Abstract
Heterotrimeric-G-protein-mediated signaling pathways modulate the expression of the essential genes in many fundamental cellular processes in fungi at the transcription level. However, these processes remain unclear in Penicillium oxalicum. In this study, we generated knockout and knockout-complemented strains of gng-1 (POX07071) encoding the Gγ protein and found that GNG-1 modulated the expression of genes encoding plant-biomass-degrading enzymes (PBDEs) and sporulation-related activators. Interestingly, GNG-1 affected expression of the cxrB that encodes a known transcription factor required for the expression of major cellulase and xylanase genes. Constitutive overexpression of cxrB in ∆gng-1 circumvented the dependence of PBDE production on GNG-1. Further evidence indicated that CxrB indirectly regulated the transcription levels of key amylase genes by controlling the expression of the regulatory gene amyR. These data extended the diversity of Gγ protein functions and provided new insight into the signal transduction and regulation of PBDE gene expression in filamentous fungi.
Key points
• GNG-1 modulates the expression of PBDE genes and sporulation-related genes.
• GNG-1 controls expression of the key regulatory gene cxrB.
• Overexpression of cxrB circumvents dependence of PBDE production on GNG-1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Filamentous fungi are widely distributed in soil (Powers-Fletcher et al. 2016; Zhang et al. 2014) and have a strong capacity to degrade macropolymers such as cellulose, xylan, and starch in plant biomass. This ability is dependent upon the secretion of carbohydrate-active enzymes (CAZymes), which are essential for the carbon cycle and climatic changes (Gougoulias et al. 2014). The secretion of CAZymes by filamentous fungi is strictly controlled, specifically at the transcription level (Benocci et al. 2017). With decades of effort, several transcription factors (TFs) involved in the regulation of PBDE (plant-biomass-degrading enzyme) gene expression in fungi have been identified, including AmyR and CxrB (Li et al. 2017; Schmoll 2018). CxrB positively regulates the expression of cellulase and xylanase genes in Penicillium oxalicum (Schmoll et al. 2009; Yan et al. 2017), and AmyR activates the expression of major amylase genes and represses the transcription of cellulase genes in this fungus (Li et al. 2015).
G-protein-mediated signaling pathways modulate multiple biological processes, including growth, development, and virulence (Chakravorty and Assmann 2018). The conventional G protein consists of subunits Gα, Gβ, and Gγ, which form the heterotrimeric complex Gαβγ in the inactive state. In most filamentous fungi, the Gα proteins are classified into three groups. Groups I and III Gα proteins contribute to vegetative growth, development, and pathogenesis, whereas Group II proteins play minor roles (Li et al. 2007; Liu et al. 2018). Under environmental stimulation, G-protein-coupled receptors are activated, and then bind and activate G proteins, leading to the separation of Gα from the heterodimer Gβγ and GTP-GDP exchange on Gα. The separated Gα and heterodimer Gβγ then drive specific downstream effector proteins, mainly including adenylate cyclase/cAMP, mitogen-activated protein (MAP) kinases, and phospholipase C (Chakravorty and Assmann 2018; Li et al. 2007).
Accumulating evidence indicates that the Gα-protein-mediated signaling pathway is linked to the regulation of cellulase and amylase genes in filamentous fungi. In Trichoderma reesei, the Gα proteins GNA1 and GNA3 play important roles in cellulase gene expression in the presence of cellulose, and their expression is light-dependent (Schmoll et al. 2009; Seibel et al. 2009). In the chestnut blight fungus Cryphonectria parasitica, the Gα protein CPG-1 is required for cellobiohydrolase I gene (cbh1) expression (Wang and Nuss 1995). In P. oxalicum, the deletion of the gene PGA3 encoding Gα protein PGA3 reduces amylase production and the transcription of the key amylase gene Amy15A, whereas it causes no significant change in cellulase production, although the expression of cellobiohydrolase gene cbh1 is increased in mutant ∆pga3 (Hu et al. 2013). These data imply that the function of Gα is species-independent. Furthermore, the Gα proteins of T. reesei and P. oxalicum are involved in the expression of cellulase and amylase genes by controlling intracellular cAMP levels (Schmoll et al. 2009; Hu et al. 2013; Schuster et al. 2012).
In contrast to the Gα proteins, few studies have reported the roles of the Gβ and Gγ proteins in the production of PBDEs in filamentous fungi. Tisch and collaborators (Tisch et al. 2011) found that the individual loss of the Gβ or Gγ proteins GNB1 or GNP1, respectively, increased cellulase production, and conversely reduced the transcription of major cellulase genes, including cbh1, in T. reesei, independently of the light status. However, how Gβγ modulates the expression of PBDE genes via TFs and whether its functions are species-specific require further research.
In this study, we showed that the Gγ subunit GNG-1 (POX07071) was required for the expression of PBDE (cellulase, xylanase, and amylase) genes and mycelial-development-related genes, which needed the contribution of an important TF CxrB in P. oxalicum.
Materials and methods
Fungal strains and their culture conditions
The P. oxalicum parental strain ∆ku70, deposited in the China General Microbiological Culture Collection (CGMCC) under accession number 3.15650, was derived from wild-type strain HP7-1 (CGMCC no. 10781) by the deletion of the ku70 gene, which is involved in the classic non-homologous end-joining pathway (Zhao et al. 2016). The other P. oxalicum mutants listed in Table 1 were generated in the parental strain ∆ku70 with a homologous double-crossover methodology. The chosen transformants were confirmed by PCR amplification with specific primers (Supplementary Table S1 and Fig. S1A) and Southern hybridization with specific probes (Supplementary Table S1 and Fig. S1B). All P. oxalicum strains were cultured on potato-dextrose agar (PDA) plates at 4°C for temporary storage. Fungal spores were collected from PDA plates precultured for 6 days at 28°C with 0.1% Tween 80, and were adjusted to a concentration of 1 × 108 per mL, before their use in fungal reproduction.
The P. oxalicum strains were cultured according to the method described by Zhao and collaborators (Zhao et al. 2016) for the measurement of cellulase and xylanase production, as well as amylase production, except that the carbon source (Avicel) was replaced with soluble corn starch (SCS) (Sigma-Aldrich, St. Louis, MO, USA).
For RNA-Seq and RT–qPCR analyses, the P. oxalicum strains were cultured for 4–48 h as described above. The mycelia used for total RNA extraction were collected every 12 h.
The P. oxalicum strains were cultured on MMM plates containing glucose, Avicel, or soluble corn starch (Sigma-Aldrich, St. Louis, MO, USA) or on PDA plates at 28°C, for 20 h for microscopic analyses and for 3 days for colony analyses.
Extraction of fungal DNA and RNA
Total DNA and RNA were extracted from the P. oxalicum strains as described by Zhao et al. (2016). The quality and quantity of the extracted DNA and RNA were evaluated with electrophoresis on 1.0% agarose gel and a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Construction of deletion mutants and complementation strains
The methods used to construct the deletion mutants and their corresponding complementation strains in the P. oxalicum parental strain ∆ku70 were reported by Yan et al. (2017).
Construction of overexpression strains
The overexpression strains of P. oxalicum were constructed as described for the complementation strains, except that the encoding sequence of the complementation gene was replaced by the overexpressed gene with the native promoter or the constitutive promoter pTEF1.
RNA-Seq and data analysis
RNA sequencing (RNA-Seq) of the P. oxalicum strains was as previously reported (Li et al. 2020). Total RNAs were extracted from each P. oxalicum strain cultivated on Avicel for 24 h after a transfer. Three biological replicates of each sample were sequenced and further analyzed. The appropriate RNA was used to construct a library of single-stranded circular DNAs, which were then sequenced on the BGI-SEQ-500 platform at BGI, Shenzhen, China. The raw reads generated were filtered with SOAPnuke v1.5.2 (Chen et al. 2018) to generate clean reads. The clean reads were mapped onto the genome of P. oxalicum strain HP7-1 and annotated with Hierarchical Indexing for Spliced Alignment of Transcripts v2.0.4 (Kim et al. 2015) and Bowtie2 v2.2.5 (Langmead and Salzberg 2012). The transcript levels of the genes were represented as fragments per kilobase of exon per million mapped reads (FPKM) values, which were obtained with RSEM v1.2.12 (Li and Dewey 2011). The differentially expressed genes (DEGs) were detected with DESeq tool (Love et al. 2014), with cutoff values of q ≤ 0.05 and |log2(fold change)| ≥ 1.
RT-qPCR analysis
RT-qPCR was used to compare the gene transcription levels in different fungal strains based on a previously described method (Yan et al. 2017). The specific primers corresponding to each target gene were shown in Supplementary Table S1. The relative expression of each target gene was calculated with the 2−ΔΔCT method (Livak and Schmittgen 2001). The actin-encoding gene POX09428 was used as the internal control gene, and the expression of all the target genes was normalized to the expression level in the parental strain Δku70. Three independent biological replicates of each RT–qPCR experiment were performed.
Measurement of PBDE production
The production of PBDEs, including cellulases, xylanases, raw-cassava-starch-degrading enzymes (RSDEs), and soluble-starch-degrading enzymes (SSDEs), were measured with the methods reported by Yan et al. (2017) and Wang et al. (2018). The enzymatic activity units (U) for filter paper cellulase (FPase), carboxymethylcellulase (CMCase), xylanase, SSDEs and RSDEs were defined as the amount of enzyme that produced 1 μmoL of reducing sugar per minute from the appropriate substrate, whereas 1 unit of p-nitrophenyl-β-cellobiosidase (pNPCase) or p-nitrophenyl-β-glucopyranosidase (pNPGase) was the amount of enzyme that produced 1 μmoL of p-nitrophenol per minute from the appropriate substrate. Each experiment was performed independently at least three times.
Southern hybridization analysis
The deletion mutant of P. oxalicum was analyzed with Southern hybridization to confirm the insertion of the knockout DNA cassette at a single locus in the chromosome. Southern hybridization was performed as previously described (Zhao et al. 2016).
Measurement of P. oxalicum biomass
The accumulated mycelial weights of P. oxalicum cultured in MMM containing either glucose or SCS were quantified after the mycelia were dried at 50°C. When the P. oxalicum strains were cultured in MMM containing Avicel as the sole carbon source, the intracellular proteins were extracted from the collected mycelia, and represented the true mycelial accumulation. The intracellular proteins were extracted with protein extraction buffer (g/L: NaCl 8.5, Na2HPO4 2.2, NaH2PO4 0.2, phenylmethylsulfonyl fluoride 0.87, ethylenediaminetetraacetic acid 1.86, pH 7.4] based on the method of Li and collaborators (Li et al. 2020). The mycelia were collected from the P. oxalicum cultures every 12 h until 72 h. All experiments were performed independently in triplicate.
Determination of asexual spore numbers
Fresh spores (1 × 105) from the P. oxalicum strains were spread on plates containing Avicel, SCS, or glucose, or PDA plates. The inoculated Avicel- and SCS-containing plates were incubated at 28°C for 10 days, whereas the inoculated glucose- and PDA-containing plates were incubated for 15 and 3 days, respectively. More than 10 samples (1 cm2) of each strain were taken with a hole puncher. The asexual spores were washed with 0.1% Tween 80 and counted with a hemocytometer after the appropriate dilution. Triplicate biological repetitions of each experiment were performed.
In vitro electrophoretic mobility shift assay (EMSA) and competitive EMSA
In vitro EMSA and competitive EMSA were performed as described by Yan et al. (2017). The tested genes were amplified by PCR with specific primers (Supplementary Table S1).
Bioinformatic analysis
Homologous protein sequences of GNG-1 were screened on the National Center for Biotechnology Information BlastP website (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and then downloaded for further analysis. A phylogenetic tree was constructed with MEGA version X (Kumar et al. 2018) with the neighbor-joining method and a Poisson correction model. Bootstrap values were calculated from 1000 replicates. A sequence alignment was constructed in MUSCLE online with Clustal W (https://www.ebi.ac.uk/Tools/msa/muscle/).
Investigation of colony phenotypes and hyphae
P. oxalicum colonies on solid plates were directly photographed with a Canon EOS 6D camera (Canon Inc., Tokyo, Japan). The hyphae of P. oxalicum were examined with light microscopy (Olympus DP480; Olympus, Tokyo, Japan). The harvested hyphae were transferred onto microscope slides with phosphate-buffered saline. The photomicrographs were analyzed with cellSens Dimension digital imaging software (Olympus).
Subcellular localization of protein CxrB
GFP was used as the reporter protein to visualize the subcellular localization of CxrB in P. oxalicum hyphae. The expression of gfp-labeled cxrB was enhanced by using the inducible promoter pPoxEGCel5B (Wang et al. 2018).
Statistical analysis
All experimental data were analyzed statistically with Student’s t test in Microsoft Excel (Office 2016) (Microsoft, Redmond, WA, USA).
Accession number
Sequence of GNG-1 was uploaded in GenBank with accession number MT993961. Transcriptomic data of P. oxalicum strains have been deposited in the Sequence Read Archive database (Accession No. GSE154704)
Results
Sequence analysis of Gγ protein GNG-1 in P. oxalicum
When we screened the annotated proteins encoded in the P. oxalicum strain HP7-1 genome (Zhao et al. 2016), POX07071 was predicted to encode the G protein subunit Gγ. The protein POX07071 contained 90 amino acids, a Gγ-like domain (GGL; amino acids 20–90; E-value = 2.39e−14) (Fig. 1A), and the CCAAX (cysteine, cysteine, aliphatic, aliphatic, X [any residue]) box CCTVM at the C-terminus (Fig. 1B). A BlastP alignment indicated that POX07071 shared 100%, 95.6%, 67.7%, 65.6%, and 30.95% identity with Gγ proteins in P. oxalicum strain 114-2, Aspergillus clavatus NRRL 1, T. reesei QM6a, Neurospora crassa OR74A, and Saccharomyces cerevisiae S288C, respectively.
Sequence analysis of G protein gamma subunit GNG-1 (POX07071) of P. oxalicum. (A) Conserved domain of GNG-1: GGL, G-protein gamma-like domain. (B) Multiple alignment of GNG-1 and its orthologues. CAA89613.1: Ste18p from Saccharomyces cerevisiae S288C; XP_00126964.1: Ste18/GpgA from Aspergillus clavatus NRRL 1; XP006963419.1: Gpg1 from Trichoderma reesei QM6a; XP_956152.2: Gpg from Neurospora crassa OR74A. Black frame marks the conserved CCAAX box (cysteine, cysteine, aliphatic, aliphatic, X [any residue]). Red residues indicate small amino acids (small + hydrophobic, including aromatic tyrosine). Blue residues indicate acidic amino acids. Magenta residues indicate basic amino acid histidine. Green residues indicate hydroxyl + sulfhydryl + amine + glycine. “*” indicates that the amino acids are identical in all the aligned sequences. “:” indicates conserved amino acids with very similar properties. “.” indicates conserved amino acids with weakly similar properties. (C) Phylogenetic tree constructed with MEGA version X software. The neighbor-joining method was used with a Poisson model. Bootstrap values were calculated from 1000 replicates
A neighbor-joining phylogenetic analysis showed that POX07071 had close evolutionary relationships with homologues in some Aspergillus, but more distant relationships with those of T. reesei and S. cerevisiae (Fig. 1C). To facilitate further study, POX07071 was redesignated GNG-1.
GNG-1 modulates the production of PBDE in P. oxalicum
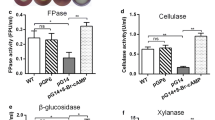
To investigate the effects of GNG-1 on PBDE (cellulase, xylanase, and amylase) production in P. oxalicum, the deletion mutant ∆gng-1 was constructed (Supplementary Fig. S1A), and confirmed with PCR and Southern hybridization analyses (Supplementary Fig. S1A and B). After preculture in glucose medium for 24 h, the mutant ∆gng-1 and the parental strain ∆ku70 were cultured for 2–4 days in MMM containing Avicel (Sigma-Aldrich) as the sole carbon source, and their cellulase and xylanase production were determined. The production of cellulases (FPase, CMCase, and pNPCase) by the mutant ∆gng-1 was 50.8–96.1% lower than that of ∆ku70 and the production of xylanase was 40.9–80.6% lower than that of ∆ku70 (all P < 0.01; Fig. 2A–C and E). Notably, the deletion of gng-1 caused a 29-fold increase in pNPGase production after culture for 2 days, and a 10.7% increase after 4 days (P < 0.05; Fig. 2D).
PBDE production in P. oxalicum strains. Mutant strain ∆gng-1, parental strain ∆ku70, complementation strain ∆gng-1::gng-1, and truncated complementation strain ∆gng-1::gng-11–85 were cultured in MMM containing Avicel for cellulase and xylanase production or soluble corn starch for amylase production for 2–4 days at 28°C with shaking at 180 rpm after their transfer from glucose. (A) FPase; (B) CMCase; (C) pNPCase; (D) pNPGase; (E) xylanase; (F) SSDE; (G) RSDE. FPase, filter paper cellulase; CMCase, carboxymethylcellulase; pNPCase, p-nitrophenyl-β-cellobiosidase; pNPGase, p-nitrophenyl-β-glucopyranosidase; MMM: modified minimal medium; SSDE: soluble-starch-degrading enzyme; RSDE: raw-cassava-starch-degrading enzyme. Data points represent means ± standard deviations. **P ≤ 0.01 on Student’s t-test, indicating significant differences between each mutant or complementation strain and the parental strain ∆ku70
After preculture in glucose medium for 24 h, the deletion mutant ∆gng-1 and the parental strain ∆ku70 were cultured for 2–4 days in MMM containing SCS at 28 °C with shaking at 180 rpm, and their production of SSDEs and RSDEs was measured. SSDE and RSDE production in ∆gng-1 was 49.3–77.8% lower than that in the parental strain ∆ku70 (all P < 0.01; Fig. 2F and G).
To confirm that the change in PBDE production resulted from the deletion of gng-1 in P. oxalicum, the complementation experiment was performed. The complementation strain ∆gng-1::gng-1 was constructed, with gng-1 expressed under the native promoter inserted into a protease gene (POX05007) locus (Supplementary Fig. S1C). The complementation strain ∆gng-1::gng-1 restored PBDE production to the levels observed in ∆ku70 (P > 0.05; Fig. 2). These data demonstrated that the changes in PBDE production in Δgng-1 resulted from the deletion of gng-1.
The CCAAX motif at the C-terminus of Gγ can be modified with a farnesyl or geranylgeranyl moiety, which is required for the membrane association of the heterodimer Gβγ (Alvaro and Thorner 2016). To investigate effects of the CCAAX motif on PBDE production, we constructed the strain ∆gng-1::gng-11–85 in which the introduced gng-1 lacked the sequence encoding the CCAAX motif (Supplementary Fig. S1D). When cultured for 4 days in MMM containing Avicel as the sole carbon source, ∆gng-1::gng-11–85 partly lost the ability to produce cellulase, producing only 60.8%–73.7% of cellulase produced by ∆ku70 (P < 0.01; Fig. 2A–D). Surprisingly, the production of xylanase by ∆gng-1::gng-11–85 was normal (Fig. 2E). As we expected, SSDE and RSDE production by ∆gng-1::gng-11–85 decreased by 35.8% and 32.4% in comparison with those of ∆ku70, respectively (P < 0.01; Fig. 2F and G). These results indicated that the CCAAX motif modified with a farnesyl or geranylgeranyl moiety was required for cellulase and amylase production.
GNG-1 positively affects mycelial growth and conidiation in P. oxalicum
To determine the effects of GNG-1 on fungal mycelial growth, solid MMM plates containing glucose, Avicel, or SCS or PDA plates were inoculated with the P. oxalicum mutant ∆gng-1, the parental strain ∆ku70, or the complementation strain ∆gng-1::gng-1 and ∆gng-1::gng-11–85, and incubated at 28 °C for 3 days. The ∆gng-1 colonies showed irregular edge on PDA, glucose and SCS, while colonies of the ∆ku70, ∆gng-1::gng-1, and ∆gng-1::gng-11–85 exhibited regular edge. The ∆gng-1 colony on SCS was smaller than those of the others. The color of the ∆gng-1 on all tested plates showed some degree of alteration compared with those of the ∆ku70 and ∆gng-1::gng-1. The ∆gng-1::gng-1 colonies were slightly larger than those of ∆ku70, whereas their color was similar (Fig. 3).
Phenotypic analysis of P. oxalicum strains. (A) Colony characteristics. P. oxalicum mutant strain ∆gng-1, parental strain ∆ku70, complementation strain ∆gng-1::gng-1, and truncated complementation strain ∆gng-1::gng-11–85 were grown directly on solid plates containing MMM with different carbon sources for 3 days at 28°C. Scale bar = 0.9 cm. MMM: modified minimal medium; PDA: potato dextrose agar; SCS: soluble corn starch
After the four P. oxalicum strains were cultured on solid medium plates for 20 h, the hyphae were collected for microscopic evaluation. The hyphae of ∆gng-1 and ∆gng-1::gng-11–85 produced conidiophores, whereas those of ∆ku70 and ∆gng-1::gng-1 did not (Fig. 4A and B). However, at 48 h, the hyphae of all the P. oxalicum strains produced conidiophores (Supplementary Fig. S2). It is noteworthy that the expression of gng-11–85 in mutant ∆gng-1 did not restore conidiospore production to the level in ∆ku70, suggesting that the deleted CCAAX motif controlled the time at which hyphae produce conidiospores. In addition to this difference in conidiospore production, the branches of the ∆gng-1 hyphae were less abundant than those of ∆ku70, ∆gng-1::gng-1, and ∆gng-1::gng-11–85 during the whole culture period. However, the hyphal branches of ∆ku70, ∆gng-1::gng-1, and ∆gng-1::gng-11–85 were similar (Fig. 4; Supplementary Fig. S2).
Microscopic investigation of hyphae and asexual spores. (A) P. oxalicum hyphae. Mutant strain ∆gng-1, parental strain ∆ku70, complementation strain ∆gng-1::gng-1, and truncated complementation strain ∆gng-1::gng-11–85 were cultured on solid medium at 28°C for 20 h. Mutant ∆gng-1 displayed phialides, marked with red arrowheads, whereas neither the parental strain ∆ku70 nor the complementation strain ∆gng-1::gng-1 did. Scale bar = 100 μm. (B) Magnified phialides are marked with red arrowheads. Scale bar = 25 μm. (C) Numbers of asexual spores produced by P. oxalicum. Fungal strains were cultured on plates containing Avicel or SCS for 7 days, glucose for 8 days, or PDA for 4 days. Each data point is a mean ± standard deviation. **P < 0.01 indicates differences between the mutant ∆gng-1 and parental strain ∆ku70, complementation strain ∆gng-1::gng-1, or ∆gng-1::gng-11–85 on Student’s t test. PDA: potato dextrose agar; MMM: modified minimal medium; SCS: soluble corn starch
The asexual spores produced by the mutant ∆gng-1 were counted grown on PDA plates and plates containing Avicel, glucose, or SCS. Both ∆ku70 and ∆gng-1::gng-1 were used as controls. The number of spores produced by ∆gng-1 was only 33.3–50.1% of the control values (P < 0.01), although there was no significant difference between ∆ku70 and ∆gng-1::gng-1 (Fig. 4C), suggesting that GNG-1 is positively involved in spore production in P. oxalicum. The number of spores produced by ∆gng-1::gng-11–85 was also lower than the number produced by ∆ku70, indicating that the CCAAX motif of GNG-1 was involved in spore production by P. oxalicum.
Liquid MMM containing glucose, Avicel, or SCS was inoculated with strain ∆gng-1 or ∆ku70 and incubated at 28 °C for 72 h to construct their growth curves. The biomass accumulation in the ∆gng-1 culture in MMM containing either glucose or SCS as the sole carbon source decreased markedly by 35.1–50.2% or 11.3–57.2%, respectively, of the control (∆ku70) value (both P < 0.05) after 12 h in culture (Fig. 5A and B). However, at 12 h, the mutants ∆gng-1 and ∆ku70 showed similar biomass accumulation in glucose (Fig. 5A). However, the biomass accumulated by ∆gng-1 in SCS medium was reduced to 49.9% of the control value (Fig. 5B). By contrast, in medium containing Avicel, the amount of intracellular proteins in ∆gng-1 was less than that in ∆ku70 at 12 h but similar to that in ∆ku70 at 24–48 h, although this was followed by a 22.4–30.3% reduction relative to that in the ∆ku70 (P < 0.05; Fig. 5C). Therefore, GNG-1 was involved in the growth of P. oxalicum, independently of the carbon source.
Growth curves of P. oxalicum mutant ∆gng-1 and parental strain ∆ku70. (A) Growth curves measured in MMM containing glucose; (B) growth curves measured in MMM containing SCS; (C) growth curves measured in MMM containing Avicel. The appropriate medium was directly inoculated with the asexual spores (1 × 108) of each P. oxalicum strain and incubated for 72 h at 28°C with shaking at 180 rpm. Data points represent means ± standard deviations. MMM: modified minimal medium; SCS: soluble corn starch
GNG-1 modulates the mRNA levels of major PBDE genes, known regulatory genes, fungal development-related genes, and sugar-transporter-encoding genes in P. oxalicum
RNA-Seq was used to investigate the genome-wide changes in mRNA expression that resulted from the deletion of the gng-1 gene in P. oxalicum. After preculture in glucose for 24 h, strains ∆gng-1 and ∆ku70 were cultured in MMM containing Avicel for 24 h, and their total RNAs were extracted and sequenced. The transcriptomic data generated were reliable, with a Pearson’s correlation coefficient of R2 > 0.96 and a principal components analysis for three biological repetitions of each strain (Supplementary Fig. S3). A comparative analysis of these transcriptomes identified 1329 differentially expressed genes (DEGs) (Supplementary Table S2), with thresholds of |log2(fold change)| ≥ 1.0 and q ≤ 0.05. These consisted of 739 upregulated and 590 downregulated genes. Kyoto Encyclopedia of Genes and Genomes (KEGG) annotation showed that approximately 80% of the DEGs were involved in metabolism, specifically carbohydrate metabolism and amino acid metabolism (Fig. 6A).
Transcriptomic analysis of P. oxalicum mutant Δgng-1 and the parental strain Δku70 grown in MMM containing Avicel as the sole carbon source. (A) Differentially expressed genes (DEGs) in Δgng-1 annotated with the KEGG database. DEGs were detected with thresholds of |log2(fold change)| ≥ 1 and q ≤ 0.05. (B) DEGs encoding PBDEs, putative transcription factors, and sugar transporters are shown with green, purple, and dark red lines, respectively. Red and blue circles indicate up- and downregulated DEGs in mutant Δgng-1. Sizes of circles were calculated as 10 × log2 (fold change). MMM: modified minimal medium; KEGG: Kyoto Encyclopedia of Genes and Genomes
Screening the 1329 DEGs identified 146 genes encoding putative CAZymes. The transcript levels of 63% of these genes reduced in ∆gng-1, with log2 (fold change) ranging from −5.5 to −1.0. Strikingly, among these 146 DEGs, 17 cellulase genes were detected, including cellobiohydrolase gene POX05587/Cel7A-2, endo-β-1,4-glucanase genes POX05571/Cel7B and POX07535/Cel12A, β-glucosidase gene POX06835/Cel3A, and six endo-β-1,4-xylanase genes, such as POX00063/Xyn10A and POX06783/Xyn11A. The transcripts levels of all these genes in ∆gng-1 showed 50.8 to 97.8% reduced transcripts in the ∆gng-1 compared with that in the parental strain ∆ku70 (Fig. 6B).
Interestingly, three amylase genes (raw-starch-degrading glucoamylase gene PoxGA15A [POX01356] and α-glucosidase genes POX03741 and POX08147) were also detected among the DEGs, and their transcript levels were reduced by 81.2%, 50.2%, and 73.0% in ∆gng-1, respectively (Fig. 6B).
It should be noted that the deletion of gng-1 must have altered the expression of TF-encoding genes because TFs directly control gene expression. A total of 41 TF-encoding DEGs were identified, including 19 upregulated (1.1 < log2 [fold change] < 7.3) and 22 downregulated genes (−2.9 < log2 [fold change] < -1.0). Nine genes (POX00972, POX02484, cxrB [POX04420], POX05145, POX05726, brlA [POX06534], flbD [POX07099], POX09124, and POX09469) have previously been shown to regulate PBDE production to various extents (Yan et al. 2017; Zhao et al. 2016, 2019a, 2019b; Qin et al. 2013). Of these, the mRNA levels of three genes (POX00972, POX02484, and cxrB) were downregulated by 66.3–81.5% in ∆gng-1 compared with their expression in ∆ku70, whereas the others were upregulated 2.2–85.7-fold in ∆gng-1 (Fig. 6B). CxrB positively regulates cellulase and xylanase production (Yan et al. 2017). BrlA negatively controls cellulase production and positively regulates fungal conidiation (Qin et al. 2013). In addition to brlA, another two genes that are required for fungal conidiation, abaA (POX07025) and flbD (Ojeda-López et al. 2018), showed 16-fold increases in their transcript levels in ∆gng-1.
Sugar transporters are required for the expression of PBDE genes in filamentous fungi, specifically cellodextrin transporters (Li et al. 2013). Therefore, the effects of gng-1 on the expression of genes encoding putative sugar transporters were investigated. We screened the 1329 DEGs annotated with the InterPro database using IPR005828 (major facilitator, sugar transporter-like), IPR005829 (sugar transporter, conserved site), and IPR003663 (sugar/inositol transporter). A total of 28 DEGs encoding putative sugar transporters were altered at the transcription level in ∆gng-1, with log2 (fold change) ranging from −2.7 to 6.6. Remarkably, the transcripts of the POX05915 gene encoding the cellodextrin transporter CdtD were reduced by 54.3%, whereas the POX07227 and POX07576 genes, encoding the homologues of the glucose transporters RCO-3 (Madi et al. 1997) and GLT1 (Wang et al. 2017a, b) of N. crassa, respectively, were upregulated by 20.4- and 53.7-fold, respectively (Fig. 6B).
Strikingly, transcripts of the POX00158 gene, which encodes a FUS3/FSS1-like MAP kinase, were reduced by 66.1% in the mutant ∆gng-1 compared with their expression in ∆ku70 (Supplementary Table S2).
To confirm the effects of gng-1 on the expression of PBDE genes in P. oxalicum, reverse transcription (RT)-real-time quantitative PCR (RT-qPCR) was used. P. oxalicum strains ∆gng-1 and ∆ku70 were cultured for 4–48 h in MMM containing Avicel at 28°C with shaking at 180 rpm. The tested genes are shown in Fig. 7. The expression of all the tested genes was downregulated by 27.7–99.5% in the mutant ∆gng-1 at 4–24 h of Avicel induction compared with their expression in ∆ku70. In contrast, at 48 h, the transcripts of POX06783/Xyn11A had decreased by 87.1%, whereas the transcript levels of POX01166/Cel5B, POX05571/Cel7B, POX03641, and POX06079 had increased by 23.8–116.1% (Fig. 7A).
Regulation of gene mRNA levels by GNG-1 in P. oxalicum detected with RT–qPCR. (A) The mRNA levels of key cellulase and xylanase genes in the mutant ∆gng-1 cultured in the presence of Avicel for 4–48 h. cbh: cellobiohydrolase gene; eg: endo-β-1,4-glucanase gene; bgl: β-1,4-glucosidase gene; xyn: endo-xylanase gene. (B) mRNA levels of known regulatory genes in mutant ∆gng-1 cultured in the presence of Avicel for 4–48 h. CxrB regulates the expression of cellulase and xylanase genes in P. oxalicum, whereas BrlA, AbaA, and FlbD are involved in asexual development. (C) mRNA levels of major amylase genes and their regulatory gene in mutant ∆gng-1 cultured in the presence of soluble corn starch for 4–48 h. Raw-starch-degrading enzyme gene, PoxGA15A; glucoamylase gene, POX02412; α-amylase gene, amy13A; regulatory gene, amyR. (D) cxrB mRNA levels in ∆gng-1::cxrB cultured in the presence of Avicel for 4–48 h. Gene mRNA levels in mutant ∆gng-1::cxrB were normalized against the levels in the parental strain Δku70. Data points represent means ± standard deviations. **P ≤ 0.01 and *P ≤ 0.05 on Student’s t test, indicating significant differences between the deletion mutant and the parental strain ∆ku70
The transcript levels of four key regulatory genes, cxrB, brlA, abaA, and flbD, were also investigated in P. oxalicum with RT–qPCR assays. The transcripts of cxrB were downregulated in the mutant ∆gng-1 during the whole period of culture, whereas the transcripts of abaA were upregulated. The transcripts of both brlA and flbD increased at 12–24 h but decreased again at 48 h (Fig. 7B).
Whether the expression of major amylase genes is affected by GNG-1 was analyzed with RT-qPCR. After preculture in glucose medium, P. oxalicum strains ∆gng-1 and ∆ku70 were cultured for 4–72 h in MMM containing SCS as the sole carbon source and their total RNA was extracted. The genes tested included PoxGA15A, POX02412, Amy13A (POX09352), and their regulatory gene amyR (POX03890). The mRNA levels of all the tested amylase genes were 2.2–22.6-fold higher in ∆gng-1 than in ∆ku70 at 4 h after induction. The expression of amyR was reduced by 35.4%. At 12 h, the transcripts of PoxGA15A and POX02412 had increased by 82.2% and 1.2-fold, respectively, in ∆gng-1, whereas those of amyR and Amy13A had decreased by 45.5% and 39.2%, respectively. After 12 h, the transcripts of all the tested genes decreased in ∆gng-1 by 18%–85.7% (Fig. 7C).
The requirement of GNG-1 for PBDE production, but not for fungal conidiation, is replaced by constitutive overexpression of the regulatory gene cxrB
The known regulatory gene cxrB was among the DEGs controlled by gng-1. Therefore, we speculated that the involvement of gng-1 in PBDE gene expression might depend on cxrB. To test this hypothesis, cxrB was constitutively overexpressed in the mutant ∆gng-1. The constructed strain ∆gng-1::cxrB was confirmed (Supplementary Fig. S1D) with PCR using specific primers (Supplementary Table S1). In ∆gng-1::cxrB, the transcription of cxrB increased by 45.5% to 3-fold after Avicel induction for 4–48 h compared with that in the ∆ku70 (Fig. 7D). Interestingly, the production of all cellulases and xylanases by ∆gng-1::cxrB in the presence of Avicel, and the production of SSDEs and RSDEs on SCS, were restored to the levels in both the parental strain ∆ku70 and the complementation strain ∆gng-1::gng-1 (P > 0.05; Fig. 2).
The phenotypes of ∆gng-1::cxrB on plates containing glucose, SCS, or Avicel or on PDA plates were similar to those of ∆ku70, except with a slight change in colony color (Fig. 3). Strangely, ∆gng-1::cxrB displayed early conidiophore production compared with that in ∆ku70 and ∆gng-1::gng-1 after culture for 20 h on all the carbon sources tested (Avicel, starch, and glucose) and PDA plates, similar to that in mutant ∆gng-1. The mutant ∆gng-1::cxrB showed fewer hyphal branches than ∆ku70 or ∆gng-1::gng-1 but more than ∆gng-1 (Fig. 4; Supplementary Fig. S2). Moreover, the numbers of asexual spores produced by mutant ∆gng-1::cxrB was restored to the level of spore production by ∆ku70 (Fig. 4C). These results indicate that CxrB, acting downstream from GNG-1, and its overexpression (partially) compensated for the deletion of gng-1 in PBDE production and fungal development.
CxrB positively regulates the production of RSDEs and SSDEs in P. oxalicum
GNG-1 affects amylase production of P. oxalicum, and constitutively expressed cxrB restored the amylase production of the mutant ∆gng-1 to the level in the parental strain ∆ku70, suggesting that CxrB regulates the production of amylase in P. oxalicum. To confirm this, the mutant ∆cxrB, parental strain ∆ku70, and complementation strain ∆cxrB::cxrB (Yan et al. 2017) were cultured at 28°C for 2–4 days in MMM containing SCS as the sole carbon source. Both SSDE and RSDE production in mutant ∆cxrB reduced by 32.6%–53.5% compared with that in the parental strain ∆ku70 (P < 0.01). The complementation strain ∆cxrB::cxrB secreted similar amounts of SSDEs and RSDEs to ∆ku70 (Fig. 8A), verifying that CxrB regulated amylase production in P. oxalicum.
Effects of CxrB on amylase production (A) and mRNA levels of essential amylase genes (B) and asexual-development-related genes (C) in P. oxalicum. After their transfer from glucose, fungal strains were cultured in MMM containing soluble corn starch for 2–4 days to test amylase production (A), for 4–48 h to test gene expression (B), or in MMM containing Avicel for 4–48 h (C) at 28°C with shaking at 180 rpm. Gene mRNA levels in mutant Δgng-1 were normalized against the levels in the parental strain Δku70. **P ≤ 0.01 and *P ≤ 0.05 by Student’s t-test, indicate significant differences between the deletion mutant and the parental strain ∆ku70. MMM: modified minimal medium; SSDE: soluble-starch-degrading enzyme; RSDE: raw-cassava-starch-degrading enzyme. Data points represent means ± standard deviations
CxrB indirectly regulates the mRNA levels of major amylase genes by controlling the regulatory gene amyR and directly regulates the expression of fungal development-related genes
When the mutant ∆cxrB was cultured in MMM containing SCS as the sole carbon source, the transcription levels of major amylase genes, including PoxGA15A, Amy13A, POX02412, and amyR, was investigated with RT-qPCR. The parental strain ∆ku70 was used as the control. At 4 h after induction, the mRNA levels of all the genes tested were upregulated by 92.0% to 7.3-fold in ∆cxrB compared with that in ∆ku70. At 12 h, the transcript levels of PoxGA15A and amy13A had increased by 180.1% and 70.1%, respectively, in ∆cxrB, whereas the other genes were downregulated by 50.8–51.8%. After induction for 12 h, all the tested genes in ∆cxrB were downregulated by 38.2–92.1%, except Amy13A at 24 h (P < 0.05; Fig. 8B).
An in vitro electrophoretic mobility shift assay (EMSA) was used to determine whether CxrB binds the promoter regions (500–1000 bp upstream) of major amylase genes and their regulatory gene amyR. A DNA fragment from gene POX05989, encoding β-tubulin, was used as the control. The putative DNA-binding domain at CxrB194–302, which contains two C2H2-type zinc fingers, was recombinantly expressed in Escherichia coli and purified, fusing with thioredoxin (Trx)-, a histidine (His)- and S-tag (Yan et al. 2017; Supplementary Fig. S4). No delayed band was observed when the recombinant protein rCxrB194–302 was tested with 6-FAM (fluorescein azide) -labeled probes from the three amylase genes, or the control proteins bovine serum albumin (BSA) and Trx–His–S, and DNA fragments competitive probes and control probe (Supplementary Fig. S5). Interestingly, delayed bands appeared when the amyR probe was added to 1.6–2.0 μg of rCxrB194–302. Neither BSA nor Trx–His–S interacted with the amyR probe. The competitive amyR probe, without 6-FAM, considerably impeded the interaction between rCxrB194–302 and the amyR probe (Fig. 9A). These data suggested that CxrB specifically bound the promoter region of amyR.
Interaction between CxrB and target genes revealed by in vitro electrophoretic mobility shift assay (EMSA). (A) Regulatory gene amyR. (B) Regulatory gene brlA. (C) Regulatory gene flbD. Recombinant protein rCxrB194–302 (0–2.0 μg) was mixed with 50 ng of FAM-labeled probes. In each EMSA reaction, nonspecific sheared salmon sperm DNA was added to prevent nonspecific binding between the protein and probes. Probes without the FAM label were used for the competitive EMSA. BSA, Trx–His–S fusion protein, or the promoter region of the β-tubulin gene alone were used as controls. FAM, 6-carboxyfluorescein; BSA, bovine serum albumin
In addition to amylolytic genes, the effects of CxrB on the mRNA levels of the fungal development-related genes brlA and flbD were observed under Avicel induction. The expression of brlA and flbD in the mutant ∆cxrB increased by 40.3% to 28-fold during the whole culture period compared with that in the parental strain ∆ku70 (P < 0.05; Fig. 8C). In vitro EMSA and competitive EMSA also confirmed that recombinant rCxrB194–302 specifically bound the promoter regions of brlA and flbD (Fig. 9B and C).
CxrB localizes in the nucleus
To visualize the subcellular localization of CxrB in P. oxalicum, recombinant strains carrying the green fluorescent protein (GFP)-labeled fusion protein CxrB–GFP were constructed with homologous recombination (Supplementary Fig. S6A). The expression of the fusion gene cxrB–gfp was controlled by the Avicel-induced promoter pPoxEGCel5B (Wang et al. 2018). The recombinant strains generated were confirmed with PCR (Supplementary Fig. S6B–F) using specific primers (Supplementary Table S1). When the various strains were cultured in MMM containing Avicel as the sole carbon source, the fluorescent GFP signals were predominantly detected in the cell nuclei and merged well with the fluorescence of 4′,6-diamidino-2-phenylindole (DAPI) (Fig. 10), which is used for the microscopic detection of nuclei and nuclear DNA in live cells (Tarnowski et al. 1991).
Subcellular localization of CxrB in P. oxalicum. Transformants expressing CxrB–GFP were precultured in MMM with Avicel as the sole carbon source for 48 h at 28°C with a shaking at 180 rpm, and the parental strain Δku70 was used as a control. The localization of CxrB was monitored by recording the GFP signal. Fungal nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI). The samples were observed under a fluorescence microscope (Olympus DP480; Olympus, Tokyo, Japan). Scale bar = 25 μm
Discussion
In this study, we characterized the roles of putative G protein γ subunit GNG-1 in P. oxalicum in detail. GNG-1 modulated PBDE (cellulase, xylanase and amylase) production in P. oxalicum under Avicel or SCS induction and the expression of major PBDE genes. Importantly, we have shown for the first time that GNG-1 affects the production of amylase and xylanase and the expression of major amylase and xylanase genes. GNG-1 modulates the expression of PBDE genes might through affecting the expression of regulatory gene cxrB. CxrB directly regulates the expression of major cellulase and xylanase genes on Avicel (Yan et al. 2017) and indirectly regulates the transcripts of major amylase genes through controlling the expression of another regulatory gene amyR under SCS induction in P. oxalicum (Fig. 11). AmyR is required for amylase production by directly regulating the major amylolytic genes, such as the α-amylase gene amy13A and the glucoamylase gene amy15A, in P. oxalicum, Aspergillus sp., and Talaromyces pinophilus under starch induction (Li et al. 2015; Zhang et al. 2017; Ma et al. 2021).
Schematic model of the proposed roles of G protein gamma subunit GNG-1 modulated by the downstream transcription factor CxrB in P. oxalicum. Dashed line indicates that how GNG-1 modulates the expression of cxrB is unknown. Bold lines with arrows represent activation and barred lines represent inhibition
A question attacks our attention that how GNG-1 mediates the expression of cxrB. A possible mechanism is that GNG-1 activates downstream MAP kinase and cAMP-dependent signal cascades that modulate the expression of cellulase and xylanase genes (Schmoll 2018; Ma et al. 2021; Wang et al. 2017a, b). The Gβ could interact with a MAPK scaffold Ste5 that anchored MAPKKK Ste11, MPKK Ste7 and MAPK Fus3 together (Alvaro and Thorner 2016). Recently, MAP kinase PoxMK1 as a homolog of Fus3 positively controlled the expression of cxrB in P. oxalicum (Ma et al. 2021). However, the exact signal pathway of G protein on regulation of cellulase and xylanase under Avicel induction still needs to be further studied.
It should also be noted that the deletion of gng-1 in P. oxalicum reduced cellulase production, whereas the deletion of its orthologue gng-1 in T. reesei enhanced cellulase production (Tisch et al. 2011). In T. reesei, the Gβ and Gγ with their putative chaperone PhLP1 negatively influence the abundance of rgs1 transcript, which leads to improved activity of the Gα GNA1. Deletion of gna-1 abolishes the expression of major cellulase gene in light but enhances it in darkness. Surprisingly, constitutive activation of GNA1 only enhanced the expression of cellulase genes in the presence of both cellulose and light (Schmoll 2018). Surprisingly, mutant ∆gng-1 of T. reesei reduced the transcriptional levels of major cellulase genes but increased cellulase secretion, which might be due to post-transcriptional regulation (Tisch et al. 2011). However, there are insufficient evidence supporting those conclusions thus far, and the detail studies must be carried out in future. Clearly, signal transduction and regulation of cellulase gene expression in T. reseei are different from that in P. oxalicum.
Moreover, the C-terminal CCAAX motif contributed to the production of cellulase, hyphal conidiospores, and asexual spores, but not to hyphal branching in P. oxalicum. When Gβγ is released from the activated heterotrimeric G protein, it activates the downstream MAP kinase cascade, which occurs at the plasma membrane because Gβγ is firmly tethered there by the CCAAX motif in Gγ, with the aforementioned lipophilic moieties (Alvaro and Thorner 2016). The lack of the CCAAX motif affects the anchoring of Gβγ to the plasma membrane, altering the downstream signal transduction pathways. The mutant ∆gng-1::gng-11–85 retained approximately 70% of the cellulase production of its parental strain ∆ku70, implying that an alternative pathway activated by Gγ or an Gγ-independent pathway is also involved in the regulation of cellulase gene expression in P. oxalicum. Strangely, the C-terminal CCAAX motif did not contribute to xylanase production, which may be attributable to the balance of the regulatory network formed by downstream TFs. It should be noted that these results may also be attributable to the different levels of mRNA and protein expressed from an alternative locus (POX05007) or the involvement of the cAMP pathway, which is modulated by Gα, but these possibilities require further study.
In addition to PBDE production, GNG-1 was also required for the vegetative growth and conidiation of P. oxalicum and the expression of related genes, such as brlA and flbD, as has been reported in A. nidulans, T. reesei, and N. crassa (Tisch et al. 2011; Krystofova and Borkovich 2005; Seo et al. 2005). Importantly, in this study, we have also shown that CxrB directly bound the promoter regions of both brlA and flbD to repressed their expression under Avicel induction (Fig. 11). BrlA and FlbD are essential for asexual sporulation in filamentous fungi, and FlbD activates the expression of brlA (Qin et al. 2013; Arratia-Quijada et al. 2012). The deletion of either cxrB or brlA affects the vegetative growth of P. oxalicum (Yan et al. 2017; Qin et al. 2013). Although the early conidiation in ∆gng-1 may result from the increased expression of brlA and flbD, the number of asexual spores produced by ∆gng-1 significantly reduced, which might be attributable to the reduced mycelial biomass of ∆gng-1. The overexpression of cxrB in the mutant ∆gng-1 partly restored hyphal branching, but did not restore conidiation to the level in ∆ku70, thus leading to spore production similar to that in ∆ku70. Other possible mechanisms still require investigation.
In summary, we found that GNG-1 modulated the expression of PBDE genes and mycelial-development-related genes, which depends on the implementation of the downstream transcription factor CxrB, and in this way, positively affected PBDE production and fungal development. The generated results extended the diversity of Gγ protein functions and provided new insight into the signal transduction and regulation of PBDE gene expression in filamentous fungi.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Alvaro CG, Thorner J (2016) Heterotrimeric-G-protein-coupled receptor signaling in yeast mating pheromone response. J Biol Chem 291:7788–7795
Arratia-Quijada J, Sánchez O, Scazzocchio C, Aguirre J (2012) FlbD, a Myb transcription factor of Aspergillus nidulans, is uniquely involved in both asexual and sexual differentiation. Eukaryot Cell 11:1132–1142
Benocci T, Aguilar-Pontes MV, Zhou M, Seiboth B, de Vries RP (2017) Regulators of plant biomass degradation in ascomycetous fungi. Biotechnol Biofuels 10:152
Chakravorty D, Assmann SM (2018) G protein subunit phosphorylation as a regulatory mechanism in heterotrimeric G protein signaling in mammals, yeast, and plants. Biochem J 475:3331–3357
Chen YX, Chen YS, Shi CM, Huang ZB, Zhang Y, Li SK, Li Y, Ye J, Yu C, Li Z, Zhang XQ, Wang J, Yang HM, Fang L, Chen Q (2018) SOAPnuke. a MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 7:1–6
Gougoulias C, Clark JM, Shaw LJ (2014) The role of soil microbes in the global carbon cycle: tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J Sci Food Agric 94:2362–2371
Hu YB, Liu GD, Li ZH, Qin YQ, Qu YB, Song X (2013) G protein-cAMP signaling pathway mediated by PGA3 plays different roles in regulating the expressions of amylases and cellulases in Penicillium decumbens. Fungal Genet Biol 58–59:62–70
Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12:357–360
Krystofova S, Borkovich KA (2005) The heterotrimeric G-protein subunits GNG-1 and GNB-1 form a Gβγ dimer required for normal female fertility, asexual development, and Ga protein levels in Neurospora crassa. Eukaryot Cell 4:365–378
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359
Li B, Dewey CN (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12:323
Li LD, Wright SJ, Krystofova S, Park G, Borkovich K (2007) Heterotrimeric G protein signaling in filamentous fungi. Annu Rev Microbiol 61:423–452
Li J, Liu GD, Chen M, Li ZH, Qin YQ, Qu YB (2013) Cellodextrin transporters play important roles in cellulase induction in the cellulolytic fungus Penicillium oxalicum. Appl Microbiol Biotechnol 97:10479–10488
Li ZH, Yao GS, Wu RM, Gao LW, Kan QB, Liu M, Yang P, Liu GD, Qin YQ, Song X, Zhong YH, Fang X, Qu YB (2015) Synergistic and dose-controlled regulation of cellulase gene expression in Penicillium oxalicum. PLoS Genet 11(9):e1005509
Li ZH, Liu GD, Qu YB (2017) Improvement of cellulolytic enzyme production and performance by rational designing expression regulatory network and enzyme system composition. Bioresour Technol 245:1718–1726
Li CX, Zhao S, Luo XM, Feng JX (2020) Weighted gene co-expression network analysis identifies critical genes for the production of cellulase and xylanase in Penicillium oxalicum. Front Microbiol 11:520
Liu YH, Yang KL, Qin QP, Lin GN, Hu TR, Xu ZL, Wang S (2018) G protein α subunit GpaB is required for asexual development, aflatoxin biosynthesis and pathogenicity by regulating cAMP signaling in Aspergillus flavus. Toxins 10:117
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods 25(4):402–408
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550
Ma B, Ning YN, Li CX, Tian D, Guo H, Pang XM, Luo XM, Zhao S, Feng JX (2021) A mitogen-activated protein kinase PoxMK1 mediates regulation of the production of plant-biomass-degrading enzymes, vegetative growth, and pigment biosynthesis in Penicillium oxalicum. Appl Microbiol Biotechnol 105:661–678
Madi L, McBride SA, Bailey LA, Ebbole DJ (1997) rco-3, a gene involved in glucose transport and conidiation in Neurospora crassa. Genetics 146:499
Ojeda-López M, Chen W, Eagle CE, Gutiérrez G, Jia WL, Swilaiman SS, Huang Z, Park HS, Yu JH, Cánovas D, Dyer PS (2018) Evolution of asexual and sexual reproduction in the aspergilli. Stud Mycol 91:37–59
Powers-fletcher MV, Kendall BA, Griffin AT, Hanson KE (2016) Filamentous fungi. Microbiol Spectr 4(3):DMIH2-0002-2015
Qin YQ, Bao LF, Gao MR, Chen M, Lei YF, Liu GD, Qu YB (2013) Penicillium decumbens BrlA extensively regulates secondary metabolism and functionally associates with the expression of cellulase genes. Appl Microbiol Biotechnol 97:10453–10467
Schmoll M (2018) Regulation of plant cell wall degradation by light in Trichoderma. Fungal Biol Biotechnol 5:10
Schmoll M, Schuster A, Silva RN, Kubicek CP (2009) The G-alpha protein GNA3 of Hypocrea jecorina (anamorph Trichoderma reesei) regulates cellulase gene expression in the presence of light. Eukaryot Cell 8(3):410–420
Schuster A, Tisch D, Seidl-Seiboth V, Kubicek CP, Schmoll M (2012) Roles of protein kinase A and adenylate cyclase in light-modulated cellulase regulation in Trichoderma reesei. Appl Environ Microbiol 78:2168–2178
Seibel C, Gremel G, Silva RN, Schuster A, Kubicek CP, Schmoll M (2009) Light-dependent roles of the G-protein alpha subunit GNA1 of Hypocrea jecorina (anamorph Trichoderma reesei). BMC Biol 7:58
Seo JA, Han KH, Yu JH (2005) Multiple roles of a heterotrimeric G-protein γ-subunit in governing growth and development of Aspergillus nidulans. Genetics 171:81–89
Tarnowski BI, Spinale FG, Nicholson JH (1991) DAPI as a useful stain for nuclear quantitation. Biotech Histochem 66(6):297–302
Tisch D, Kubicek CP, Schmoll M (2011) The phosducin-like protein PhLP1 impacts regulation of glycoside hydrolases and light response in Trichoderma reesei. BMC Genomics 12:613
Wang P, Nuss DL (1995) Induction of a Cryphonectria parasitica cellobiohydrolase I gene is suppressed by hypovirus infection and regulated by a GTP-binding-protein-linked signaling pathway involved in fungal pathogenesis. Proc Natl Acad Sci U S A 92:11529–11533
Wang B, Li J, Gao J, Cai P, Han X, Tian C (2017a) Identification and characterization of the glucose dual-affinity transport system in Neurospora crassa: pleiotropic roles in nutrient transport, signaling, and carbon catabolite repression. Biotechnol Biofuels 10:17
Wang MY, Zhang ML, Li L, Dong YM, Jiang Y, Liu KM, Zhang RQ, Jiang BJ, Niu KL, Fang X (2017b) Role of Trichoderma reesei mitogen-activated protein kinases (MAPKs) in cellulase formation. Biotechnol Biofuels 10:99
Wang L, Zhao S, Chen XX, Deng QP, Li CX, Feng JX (2018) Secretory overproduction of a raw starch-degrading glucoamylase in Penicillium oxalicum using strong promoter and signal peptide. Appl Microbiol Biotechnol 102(21):9291–9301
Yan YS, Zhao S, Liao LS, He QP, Xiong YR, Wang L, Li CX, Feng JX (2017) Transcriptomic profiling and genetic analyses reveal novel key regulators of cellulase and xylanase gene expression in Penicillium oxalicum. Biotechnol Biofuels 10:279
Zhang Z, Liu JL, Lan JY, Duan CJ, Ma QS, Feng JX (2014) Predominance of Trichoderma and Penicillium in cellulolytic aerobic filamentous fungi from subtropical and tropical forests in China, and their use in finding highly efficient beta-glucosidase. Biotechnol Biofuels 7:107
Zhang T, Zhao S, Liao LS, Li CX, Liao GY, Feng JX (2017) Deletion of TpKu70 facilitates gene targeting in Talaromyces pinophilus and identification of TpAmyR involvement in amylase production. World J Microbiol Biotechnol 33:171
Zhao S, Yan YS, He QP, Yang L, Yin X, Li CX, Mao LC, Liao LS, Huang JQ, Xie SB, Nong QD, Zhang Z, Jiang L, Xiong YR, Duan CJ, Liu JL, Feng JX (2016) Comparative genomic, transcriptomic and secretomic profiling of Penicillium oxalicum HP7-1 and its cellulase and xylanase hyper-producing mutant EU2106, and identification of two novel regulatory genes of cellulase and xylanase gene expression. Biotechnol Biofuels 9:203
Zhao S, Liao XZ, Wang JX, Ning YN, Li CX, Liao LS, Liu Q, Jiang Q, Gu LS, Fu LH, Yan YS, Xiong YR, He QP, Su LH, Duan CJ, Luo XM, Feng JX (2019a) Transcription factor Atf1 regulates expression of cellulase and xylanase genes during solid-sate fermentation of ascomycetes. Appl Environ Microbiol 85:e01226–e01219
Zhao S, Liu Q, Wang JX, Liao XZ, Guo H, Li CX, Zhang FF, Liao LS, Luo XM, Feng JX (2019b) Differential transcriptomic profiling of filamentous fungus during solid-state and submerged fermentation and identification of an essential regulatory gene PoxMBF1 that directly regulated cellulase and xylanase gene expression. Biotechnol Biofuels 12:103
Acknowledgements
We thank Core Facility Center, specifically Mass Spectrometry Technology Platform from State Key Laboratory for Conservation and Utilization of Subtropical Agro-bioresources, Guangxi University, for technological support.
This work was financially supported by the grants from the National Natural Science Foundation of China (grants 31760023 and 31660305) to JXF and SZ, the Autonomous Research Project of State Key Laboratory for Conservation and Utilization of Subtropical Agro-bioresources (SKLCUSA-a201902 and SKLCUSA-a201923) to JXF, Training Program for 1000 Young and Middle-aged Key Teachers in Guangxi at 2019, and the One Hundred Person Project of Guangxi to SZ.
Author information
Authors and Affiliations
Contributions
SZ designed, supervised this study, and revised the manuscript. XMP performed mutant construction and measurement of enzymatic production, analysis of gene expressional levels and manuscript preparation. DT, TZ, and LSL were involved in mutant construction and data discussion. CXL carried out bioinformatic analyses. XML was involved in preparation of experimental materials. JXF was involved in data analysis and revised manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 1751 kb)
Rights and permissions
About this article
Cite this article
Pang, XM., Tian, D., Zhang, T. et al. G protein γ subunit modulates expression of plant-biomass-degrading enzyme genes and mycelial-development-related genes in Penicillium oxalicum. Appl Microbiol Biotechnol 105, 4675–4691 (2021). https://doi.org/10.1007/s00253-021-11370-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11370-3