Abstract
The novel β-agarase gene aga575 from the agarolytic bacterium Aquimarina agarilytica ZC1 is composed of 2142 bp, and the encoded protein Aga575 has the highest amino acid sequence homology of only 65.2% with known agarases. Though carrying a domain of glycoside hydrolase family 42 in the C-terminal, Aga575 should belong to glycoside hydrolase family 50 according to the phylogenetic analysis. Gene aga575 was successfully cloned and overexpressed in Escherichia coli Rosetta (DE3) cells. The recombinant protein had the maximal agarase activity at pH 8.0 and 37 °C. The values Km and Vmax toward agarose were 8.4 mg/mL and 52.2 U/mg, respectively. Aga575 hydrolyzed agarose and neoagarooligosaccharides to yield neoagarobiose as the sole product. The agarose hydrolysis pattern of Aga575 indicated that it was an exo-type β-agarase. Random mutagenesis was carried out to obtain two beneficial mutants M1 (R534G) and M2 (S4R-R424G) with higher activities. The results showed that the agarase activity of mutant M1 and M2 reached 162% and 192% of the wild-type agarase Aga575, respectively. Moreover, the activity of the mixed mutant M1/M2 (S4R-R424G-R534G) increased to 227%.
Key points
• Aga575 is a novel exo-type β-agarase degrading agarose to yield neoagarobiose as the sole product.
• Though owning a domain of glycoside hydrolase family GH42, Aga575 should belong to family GH50.
• The agarase activity of one mutant increased to 227% of the wild-type Aga575.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The seaweed polysaccharides are the main ingredients in cell walls of marine algae, which is crucial to industrial application as well as biological cycling owing to its unique bioactive function (Henshaw et al. 2006). Agar, as an important heterogeneous polysaccharide, is the major cell wall component of marine red algae (Renn 1997) and mainly consists of agarose and agaropectin (Rochas et al. 1986). Agarose is a polymer composed of repeating linear polysaccharide units of 3,6-anhydro-L-galactopyranose- α-1,3-D-galactopyranose (L-AHG-α-1,3-D-Gal) that are linked with β-1,4 glycosidic bonds (Rees 1969). Agarose can be hydrolyzed by either α-agarases (E.C. 3.2.1.158) at the α-1,3 linkage producing agaro-oligosaccharides (AOS) (Rochas et al. 1994) or β-agarases (E.C.3.2.1.81) at the β-1,4 linkage (Morrice et al. 1983) producing neoagarooligosaccharides (NAOS). To date, diverse bioactivities of NAOS have been reported, such as antioxidation activities (Jonnadula et al. 2020; Xu et al. 2018; Zhang et al. 2019), whitening and moisturizing effects on skin (Kim et al. 2017; Kobayashi et al. 1997), prebiotic effects (Zhang et al. 2019, 2020), anti-fatigue effects (Zhang et al. 2017), anti-inflammatory effects (Wang et al. 2017), antitumor activity (Lee et al. 2017), and treatment potential on type II diabetes (Lin et al. 2019). Therefore, NAOS have great potential applications in food, medicine, and cosmetic industries.
Nowadays, most of the reported agarases belong to β-agarases (Jahromi and Barzkar 2018; Kwon et al. 2020). Based on the similarity of amino acid sequence, β-agarases are mainly divided into four glycoside hydrolase (GH) families in the CAZy database including GH16, GH50, GH86, and GH118. Most of the reported β-agarases display endo-lytic activity and hydrolyze agarose to produce mixed NAOS with various degrees of polymerization (DPs). Only a few β-agarases display exo-lytic activity producing single product, especially those exo-type agarases in the family GH50 which degrade agarose to yield neoagarobiose (NA2) as the sole product. Generally, the agarolytic bacteria produce agarases of family GH118 to decompose agarose into NAOS with high DPs and produce GH16 and GH86 agarases to decompose agarose into NAOS with mediate DPs. And NAOS is further decomposed into NA2 by exo-type agarases of family GH50. NA2 is finally degraded by NA2 hydrolase into D-galactose and 3,6-anhydro-L-galactose which will be utilized by the agarolytic bacteria.
The agarolytic bacterium Aquimarina agarilytica ZC1 was isolated from the surface of fresh porphyra (Lin et al. 2012a), and some putative agarase genes had been found in its genome (Lin et al. 2012b). A phylogenetic tree containing all the putative agarases of strain ZC1 and the reported agarases from different GH families was constructed (data not shown). In the phylogenetic tree, the putative agarase Aga575 was grouped with characterized GH50 β-agarases but formed an independent branch. Therefore, it indicated that the putative agarase Aga575 should belong to family GH50 and should be different with the reported GH50 β-agarases. In order to characterize the putative agarase Aga575, gene cloning and expression of Aga575 were performed in this study, and it was finally found that Aga575 was a novel exo-lytic β-agarase of family GH50. Random mutagenesis using error-prone PCR was also performed to obtain mutants with enhanced agarase activity.
Materials and methods
Bacterial strains and chemicals
The agarolytic bacterium Aquimarina agarilytica ZC1 was kept in our laboratory and had been deposited at the NITE Biological Resource Center of Japan (NBRC) under the accession number NBRC 107,695. Escherichia coli DH5α and E. coli Rosetta (DE3) pLyS were used for routine cloning and protein expression, respectively. Unless otherwise stated, all chemicals were of analytical grade or higher.
Sequence analysis
The nucleotide sequence of gene aga575 had been deposited at GenBank with accession number NZ_JH621258. The signal peptide of agarase gene was predicted using the SignalP 5.0 server (Almagro et al. 2019). Homology analysis of amino acid sequence was carried out with the Blastp program (http://blast.ncbi.nlm.nih.gov). Protein domain search was performed using MOTIF (https://www.genome.jp/tools/ motif/) and InterPro (https://www.ebi.ac.uk/interpro/). A phylogenetic neighbor-joining tree to analyze the evolutionary relationship of Aga575 with characterized β-agarases from different glycoside hydrolase (GH) families was constructed using the software MEGA. Multiple sequence alignment for Aga575 and its mutants were performed by using DNAMAN 6.0 software.
Gene expression and purification of recombinant proteins
The agarase gene aga575 without the signal peptide was obtained by PCR from the genomic DNA of Aquimarina agarilytica ZC1 using the specific primers 575F and 575R (Table S1) with restriction enzyme sites of Nde I and Xho I at the N-terminus and C-terminus, respectively. A poly-6-histidine tag was added to the C-terminus of the protein in order to purify the protein by affinity chromatography. The agarase gene was then ligated to the pET-32a ( +) vector to construct the recombinant plasmid which was finally transformed into E. coli Rosetta (DE3) pLyS cells.
The transformed E. coli cells were incubated at 37 °C until the OD600nm of the culture reached to 0.6–0.7 in Luria–Bertani broth with 50 μg/mL of ampicillin. Then the protein expression was induced by 0.1 mM isopropyl-β-D-thiogalactopyranoside at 16 °C for 24 h. After induction, the E. coli cells were collected by centrifugation at 4 °C and then were resuspended in the 20 mM Tris–HCl buffer containing 0.25 mM NaCl (pH 7.8) and disrupted on ice by sonication. The histidine-tagged recombinant proteins were purified by a Ni–NTA His Band resin (Merck) and then analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The protein determination was performed with a BCA protein assay kit (Genstar, Beijing).
Random mutagenesis and site-directed mutagenesis
Error-prone PCR was performed to induce the random mutagenesis of gene aga575. Error-prone PCR was carried out with specific primers (575F and 575R, Table S1) and 0.05 mmol/L of Mn2+ in the reaction mixture. The site-directed mutagenesis PCR was carried out with the PrimeSTAR HS DNA polymerase (TaKARA Co., Ltd) and primers M-575F and M-575R according to the three-step method reported by Wu et al. (2017). The expression of mutant genes in the E. coli cells and purification of recombinant mutant proteins were the same with that of the wild-type agarase.
Degradation of agarose and NAOS
The agarose hydrolysis by agarase Aga575 was performed in 20 mM pH8.0 Tris–HCl buffer at 40 °C containing purified Aga575 and 1% agarose for 15 min, 30 min, 1 h, 4 h, 8 h, and 18 h, respectively. The degradation of NAOS with various degrees of polymerization (NA4, NA6, and NA8) by agarase Aga575 was also carried out at 40 °C for 24 h. All the above reactions were stopped by heating in boiling water for 5 min, and then the reaction mixtures were centrifuged at 12,000 g for 10 min. The supernatant was applied to a Silica Gel 60 thin-layer chromatography (TLC) plate, and the plate was then developed with a solvent mixture composed of n-butanol/acetic acid/H2O (2:1:1, by volume). The oligosaccharides on the plate were visualized by spraying with 10% (v/v) H2SO4 and followed by heating at 85 °C. For the carbon-13 nuclear magnetic resonance (13C NMR) spectrum analysis, the agarose degradation products were lyophilized (dissolved in D2O, Sigma, USA) recorded on AVANCE 400 MHz (Brucker Biospin, Swiss).
Enzymatic activity assay
Unless otherwise stated, the activity of agarase was measured by the DNS (3,5-dinitrosalicylic acid) method following the description by Lin et al. with some revisions (2017). Two hundred microliters of enzyme solution was mixed with 800 μL Tris–HCl buffer (20 mM, pH8.0) including 0.3% agarose. After incubation at 40 °C for 20 min, the mixture was heated in boiling water for 10 min to stop the reaction. Then 1 mL DNS reagent solution was added, and the mixture was incubated in boiling water for 15 min to develop the color. The absorbance at 540 nm was recorded subsequently. The amounts of reducing sugars generated were determined using D-galactose as a standard. One unit of the enzyme activity was defined as the amount of enzyme required to produce 1 μmol reducing sugar per minute. The Km and Vmax values were calculated using the Lineweaver–Burk equation and low-melting point agarose ranging from 1 to 5 mg/mL in 20 mM Tris–HCl buffer (pH 7.0) at 37 °C.
The effects of pH, temperature, and chemical on agarase activity
The effect of pH on the agarase activity of Aga575 was measured at 37 °C in various buffers: pH 4.0–6.0 (50 mM sodium citrate-citric acid), pH 7.0–8.0 (50 mM Tris–HCl), pH 9.0–10.0 (50 mM Tris–glycine), and pH 11.0 (50 mM Na2HPO4-NaOH). The pH stability was assessed by pre-incubating the enzyme solution in various pH buffers ranging from 4.0 to 11.0 at 4 °C for 12 h. The effect of temperature on the agarase activity was determined at pH 8.0 from 25 to 65 °C. In the thermal stability test, agarase Aga575 was pre-incubated in a temperature range of 25–65 °C at pH 8.0 for 1 h, and the residual enzyme activity was measured subsequently.
The effects of metal ions, chelators, denaturants, and reducing reagents on agarase activity were studied. Each chemical with two final concentrations of 10 mM and 50 mM was added to the reaction mixture, respectively. The relative activity was defined as 100% with respect to that measured under the standard assay conditions without adding any reagent.
Results
Sequence analysis of agarase Aga575
Agarase Aga575 was encoded by the gene aga575 of 2142 bp. There was a signal peptide of 22 amino acids at the N-terminus of Aga575 predicted by the SignalP Server. The MOTIF search showed that Aga575 had an agarase CBM-like domain at the N-terminus (aa 47–124) and a GH42 family domain in the C-terminus (aa 439–598). The Blastp search analysis revealed that Aga575 had the highest amino acid identity of 65.2% with the putative β-agarase (GenBank accession no. WP_149627357) from strain Aquimarina sp. RZ0 and 60.7% with the putative β-agarase (QCX40303) from Flavobacteriaceae bacterium 10Alg115. Based on the phylogenetic tree containing characterized enzymes from different GH families (Fig. 1), the two previously reported exo-type GH42 β-agarases and Aga575 were grouped with characterized GH50 β-agarases, but not with the typical GH42 family enzymes. It indicated that β-agarases carrying the GH42 family domain might be actually close to β-agarases of family GH50. Besides, Aga575 formed an independent branch on the phylogenetic tree (Fig. 1) with the GH50 exo-type agarase AgaO of Flammeovirga sp. MY04 (AME16507) with which Aga575 had an amino acid identity of only 51%. All the generated data suggest that Aga575 belongs to family GH50 and should be different with other GH50 agarases.
Phylogenetic tree analysis of Aga575 with other reported agarases and beta-galactosidases. All the amino acid sequences were obtained from the database of CAZy and NCBI. The accession numbers and GH family names were shown on the tree. The bootstrap value was set as 1000. Bar, 0.2 substitutions per nucleotide position
Expression and purification of recombinant Aga575
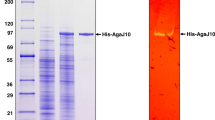
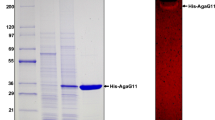
The recombinant Aga575 was overexpressed in the E. coli Rosetta (DE3) cells. After IPTG induction at the temperature of 16 °C, agarase activity of the recombinant protein was detected in the supernatant of the cell lysates. The recombinant protein was present mostly in a soluble form instead of inclusion bodies. The recombinant protein was successfully purified by Ni–NTA affinity chromatography. The purified recombinant Aga575 formed a single band in the SDS-PAGE, and the observed molecular weight of the recombinant protein was estimated to 79.8 kDa including the 6-histidine tag, which was close to the theoretical molecular mass of 79.6 kDa (Figure S1).
Analysis of enzymatic products
The enzymatic hydrolysis products of agarase Aga575 were analyzed by TLC. As shown in Fig. 2A, there was no reaction intermediate in the time course agarose degradation of agarase Aga575; the sole product was NA2. Besides, agarase Aga575 also hydrolyzed NAOS with various degrees of polymerization (NA4, NA6, and NA8) to yield NA2 as the sole product (Fig. 2B). The end products of time course agarose degradation were further identified by 13C NMR spectrum (Fig. 3). There was no resonance observed at around 90.7 ppm which is the typical signal of AOS produced by agarose degradation of α-agarases. However, there were obvious resonance signals around 92.2 and 96.2 ppm that were characteristics of the reducing end of NA2. And the signal corresponding to the non-reducing 3,6-anhydro-L-galactopyranose was detected at 97.75 ppm. These results demonstrated that Aga575 was an exo-lytic β-agarase which can hydrolyze agarose into NA2.
TLC analysis on the degradation of agarose and NAOS by agarase Aga575. A Time course degradation of agarose. Time intervals were as follows: lane 1, 15 min; lane 2, 30 min; lane 3, 1 h; lane 4, 4 h; lane 5, 8 h; lane 6, 18 h; lane 7, NA2. B Degradation of NAOS. Lane 11, NA2; lane 12, NA4; lane 13, NA4 + Aga575; lane 14, NA6; lane 15, NA6 + Aga575; lane 16, NA8; lane 17, NA8 + Aga575
Enzymatic properties of recombinant Aga575
The pH and temperature effects on the agarase activity of Aga575 were studied (Fig. 4). The enzyme displayed the optimal activity at pH 8.0, and the activities at pH 6.0–10.0 were over 65% of the optimal activity. It retained more than 75% of the maximal activity after being incubated at a pH range of 5.0–10.0 for 12 h (Fig. 4A). Aga575 displayed the optimal agarase activity at 37 °C. It was stable and retained 95% of the maximum activity at temperatures below 37 °C for 1 h. And it still kept 70% of the maximal activity at 40 °C. However, its agarase activity declined dramatically over 40 °C (Fig. 4B). The Km and Vmax values of Aga575 were 8.4 mg/mL and 52.2 U/mg based on the Lineweaver–Burk plot, respectively. The effects of various chemicals on the agarase activity of Aga575 were carried out at two concentrations (10 and 50 mM) under the standard assay conditions. As shown in Table 1, the agarase activity of Aga575 was significantly enhanced by Ca2+. Especially, 50 mM Ca2+ activated the enzyme activity 2.35 times. However, the activity was inhibited by Mg2+, Al3+, Fe3+, EDTA, and SDS.
Characterization of recombinant Aga575 mutants
Two mutants (designated M1 and M2) of Aga575 with higher agarase activity were obtained by random mutagenesis. The expression analysis and purification of recombinant mutant proteins were shown in Figure S2. The sequences of mutants were determined. The amino acid sequences of wild-type Aga575 and its mutants were shown in Fig. 5. The results showed that compared with the wild-type Aga575, the agarase activity of M1 (R534G) and M2 (S4R-R424G) reached 162% and 192%, respectively (Table 2). Besides, a mixed mutant designated as M1/M2 (S4R-R424G-R534G) was constructed by site-directed mutagenesis and its agarase activity was as 227% as that of the wild-type agarase Aga575 (Table 2).
Discussion
It is generally well known that β-agarases are divided into four glycoside hydrolase families including GH16, GH50, GH86, and GH118. In recent years, to our knowledge, three β-agarases carrying other GH family domains were reported, including the exo-type GH42 β-agarase Aga1161 from Pseudoalteromonas sp. NJ21 (Li and Sha 2015), the exo-type GH42 β-agarase AgaJ10 (Choi et al. 2019), and the GH39 β-agarase AgaJ9 from Gayadomonas joobiniege G7 (Jung et al. 2017). In this study, the MOTIF search showed that Aga575 also had a GH42 family domain in the C-terminal. However, on the phylogenetic tree (Fig. 1), the two previously reported exo-type GH42 β-agarases and Aga575 were grouped with characterized GH50 β-agarases, but not with the typical GH42 family enzymes. It indicated that β-agarases carrying the GH42 family domain might be actually close to β-agarases of family GH50. According to phylogenetic analysis, agarase Aga575 in this study should belong to family GH50.
The characteristics of β-agarases from different GH families are varied (Park et al. 2020; Veerakumar and Manian 2018). However, β-agarases from the same GH family seem to own some similar characteristics, such as the molecular mass and the agarose degradation products. The agarases of family GH16 and GH118 generally have smaller molecular masses compared with other GH family agarases. That is mostly less than 60 kDa. In contrast, the GH50 and GH86 agarases used to own larger molecular weights. Especially, the endo-type GH50 agarases and some GH86 agarases usually have molecular weights over 100 kDa, while the exo-type GH50 agarases mostly have molecular masses from 80 to 90 kDa. As for the agarose degradation products, exo-type GH50 agarases degrade agarose to yield NA2 as the sole product, while endo-type GH50 agarases usually decompose agarose into NA2 and NA4. And the agarose degradation products of GH16 and GH86 agarases are mainly NA4 and NA6. The GH118 agarases usually degrade agarose to produce larger NA (NA8, NA10, and NA12). In this study, the molecular weight of agarase Aga575 is about 80 kDa, and it degrades agarose to yield NA2 as the sole product. In this sense, agarase Aga575 displays characteristics of exo-type GH50 agarases. Based on the time course degradation of agarose and the degradation of NAOS, agarase Aga575 should be an exo-type GH50 agarase.
In general, many GH16 agarases have Ca2+ ion-binding sites; their agarase activities can be stimulated by CaCl2 (Veerakumar and Manian 2018). Among the several reported exo-type GH50 agarases, only Aga50D from Saccharophagus degradans 2–40 can be enhanced by 100 mM CaCl2 (Kim et al. 2010). In this study, Ca2+ could significantly enhance the enzymatic activity of the exo-type GH50 agarase Aga575 both at 10 mM and 50 mM, especially 50 mM Ca2+ activated the enzyme activity 2.35 times. However, the activity of GH50 exo-type agarase AgaO which formed an independent branch with Aga575 in the phylogenetic tree could be inhibited by Ca2+ (Han et al. 2016). This indicates that Aga575 is different from those several reported exo-type GH50 agarases.
Random mutagenesis is commonly employed to improve the thermal stability, catalytic activity, and substrate specificity of enzymes (Baek et al. 2017; Liu et al. 2017; Niu et al. 2006). In fact, the binding stability between substrate and enzyme is supported by a panel of hydrophobic residues located nearby the catalytic site (Lammens et al. 2009). The enhancement in the catalytic activities of mutants might derive from the substitution of Arg by Gly, which can develop a significant effect on hydrophobic interactions due to the property of aliphatic amino acid (Yennamalli et al. 2011).
NA2 has been reported to have a better moisturizing effect than the typical moisturizing reagents glycerol and hyaluronic acid (Kobayashi et al. 1997). NA2 also exhibited skin-whitening activity in B16 murine melanoma cells and showed low cytotoxicity (Kobayashi et al. 1997; Lee et al. 2008; Yun et al. 2013). Especially, NA2 exhibited significantly lower melanin production at the concentration of 100 μg/mL compared to arbutin, a well-known skin-whitening agent (Yun et al. 2013). These findings indicate that NA2 could be used as moisturizing and whitening component in cosmetics. Besides, because of owning other diverse bioactivities which have been mentioned in the introduction section, NA2 could also have great potential applications in drug and food industries. As an exo-type β-agarase, Aga575 degrades agarose to yield NA2 as the sole product. Single product means easier purification procedures than that with multi-products. In this sense, agarase Aga575 has an advantage in producing NA2.
In summary, the novel β-agarase Aga575 encoded by 2142 bp was identified in the agarolytic bacterium Aquimarina agarilytica ZC1. Aga575 hydrolyzed agarose and neoagarooligosaccharides to yield neoagarobiose as the sole product indicating that it was an exo-type β-agarase. Random mutagenesis was carried out to obtain two beneficial mutants M1 (R534G) and M2 (S4R-R424G), the agarase activity of which increased to 162% and 192% of the wild-type agarase Aga575, respectively. Moreover, the activity of the mixed mutant M1/M2 (S4R-R424G-R534G) reached 227%. Though carrying a domain of glycoside hydrolase family 42, Aga575 together with some reported agarases of glycoside hydrolase family 42 should belong to glycoside hydrolase family 50 according to the phylogenetic analysis.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Almagro Armenteros JJ, Tsirigos KD, Sønderby CK, Petersen TN, Winther O, Brunak S, von Heijne G, Nielsen H (2019) SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat Biotechnol 37(4):420–423. https://doi.org/10.1038/s41587-019-0036-z
Baek SC, Ho TH, Lee HW, Jung WK, Gang HS, Kang LW, Kim H (2017) Improvement of enzyme activity of β-1, 3–1, 4-glucanase from Paenibacillus sp. X4 by error-prone PCR and structural insights of mutated residues. Appl Microbiol Biotechnol 101(10):4073–4083. https://doi.org/10.1007/s00253-017-8145-4
Choi U, Jung S, Hong SK, Lee CR (2019) Characterization of a novel neoagarobiose-producing GH42 β-agarase, AgaJ10, from Gayadomonas joobiniege G7. Appl Biochem Biotechnol 189(1):1–12. https://doi.org/10.1007/s12010-019-02992-5
Han W, Cheng Y, Wang D, Wang S, Liu H, Gu J, Wu Z, Li F (2016) Biochemical characteristics and substrate degradation pattern of a novel exo-type β-agarase from the polysaccharide-degrading marine bacterium Flammeovirga sp. Strain MY04. Appl Environ Microbiol 82(16):4944–4954. https://doi.org/10.1128/aem.00393-16
Henshaw J, Horne-Bitschy A, van Bueren AL, Money VA, Bolam DN, Czjzek M, Ekborg NA, Weiner RM, Hutcheson SW, Davies GJ, Boraston AB, Gilbert HJ (2006) Family 6 carbohydrate binding modules in β-agarases display exquisite selectivity for the non-reducing termini of agarose chains. J Biol Chem 281(25):17099–17107. https://doi.org/10.1074/jbc.M600702200
Jahromi ST, Barzkar N (2018) Future direction in marine bacterial agarases for industrial applications. Appl Microbiol Biotechnol 102(106):6847–6863. https://doi.org/10.1007/s00253-018-9156-5
Jonnadula R, Imran M, Vashist P, Ghadi SC (2020) Production of agar-derived antioxidants and single cell detritus from Gracilaria corticata using agarase from Microbulbifer sp. CMC-5. Proc Natl Acad Sci India Sect B Biol Sci 90:73–78. https://doi.org/10.1007/s40011-019-01082-0
Jung S, Lee CR, Chi WJ, Bae CH, Hong SK (2017) Biochemical characterization of a novel cold-adapted GH39 β-agarase, AgaJ9, from an agar-degrading marine bacterium Gayadomonas joobiniege G7. Appl Microbiol Biotechnol 101(5):1965–1974. https://doi.org/10.1007/s00253-016-7951-4
Kim HT, Lee S, Lee D, Kim HS, Bang WG, Kim KH, Choi IG (2010) Overexpression and molecular characterization of Aga50D from Saccharophagus degradans 2–40: an exo-type β-agarase producing neoagarobiose. Appl Microbiol Biotechnol 86(1):227–234. https://doi.org/10.1007/s00253-009-2256-5
Kim JH, Yun EJ, Yu S, Kim KH, Kang NJ (2017) Different levels of skin whitening activity among 3,6-Anhydro-l-galactose, agarooligosaccharides, and neoagaro- oligosaccharides. Mar Drugs 15(10):321. https://doi.org/10.3390/md15100321
Kobayashi R, Takisada M, Suzuki T, Kirimura K, Usami S (1997) Neoagarobiose as a novel moisturizer with whitening effect. Biosci Biotechnol Biochem 61(1):162–163. https://doi.org/10.1271/bbb.61.162
Kwon M, Jang WY, Kim GM, Kim YH (2020) Characterization and application of a recombinant exolytic GH50A β-agarase from Cellvibrio sp. KY-GH-1 for enzymatic production of neoagarobiose from agarose. ACS Omega 5(45):29453–29464. https://doi.org/10.1021/acsomega.0c04390
Lammens W, Le Roy K, Schroeven L, Van Laere A, Rabijns A, Van den Ende W (2009) Structural insights into glycoside hydrolase family 32 and 68 enzymes: functional implications. J Exp Bot 60(3):727–740. https://doi.org/10.1093/jxb/ern333
Lee DG, Jang MK, Lee OH, Kim NY, Ju SA, Lee SH (2008) Over-production of a glycoside hydrolase family 50 β-agarase from Agarivorans sp. JA-1 in Bacillus subtilis and the whitening effect of its product. Biotechnol Lett 30(5):911–918. https://doi.org/10.1007/s10529-008-9634-4
Lee MH, Jang JH, Yoon GY, Lee SJ, Lee MG, Kang TH, Han HD, Kim HS, Choi WS, Park WS, Park YM, Jung ID (2017) Neoagarohexaose-mediated activation of dendritic cells via Toll-like receptor 4 leads to stimulation of natural killer cells and enhancement of antitumor immunity. BMB Rep 50(5):263–268. https://doi.org/10.5483/bmbrep.2017.50.5.014
Li J, Sha Y (2015) Expression and enzymatic characterization of a cold-adapted β-agarase from Antarctic bacterium Pseudoalteromonas sp. NJ21. Chin J Oceanol Limnol 33:319–327. https://doi.org/10.1007/s00343-015-4072-3
Lin B, Lu G, Zheng Y, Xie W, Li S, Hu Z (2012a) Aquimarina agarilytica sp. nov., an agarolytic species isolated from a red alga. Int J Syst Evol Micr 62:869–873. https://doi.org/10.1099/ijs.0.027136-0
Lin B, Lu G, Li S, Hu Z, Chen H (2012b) Draft genome sequence of the novel agarolytic bacterium Aquimarina agarilytica ZC1. J Bacteriol 194(10):2769. https://doi.org/10.1128/JB.00311-12
Lin B, Liu Y, Lu G, Zhao M, Hu Z (2017) An agarase of glycoside hydrolase family 16 from marine bacterium Aquimarina agarilytica ZC1. FEMS Microbiol Lett 364(4):fnx012. https://doi.org/10.1093/femsle/fnx012
Lin F, Yang D, Huang Y, Zhao Y, Ye J, Xiao M (2019) The potential of neoagaro-oligosaccharides as a treatment of type II diabetes in mice. Mar Drugs 17(10):541. https://doi.org/10.3390/md17100541
Liu X, Liang M, Liu Y, Fan X (2017) Directed evolution and secretory expression of a pyrethroid-hydrolyzing esterase with enhanced catalytic activity and thermostability. Microb Cell Fact 16(1):81. https://doi.org/10.1186/s12934-017-0698-5
Morrice LM, McLean MW, Williamson FB, Long WF (1983) β-Agarases I and II from Pseudomonas atlantica purifications and some properties. Eur J Biochem 135(3):553–558. https://doi.org/10.1111/j.1432-1033.1983.tb07688.x
Niu WN, Li ZP, Zhang DW, Yu MR, Tan TW (2006) Improved thermostability and the optimum temperature of Rhizopus arrhizus lipase by directed evolution. J Mol Catal B Enzym 43:33–39. https://doi.org/10.1016/j.molcatb.2006.04.013
Park SH, Lee CR, Hong SK (2020) Implications of agar and agarase in industrial applications of sustainable marine biomass. Appl Microbiol Biotechnol 104(7):2815–2832. https://doi.org/10.1007/s00253-020-10412-6
Rees DA (1969) Structure, conformation, and mechanism in the formation of polysaccharide gels and networks. Adv Carbohydr Chem Biochem 24:267–332. https://doi.org/10.1016/S0065-2318(08)60352-2
Renn D (1997) Biotechnology and the red seaweed polysaccharide industry: status, needs and prospects. Trends in Biotech 15(1):9–14. https://doi.org/10.1016/S0167-7799(96)10069-X
Rochas C, Lahaye M, Yaphe W, Viet MTP (1986) 13C-N.M.R.-spectroscopic investigation of agarose oligomers. Carbohyd Res 148(2):199–207. https://doi.org/10.1016/S0008-6215(00)90388-4
Rochas C, Potin P, Kloareg B (1994) NMR spectroscopic investigation of agarose oligomers produced by an α-agarase. Carbohyd Res 253:69–77. https://doi.org/10.1016/0008-6215(94)80056-1
Veerakumar S, Manian RP (2018) Recombinant β-agarases: insights into molecular, biochemical, and physiochemical characteristics. 3 Biotech 8(10):445. https://doi.org/10.1007/s13205-018-1470-1
Wang W, Liu P, Hao C, Wu L, Wan W, Mao X (2017) Neoagaro-oligosaccharide monomers inhibit inflammation in LPS-stimulated macrophages through suppression of MAPK and NF-κB pathways. Sci Rep 7:44252. https://doi.org/10.1038/srep44252
Wu YR, Zhou ZR, Zhao M, Lin B, Zhong M, Hu Z (2017) Molecular characterization of the thermostability and carbohydrate-binding module from a newly identified GH118 family agarase, AgaXa. Process Biochem 52:192–199. https://doi.org/10.1016/j.procbio.2016.10.021
Xu SY, Kan J, Hu Z, Liu Y, Du H, Pang GC, Cheong KL (2018) Quantification of neoagaro-oligosaccharide production through enzymatic hydrolysis and its anti-oxidant activities. Molecules 23:E1354. https://doi.org/10.3390/molecules23061354
Yennamalli RM, Rader AJ, Wolt JD, Sen TZ (2011) Thermostability in endoglucanases is fold-specific. BMC Struct Biol 11:10. https://doi.org/10.1186/1472-6807-11-10
Yun EJ, Lee S, Kim JH, Kim BB, Kim HT, Lee SH, Pelton JG, Kang NJ, Choi IG, Kim KH (2013) Enzymatic production of 3,6-anhydro-L-galactose from agarose and its purification and in vitro skin whitening and anti-inflammatory activities. Appl Microbiol Biotechnol 97(7):2961–2970. https://doi.org/10.1007/s00253-012-4184-z
Zhang N, Mao X, Li RW, Hou E, Wang Y, Xue C, Tang Q (2017) Neoagarotetraose protects mice against intense exercise-induced fatigue damage by modulating gut microbial composition and function. Mol Nutr Food Res 61(8):1600585. https://doi.org/10.1002/mnfr.201600585
Zhang YH, Song XN, Lin Y, Xiao Q, Du XP, Chen YH, Xiao AF (2019) Antioxidant capacity and prebiotic effects of Gracilaria neoagaro oligosaccharides prepared by agarase hydrolysis. Int J Biol Macromol 137:177–186. https://doi.org/10.1016/j.ijbiomac.2019.06.207
Zhang X, Aweya JJ, Huang ZX, Kang ZY, Bai ZH, Li KH, He XT, Liu Y, Chen XQ, Cheong KL (2020) In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohyd Polym 234:115894. https://doi.org/10.1016/j.carbpol.2020.115894
Funding
This work was supported by the Major Project of Talent Team introduction for Guangdong Provincial Laboratory of Southern Marine Science and Engineering (Guangzhou) (GML2019ZD0606), Key Project of Higher Education of Guangdong Province (2016KZDXM011), Funds for PHD researchers of Guangdong Medical University in 2017 (2XB17026), and the Talents Recruitment Grant of Yangfan Plan of Guangdong Province (4YF16003G).
Author information
Authors and Affiliations
Contributions
CD, BL, and YS performed experiments and wrote the manuscript. ZH and BL designed, supervised, and coordinated the research. TP, JL, and MZ contributed to data analysis and manuscript editing. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
All authors consent to participate.
Consent for publication
All authors consent for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dong, C., Lin, B., Song, Y. et al. Characterization and activity enhancement of a novel exo-type agarase Aga575 from Aquimarina agarilytica ZC1. Appl Microbiol Biotechnol 105, 8287–8296 (2021). https://doi.org/10.1007/s00253-021-11553-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11553-y