatments, and few studies have provided histopathological data on the potentiating or antagonistic effects of Pl. Thus, additional research supporting the development of combinational approaches is warranted.
Recent advancements in fermentation production and formulation technologies have successfully produced storage-stable Pl as an alternative to solid substrate production of poison proteins. Lower production costs encourage the development of a combined product containing Bt and Pl. However, a limited number of Bt and Pl combinations have been evaluated in toxicity interaction studies (Jung and Kim 2007; Sayed and Behle 2017). The limited data available regarding the toxicity of certain agents to Pl. xylostella hampers the identification and development of mixtures that may effective against Pl. xylostella when used in combination with biopesticides. In the present study, we evaluated a new biopesticide based on different combination times and ratios of Pl and Bt for insecticidal activity against Pl. xylostella. Various mixtures were assayed to determine the optimal ratio of the two agents for maximum insecticidal activity. Isolation, characterization, and toxicity evaluations of Bt and Pl combinations of different molecular weights were also conducted. We evaluated the possible binary antagonistic and synergistic effects of the combinations through feeding bioassays. Finally, we proposed a more effective synergistic combination of these two biological control agents by evaluating their histopathological effects and analyzing the time–mortality response of the treated Pl. xylostella.
Materials and methods
Strains and media
A Pl strain (ATCC29999) was maintained on nutrient bromothymol blue agar (NBTA) plates [2.3% nutrient agar (Difco, USA), 0.0025% bromothymol blue (Merck), and 0.004% 2,3,5-triphenyltetrazolium (Sigma-Aldrich)] at 30 °C and subcultured weekly. The Bt strain B. thuringiensis subsp. aizawai ABTS-1857 (10.8% [7000 diamond back month mortality units/mg, DBMU/mg]; Sumitomo Chemical, Taipei, Taiwan) was isolated from a commercial product, producing Cry1Aa, Cry1Ab, Cry1C, and Cry1D (Palma et al. 2014). Two media were used in this study: nutrient agar (Difco) and Luria–Bertani (LB), which contains peptone (10 g/L), yeast extract (5 g/L), and NaCl (5 g/L) (Sambrook et al. 1989).
Insect rearing
The cultivation of the Pl. xylostella colonies was conducted in incubators at 25 °C ± 1 °C and 65% ± 5% relative humidity under a 15 h:9 h (light:dark) photoperiod according to a procedure described in a previous study (Wu et al. 2020). The colony was purchased from the Taiwan Agricultural Chemicals and Toxic Substances Research Institute, Council of Agriculture, Executive Yuan. A total of 30 third instar Pl. xylostella larvae were reared separately in 9-cm sterile Petri dishes containing 1 cm3 of artificial diet.
Quantification of toxins
Pathogenicity tests were performed at 0, 12, 24, 48, 72, 96, and 120 h (h) after culturing. The Pl was cultured at 30 °C in LB agar plates. A single colony was selected to prepare a cell suspension in sterile saline solution (0.85% NaCl); the optical density (OD) of the culture at 600 nm was 0.2, equivalent to 1 × 107 log colony-forming unit (CFU)/mL. A Colony Counter 270 (SUNTEX, Taiwan) was used to count the cells. The OD and luminescence were measured at 0, 12, 24, 48, 72, 96, and 120 h with an ultraviolet–visible/near-infrared spectrophotometer (Model V-730, Jasco, Japan) and a Centro luminometer (Model 4900, Awareness Technology, USA).
To concentrate the collected Pl, 10 mL bacterial solutions were centrifuged for 10 min at 10,000 rpm. Subsequently, 4 mL of the supernatant was added to a Vivaspin 6 tube (MECO, Sartorius), and the mixture was centrifuged for 10 min at 8,000 rpm and 4 °C, with a molecular weight cutoff of 100 kDa (Kenney et al. 2019). For further bioassay analysis, we tested bacterial solutions with molecular weights of \(\ge\) 100 kDa and < 100 kDa.
The spore–crystal mixture of Bt obtained after incubation and 1 mL of bacterial solution were centrifuged for 10 min at 9,000 × g. The supernatant was discarded and each pellet was washed twice with 1 M NaCl and twice with cold distilled water. Delta-endotoxins in lyophilized spore–crystal mixtures were further solubilized in 50 mM NaOH and incubated for 2 h at room temperature with constant agitation.
Insect toxicity bioassays and sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis
Bioassays were performed using third instar larvae of Pl. xylostella, reared in our laboratory (temperature: 25 °C, relative humidity: 60%–70%, photoperiod: 15 h:9 h light:dark). Thirty larvae were placed in each 9-cm sterile Petri dish containing 1 cm3 of artificial diet. To test the effects of different culturing techniques and mixture ratios on the toxicity of the different fractions of the Pl culture, A) 50 mL each of Pl and Bt bacterial solution were mixed and cocultured (Bt whole broth and Pl whole broth, Bt + Pl), and B) Bt and Pl bacterial solutions were cultured for 12 and 120 h, respectively, and then mixed for 20 min (Bt and Pl). Pl bacterial solutions measuring 70–100 kDa provided a mortality rate of up to 88% (Kenney et al. 2019; Wu et al. 2020). To elucidate the effects of different molecular weights on the Pl bacterial solutions, the cells and supernatants were further separated using a Vivaspin 6 centrifugal concentrator with a molecular weight cutoff of 100 kDa (Denolf et al. 1993; Kenney et al. 2019). Thirty larvae were added to each dish and incubated at 25 °C. Each bioassay was performed in triplicate. The extracellular protein secreted by Pl cultivated in LB broth at different incubation times (6, 12, 18, 24, 30, 36, 42, 48, 54, 60, 66, and 72 h) and dilution ratios was analyzed using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with 6%–20% Tris–glycine polyacrylamide gels (Bio-Rad, Taiwan). Native PAGE was conducted for the electrophoretic mobility shift assays by using precast NuPAGE Novex 3%–8% Tris–acetate gels (Invitrogen) and applying 150 V for 3 h.
Preparation and sectioning of P. xylostella midgut tissues
The third instar larvae of Pl. xylostella were exposed to the Pl and Bt whole broth with a cell concentration of 107 cells/mL or the supernatant for a specific length of time. The samples were fixed in 10% neutral buffered formalin solution for 24 h, incubated in a 30% sucrose–phosphate-buffered saline (PBS) solution for 24 h, and saturated in FSC 22 embedding medium (Leica Surgipath, 3801480S), a mixture of polyvinyl alcohol and polyethylene glycol that surrounds but does not infiltrate tissue. Thereafter, the samples were mounted on a cryostat base at –20 °C. Sections (10 μm) were prepared using a cryostat microtome (Leica CM1520). For immune labeling, the sections were warmed at room temperature for 10 min, resaturated, and permeabilized in blocking solution (1:19, glacial acetic acid: methanol) for 10 secs. The sections were then washed and stained with hematoxylin and eosin (Okech et al. 2008) for the histopathological localization of toxin effects.
Statistical analysis
The Pl cell toxicity levels are presented as the average of the measurements from the three repetitions of each experiment. All statistical analyses were performed using R software (R Core Team 2021). The mortality data were arcsine-transformed, and a one-way analysis of variance was conducted, with the significance level set at p < 0.05. A Tukey’s honestly significant difference test (HSD) was conducted to compare within-group means; all values are presented as mean ± standard error of the mean. The time–mortality data were employed for survival analysis (using Kaplan–Meier estimators and a log-rank test) with the Survival and Survminer R packages (R Core Team 2021). The interactions between Bt and Pl were evaluated using the method described by Tabashnik (1992). The expected LT50 of a mixture was calculated using the following mathematical equation (modified from Tabashnik 1992):
where LT50(m) represents the expected LT50(m) of the mixture of toxins A and B; LT50(A) is the observed LT50 for toxin A alone; LT50(B) is the observed LT50 for toxin B alone; and RA and RB represent the proportions of toxin A and toxin B in the mixture, respectively. To assist in evaluating both the type and magnitude of each interaction, we determined the synergism factor (SF) for each mixture, which is calculated by dividing the expected LT50(m) by the observed LT50(m). Additive interactions with SF values of more than 1.5 were classified as synergistic (Li and Bouwer 2014).
Results
Quantification of toxins
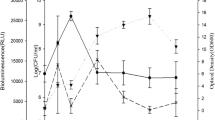
Regarding the bacterial growth of the Pl colonies in this study, the first 2 h of cultivation represented the lag phase, 2–12 h represented the exponential phase, and 12–24 h represented the stable phase (stationary phase). The period after 72 h was the death phase (decline phase). The highest colony number of the Pl culture in the LB medium was 9.84 ± 0.35 log CFU/mL and was observed at 72 h; two bioluminescence expression peaks were observed at 12 and 72 h. The protease activity, however, exhibited expression peaks at 120 h, indicating that protease could be secreted extracellularly; this results was in contrast to the bioluminescence expression peaks and the number of bacterial colonies intracellularly (Fig. 1).
To prepare the single cultures and the coculture, the Pl and Bt were inoculated into LB liquid media. The numbers of luminescent colonies in the darkroom were counted, and the colony numbers of Pl and Bt + Pl peaked at 72 h (9.84 ± 0.35 and 9.53 ± 0.03 log CFU/mL, respectively). The colony numbers of Bt peaked at 120 h (10.85 ± 0.01 log CFU/mL, F2,6 = 99.32, p < 0.001, Fig. 2a). The protease activity of the Pl and Bt + Pl treatments peaked at 120 h, with 1.95 ± 0.02 U and 0.83 ± 0.13 U, respectively (F2,6 = 501.3, p < 0.001, Fig. 2b).
Quantification of toxins for cocultured Bacillus thuringiensis subsp. aizawai (Bt) and Photorhabdus luminescens ATCC 29,999 (Pl). a Bacteria population; (b) protease of Pl; arcsine-transformed mortality at (c) 50 × dilution and (d) 100 × dilution among single bacterium treatments (Pl and Bt) and Bt and Pl coculture after 120-h incubation (Bt + Pl). Different letters (a, b, c) indicate significant differences (p < 0.05), as measured by honestly significant difference (Tukey’s HSD) test
The samples containing the Pl, Bt, and Bt + Pl treatments were diluted 50 × and 100 × . The arcsine-transformed mortality of the Bt + Pl culture treatments were 1.42 ± 0.13 and 0.82 ± 0.07 at 50 × and 100 × dilution, respectively, which were significantly higher than those of the Pl treatments (0.54 ± 0.04 and 0.40 ± 0.03 under 50 × and 100 × dilution, respectively; F2,6 = 143.3 and 602.3, respectively; both p < 0.001; Fig. 2c and 2d), indicating that the addition of Bt might synergistically enhanced the insecticidal activity. However, at both levels of dilution, the Bt treatments resulted in the highest mortality rates, indicating that coculturing exerts an antagonistic effect on the insecticidal activity of Pl.
Insect toxicity bioassays of two bacteria cultured separately then mixed and SDS-PAGE analysis
The Bt was mixed with the Pl, supernatant, and isolated protein separately. In the whole-broth treatment, the combination of the Bt and the Pl supernatant (+ Pls) exhibited the highest insecticidal activity (1.45 ± 0.11), although this value was not significantly different from the insecticidal activity of the Pl whole broth (+ Pl, 1.45 ± 0) or the Pl proteins with molecular masses \(\ge\) 100 kDa (+ Plo, 1.32 ± 0.22, F5,12 = 52.7, p < 0.001, Fig. 3). These three combination treatments may effectively enhance the insecticidal effect of Pl. By contrast, the isolated protein with a molecular mass < 100 kDa (+ Plb, 0.26 ± 0.07) decreased the insecticidal activity of both bacteria. Therefore, the main insecticidal factor of the combination treatment is the protein with a molecular mass \(\ge\) 100 kDa; the isolated proteins with a molecular mass < 100 kDa (mostly proteases) reduced the insecticidal effect of Bt (Fig. 3).
Arcsine-transformed mortality among (a) single bacterium treatments, Photorhabdus luminescens (ATCC 29,999; 120-h incubation, Pl) and Bacillus thuringiensis subsp. aizawai (120-h incubation, Bt); (b) Bt mixed with the Pl whole broth, (120-h incubation, + Pl); the Pl supernatant (120-h incubation, + Pls); the Pl protein with molecular weight \(\ge\) 100 kDa (120-h incubation, + Plo); and the Pl protein with molecular weight < 100 kDa (120-h incubation, + Plb). (c) The extracellular proteins secreted by Pl cultivated in LB broth in different treatment combinations were separated by SDS-PAGE; the markers and lanes 1–6 indicate the results for Pl, Bt, + Pl, + Pls, + Plo, and + Plb, respectively. Different letters indicate significant differences as measured by HSD test with p < 0.05
In the SDS-PAGE analysis, as indicated in Fig. 3c (Bt), the crystal protoxins of Bt were mostly concentrated in the range of 130–140 kDa. When the fermentation broth of Pl was mixed with Bt, the 140-kDa protoxin protein band lightened and disappeared (Fig. 3c, + Pl, + Plo, + Plb), indicating the decomposing effect of the Pl protease on Bt.
Histopathological midgut tissue sectioning of Pl. xylostella
In the nontreatment group (CK, 120 h), the midgut tissue cells, the apical membranes (Ams), and the intestines (lumens) of the healthy Pl. xylostella (Fig. 4a) exhibited high integrity with few vesicles. In the pathological tissue section of the Pl. xylostella treated with the Pl fermentation broth (Fig. 4b), the lumen and Am were severely damaged because the Pl in the insect intestine destroyed the columnar cells and thinned the intestinal wall. In the pathological tissue section of Pl. xylostella orally treated with Bt (Fig. 4c), the tissues were destroyed by diffusion, and many vesicles and cracks appeared from the Am to the basal membrane (Bm). This phenomenon was caused by perforation due to the interaction of the Bt toxin and specific receptors on the intestinal surface.
Histopathological effects of (a) nontreatment on midgut tissue of the third instar of Plutella xylostella larvae; (b) Photorhabdus luminescens (ATCC 29,999; Pl) whole broth (after 120-h incubation), (c) Bacillus thuringiensis subsp. aizawai (Bt, after 120-h incubation); (d) Bt and Pl cocultured (after 120-h incubation); (e) Bt and Pl (after 120-h separate incubation and mixing); (f) Bt and Pl cells (after 120-h separate incubation and mixing); (g) Bt and Pl supernatant (after 120-h separate incubation and mixing); (h) Bt and Pl protein with a molecular weight \(\ge\) 100 kDa, (after 120-h separate incubation and mixing); and (i) Bt and Pl protein with a molecular weight < 100 kDa (after 120-h separate incubation and mixing). Am, apical membrane; Bm, basal membrane; Lu, lumen; V, vacuole formation
In the pathological tissue section of the cocultured Bt and Pl, many vesicles and rifts were visible near the Am, and the columnar cells of the intestinal wall were severely thinned (Fig. 4d). Compared with the pathological tissue treated with the whole-broth Bt and Pl (in which the two types of bacteria were cultured separately then mixed, Fig. 4e), the Am treated with the coculture maintained higher integrity; however, the junction of the Am and Bm decomposed and peeled off (Fig. 4e). The Pl caused degradation and irregular peeling that did not appear in the cocultured tissue sections (Fig. 4d). Both sections exhibited many cracks on the Am, which were caused by the perforation of the Bt toxins. In the pathological tissue section of the oral Bt fermentation broth mixed with Pl cells only (Fig. 4f), the intestinal wall was not markedly thinned, but many vesicle gaps were visible near the intestinal Am.
Figure 4g and 4h displays pathological tissue slices treated with the Bt fermentation broth mixed with the supernatant of Pl and the isolated protein of Pl with a molecular mass \(\ge\) 100 kDa, respectively. The Am was severely damaged, and the cracks on the lumen were clearly visible, which indicates that the mixed treatment was effective in destroying the intestines. By contrast, as presented in Fig. 4i, the pathological tissue section treated with a Bt fermentation broth and Pl isolate protein (with a molecular weight < 100 kDa) exhibited higher Am integrity than did sections of tissues that received other treatments. Some vesicle gaps were visible near the lumen because of the effect of the Bt. The results indicated that photobacterial proteins with molecular masses \(\ge\) 100 kDa exert a dominant effect on intestinal tissue damage.
Time–mortality analysis
Regarding time–mortality analysis, the fermentation broth of Bt mixed with that of Pl (+ Pl, Fig. 5a), the supernatant (+ Pls), and the isolated protein with a molecular mass \(\ge\) 100 kDa (+ Plo, Fig. 5b), caused high mortality of the Pl. xylostella at 18–24 h, which is much earlier than when Bt, Pl, and isolated protein with a molecular weight < 100 kDa (+ Plb) were applied. The insecticidal mortality peaked at 30 h. Thus, the presence of the large Pl proteins can enhance insecticidal activity and accelerate the insecticidal effect on Pl. xylostella. A slight synergistic interaction between Bt and Pl was observed in the 1:1 mixture; by contrast, weak antagonistic interactions were observed in the 4:1 and 1:4 mixtures (Table 1).
Kaplan–Meier estimators and log-rank tests among (a) Photorhabdus luminescens (ATCC 29,999; 120-h incubation, Pl), and Bacillus thuringiensis subsp. aizawai, (120-h incubation, Bt), and Bt mixed with the Pl whole broth (120-h incubation, + Pl); (b) the Pl supernatant (120-h incubation, + Pls); the Pl protein with a molecular weight \(\ge\) 100 kDa (120-h incubation, + Plo); and the Pl protein with a molecular weight < 100 kDa (120-h incubation, + Plb). The shaded area indicates the confidence interval estimated using the Survival and Survminer R packages (R Core Team 2021)
Discussion
To understand the interactions between Bt and Pl and their effects on the mortality of Pl. xylostella, cocultivation and separate cultivation with subsequent mixing were adopted. In the cocultivation mode, the Pl and Bt interfered with each other, and the Pl exerted a strong antagonistic effect on the Bt population. The Bt + Pl treatment exhibited significantly higher mortality than did Pl alone. At 72 h, the Pl. xylostella pathological tissue sections exhibited many vesicles and rifts near the Am (a typical symptom of Bt) and severe thinning of the columnar cells in the intestinal wall (a symptom of Pl). Both pathological symptoms appeared simultaneously, which may suggest a complementary effect (BenFarhat et al. 2013; Jallouli et al. 2018). However, because Pl inhibited the growth of Bt (Fig. 2a), the insecticidal effect of the Bt + Pl treatment was not as strong as that of Bt alone, indicating possible resource or growth competition. Therefore, the coculture treatments were not ideal.
When Bt and Pl were cultivated separately, the mixed fermentation broth exhibited insecticidal activity that was higher than that of either monoculture treatment, with high mortality of Pl. xylostella at 24–30 h. The average lethal time of the mixed broth was earlier than that of either monoculture treatment (Fig. 5), which is consistent with the results of BenFarhat et al. (2013) and Bishop (2014). In the pathological tissue sections of the mixed broth, the junction of the Am and Bm decomposed and peeled off reflecting the destructive synergetic effects of the treatment on the intestines (ffrench-Constant et al. 2007; Jung and Kim 2007; Bishop 2014; Palma et al. 2014; Kenney et al. 2019; Wu et al. 2020).
Analysis of the pathological tissue sections of the samples treated with the Bt + Pl cocultured treatment (Fig. 4d), the Bt fermentation broth mixed with the Pl cells only (Fig. 4f), and the Bt fermentation broth mixed with the Pl isolate protein with a molecular weight < 100 kDa (Fig. 4i), as well as SF analysis, demonstrated the antagonistic effects of the Pl and Pl protease on the Bt cells. The compromised Am integrity of Pl. xylostella, which is a symptom of exposure to Bt, was rarely observed in the treatments containing Pl, indicating that the insecticidal activity of Bt was inhibited by Pl (Estruch et al. 1996; Shrestha et al. 2010).
The protoxin of the Bt cultivated for 120 h was mostly concentrated in the 140 kDa position (Fig. 3c). When the Pl and the Bt were cultured separately and then mixed in equal volume, the protein band at 140 kDa lightened and disappeared, the protein bands at 60–70 kDa became considerably more prominent, and the insecticidal activity against Pl. xylostella significantly increased (Fig. 3b and 3c). Pl protease may therefore play a role in the decomposition of Bt protoxins (Sayed and Behle 2017; Jallouli et al. 2018). The SF of the mixture with the 1:1 Bt:Pl ratio was the highest, whereas those of the mixtures with 4:1 and 1:4 ratios revealed antagonistic effects. Thus, the synergistic interaction of the two bacteria may be related to Pl infection of the hemocoel, which is potentiated by the activity of Bt against the midgut epithelia of Pl. xylostella (Jung and Kim 2006; da Silva et al. 2020). Previously, secondary metabolites, namely oxindole and benzylideneacetone, produced by P. temperata have also been reported as potentiators of Bt toxicity, which suppress the immune responses of their insect hosts (Seo et al. 2012). Wu et al. (2020) reported that a bacterial solution of Pl (50 × dilution) exhibited the highest insecticidal activity among the 5 × , 10 × , 50 × and 100 × dilution treatments; in the current study, combination treatments containing much lower concentrations of bacterial solutions exhibited effects consistent with those of treatments containing Pl (50 ×) or Bt (1000 ×) alone.
To sum up, an appropriate ratio of Bt to Pl or Pl supernatant serves as an optimal candidate for use as an insecticidal agent with a rapid lethal effect by drawing on the synergistic effect of its components. In the future, combination treatments can be employed to substantially increase efficiency and reduce production costs while preventing the ubiquitous resistance of economically important pests to Bt pesticides (Sayed and Behle 2017). If the optimal combination formula is determined, and precise lifecycle assessments are conducted, the use of such treatments will assist in the transition to sustainable agriculture in a low-carbon society.
References
BenFarhat D, Dammak M, Khedher SB, Mahfoudh S, Kammoun S, Tounsi S (2013) Response of larval Ephestia kuehniella (Lepidoptera: Pyralidae) to individual Bacillus thuringiensis kurstaki toxins mixed with Xenorhabdus nematophila. J Invertebr Pathol 114:71–75. https://doi.org/10.1016/j.jip.2013.05.009
Bishop AH (2014) Expression of prtA from Photorhabdus luminescens in Bacillus thuringiensis enhances mortality in lepidopteran larvae by sub-cutaneous but not oral infection. J Invertebr Pathol 121:85–88. https://doi.org/10.1016/j.jip.2014.07.001
Bravo A, Gill SS, Soberón M (2007) Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49:423–435. https://doi.org/10.1016/j.toxicon.2006.11.022
Bravo A, Likitvivatanavong S, Gill SS, Soberón M (2011) Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochem Mol Biol 41:423–431. https://doi.org/10.1016/j.ibmb.2011.02.006
da Silva WJ, Pilz-Júnior HL, Heermann R, da Silva OS (2020) The great potential of entomopathogenic bacteria Xenorhabdus and Photorhabdus for mosquito control: a review. Parasit Vectors 13:376. https://doi.org/10.1186/s13071-020-04236-6
Denolf P, Jansens S, Van Houdt S, Peferoen M, Degheele D, Van Rie J (1993) Biotinylation of Bacillus thuringiensis insecticidal crystal proteins. J Appl Environ Microbiol 59:1821–1827. https://doi.org/10.1128/aem.59.6.1821-1827.1993
Donovan WP, Engleman JT, Donovan JC, Baum JA, Bunkers GJ, Chi DJ, Clinton WP, English L, Heck GR, Ilagan OM, Krasomil-Osterfeld KC, Pitkin JW, Roberts JK, Walters MR (2006) Discovery and characterization of Sip1A: a novel secreted protein from Bacillus thuringiensis with activity against coleopteran larvae. Appl Microbiol Biotechnol 72:713–719. https://doi.org/10.1007/s00253-006-0332-7
Duchaud E, Rusniok C, Frangeul L, Buchrieser C, Givaudan A, Taourit S, Bocs S, Boursaux-Eude C, Chandler M, Charles JF, Dassa E, Derose R, Derzelle S, Freyssinet G, Gaudriault S, Médigue C, Lanois A, Powell K, Siguier P, Vincent R, Wingate V, Zouine M, Glaser P, Boemare N, Danchin A, Kunst F (2003) The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat Biotechnol 21:1307–1313. https://doi.org/10.1038/nbt886
Estruch JJ, Warren GW, Mullins MA, Nye GJ, Craig JA, Koziel MG (1996) Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc Natl Acad Sci USA 93:5389–5394. https://doi.org/10.1073/pnas.93.11.5389
Ffrench-Constant RH, Dowling A, Waterfield NR (2007) Insecticidal toxins from Photorhabdus bacteria. Toxicon 49:436–451. https://doi.org/10.1016/j.toxicon.2006.11.019
Furlong MJ, Wright DJ, Dosdall LM (2013) Diamondback moth ecology and management: problems, progress, and prospects. Annu Rev Entomol 58:517–541. https://doi.org/10.1146/annurev-ento-120811-153605
Gao Y, Oppert B, Lord JC, Liu C, Lei Z (2012) Bacillus thuringiensis Cry3Aa toxin increases the susceptibility of Crioceris quatuordecimpunctata to Beauveria bassiana infection. J Invertebr Pathol 109:260–263. https://doi.org/10.1016/j.jip.2011.12.003
Jallouli W, Boukedi H, Sellami S, Frikha F, Abdelkefi-Mesrati L, Tounsi S (2018) Combinatorial effect of Photorhabdus luminescens TT01 and Bacillus thuringiensis Vip3Aa16 toxin against Agrotis segetum. Toxicon 142:52–57. https://doi.org/10.1016/j.toxicon.2017.12.054
Jung SC, Kim YG (2006) Synergistic effect of entomopathogenic bacteria (Xenorhabdus sp. and Photorhabdus temperata ssp. temperata) on the pathogenicity of Bacillus thuringiensis ssp. aizawai against Spodoptera exigua (Lepidoptera: Noctuidae). Environ Entomol 35:1584–1589. https://doi.org/10.1093/ee/35.6.1584
Jung SC, Kim YG (2007) Potentiating effect of Bacillus thuringiensis subsp. kurstaki on pathogenicity of entomopathogenic bacterium Xenorhabdus nematophila K1 against diamondback moth (Lepidoptera: Plutellidae). J Econ Entomol 100:246–250. https://doi.org/10.1603/0022-0493(2007)100[246:PEOBTS]2.0.CO;2
Kenney E, Hawdon JM, O’Halloran D, Eleftherianos I (2019) Heterorhabditis bacteriophora excreted-secreted products enable infection by Photorhabdus luminescens through suppression of the Imd pathway. Front Immunol 10:2372. https://doi.org/10.3389/fimmu.2019.02372
Li H, Bouwer G (2014) Evaluation of the synergistic activities of Bacillus thuringiensis Cry proteins against Helicoverpa armigera (Lepidoptera: Noctuidae). J Invertebr Pathol 121:7–13. https://doi.org/10.1016/j.jip.2014.06.005
Mantzoukas S, Milonas P, Kontodimas D, Angelopoulos K (2013) Interaction between the entomopathogenic bacterium Bacillus thuringiensis subsp. Kurstaki and two entomopathogenic fungi in bio-control of Sesamia nonagrioides (Lefebvre) (Lepidoptera: Noctuidae). Ann Microbiol 63:1083–1091. https://doi.org/10.1007/s13213-012-0565-x
Mohan M, Sushil SN, Selvakumar G, Bhatt JC, Gujar GT, Gupta HS (2009) Differential toxicity of Bacillus thuringiensis strains and their crystal toxins against high-altitude Himalayan populations of diamondback moth, Plutella xylostella L. Pest Manag Sci 65:27–33. https://doi.org/10.1002/ps.1639
Okech BA, Meleshkevitch EA, Miller MM, Popova LB, Harvey WR, Boudko DY (2008) Synergy and specificity of two Na+- aromatic amino acid symporters in the model alimentary canal of mosquito larvae. J Exp Biol 211:1594–1602. https://doi.org/10.1242/jeb.017244
Palma L, Muñoz D, Berry C, Murillo J, Caballero P (2014) Bacillus thuringiensis toxins: an overview of their biocidal activity. Toxins 6:3296–3325. https://doi.org/10.3390/toxins6123296
Park Y, Kim Y (2000) Eicosanoids rescue Spodoptera exigua infected with Xenorhabdus nematophilus, the symbiotic bacteria to the entomopathogenic nematode Steinernema carpocapsae. J Insect Physiol 46:1469–1476. https://doi.org/10.1016/S0022-1910(00)00071-8
R Core Team 2021. R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria
Sambrook J, Frisch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, second ed. Spring Harbor Laboratory, Cold Spring Harbor, New York
Sayed AMM, Behle RW (2017) Evaluating a dual microbial agent biopesticide with Bacillus thuringiensis var. kurstaki and Beauveria bassiana blastospores. Biocontrol Sci Technol 27:461–474. https://doi.org/10.1080/09583157.2017.1303662
Seo S, Lee S, Hong Y, Kim Y (2012) Phospholipase A2 inhibitors synthesized by two entomopathogenic bacteria, Xenorhabdus nematophila and Photorhabdus temperata subsp. temperata. Appl Environ Microbiol 78:3816–3823. https://doi.org/10.1128/AEM.00301-12
Shrestha S, Kim Y (2008) Eicosanoids mediate prophenoloxidase release from oenocytoids in the beet armyworm Spodoptera exigua. Insect Biochem Mol Biol 38:99–112. https://doi.org/10.1016/j.ibmb.2007.09.013
Shrestha S, Kim Y (2009) Biochemical characteristics of immune-associated phospholipase A2 and its inhibition by an entomopathogenic bacterium, Xenorhabdus nematophila. J Microbiol 47:774–782. https://doi.org/10.1007/s12275-009-0145-3
Shrestha S, Hong YP, Kim Y (2010) Two chemical derivatives of bacterial metabolites suppress cellular immune responses and enhance pathogenicity of Bacillus thuringiensis against the diamondback moth, Plutella xylostella. J Asia-Pac Entomol 13:55–60. https://doi.org/10.1016/j.aspen.2009.11.005
Tabashnik BE (1992) Evaluation of synergism among Bacillus thuringiensis toxins. Appl Environ Microbiol 58:3343–3346. https://doi.org/10.1128/aem.58.10.3343-3346.1992
Thomas GM, Poinar GO (1979) Xenorhabdus gen. nov., a genus of entomopathogenic, nematophilic bacteria of the family Enterobacteriaceae. Int J Syst Evol Microbiol 29:352–360. https://doi.org/10.1099/00207713-29-4-352
Wu LH, Wang YT, Hsieh FC, Hsieh C (2020) Insecticidal activity of Photorhabdus luminescens 0805–P2R against Plutella xylostella. Appl Biochem Biotechnol 191:191–200. https://doi.org/10.1007/s12010-020-03289-8
Zalucki MP, Shabbir A, Silva R, Adamson D, Shu-Sheng L, Furlong MJ (2012) Estimating the economic cost of one of the world’s major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): just how long is a piece of string? J Econ Entomol 105:1115–1129. https://doi.org/10.1603/EC12107
Zhu H, Grewal PS, Reding ME (2011) Development of a dessicated cadaver deliver system to apply entomopathogenic nematodes for control of soil pests. Appl Eng Agric 27:317–324. https://doi.org/10.13031/2013.37065
Acknowledgements
We thank two anonymous reviewers for their constructive comments on the manuscript. This work was supported by the Ministry of Science and Technology, Taiwan through grants (MOST 108-2313-B-020-010-MY3) & (MOST 109-2221-E-017-003-). This manuscript was edited by Wallace Academic Editing.
Funding
This work was funded by the Ministry of Science and Technology, Taiwan through grants (MOST 108–2313-B-020–010-MY3) and (MOST 109–2221-E-017–003-).
Author information
Authors and Affiliations
Contributions
LHW was involved in methodology, validation, formal analysis, investigation, data curation, and writing the original draft. YZC carried out validation and formal analysis. FCH: provided ATCC 29,999, Pl strain, and suggestion for experimental design and data analysis. CTL was responsible for section and analysis, and investigation. CYH took part in conceptualization, methodology, data curation, supervision, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Conflict of Interest
L. H. Wu declares that he has no conflict of interest. Y. Z. Chen declares that she has no conflict of interest. Feng-Chia Hsieh declares that he has no conflict of interest. C. T. Lai declares that he has no conflict of interest. And Chienyan Hsieh declares that he has no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, L.H., Chen, Y.Z., Hsieh, FC. et al. Combined effect of Photorhabdus luminescens and Bacillus thuringiensis subsp. aizawai on Plutella xylostella. Appl Microbiol Biotechnol 106, 2917–2926 (2022). https://doi.org/10.1007/s00253-022-11905-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-11905-2