Abstract
Salmonella Enteritidis (SE) causes both horizontal and vertical transmission of diseases in poultry industry and is also one of the main causes of human food poisoning. Sequence analysis of the sef operon of poultry-derived Salmonella serotypes showed the presence of an entire sef operon in SE, whereas only sef pseudogenes were found in Salmonella Gallinarum and Salmonella Pullorum. Subsequently, the sef operon of SE was cloned into the pBR322 plasmid and expressed in a modified Escherichia coli strain SE5000. sef operon expression was demonstrated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, western blot, agglutination assay, and transmission electron microscopy. The results showed that SE5000+Sef, but not SE5000+pBR322, could specifically react with SE-positive chicken serum in an agglutination assay, which could be clearly visualized by the naked eye within less than 2 min. In contrast, SE5000+Sef could not be recognized in Salmonella Gallinarum– and Salmonella Pullorum–positive chicken sera. Next, taking advantage of the exclusive presence of an entire sef operon in SE, we set up an agglutination-based detection system to monitor the dynamics of Sef-targeted antibody from SE-infected chicks for 47 days. Using the proposed detection method, SE was readily detectable starting from 2 weeks post-infection. Finally, we compared the proposed SE5000+Sef-based detection system with commercially available agglutination antigen using the classical bacterial isolation and identification procedure as reference. The results showed that the SE5000+Sef system was more consistent with the results of bacterial isolation and identification with almost 100% accuracy. We established a simple, sensitive, and cheap agglutination method for rapid and specific detection of SE-infected chickens, which can facilitate epidemiological investigation and eradication of SE infections.
Key points
• Only the Salmonella Enteritidis serotype expressed Sef fimbriae in chicken infected with SE.
• A rapid, large-scale method of detection by the naked eye of detection of SE-infected chicken is presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salmonella Enteritidis (SE) is a pathogen of a broad range of host organisms; it is also the main causative agent of human food poisoning and can lead to nontyphoid gastroenteritis in humans, livestock, and poultry (Gal-Mor et al. 2014). Every year, about 93.8 million people in the world are infected with Salmonella, and around 155,000 die of the Salmonella infection (Majowicz et al. 2010). In China, gastroenteritis caused by Salmonella accounts for about 40% of bacterial food poisoning (Song et al. 2016), ranking first among bacterial food poisoning. SE is the main pathogen of Salmonella food poisoning. Livestock and poultry products are the main infection sources of SE, and eggs are the primary causes of human Salmonella infections (Gantois et al. 2009). In case of egg contamination with SE, the entire production must be discarded. For example, in 2010, 500 million “problem eggs” were recalled because of an SE outbreak in the USA (Kuehn 2010). Moreover, SE is not only the cause of human food poisoning but also the cause of great losses in livestock and poultry breeding. Therefore, diagnostic and detection methods are urgently needed as a primary step to eradicate Salmonella infections, especially considering that the current methods lack sensitivity and efficiency.
At present, the many types of detection methods of Salmonella include traditional biological and biochemical identification, immunological diagnosis, and molecular biological identification (Eriksson and Aspan 2007). Conventional microbiological methods include enrichment culture, preliminary culture, isolation from pure culture, and determination with a series of biochemical reactions, serological grouping, and bacterial types (Miao et al. 2017). Molecular biology protocols also have been performed for Salmonella detection; these involve nucleic acid probe detection technology (Machado et al. 2019), polymerase chain reaction (PCR) technology (Yang et al. 2020), and gene chip (Ricke et al. 2013). Other immunological diagnostic methods of Salmonella include enzyme-linked immunosorbent assay (ELISA) (Oliveira et al. 2006), Dot-ELISA (Roychoudhury et al. 2009), immunofluorescence (Stoica et al. 2018), and immunomagnetic separation (Lynch et al. 2004). These methods are limited, however, by their time-consuming and laborious nature (Li et al. 2020). Thus, optimized methods for pathogenic Salmonella detection are urgently needed.
Since the 1930s, scientists have developed a series of serological methods for detection of SE-infected chickens; these techniques include the serum tube agglutination test (STAT) (Gast 1997), latex agglutination test (LAT) (Margot et al. 2013), and agglutination test with staining antigen for whole blood tests (Yang et al. 2019). Among them, the slide agglutination test with staining antigen has the advantages of simplicity and a time-saving but moderate level of specificity. Currently, commercial agglutination antigen targeted for somatic antigen consists of the whole bacterial cell, which can be the source of nonspecific cross-reactions with many blood or serum components (Yan et al. 2011); consequently, it can be the origin of many false-positive and false-negative reactions with poor reproducibility (Fasano et al. 2017). Therefore, this method lacks both specificity and sensitivity in detection of SE-infected chickens.
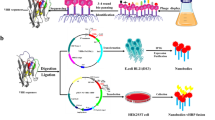
Sef fimbriae are a type of fimbriae that are encoded by a sefABCD operon. They are an important virulence factor involved in the initial attachment, which is an important step in the SE infection process (Zhu et al. 2009; Yue et al. 2012). Turcotte and Woodward (1993) reported that the sefA gene, which encodes the major subunit of Sef fimbriae, is widely conserved within Salmonella serotype D; the authors developed latex particles coated with anti-SefA monoclonal antibodies as a diagnostic system to successfully detect SE and Salmonella Dublin, but not Salmonella Gallinarum and Salmonella Pullorum, which are lacking a functional Sef. Our study found that only SE had a complete sef operon among the Salmonella serotypes isolated from infected chickens. In Salmonella Gallinarum and Salmonella Pullorum, the sef operon is only represented by pseudogenes and it is completely missing in Salmonella Typhimurium. With the exception of SE, none of the Salmonella serotypes express Sef fimbriae in vivo and therefore do not induce the production of antibodies against Sef in infected chickens. On the basis of this genetic divergence between SE and other Salmonella serogroups, this study was undertaken to describe a novel agglutination method using a recombinant Escherichia coli SE5000+Sef strain (i.e., the SE5000 expressing Sef fimbriae in the optimal condition) for the detection of SE in chickens. The proposed method combines all requested qualities, such as specificity, sensitivity, rapidity, and low cost, and can be generalized as a method of choice for future SE detection in poultry farming.
Materials and methods
Bacterial strains and plasmids
The modified E. coli SE5000 strain (Schifferli et al. 1991) obtained from Dr. Dieter Schifferli (University of Pennsylvania School of Veterinary Medicine, Philadelphia, PA, USA) was stored in our laboratory (Xia et al. 2015). The standard strain C7920, used as an SE cloning strain, was purchased from China Institute of Veterinary Drug Control (Beijing, China) (Liquan Huang 2006). Salmonella Gallinarum strains, including vaccine SG9R (purchased from China Institute of Veterinary Drug Control, Beijing, China) and isolate SG01 (isolated from our laboratory, and kept in China Institute of Veterinary Drug Control, Beijing, China), and Salmonella Pullorum strains, including reference CVCC523 (purchased from China Institute of Veterinary Drug Control, Beijing, China) and isolate S08 (isolated from our laboratory, and kept in China Institute of Veterinary Drug Control, Beijing, China), were stored in our laboratory. Plasmid pBR322 purchased from New England Biolabs (NEB; Beverly, MA, USA) was used as the expression vector. All bacterial strains were grown in Luria-Bertani (LB) broth at 37 °C.
Bioinformatics analysis
We performed multiple sequence alignments using sef operon sequences retrieved from the National Center for Biotechnology Information (NCBI; Bethesda, MD, USA) for different Salmonella Pullorum, Salmonella Gallinarum, and SE strains. The sef operon nucleotide sequence from SE strain SE95 (CP050716) was selected as a reference sequence. We used the NCBI Nucleotide BLAST tool to look for the homologous sef operon in Salmonella Pullorum, Salmonella Gallinarum, and SE. The presence of the sef operon was verified in Salmonella Pullorum/Gallinarum strains, including Salmonella Gallinarum strains (CP019035 and AM933173) and Salmonella Pullorum strains (CP003786, CP003047, CP006575, LK931482, CP022963, and CP012347). The sequence data were analyzed and aligned by the ClustalW method using the MEGA 7 software (version 7.0.26) and Jalview (version 2.11.0) (Waterhouse et al. 2009).
PCR, DNA cloning, and construction of expression vector
The sef operon was amplified from the genome of SE standard strain C7920 (NCBI: MW594401) using the upstream primer sef-up: 5′- G GAT CC AAA ATg gCg TgA gTA TAT TAg CAT CCg CA -3′ and the downstream primer sef-lo: 5′- G TCG ACTT ATT ATA ATT CAA TTT CTG TCG CAT AT -3′. The underlined sequences represent BamHI and SalI restriction enzyme sites, respectively. PCR was performed using Phanta Max Super-Fidelity DNA Polymerase (P505-d1/d2/d3, Vazyme Nanjing, China) (Ahmed et al. 2021). The PCR product of the sef operon and the pBR322 vector were digested by BamHI and SalI (NEB, Ipswich, MA). The linear DNA fragments were purified and ligated using T4 DNA ligase (NEB), and the recombinant plasmid named pBR-sef was confirmed by BamHI and SalI restriction enzyme/electrophoresis and DNA sequencing.
Extraction of Sef fimbriae
The pBR-sef plasmid was electroporated into the strain SE5000; the generated strain SE5000+Sef was cultured at 37 °C for 12 h. Fimbrial protein was prepared by the hot extraction method with some changes (Khan and Schifferli 1994); in brief, strains SG9R, SG01, CVCC523, S08, and SE5000 with pBR-sef cultures were centrifuged at 4 °C and 4500 rpm for 10 min, and then the sediment was washed twice with sterile phosphate-buffered saline (PBS). Then, 75 mM NaCl-0.5 mM Tris-HCl (pH 7.4) solution was added, and the mixture was incubated in a water bath at 62 °C. After 30 min, the sample was centrifuged for 20 min at 8000 rpm, and 20% saturated ammonium sulfate was added to the supernatant. After overnight precipitation, the supernatant was discarded by 14,000-rpm centrifugation for 30 min, and precipitated fimbriae were resuspended in sterile PBS.
Agglutination test
pBR322 and pBR-sef were electroporated into the SE5000 strain to generate SE5000+pBR322 and SE5000+Sef, respectively. Positive clones were screened on plates with added ampicillin. Strains SG9R, SG01, CVCC523, S08, SE5000+pBR322, and SE5000+Sef were grown at 37 °C; the amount of bacteria was adjusted to 5 × 109 colony-forming units (CFU)/mL and washed three times with sterile saline before subjecting the sample to agglutination with the laboratory-made anti-SefA monoclonal antibody (Zhu et al. 2010). Next, 10 μL of bacterial solution was mixed on a clean glass plate with the same volume of 1:40 diluted monoclonal antibody; after a 2-min incubation, the agglutination results could be seen by the naked eye.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blot
We used SDS-PAGE and western blot methods (Kurien and Scofield 2015) to identify the expression of Sef. Briefly, hot-extracted Sef fimbriae and recombinant SefA protein (including a 6 × His tag) were run onto a SDS-PAGE, followed by electro-transfer onto a nitrocellulose membrane (Biosharp, Anhui, China) using the BIO-RAD transfer system (Shanghai, China). After overnight blocking at 4 °C with 10% bovine serum albumin (BSA), the membrane was washed three times with PBS-Tween 20 (PBST) and incubated for 1 h at 37 °C with anti-SefA monoclonal antibody (diluted 1:1000 by PBST). A second washing step with PBST was made, followed by a 2-h incubation at 37 °C with a sheep anti-mouse-HRP conjugated IgG (Novus, Gudensberg, Germany). We performed a final washing step with PBST before detection with diaminobenzidine.
Transmission electron microscopy (TEM)
SE5000+pBR322 and SE5000+Sef were cultured for 12 h, and then 1 mL of each culture was centrifuged for 10 min at 4000 rpm, washed three times with the same amount of PBS, and resuspended. These samples were observed by TEM (Yang et al. 2015); briefly, a 50-μL sample was placed on a copper grid at room temperature for 15 min. The excess bacterial solution of the copper net was absorbed by a filter paper (Whatman-Xinhua, Hangzhou, China), and the copper mesh was floated on the surface of a 2% phosphotungstic acid (pH = 7.0) dye droplet. Then, we absorbed the excess dye solution and observed the sample under TEM.
Laboratory animal and animal welfare
The specific-pathogen-free (SPF) chickens used in this study were purchased from the Comparative Medical Center of Yangzhou University. All experiments were conducted in accordance with the local ethical guidelines and national legislation.
Sample collection
Sera of SPF chickens infected with Salmonella Gallinarum, Salmonella Pullorum, Salmonella Typhimurium, or SE were provided both by the China Institute of Veterinary Drug Control and by experimentally infected chickens in our laboratory. Clinically positive chicken sera of Salmonella Gallinarum, Salmonella Pullorum, Salmonella Typhimurium, and SE were confirmed as diagnostic serum samples from farms located in 10 different provinces of China. E. coli O1–, E. coli O2–, and E. coli O78–infected SPF chicken sera were obtained from Dr. Song Gao. A total of 160 sera samples were used in this study; the sources of the sera are shown in Table 1.
Specifically targeted detection for SE
To prepare SE5000+Sef agglutination antigen and SE5000+pBR322 vector as a negative control, we used a total of 160 sera samples of experimentally and clinically infected chicken (Table 1). Of the 160 samples with results provided in less than 2 min, 30 were SE-infected SPF chicken sera, 30 were SE clinically infected chicken sera, and each of the 10 SPF chicken sera samples was infected by Salmonella Gallinarum, Salmonella Pullorum, and Salmonella Typhimurium; each of the 10 positive chicken sera was clinically infected by Salmonella Gallinarum, Salmonella Pullorum, and Salmonella Typhimurium with experimental diagnosis; and 10 were SPF chicken sera. We also included O1, O2, and O78 E. coli serotype–positive SPF chicken sera.
Sensitively targeted detection for SE
Ten SPF chicks at the age of 5 days were infected orally with SE strain C7920, with the infection dose of 107 CFU in 100 μL of sterile LB broth; 10 SPF chicks were treated with the same volume of LB as control. Sera of SPF chicken were then collected every week and were subjected to agglutination testing by the SE5000+Sef system until positive detection of SefA-specific antibodies.
Isolation and identification of Salmonella strains
Salmonella strains were isolated from the tissue samples of chickens using the Chinese National Standard method (GB 4789.4–2010), with some optimized modifications. Briefly, each sample (1 g) was added to 10.0 mL selenite cystine broth (SC; Qingdao Hope Bio-Technology Ltd., Qingdao, China) and incubated at 37 °C; after 24 h of incubation, 5 μL of each culture was streaked onto xylose lysine deoxycholate selective medium (XLD; Qingdao Hope Bio-Technology Ltd.) plates and incubated at 37° C for 24 h. Next, suspected Salmonella colonies were further identified by PCR assays using universal primers for flgE (the upstream primer of flgE: ACGGACCCTGTACCGTCTAAA; the downstream primer of flgE: TGATGTTCACCGTACCGCC) for detecting all Salmonella species (Yang et al. 2020), which can conveniently and rapidly detect flagellated and nonflagellated Salmonella spp. sdfI primers (the upstream primer of sdfI: TGTGTTTTATCTGATGCAAGAGG; the downstream primer of sdfI: TGAACTACGTTCGTTCTTCTGG) were used to identify the SE serotype (Kasturi and Drgon 2017), and glgC primers (the upstream primer of glgC: CGGTGTACTGCCCGCTAT; the downstream primer of glgC: CTGGGCATTGACGCAAA) were used to identify the Salmonella Pullorum serotype (Kang et al. 2011).
Data analysis
In this study, we tested the sensitivity and specificity of the calculations using an online calculator (https://www.medcalc.org/calc/diagnostic_test.php). The number of true positives (a), false negatives (b), false positives (c), and true negatives (d) was entered into the four fields of the website, and the values of sensitivity, specificity, and corresponding 95% confidence interval were obtained.
Results
Pseudogenes found of the sef operon of Salmonella Gallinarum and Salmonella Pullorum in infected poultry
We used the NCBI database to compare the sef operon of SE with two strains of Salmonella Gallinarum and six strains of Salmonella Pullorum. The results showed the presence of substitutions and insertions in Salmonella Gallinarum CP019035 and AM933173; these mutations corresponded to either stop codons or frameshifts within the sef operon. The substitution of G to T at nt 1626 (counted from the transcriptional start site) of the sef operon caused a replacement of a glutamic acid codon with a stop codon, resulting in a truncated sefC. Other nucleotide substitutions, insertions, and deletions were found in Salmonella Pullorum CP003786, CP003047, CP006575, LK931482, CP022963, and CP012347. In the CP003786, CP003047, CP006575, and LK931482 strains, a mutation of G to A at nt 2512 (counted from the transcription start site) caused a tryptophan codon to stop codon substitution, resulting in early termination of sefC. We also noticed the presence of an insertion within the sefC open-reading frame in CP012347, in addition to the absence of a part of sefC and the entire sefD sequences in the CP022963 chromosome. Thus, we concluded that the sef operon was mainly represented by pseudogenes in Salmonella Gallinarum and Salmonella Pullorum (Fig. 1).
Amplification, cloning, and identification of the sef operon in SE
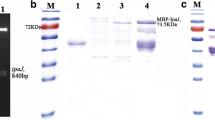
We used sef-specific primers to amplify the entire sef operon. The generated PCR product migrated as a DNA band of about 4268 bp in a 1% agarose gel (Fig. 2A), which was in agreement with the predicted size. The sef PCR product was cloned into the pBR322 plasmid generating a recombinant vector of 8212 bp (Fig. 2B).
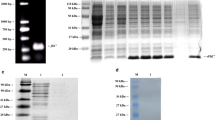
(A) PCR amplification results of the sef operon. Lane M, Trans 2K plus II DNA marker (TransGen Biotech, Beijing, China); lane 1, PCR amplification product of the sef operon; lane 2, SE5000 control. (B) The result of restriction digestion of the pBR-sef plasmid. Lane M, Trans 15K DNA marker (TransGen Biotech, Beijing, China); lane 1, circular pBR-sef plasmid; lane 2, pBR-sef linearized by digestion with SalI; lane 3, pBR-sef linearized by digestion with XbaI
Verification of the expression of Sef fimbriae
Upon transformation of the SE5000 strain with recombinant pBR322 harboring sef, we confirmed the expression of Sef fimbriae in the generated strain using an agglutination assay. The results showed a significant agglutination with the laboratory-made anti-SefA monoclonal antibody. In contrast, the SE5000 strain transformed with the empty vector did not react with the monoclonal antibody during the agglutination test (Fig. 3). We noticed that the SG9R, SG01, CVCC523, and S08 strains could not recognize the anti-SefA monoclonal antibody (results not shown). To confirm the expression of Sef, fimbriae were extracted from the SE5000 recombinant strain and subjected to both SDS-PAGE and immunoblotting using anti-SefA-specific antibody. As shown in Fig. 4, a visible band of about 14.3 kDa was detected in both Coomassie staining and western blot gels (Fig. 4A), which was consistent with the predicted size of SefA, the main subunit of fimbriae. There were no bands at 14.3 kDa by both SDS-PAGE and immunoblotting for the fimbriae extracted from the SG9R, SG01, CVCC523, and S08 strains (results not shown). For further confirmation, we subjected the SE5000 strains carrying either a pBR322-sef or the empty pBR322 to TEM analysis; we found obvious fimbria structures on the surface of strain SE5000+Sef but not on the negative control SE5000+pBR322 (Fig. 5).
SE5000+Sef system used to specifically detect SE-infected chickens
We took advantage of the genetic difference between SE and other Salmonella serogroups to design a diagnosis system consisting of the generated strains SE5000+pBR322 and SE5000+Sef as the reporter strains for agglutination assays to detect antifimbriae antibodies in serum prepared from infected chicken. We conducted a proof-of-concept study in which we tested our designed system against 160 serum samples from experimentally and clinically infected chicken. The results showed that the negative control SE5000+pBR322 did not react with any serum sample, whereas SE5000+Sef exhibited significant agglutination with 30 SPF chicken sera infected with SE and with 30 clinical SE-infected sera, with a sensitivity of 100% (95% confidence interval: 94.04% to 100%) and specificity of 100% (95% confidence interval: 96.38% to 100%). Moreover, we did not observe a cross-reaction between SE5000+Sef and samples prepared from chicken infected with Salmonella Gallinarum, Salmonella Pullorum, Salmonella Typhimurium, and E. coli (Table 2). Taken together, these data demonstrated the powerful specificity of our diagnosis tool using the SE5000+Sef system.
Infected chickens can be detected as early as 2 weeks after infection by SE5000+Sef in the agglutination assay system
Weekly antibody surveillance of SPF chicks infected with SE showed that the antibody dynamics were in accordance with the general antibody production by antigen induction (Fig. 6). In other words, we did not detect any antibody against Sef in the first week (7 days after infection). However, 2 weeks post-infection, we detected anti-Sef antibodies in two of the 10 individuals. At 6 weeks post-infection, all of the infected SPF chicks were detected positive compared with the noninfected SPF chicks.
The result of the SE5000+Sef system monitoring Salmonella Enteritidis–infected SPF chickens. Ten 5-day-old SPF chicks were infected with Salmonella Enteritidis (the infection group), while the control group included 10 SPF chicks matched for age and treated by LB as control. Antibodies of the infected chicks were tested every week, infected chickens could be detected after 2 weeks, and all infected chicks could be detected until the 6th week after infection
The SE5000+Sef system has better sensitivity and specificity than commercial agglutination antigen
For further validation of the robustness of our designed diagnosis system, we performed a comparison between a commercially available agglutination antigen and the SE5000+Sef system. The assay included subjecting forty 60-day-old laying hens selected from a chicken farm in Guangxi province to both the commercial Salmonella Pullorum–based and the SE5000+Sef system agglutination tests, in parallel to the standard bacterial isolation and identification method. Using the commercial agglutination kit, half of the population was positive and the other half was negative. Only nine SE and three Salmonella Pullorum infections of the 40 samples were isolated from mixed tissue samples of chickens using a classical bacterial isolation procedure. Data obtained from using the SE5000+Sef system showed that 10 positive samples were detected as SE-infected chickens and nine of them were consistent with the result of the bacterial isolation and identification method. Unlike the data from SE5000+Sef, however, the results of the commercial Salmonella Pullorum–based agglutination antigen did not match with the results of bacterial isolation and identification (Table 3). Taking the bacterial isolation and identification method as a standard, the sensitivity of the SE5000+Sef system was 100% (95% confidence interval: 66.37% to 100%) and the specificity was 96.88% (95% confidence interval: 83.78% to 99.92%). Thus, the SE5000+Sef system had a much better sensitivity and specificity than the commercial agglutination antigens that are still widely utilized as the main detection method.
Discussion
Sef fimbriae were believed to be unique to Salmonella serotype D. Thorns et al. (1990) first detected the expression of Sef fimbriae in SE and Salmonella Dublin isolates; then, they reported that Sef was expressed by organisms only within group D serotypes of SE, including Salmonella Dublin and very rare Salmonella Blegdam and Salmonella Moscow serovars. A similar conclusion was reported by Turcotte and Woodward (1993), who found that the sefA gene was also conserved in Salmonella Gallinarum and Salmonella Pullorum, but it was not clear why Sef was not expressed in Salmonella Gallinarum and Salmonella Pullorum. According to bioinformatics analysis, the sefC and sefD genes of the sef operon in Salmonella Gallinarum and Salmonella Pullorum variably harbor different point mutations, a fragment insertion, and a deletion. The SE5000+Sef detection system did not agglutinate with antibodies produced in chickens infected both naturally and experimentally by Salmonella Gallinarum and Salmonella Pullorum, and no Sef fimbriae could be detected from the extracts of Salmonella Gallinarum and Salmonella Pullorum strains, which was consistent with our bioinformatics data analysis showing the presence of pseudogenes in the sef operon of these strains.
At present, the main serotypes of Salmonella isolates from chicken flocks are Salmonella Gallinarum and Salmonella Pullorum, SE, and Salmonella Typhimurium (Wang et al. 2020). Unlike both Salmonella Gallinarum and Salmonella Pullorum, which are isolated from sick chickens, SE exists only in healthy-looking chicken (Wang et al. 2020). Importantly, the traditional serotyping is determined by somatic antigen O, and SE cannot be differentiated from Salmonella Gallinarum and Salmonella Pullorum because they share the same O antigens (O1, O9, and O12) (Yang et al. 2019). Therefore, specific and rapid detection methods for SE in chicken are very important. At present, although laboratory-manipulated methods include microbial isolation and identification, PCR and other molecular biology methods, and ELISA and other immunological methods, they all fail to perform as a rapid and low-cost screening for SE in large-scale chicken farms, or in eradicating SE (Eriksson and Aspan 2007). The traditional agglutination method has the advantages of being fast and economical, and it can be used for large-scale detection of pathogens on the spot; however, it has been criticized for being less specific and less sensitive because of confusing false-positive and false-negative results (Schreier et al. 2013). This confusion is due to the fact that commercial agglutination antigens use somatic antigens that are produced by all Salmonella species. Therefore, the diversity and complexity of the used antigens are a source of undesired nonspecific cross-reactions with different chicken sera, especially with obvious cross-reactions clinically by certain Enterobacteriae-infected chicken sera (Yang et al. 2019). We also noticed that the traditional agglutination method detected somatic antigen-based antibodies with low sensitivity because of the weak immunogenicity of the somatic antigen. Therefore, an alternative method for chicken SE detection is urgently needed to meet the requirements of a rapid, specific, sensitive, and economic agglutination detection test.
Considering the unique expression of Sef fimbriae in SE among Salmonella serotype D, Sef can be used for exclusive detection of SE in infected poultry by serological methods. For instance, a monoclonal antibody–coated latex reagent against the major subunit SefA (molecular weight of 14.3 kDa) was used to identify SE but came with a high number of other bacteria (Thorns et al. 1994). Rajashekara et al. (1998) improved LAT by using recombinant SefA protein instead of monoclonal antibody–coated latex particles for detecting anti-SefA antibodies. Their method was not tested against SE-infected samples, however, and little is known about the sensitivity of the developed LAT method. We wondered whether an alternative method could be designed to detect patients or sick animals infected with SE. In this study, we cloned the sef operon of SE and confirmed its expression in SE5000. We then validated the proof-of-principle of the designed SE5000+Sef system for SE detection. Unlike the classical agglutination and LAT methods, our SE5000+Sef detection system included an internal negative control, SE5000+pBR322, with zero detectable agglutination with serum samples tested. We next tested the efficiency of the SE5000+Sef detection system; the obtained results showed that the infected SPF chicks could be detected relatively early by the SE5000+Sef system, starting from 2 weeks post-infection. In addition, the detection system showed strong specificity and sensitivity compared with the commercial Salmonella Pullorum–based agglutination antigens, and the results were completely consistent with the results of bacterial isolation and identification.
By optimizing the traditional plate agglutination reaction with much more specificity and sensitivity, this study proposed a method for large-scale, rapid, and on-site detection of SE in chickens. Considering its convenience for epidemiological investigation and disease control and prevention of SE in chickens, our Sef fimbriae–based method is suggested as a promising and robust technique that can be generalized for future SE detection in poultry farming.
Data availability
The authors declare that the data supporting the findings of this study are available within the article.
References
Ahmed A, Wang M, Khan R, Shah AA, Guo H, Malik S, Xia K, Hu Z (2021) A splice-site variant (C.3289-1G>T) in OTOF underlies profound hearing loss in a Pakistani kindred. BMC Med Genet 14:2. https://doi.org/10.1186/s12920-020-00859-x
Eriksson E, Aspan A (2007) Comparison of culture, ELISA and PCR techniques for Salmonella detection in faecal samples for cattle, pig and poultry. BMC Vet Res 3:21. https://doi.org/10.1186/1746-6148-3-21
Fasano RM, Sullivan HC, Bray RA, Gebel HM, Meyer EK, Winkler AM, Josephson CD, Stowell SR, Duncan A, Roback JD (2017) Genotyping applications for transplantation and transfusion management: the Emory experience. Arch Pathol Lab Med 141(3):329–340. https://doi.org/10.5858/arpa.2016-0277-SA
Gal-Mor O, Boyle EC, Grassl GA (2014) Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front Microbiol 391(5):1–10. https://doi.org/10.3389/fmicb.2014.00391
Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Gast R, Humphrey TJ, Van Immerseel F (2009) Mechanisms of egg contamination by Salmonella Enteritidis. FEMS Microbiol Rev 33(4):718–738. https://doi.org/10.1111/j.1574-6976.2008.00161.x
Gast RK (1997) Detecting infections of chickens with recent Salmonella Pullorum isolates using standard serological methods. Poult Sci 76(1):17–23. https://doi.org/10.1093/ps/76.1.17
Kang M-S, Kwon Y-K, Jung B-Y, Kim A, Lee K-M, An B-K, Song E-A, Kwon J-H, Chung G-S (2011) Differential identification of Salmonella enterica subsp enterica serovar Gallinarum biovars Gallinarum and Pullorum based on polymorphic regions of glgC and speC genes. Vet Microbiol 147(1-2):181–185. https://doi.org/10.1016/j.vetmic.2010.05.039
Kasturi K, Drgon T (2017) Real-time PCR method for detection of Salmonella spp. in environmental samples. Appl Environ Microbiol 83(14). https://doi.org/10.1128/aem.00644-17
Khan AS, Schifferli DM (1994) A minor 987P protein different from the structural fimbrial subunit is the adhesin. Infect Immun 62(10):4233–4243. https://doi.org/10.1128/iai.62.10.4233-4243
Kuehn BM (2010) Salmonella cases traced to egg producers findings trigger recall of more than 500 million eggs. JAMA-J AM Med Assoc 304(12):1316. https://doi.org/10.1001/jama.2010.1330
Kurien BT, Scofield RH (2015) Western blotting: an introduction: methods and protocols. Methods Mol Biol 1312:17–30
Li J, Ma B, Fang J, Zhi A, Chen E, Xu Y, Yu X, Sun C, Zhang M (2020) Recombinase polymerase amplification (RPA) combined with lateral flow immunoassay for rapid detection of Salmonella in food. Foods 9(27):1–12. https://doi.org/10.3390/foods9010027
Liquan Huang YW (2006) Study on the antibacterial activity of Leontopodium leontopodioides (Willd.) Beauv. in vitro. J Traditional Chinese Vet Med 1:5–7. https://doi.org/10.13823/j.cnki.jtcvm.2006.01.001
Lynch MJB, Leon-Velarde CG, McEwen S, Odumeru JA (2004) Evaluation of an automated immunomagnetic separation method for the rapid detection of Salmonella species in poultry environmental samples. J Microbiol Methods 58(2):285–288. https://doi.org/10.1016/j.mimet.2004.04.005
Machado I, Garrido V, Hernandez LI, Botero J, Bastida N, San-Roman B, Grillo M-J, Hernandez FJ (2019) Rapid and specific detection of Salmonella infections using chemically modified nucleic acid probes. Anal Chim Acta 1054:157–166. https://doi.org/10.1016/j.aca.2018.12.027
Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O’Brien SJ, Jones TF, Fazil A, Hoekstra RM, Int Collaboration Enteric Dis B (2010) The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis 50(6):882–889. https://doi.org/10.1086/650733
Margot H, Saegesser G, Zweifel C, Stephan R (2013) Performance of a commercially available latex agglutination test kit for the identification of Salmonella. J Food Saf Food Qual 64(3):75–77. https://doi.org/10.2376/0003-925x-64-75
Miao J, Han N, Qiang Y, Zhang T, Li X, Zhang W (2017) 16SPIP: a comprehensive analysis pipeline for rapid pathogen detection in clinical samples based on 16S metagenomic sequencing. BMC Bioinformatics 18:255–259. https://doi.org/10.1186/s12859-017-1975-3
Oliveira GH, Berchieri A Jr, Montassier HJ (2006) Chicken serologic response to Salmonella enterica serotype Typhimurium assessed by ELISA. Braz J Poult Sci 8(1):51–54. https://doi.org/10.1590/s1516-635x2006000100008
Rajashekara G, Munir S, Lamichhane CM, Back A, Kapur V, Halvorson DA, Nagaraja KV (1998) Application of recombinant fimbrial protein for the specific detection of Salmonella Enteritidis infection in poultry. Diagn Microbiol Infect Dis 32(3):147–157. https://doi.org/10.1016/s0732-8893(98)00091-1
Ricke SC, Khatiwara A, Kwon YM (2013) Application of microarray analysis of foodborne Salmonella in poultry production: a review. Poult Sci 92(9):2243–2250. https://doi.org/10.3382/ps.2012-02740
Roychoudhury P, Murugkar HV, Rahman H, Subudhi PK (2009) Dot-ELISA for detection of enterotoxin production by Salmonella isolates from Japanese quail. Indian J Anim Sci 79(2):161–163
Schifferli DM, Beachey EH, Taylor RK (1991) 987P fimbrial gene identification and protein characterization by T7 RNA polymerase induced transcription and TnphoA mutagenesis. Mol Microbiol 5:61–70
Schreier S, Doungchawee G, Chadsuthi S, Triampo D, Triampo W (2013) Leptospirosis: current situation and trends of specific laboratory tests. Expert Rev Clin Immunol 9(3):263–280. https://doi.org/10.1586/eci.12.110
Song Q, Shen X, Yang Y, Zhang D, Gao H (2016) Genetically similar isolates of Salmonella enterica serotype Enteritidis persistent in China for a long-term period. J Food Sci 81(7):M1778–M1781. https://doi.org/10.1111/1750-3841.13339
Stoica C, Ancuta PN, Lucaciu IE, Banciu AR, Sorea S, Atanasescu A, Lazar MN (2018) Computerized high-tech detection technology of immunofluorescence labelled waterborne pathogenic bacteria. Rev Chim-Bucharest 69(11):4166–4170
Thorns CJ, Sojka MG, Chasey D (1990) Detection of a novel fimbrial structure on the surface of Salmonella Enteritidis by using a monoclonal antibody. J Clin Microbiol 28(11):2409–2414. https://doi.org/10.1128/jcm.28.11.2409-2414.1990
Thorns CJ, McLaren IM, Sojka MG (1994) The use of latex particle agglutination to specifically detect Salmonella Enteritidis. Int J Food Microbiol 21(1-2):47–53. https://doi.org/10.1016/0168-1605(94)90199-6
Turcotte C, Woodward MJ (1993) Cloning, DNA nucleotide sequence and distribution of the gene encoding the Sef fimbrial antigen of Salmonella Enteritidis. J Gen Microbiol 139(7):1477–1485. https://doi.org/10.1099/00221287-139-7-1477
Wang X, Wang H, Li T, Liu F, Cheng Y, Guo X, Wen G, Luo Q, Shao H, Pan Z, Zhang T (2020) Characterization of Salmonella spp. isolated from chickens in central China. BMC Vet Res 16(1):299–308. https://doi.org/10.1186/s12917-020-02513-1
Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ (2009) Jalview version 2-a multiple sequence alignment editor and analysis workbench. Bioinformatics 25(9):1189–1191. https://doi.org/10.1093/bioinformatics/btp033
Xia P, Song Y, Zou Y, Yang Y, Zhu G (2015) F4+ enterotoxigenic Escherichia coli (ETEC) adhesion mediated by the major fimbrial subunit FaeG. J Basic Microbiol 55(9):1118–1124. https://doi.org/10.1002/jobm.201400901
Yan M, Tam FCH, Kan B, Lim PL (2011) Combined rapid (TUBEX) test for typhoid-paratyphoid a fever based on strong anti-O12 response: design and critical assessment of sensitivity. PLoS One 6(9):e24743. https://doi.org/10.1371/journal.pone.0024743
Yang F-H, Zhang Q, Liang Q-Y, Wang S-Q, Zhao B-X, Wang Y-T, Cai Y, Li G-F (2015) Bioavailability enhancement of paclitaxel via a novel oral drug delivery system: paclitaxel-loaded glycyrrhizic acid micelles. Molecules 20(3):4337–4356. https://doi.org/10.3390/molecules20034337
Yang B, Niu Q, Yang Y, Dai P, Yuan T, Xu S, Pan X, Zhu G (2019) Self-made Salmonella Pullorum agglutination antigen development and its potential practical application. Poult Sci 98(12):6326–6332. https://doi.org/10.3382/ps/pez453
Yang Y, Wang P, Xia P, Yang B, Dai P, Hong T, Li J, Meng X, El Qaidi S, Zhu G (2020) Rapid detection of flagellated and non-flagellated Salmonella by targeting the common flagellar hook gene flgE. Appl Microbiol Biotechnol 104(22):9719–9732. https://doi.org/10.1007/s00253-020-10925-0
Yue M, Rankin SC, Blanchet RT, Nulton JD, Edwards RA, Schifferli DM (2012) Diversification of the Salmonella fimbriae: a model of macro- and microevolution. PLoS One 7(6):e38596. https://doi.org/10.1371/journal.pone.0038596
Zhu CH, Musa HH, Wu SL, Zhu GQ (2009) The role of Sef fimbriae in pathogenesis and enhancing the immunity of Salmonella Enteritidis. Afr J Microbiol Res 3(5):191–194
Zhu CH, Wu J, Chen WW, Hassan HM, Zhu GQ (2010) Difference and variation of the sef operon gene clusters in Salmonella Pullorum. J Basic Microbiol 50:S120–S123. https://doi.org/10.1002/jobm.200900262
Acknowledgements
The authors gratefully acknowledge Professor Song Gao in Yangzhou University, College of Veterinary Medicine, for providing the serum for this study.
Funding
This work was supported by the College Students Research & Practice Innovation Program, Graduates Research & Practice Innovation Program of Jiangsu Province (No. KYCX20_3004), International Collaboration Program from Science and Technology Agency of Jiangsu Province (2019), and Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Contributions
XG and QH performed the experiments, analyzed the data, and wrote the manuscript. JL and PX participated in the data analysis and wrote the paper. QD and GZ conceived and designed the study, participated in experimental work, and wrote the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants performed by any of the authors. The current study was approved by the Institutional Animal Care and Use Committee of the Yangzhou University College of Veterinary Medicine of China.
Consent for publication
All authors listed on this manuscript have read and agreed to the publication of this research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gu, X., Hou, Q., Liu, J. et al. Sef fimbria operon construction, expression, and function for direct rapid detection of Salmonella Enteritidis. Appl Microbiol Biotechnol 105, 5631–5641 (2021). https://doi.org/10.1007/s00253-021-11400-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11400-0