Abstract
Four independent mAb-producing CHO cell lines were grown in media supplemented with one of seven protein hydrolysates of animal and plant origin. This generated a 7x4 matrix of replicate cultures which was analysed for viable cell density and mAb productivity. In all cultures, a consistent growth rate was shown in batch culture up to 4 to 5 days. Differences between cultures appeared in the decline phase which was followed up to 7 days beyond the start of the cultures. There was a marginal but significant overall increase (x1.1) in the integral viable cell density (IVCD) in the presence of hydrolysate but a more substantial increase in the cell-specific mAb (qMab) productivity (x1.5). There were individual differences between hydrolysates in terms of enhancement of mAb productivity, the highest being a 166% increase of mAb titre (to 117 mg/L) in batch cultures of CHO-EG2 supplemented with UPcotton hydrolysate. The effect of one of the most active hydrolysates (HP7504) on antibody glycosylation was investigated. This showed no change in the predominant seven glycans produced but a significant increase in the galactosylation and sialylation of some but not all the antibodies. Overall, the animal hydrolysate, Primatone and two cotton-derived hydrolysates provided the most substantial benefit for enhanced productivity. The cotton-based hydrolysates can be viewed as valuable supplements for animal-derived component-free (ADCF) media and as a source for the investigation of chemically defined bioactive components.
Key points
• Protein hydrolysates enhanced both IVCD & qMab; the effect on qMab being consistently greater.
• Cotton-based hydrolysates showed high bioactivity and potential for use in serum-free media.
• Enhanced galactosylation and sialylation was shown for some of the Mabs tested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rapid expansion of the biopharmaceutical market provides a fruitful avenue for the fast-track drug development and wide expansion of manufacturing of recombinant proteins-based therapies (Lingg et al. 2012). Chinese ovary hamster (CHO) cells have been the workhorse of producer cell lines that show unprecedented ability to express a diverse range of therapeutic proteins with a high production efficiency and great volumetric productivity. The huge clinical demands for such therapeutic products and the expansion of the global markets necessitate building up a solid production platform that can enhance the productivity while curtailing the cost of manufacturing (O’Flaherty et al. 2020).
While the selection of a high producing host cell clone is the main step in increasing the volumetric cell yield and minimising the cost of production (Jiang et al. 2019), the development of cell culture medium is an integral step in bioproduction. It plays a crucial role not only in supporting the growth and productivity of cells in a cost effective manner, but also in minimising batch to batch variation during production (Yao and Asayama 2017).
One of the most important developments in designing culture media has been the replacement of foetal bovine serum (FBS) by non-animal-derived components that have comparable growth promoting effects. There are distinct advantages of using FBS as a sole component to add to basal media to, provide essential components including amino acids, lipids, vitamins, hormones and growth factors that can boost cells growth and productivity (Gstraunthaler et al. 2013). However, due to the presence of ill-defined components in serum, batch-to batch variation and the potential presence of microbial contamination, the use of serum has been largely disbanded in the commercial production of therapeutics (Dimasi 2011; Urbano and Urbano 2007; Doucet et al. 2005). Although serum-supplemented media can support the growth of a range of cell lines, serum-free formulations are usually specific for certain cell types (Butler 2015).
Various types of serum free media (SFM) have been developed to overcome the drawbacks of serum-containing media (Butler 2013). These include animal component-free media, protein-free media and chemically defined media (Griffiths et al. 2013). Although CD media entirely free from non-animal sources are ideal for consistency and reducing the contamination risk, their performance is often compromised. Growth factors and plant-based hydrolysates are sometimes required to allow efficient cell growth and recombinant protein production (Bauman et al. 2018; Usta et al. 2014; Ritacco et al. 2018; Butler 2013).
Protein hydrolysates are derived from the controlled acidic or enzymatic breakdown of raw material from plants, microorganisms or animals. This results in complex mixtures of nutrients and growth factors that are produced by protein sources through partial acidic or enzymatic hydrolysis. Some of the hydrolysates have been developed in the food industry as flavour enhancers prepared by acid hydrolysis (Nagodawithana et al. 2008). Generally, hydrolysates offer an inexpensive and naturally-occurring source of nutrients including peptides, carbohydrates, nucleotides, lipid, trace metals, vitamins and minerals. Several reports show the importance of hydrolysates to provide extra nutritional supports for the promotion of cell growth and mAb yield. In addition, such media supplements influence the mAb glycosylation qualitatively and quantitatively, which ultimately affects the IgG therapeutic effect (O’Flaherty et al. 2020). Bioactive peptides embedded in the sequence of proteins may be released as active components, although other small molecules contained in these hydrolysates may provide significant activity (Spearman et al. 2016). However, the composition of hydrolysates can vary from batch to batch (Ganglberger et al. 2007; Park et al. 2010). Such variation can not only alter cell productivity, but also can alter product composition, such as the IgG glycosylation, which can affect the therapeutic responses (Landauer 2014).
Protein hydrolysates have been known for some time to support mammalian cell growth and productivity. The major sources of hydrolysates include meat, milk, soy, cottonseed, rice, wheat, chickpea, corn and algae (Galanakis 2019; Chalamaiah et al. 2018; Bhat et al. 2015; Hou et al. 2017). One of the original protein hydrolysates used in mammalian cell culture was A tryptic meat digest (Primatone) which was shown in several studies to improve the culture of a variety of murine cells including a two-fold increase in cell density and antibody production by enhancing cell growth while inhibiting cell death (Schlaeger 1996; Bonarius et al. 1996). Primatone contains a complex mixture of short and long peptides, carbohydrates, metals, lipids and vitamins into which cells can adapt easily from serum-supplemented basal media (Schlaeger 1996; Spearman et al. 2014). However, the potential risk of animal-sourced material introducing adventitious contaminants such as viruses and prions into therapeutic products has prevented the wide-spread use of Primatone in large-scale bioprocesses (Burteau et al. 2003; Butler 2004; Gu et al. 1997; Butler 2013; Jayme and Smith 2000)
This has led to the development of several plant- and microbial-derived hydrolysates. Yeast hydrolysates have been widely used to stimulate the growth of mammalian and insect cells in serum-free media (Jagschies et al. 2018; Spearman et al. 2016). Soy hydrolysate is a good example of a low-cost plant-sourced material to supplement media in large-scale bioprocesses. This was introduced to the market in 2006 as a potential supplement for mammalian cell bioprocesses and produced by a controlled series of enzymatic steps shown to be effective in reducing batch variability(Siemensma et al. 2010). There are now a series of plant-derived hydrolysates available as supplements in serum-free media of mammalian cells. These include soy, wheat, pea, rice and cotton, many of which have shown bioactivity in enhancing the growth or productivity of specific animal cells (Ballez et al. 2004; Heidemann et al. 2000; Burteau et al. 2003; Girón-Calle et al. 2008; Kim and Lee 2009; Sung et al. 2004), This includes the widely used NSO and Chinese hamster ovary (CHO) cells (Butler and Meneses-Acosta 2012).
In the present study, we assess and compare the impact of seven different hydrolysates on three constitutive and one inducible antibody-producing CHO cell lines. We analyse the effect of these hydrolysates on the integral viable cell density in batch cultures over 7 to 9 days as well as the overall antibody titre. Finally, we assess the effect of one hydrolysate on the IgG glycosylation. Statistical analysis of this data allowed us to distinguish the independent effects of the hydrolysates on cell growth and cell-specific antibody production. This allows us to identify different types of bioactivity from these hydrolysates.
Materials and methods
Cell lines
Four different CHO cell lines that produce monoclonal antibody (mAb) were employed in this study.
-
(a)
iCHO-RTX was kindly provided by Yves Durocher (National Research Council of Canada). This cell line was developed with the glutamine synthetase (GS-) selection system for rituximab production (Poulain et al. 2017). The cells require cumate to induce gene expression to produce antibody (Mullick et al. 2006).
-
(b)
CHO-S-RTX was kindly provided by the Technical University of Denmark. This cell line was generated by transfecting of CHO-S cells with a rituximab plasmid (Pristovšek et al. 2018).
-
(c)
CHO EG2 1A7 was kindly provided by the National Research Council of Canada. It was originally derived from the CHODXB11 cell line (Agrawal et al. 2012) that produces EG2-hFc (Bell et al. 2010). The Mab product is a chimeric heavy chain antibody EG2-hFc constructed by fusing a camelid heavy chain antibody or single domain antibody to the Fc portion of a human mAb (Zhang et al. 2009).
-
(d)
CHO-Kerry was kindly provided by the Kerry group. These cells are Mab-producing and derived from CHO-DG44 with the dihydrofolate reductase (DHFR-) system.
Culture media
All cell lines were maintained in 125-ml conical shake flasks (Thermo Fisher Scientific, Grand Island, NY, USA) at a shaking speed of 120 rpm using Biogro-CHO serum-free chemically defined medium (Biogro Technologies, Waverley St, Winnipeg, MB, Canada) except CHO-Kerry which was maintained and cultured in Kerry CD serum-free chemically defined medium (Kerry, Millington Rd, Beloit, WI, USA). The cells were passaged every 3-4 days by diluting the culture to 0.3 × 106 cells/ml. Culture media were supplemented with 8 mM glutamine (Gln) (Sigma Aldrich, St. Louis, MO, USA), 0.2% commercial anti-clumping agent (A/C) (Irvin, Newtownmountkennedy, Co. Wicklow, Ireland).
Cultures of the inducible iCHO-RTX cell line were supplemented with 50 μM L-methionine sulfoximine (MSX) (Sigma Aldrich, St. Louis, MO, USA) to maintain selective pressure. Cumate (4-isopropyl benzoic acid; Ark Pharm, Arlington Heights, USA) was added at the onset of culture at a final concentration of 2 μg/ml in experimental cultures to produce mAb.
Cultures of CHO-Kerry cells were supplemented with methotrexate (Sigma Aldrich, St. Louis, MO, USA) at a final concentration of 25 nM to maintain selective pressure. Hypoxanthine (HX) and thymidine (TH) (Sigma Aldrich, St. Louis, MO, USA) were added at final concentrations of 10 mg/L each to these cells during culture experiments.
The osmolality of all media was checked using the automatic cryoscopic Osmomat 030 and following the manufacturer instructions
Hydrolysates
A range of commercial hydrolysates were provided by the Kerry group (Millington Rd, Beloit, WI, USA) (Table S1) as follows: Hypep1510 (soy-derived), Hypep7504 (cotton-derived) Hypep1512 (soy-derived), Hypep4601n (wheat-derived), Ultrapep Cotton (cotton-derived), Hypep5603n (rice-derived) and Primatone P37 (animal/meat-derived). Following preliminary experiments to determine the optimal hydrolysate concentration for cell growth, each hydrolysate was supplemented to experimental cultures at 5 g/L from stock solutions of 20 g/L made up in the corresponding medium. The hydrolysate stock solutions were filtered and sterilised using 0.2-μM filter (Thermo Fisher Scientific, Grand Island, NY, USA).
Experimental cultures supplemented with the hydrolysates
Cells were seeded at inoculation density of 0.4 × 106 cells/ml in 50-ml cell culture tubes (Greiner CELLSTAR, Cruinn, Cherry Orchard, Dublin, Ireland) containing 6 ml of culture media alone or with added 5 g/l of the indicated hydrolysates in duplicates. The tubes were incubated at 5% CO2 incubator at a shaking speed of 250 rpm. Viable cell density was determined at daily intervals from cell suspension samples (11 μl) mixed with trypan blue reagent (1:1 v/v) (Sigma Aldrich, St. Louis, MO, USA) with a Luna II automated cell counter (Dongan-gu Anyang-si, Gyeonggi-do 14055, South Korea) and evaluated by a standard method (Patterson Jr 1979).
Metabolite analysis
Media substrates and metabolic by-products including mAb, lactate, glutamine, glutamate, ammonia and lactate were measured using the bioprocess analyser CEDEX Bio (Roche, Mannheim, Germany) following the manufacturer’s instructions after centrifugation of the sample for 5 min at 10,000 rpm. Total mAb were measured at days 4, 5, 6 and 7, which represented the period from the beginning of the stationary phase until the end of the culture for the cell lines (iCHO-RTX, CHO-S-RTX and CHO-EG2). For CHO-Kerry, samples were measured at days 4 and 5 (beginning of stationary phase) and days 8 and 9 (the end of the culture)
Specific rate of production
Cell-specific productivity (qMab) values were determined from the linear gradients of plots of product concentration against integral of viable cell density time (IVCD) from time zero (Renard et al. 1988; Rodriguez et al. 2005). These values as well as specific growth rate μ and doubling times Td were determined as previously shown (Chusainow et al. 2009; Chida et al. 2013).
Glycan analysis
The glycans of IgGs produced by the cells in the control and hydrolysate HP7504-supplemented media were characterised to assess the influence of the hydrolysate on glycosylation pattern. The IgG samples were collected on day 5 of culture growth.
To characterise the glycan profile, 50 μg of each IgG was loaded on a protein A HP SpinTrap™ column (Cytiva, Freiburg, Germany) with phosphate buffer saline (PBS) pH 7.4 and incubated at room temperature for 15 min on a thermomixer (Eppendorf, Hamburg, Germany). The columns were centrifuged to remove the buffer and washed three times with PBS. Fresh PBS buffer was loaded onto the columns, a volume of 2 μL PNGase F glycerol free, recombinant (New England Biolabs, Ipswich, MA, USA) was loaded and the columns were incubated for 24 h at 37 °C on a thermomixer at 600 rpm. The released glycans were collected, filtered using a 10 K centrifugal filter (EMD Millipore Corporation, Billerica, MA, U.S.A.) dried and then labelled with 5uL of procainamide labelling solution (40 mg of procainamide, 25 mg of NaBH3CN, 280 μl DMSO, 120 μl acetic acid and 100 μl water) at 65 °C for 1 h. The labelled samples were further purified with 6 × 1 mL of acetonitrile using a HyperSep Diol™ 50 mg/1 mL 96-Removable Well Plate (Thermo Fischer Scientific) to remove the excess of procainamide. The cleaned labelled glycans were eluted with 1 mL of water and dried.
In sequence the glycans were analysed using a Waters ACQUITY I-class Bio UPLC system (Waters Corporation, Milford, MA, USA) consisting of a bi-quaternary solvent manager, a sample manager, set to 5 °C, a column manager, operating at 40 °C, and a fluorescence (FLR) detector (310 nm excitation wavelength, 370 nm emission wavelength). The glycans were resuspended in 30:70 μL of water/acetonitrile and 1 μL injected for analysis in a Waters ACQUITY UPLC using a Glycan BEH Amide column (2.1 × 150 mm, packed with 1.7 um particle size, 130 Å pore size) (Waters Corporation, Milford, MA, USA). Mobile phase A was 50 mM ammonium formate solution (pH 4.4) and Mobile phase B was acetonitrile (ACN). Analyte separation was accomplished by gradient elution using a gradient running from 70%-53% (v/v) Mobile phase B over 25 min at a flow rate of 0.5 mL/min.
Procainamide labelled standard (Ludger, Oxfordshire, UK) was used as a standard to assign the GU values for sample peaks. The standard was reconstituted in 300 μL of water, followed by a second dilution of 1:5 in water. Six microlitres of this solution were diluted in 14 μL of ACN and 10 μL were injected for UPLC analysis.
Data was analysed using Empower 3 software. Glycostore database was used to correlate the sample GU values with corresponding glycan structures in the database (www.glycostore.org).
The galactosylation index (GI) was calculated as the number of galactose residues on glycan structures: G0—no galactose, G1—one galactose and G2—2 galactose residues.
-
The sialylation Index was calculated by including all the sialic acid molecules on the glycan structure. S0—no sialic acid, S1—one sialic acid and S2—2 sialic acid residues:
$$ \boldsymbol{SI}=\frac{\left(\boldsymbol{S}\mathbf{1}\ast \mathbf{0}.\mathbf{5}+\boldsymbol{S}\mathbf{2}\right)}{\boldsymbol{S}\mathbf{0}+\boldsymbol{S}\mathbf{1}+\boldsymbol{S}\mathbf{2}} $$
Statistical analysis
Statistical analysis was performed using Graphpad Prism 5.0 (Obaidi et al. 2018). Data were analysed using paired t-test to compare the overall effect of each individual hydrolysate and control. One-way analysis of variance (Fujisawa et al. 2014) was also employed to compare control and hydrolysate-treated groups using the Dunnett multiple comparison post-test. Results were expressed as the mean ± standard error (SEM) of three independent experiments. A probability of 0.05 or less was considered statistically significant.
Results
The impact of the hydrolysates on CHO cells’ growth profile
The impact of seven hydrolysates on the growth of four different CHO cell lines was evaluated (Fig. 1). The cells were seeded into 4 different media formulations supplemented with each of the 7 hydrolysates (5 g/L). The cultures were monitored daily for cell density and viability through growth, stationary and decline phases until the population viability decreased to around 30-50% which occurred after 7 to 9 days. There was no evidence of a significant lag phase in any of the cultures and exponential growth proceeded at a mean specific growth rate of 0.032 ± 0.003 day−1 that corresponds to a doubling time of 22 h. There was no significant difference in the maximum specific growth rate measured between any of the cultures as determined by paired t-test except for the CHO-S-RTX cells with HP4601n, where we measured a slightly but significantly (p < 0.05) higher doubling time compared with control and HP7504-supplemented culture (Table S2).
Effects of the hydrolysates on CHO cell growth. CHO cells (a iCHO-RTX, b CHO-S-RTX, c CHO-EG2 and d CHO-Kerry) were seeded at a density of 0.4 × 106 cells/ml in Biogro medium (a–c) or Kerry CD medium (d) with added 5 g/L hydrolysate as indicated. Cultures were continued for 7–9 days until the viability reduced to ~ 30%. Samples (11 μl) were taken at daily intervals to determine viable cell density and viability. Data are presented as mean ± SEM of three independent experiments, and analysed using paired t-test; *, **, ***Statistically significant differences relative to the untreated control at p < 0.05, 0.01 and 0.001 respectively
For 3 of the 4 cell lines, a plateau of cell density was reached after ~ 4 days with the CHO-Kerry cells continuing growth until day 7. The peak of VCD varied between cell lines from 2.2 × 106 cells/ml to 8.0 × 106 cells/ml, the higher value occurring with the CHO-Kerry cell line because of the longer growth phase up to day 7. After the maximum VCD, the cells entered a decline phase the steepness of which was highly dependent upon the hydrolysate supplement. The viability of the cell populations was > 98% prior to the decline phase in all cultures. The bioactivity of the supplemented hydrolysates appeared in their ability firstly to allow continued cell growth to a higher VCD and secondly to reduce the rate of the decline phase. Either of these activities would lead to a higher overall integrated viable cell density (IVCD) which is a measure of the sustainability of VCD and viability over the period of culture. The growth profiles show a decline in viability and total viable cell density after 4 days in all cases but at different rates that were reflected in the overall measured IVCD (Fig. 1 and Fig. S1).
The CHO-Kerry culture showed the highest enhanced VCD of 8.5 × 106 cells/ml which was produced in the presence of HP1510. This compared with peak VCDs 4.7 × 106, 4.1 × 106 and 3.7 × 106 cells/ml for the iCHO-RTX, CHO-S-RTX and CHO-EG2 cell lines respectively in the presence of HP7504 or Primatone which substantially enhanced values compared with controls.
Although there were clear differences in the effects of specific hydrolysates on the growth profiles between cell lines there was an enhancement of IVCD in almost all cases ( Fig. S1). Primatone showed the highest activity overall in increasing the maximum VCD and IVCD of iCHO-RTX, CHO-S-RTX and CHO-EG2 cells (Table 1). Primatone and HP1512 hydrolysates enhanced IVCD of the EG2 cells by × 1.6 and × 1.4 respectively. On the other hand, the wheat HP4601n and rice H5603 hydrolysates seemed to be the least beneficial hydrolysates with IVCD values not significantly different from control in most cases or just marginally higher in the EG2 cultures (Table 1).
The impact of the hydrolysates on total mAb titre
The hydrolysates enhanced mAb titre significantly in all cell lines (p < 0.001) except for UP cotton and HP7504 on iCHO-RTX. The most substantial increase in Mab productivity overall was for UPcotton hydrolysate that increased the EG2 antibody from CHO-EG2 cells to 117 mg/L from 44 mg/L. Primatone increased the Mab of CHO-Kerry to 661 mg/L from 378 mg/L. Rituximab concentrations were enhanced by Primatone and HP5603n to 145 mg/L and 56 mg/L from lower levels (58 and 37 mg/L) in CHO-S-RTX and iCHO-RTX cells respectively (Fig. 2).
Effects of the hydrolysates on total mAb. Samples (300 μl) of cell suspension were taken from the cultures described in Fig. 1, centrifuged at 10,000 rpm for 5 min. The total mAb concentration in the resulting supernatants was measured using the Cedex Bio-based turbidimetric immunoassay system at the indicated time points. Data were presented as mean ± SEM of three independent experiments and analysed using paired t-test. *, **, ***Statistically significant differences relative to the untreated control at p < 0.05, 0.01 and 0.001 respectively
The positive effects of the hydrolysates for enhancement of antibody titres were ranked differently for each cell line tested but an overall ranking was as follows: Primatone (meat-derived) > (cotton-derived) HP7504/UPcotton > (soy-derived) HP1510/HP1512 > (rice-derived) HP5603n > (wheat-derived) HP4601n. The ranking indicated Primatone to have the highest bioactivity and HP4601n the lowest (Table 3).
Effects of hydrolysates on IVCD and qMab
Tables 1, 2 and 3 outline the effect of hydrolysates on CHO cells on the growth and productivity parameters, IVCD, mAb and qMab as the fold increase of each culture compared with the corresponding control culture without a hydrolysate supplement. The qMab is a measure of the intrinsic cell-specific productivity of the viable cells that is dependent upon the metabolic activity in each cell to synthesise an antibody and can also be stimulated by the supplemented hydrolysates (Fig. S2). Both IVCD and qMab may be modulated independently but the final Mab titre is the product of these two parameters ([Mab] = qMab*IVCD).
The hydrolysates increased both qMab and IVCD (Table 1 and 2) with just two exceptions. The UPcotton and HP7504 hydrolysates inhibited both qMab and IVCD of the iCHO-RTX system which accounted for a reduction of Mab titre by nearly 50%. HP5603n enhanced qMab (× 1.5) of CHO-S-RTX cells but had no significant effect on IVCD. On the other hand, HP1512 enhanced the IVCD of CHO-EG2 cells by × 1.4 but had no effect on qMab. Nevertheless, apart from the negative effect of UPcotton and HP7504 hydrolysates, in all other cases, the hydrolysates had a positive effect on the specific and total Mab produced (Table 3).
To investigate these parameters further we performed a statistical analysis of the overall data using the 28 values of the 7 × 4 matrix of hydrolysates and cell lines. This showed that the qMab was enhanced by a mean of × 1.45 ± 0.07 compared with the matched controls, whereas the IVCD was enhanced by × 1.09 ± 0.04. This difference is statistically significant (p<0.0001) and indicates that overall protein hydrolysates have a greater effect on the enhancement of qMab compared with IVCD.
Metabolic analysis of the CHO-S-RTX cells in the presence of hydrolysates
The utilisation rates of the major energy substrates (glucose and glutamine) were analysed along with the corresponding production rates of the metabolic by-products (lactate and ammonia) for the CHO-S-RTX cells in the presence of each hydrolysate (Table 4). The growth phase data (days 0–4) shows typical glucose uptake rates within a narrow range of 5.4 to 6.2 pmol/cell/day, but there were some significant differences.
The glucose utilisation and lactate production rates were significantly different between the control and HP7504-containing cultures, which was also reflected by a lower metabolic coefficient (lac/glu) (p < 0.01) that is indicative of a more oxidative metabolism in the presence of the hydrolysate. Similar significant reductions in metabolic coefficients were shown in Primatone and HP1512 containing cultures (p < 0.05).
During the decline phase (days 5–7) the glutamine was completely consumed and there was evidence of a switch from lactate production to consumption. The glucose consumption rate was variable between cultures, ranging from 1.5 to 6.0 pmol/cell/day, probably reflecting the different metabolic states of the cells at maximum VCD. While Primatone and cotton-derived and soy-derived hydrolysates reduced the cell-specific glucose consumption rate significantly during the growth phase, they increased the consumption rate during the decline phase.
Lactate metabolism was altered during the shift in the growth phase of CHO-S-RTX to the decline phase, with a decrease of qLac from 8.2 to 0.8 pmol/cell/day in the control. Reduced lactate production or consumption rates (as shown by negative values) were also shown by the hydrolysate-supplemented cultures in the decline phase (Table 4). The mean calculated lactate/glucose metabolic coefficient was 1.27, which is within the range of values commonly documented in other publications (Mulukutla et al. 2012).
The control cultures showed a glutamine utilisation rate of 0.78 pmol/cell per day from time zero to almost complete depletion at day 4 with no significant difference in the profiles between cultures. Similar values of qGln were shown for hydrolysate-supplemented cultures except for Primatone, which showed a significantly lower rate (p < 0.05). However, this was not reflected in the metabolic coefficient (NH3/Gln), which for Primatone increased to a value higher than the control.
The ammonia production rate for the control cultures was 1.7 pmol/cell per day up to day 4 followed by a reduced rate up to day 7. Four of the hydrolysate supplemented cultures showed significantly higher qNH3 and NH3/gln values compared with the control. The most significant increases were observed in HP4601n, UPcotton, HP5603n and Primatone containing media (p<0.05). Glutamine was totally consumed by the cells at day 4, although ammonia production continued in the decline phase up to day 7. There were differences in the rate of ammonia production at this phase that could be accounted for by variable cell lysis.
The significantly higher levels of ammonia found in the HP4601n-supplemented culture prompted an examination of the ammonia levels in the hydrolysates. Table 5 shows the initial and final ammonia/ ammonium concentrations in the various cultures. This analysis showed significant differences in the ammonia content in the hydrolysates themselves. The highest initial level of ammonia in the hydrolysates was in HP4601n at 3.08 mM whereas the lowest level was in HP7504 (0.36 mM). The higher initial concentration in the HP4601n-supplemented culture was reflected in the final ammonia concentration of 12.57 mM, a level that could be inhibitory to cell growth (Doyle and Butler 1990). It was noted that this concentration was also attained in the UPcotton-supplemented cultures even though the initial concentration of ammonia in this hydrolysate was lower
To address the question whether the concentration of ammonia in the hydrolysates was the key factor in determining bioactivity, an experiment was set up to spike the high-performing HP7504-supplemented culture with ammonia at the same concentration as determined in HP4601n, which had the highest starting ammonia concentration. Ammonium sulphate was added to the HP7504-supplemented culture to increase the ammonia to 3 mM which was the initial concentration in the HP4601n-supplemented culture. No change in pH and osmolality was observed following this addition. The resulting growth curves are shown in Fig. 3. This shows that the growth of cells up to day 6 in the HP7504 culture was not affected appreciably by the extra ammonia, although the decline phase beyond day 6 occurred at a faster rate. The IgG concentration determined in these cultures showed that there was a significant decrease (~ 10%) in the maximum concentration as a result of the presence of extra ammonia. However, although adding ammonia to the HP7504 reduced IVCD and total IgG significantly compared with HP7504 alone, it did not reduce the bioactivity of the best performing hydrolysate (HP7504) to the level of the least performing hydrolysate (HP4601n).
The effect of added ammonia on bioactivity. CHO-Kerry cells were seeded at a density of 0.4 × 106 cells/ml in Kerry-CD CHO medium supplemented with 5 g/L hydrolysate as indicated. Ammonium sulphate was added to increase the concentration of ammonia in the control and H7504 cultures to the same concentration as the initial concentration in the H4601n culture (3 mM). Cultures were continued for 8 days until the viability reduced to ~ 40%. Samples (11 μl) were taken on days 5 to 8 to determine viable cell density (a). Samples (300 μl) of cell suspension were taken from the same culture, centrifuged at 10,000 rpm for 5 min. The total mAb concentration in the resulting supernatants was measured using the Cedex Bio-based turbidimetric immunoassay system at the indicated time points (b). Data are presented as mean ± SEM of three independent experiments, and analysed using paired t-test.***Statistically significant differences relative to the untreated control at p< 0.001
The impact of the hydrolysate HP7504 on the IgGs glycosylation
The hydrolysate HP7504 presented the most positive effect on antibody titre for the cells CHO-S-RTX, CHO-EG2 and CHO-Kerry. The hydrolysate supressed IgG production only in the iCHO cells. To assess if the hydrolysate also influences the IgG glycosylation, the glycans from the IgGs produced in supplemented and control media were characterised and compared (Fig. 4).
Comparison of glycan profiles of IgGs produced in control and hydrolysate supplemented media. Glycan profile of IgGs produced by a iCHO-RTX, b CHO-S-RTX, c CHO-EG2 and d CHO-Kerry cells in control (upper chromatogram) and HP7504-supplemented (lower chromatogram) media. The monosaccharides N-acetylglucosamine, fucose, mannose, galactose and N-acetylneuraminic acid compose the most abundant glycan structures detected
A comparison was made between the glycan profiles of IgG produced in the presence of HP7504 hydrolysate and control cultures for the four producer cells. The HP7504 was chosen because it had the greatest impact on antibody titre in these cells compared with the other hydrolysates.
Seven predominant glycan structures were found in the IgG (Fig. 4). These included, FA2 (a fucosylated diantennary), M5 (pentamannosylated), FA2G1 and FA2G1′ (a monofucosylated monogalactosylated with galactose linked to either of the glycan antenna), FA2G2 (fucosylated and digalactosylated), FA2G2S1 (monosialylated) and FA2G2S2 (disialylated). The percentage of each of these structures in relation to the total glycan composition is shown in Table 6. There were differences in the baseline profiles of these 4 antibodies, the most notable being the high galactosylation and sialylation of the EG2 antibody as has been previously noted (Liu et al. 2014).
As seen in Table 6, the galactosylation and sialylation of the IgGs increased significantly with hydrolysate supplementation in 2 of the cells (CHO-S-RTX and CHO-EG2). The galactosylation and sialylation indices (GI and SI) were calculated as indicated in the “Materials and methods” section. The GI value was particularly high for the EG2 antibody and increased from 0.65 to 0.77 in the presence of the hydrolysate. Similarly, high values of sialylation were shown in this antibody with SI increasing to 0.24 in the presence of hydrolysate. As would be expected in this case, the peak for FA2 glycan which is the precursor of galactosylation and sialylation decreased with the increase in galactosylated and sialylated species (Fig. 4a, b). On the other hand, the presence of the hydrolysate decreased the galactosylated and sialylated species of IgGs produced by iCHO cells (Fig. 4c). In the case of the CHO Kerry IgGs, there was no apparent change in the measured quantity of galactosylated or sialylated glycans. However, in the presence of the hydrolysate, there was a decrease in the amount of FA2 and an increase in the amount of M5 (Fig. 4d).
Discussion
The composition of serum-free media is a key factor in the ability to produce biologicals such as monoclonal antibodies with high productivity in a bioprocess (Butler 2015). The nutrients and growth-promoting factors in media can support cell growth to a high cell density and maintain cell viability over a prolonged period. The gradual incremental improvement of media composition over time has accounted for significant productivity increases in commercial bioproduction processes (Wurm 2004). A second and independent factor in this productivity increase has been the isolation of cell clones with high cell-specific productivity which is usually measured as pg/cell/day. This is largely a function of appropriate genetic engineering of the producer cells but can also be affected in turn by the composition of the media.
Six of the hydrolysates studied in this paper are derived from plants including cotton, soy, wheat and rice. They contain a variety of small molecular weight components that are bioactive. These may be peptides resulting from the controlled enzymatic breakdown or other materials such as trace metals or polyamines that have been identified in some hydrolysate sources (Spearman et al. 2014; Spearman et al. 2016; Schlaeger 1996). Batch to batch variability of the composition of hydrolysates is one problem that can arise from the use of the raw material. To address this manufacturers such as the Kerry Group have attempted to tightly control the process conditions during hydrolysis and utilise ultrafiltration to limit the size of components based on the molecular weight cut off of the filter (Siemensma et al. 2010).
Batch-to-batch variation in the composition of hydrolysates represents one of the perceived difficulties in the routine utilisation of hydrolysates as media supplements in bioprocessing (Gupta et al. 2013). In a preliminary experiment we investigated, the effect of two to three batches of four of the hydrolysates (HP1512, UP Cotton, HP7504 and HP5603n) on CHO-S-RTX cells’ IVCD, qMab and total Mab titre. However, no significant differences in these measured parameters were determined among the tested batches of the hydrolysates (Fig. S3).
The data presented here involves the analysis of the growth and antibody production of 4 independent cell lines in cultures supplemented with 7 hydrolysates. Although the cell lines used are all designated as CHO cells, they are of different origin and were transfected to produce mAbs by different techniques. Three of the cell lines are stable constitutive mAb producers, and in one case (iCHO-RTX), the mAb production requires induction by a small molecule. Furthermore, one cell line was grown in a different basal media from the other three. CHO cells are the most widely used cell lines for commercial production of mAbs and other recombinant proteins, although the variability of the genetics of different CHO cell lines has been well documented (Lewis et al. 2013). Nevertheless, using 4 CHO lines of different origins has allowed a comprehensive analysis of the generic bioactive properties of the 7 hydrolysates used in this study.
Using the representative CHO cell lines, we determined the stimulatory effects of the hydrolysates on the productivity enhancement of mAbs in relation to viable cell density and cell-specific productivity. Overall the hydrolysates were shown to be bioactive in enhancing the productivity of the Mab titre in these diverse cultures with an overall × 1.73 ± 0.09 increase. We were able to analyse the data further to determine the specific effects of the hydrolysates on the overall IVCD in units of cell-day and cell-specific antibody productivity as pg/cell/day. Statistical analysis of the 7 × 4 matrix of growth experiments showed a marginal but significant overall increase (× 1.1) in IVCD in the presence of hydrolysate but a more substantial increase in the cell-specific mAb (qMab) productivity (× 1.5).
Despite the overall positive effect of hydrolysate supplements, there were significant differences in the bioactivity of individual hydrolysates. Of the plant hydrolysates tested, the cotton derivative HP7504 resulted in the highest increase in mAb titre followed by the soy hydrolysates (HP1510 and HP1512) whereas the wheat gluten hydrolysate (HP4601n) showed the lowest enhancement of titre. In contrast, a study involving mAb production from Sp2/0 cells showed that wheat gluten hydrolysate, HP4601n enhanced productivity to a greater extent than other hydrolysates (Ganglberger et al. 2007). However, similarly to our results, this occurred through a combination of enhanced IVCD (25%) and qMab (36%). Other reported data have shown the value of soy hydrolysates to support the growth of isolated human cells (Essers et al. 2011). Wheat hydrolysate has also been shown to support the production of recombinant human-interferon-γ from CHO cells (Burteau et al. 2003). In some cases, cocktails of hydrolysates are important in providing the required support as in anchorage-dependent cells (Rourou et al. 2009).
There are a variety of factors that may allow protein hydrolysates to support cell growth and mAb production. The pool of short peptides could include those that act as exogenous growth factors by binding cell receptors to activate growth pathways or reduce apoptosis has been explored with those containing lysine and arginine shown to be particularly active (Franek 2010). The possibility that protein hydrolysates provide protection against the shear stress has been suggested as a similar role as albumin in serum (Siemensma et al. 2010).
Changes to the energy metabolism of the cells have been shown to be affected by hydrolysates with increased ratios of glucose to glutamine oxidation and an increased respiration quotient leading to reduced by-product formation (Siemensma et al. 2010). Maximum VCD was recorded in our study at day 4 when the cells consumed all supplemented glutamine. This was followed by cells entering a decline phase with no obvious stationary phase. This suggests an essential requirement for glutamine during the growth phase of the cells. We speculate that adding fresh glutamine to the culture (fed-batch) at this point might prolong cellular longevity and delay the entrance of cells into a stage of nutrient-deprivation. The decline phase was accompanied by a metabolic switch of lactate to consumption rather than production. Such a phenomenon was recognised in other studies as desirable in a bioprocess (Hartley et al. 2018) because the accumulation of lactate can limit cell growth, alter the pH and increase the osmolality of the media (Fu et al. 2016; Gagnon et al. 2011). Depletion of glucose or glutamine, reduced glycolytic flux, temperature and pH shifts are the reported triggers of the lactate switch (Hartley et al. 2018) and associated with an improved metabolic efficiency (Liste-Calleja et al. 2015; Luo et al. 2012). The presence of hydrolysates in the media, particularly Primatone, and cotton-derived and soy-derived hydrolysates drastically increased lactate consumption and showed a significant alteration of the cell metabolism during the decline phase. Importantly, the significant reduction of lactate production by the cells grown in HP7504 containing medium during the growth phase might indicate a switch to a more oxidative metabolism of glucose and thus less lactate production. The promotion of oxidative metabolism by hydrolysates was documented by another study (Mosser et al. 2013). In our study, we measured the metabolic coefficients of qLac/qGlu and qNH3/qGln as indicators of energy metabolism. Lower values of these coefficients result in reduced accumulation of metabolic by-products and a more efficient cellular metabolism (Toussaint et al. 2016). The presence of hydrolysates, particularly HP7504 reduced those ratios and indicated a more efficient metabolism of the cells in the presence of the hydrolysate compared with the control.
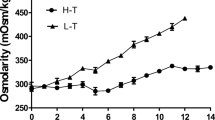
Although we observed a slight increase in osmolality after the addition of hydrolysates, this was unlikely to account for the observed increases in mAb titre. The highest osmolality value was recorded with Primatone (0.415 Osmol/kg) compared with the control (0.376 Osmol/kg) (data not shown). It has been reported that increased osmolality can negatively affect cell growth but significantly improve cell productivity (Ryu et al. 2000; Kim and Lee 2002). However, adding hydrolysates to our media did not inhibit cell growth rate and the osmolality change was within the optimum range as defined in other studies (Davami et al. 2014; Li et al. 2010).
The level of ammonia in HP4601n supplemented media was significantly higher (up to × 8.6) than other hydrolysates such as HP7504 and HP1510. However, the rate of ammonia production during culture was not different between cultures but because of the higher starting point increased to 12.6 mM at day 7 in the HP4601n-supplemented cultures. The possibility was considered that the higher concentration of ammonia might explain the lower performance of HP4601n compared with the other hydrolysates. However, the performance of the HP7504 did not decrease to the level of HP4601n when ammonia was added to HP7504 at the same initial concentration of HP4601n. This suggests that despite differences in the ammonia levels found in the hydrolysates this is not the predominant factor to explain the differences in bioactivity.
The IgG glycosylation is influenced by variables such as the metabolic state of the cell, oxygen levels, culture pH, availability of substrates, enzymes and co-factors (Villacrés et al. 2015). The overall effect of the hydrolysates on glycosylation was variable with respect to the 4 culture systems tested. As shown the hydrolysate HP7504 enhanced the galactosylation of CHO-S-RTX and CHO-EG2 cells. One possible reason is that this hydrolysate may contain co-factors such as manganese that support the activity of galactosyl transferases. The particularly high galactosylation and sialylation levels of the EG2 antibody have been previously noted and are undoubtedly related to its unusual chimeric structure which lacks light chains and the CH1 heavy chain domain (Liu et al. 2014). The negative influence of the hydrolysate on iCHO-RTX cell growth did not only affect IgG production, but had a slight but significant effect in decreasing galactosylation and increasing the non-galactosylated, FA2. On the other hand, the hydrolysate had a the neutral effect on the galactosylation of CHO-Kerry IgGs, but an increase in the high mannose (M5) and a concomitant decrease in FA2. The increase in high mannose glycans has been previously attributed to changes in pH, osmolality, increased specific productivity and nutrient limitation that could result in inefficient processing of M5 in the ER or Golgi (Slade et al. 2016)
In conclusion, we provide data to show the value of various protein hydrolysates in enhancing both the integral of viable cell density and specific-cell productivity in the production of mAbs from CHO cells. The plant hydrolysates identified, particularly cotton hydrolysates, are valuable supplements to animal component-free media. Furthermore, work on the identification of the bioactive components in these hydrolysates is on-going. This information would provide components that then could be used in chemically defined medium for enhanced cell growth and productivity.
Data availability
Glycan structures were validated by reference to the Glycostore database (www.glycostore.org).
References
Agrawal V, Slivac I, Perret S, Bisson L, St-Laurent G, Murad Y, Zhang J, Durocher Y (2012) Stable expression of chimeric heavy chain antibodies in CHO cells Single Domain Antibodies (pp. 287-303): Springer.
Ballez JS, Mols J, Burteau C, Agathos SN, Schneider Y-J (2004) Plant protein hydrolysates support CHO-320 cells proliferation and recombinant IFN-γ production in suspension and inside microcarriers in protein-free media. Cytotechnology 44(3):103–114
Bauman E, Granja PL, Barrias CC (2018) Fetal bovine serum-free culture of endothelial progenitor cells—progress and challenges. J Tissue Eng Regen Med 12(7):1567–1578
Bell A, Wang ZJ, Arbabi-Ghahroudi M, Chang TA, Durocher Y, Trojahn U, Baardsnes J, Jaramillo ML, Li S, Baral TN, O’Connor-Mccourt M, Mackenzie R, Zhang J (2010) Differential tumor-targeting abilities of three single-domain antibody formats. Cancer Lett 289(1):81–90
Bhat Z, Kumar S, Bhat HF (2015) Bioactive peptides of animal origin: a review. J Food Sci Technol 52(9):5377–5392
Bonarius HP, Hatzimanikatis V, Meesters KP, de Gooijer CD, Schmid G, Tramper J (1996) Metabolic flux analysis of hybridoma cells in different culture media using mass balances. Biotechnol Bioeng 50(3):299–318
Burteau CC, Verhoeye FR, Molsl JF, Ballez J-S, Agatho SN, Schneider Y-J (2003) Fortification of a protein-free cell culture medium with plant peptones improves cultivation and productivity of an interferon-γ-producing CHO cell line. Vitro Cell Dev Biol-Anim 39(7):291–296
Butler M (2004) Animal cell culture and technology: Taylor & Francis.
Butler M (2013) Serum-free media: standardizing cell culture system. Pharm Bioproces 1(4):315–318
Butler M (2015) Serum and protein free media Animal cell culture (pp. 223-236): Springer.
Butler M, Meneses-Acosta A (2012) Recent advances in technology supporting biopharmaceutical production from mammalian cells. Appl Microbiol Biotechnol 96(4):885–894
Chalamaiah M, Yu W, Wu J (2018) Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem 245:205–222
Chida Y, Takagi K, Terada S (2013) Establishment of a mammalian cell line suitable for industrial production of recombinant protein using mutations induced by high-energy beam radiation. Cytotechnology 65(6):955–965
Chusainow J, Yang YS, Yeo JHM, Toh PC, Asvadi P, Wong NSC, Yap MGS (2009) A study of monoclonal antibody-producing CHO cell lines: What makes a stable high producer? Biotechnol Bioeng 102(4):1182–1196
Davami F, Baldi L, Rajendra Y, Wurm F (2014) Peptone Supplementation of Culture Medium Has Variable Effects on the Productivity of CHO Cells. Int J Mol Cell Med 3(3):146–156
Dimasi L (2011) Meeting increased demands on cell-based processes by using defined media supplements. Bioprocess J 9(8):9
Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X (2005) Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol 205(2):228–236
Doyle C, Butler M (1990) The effect of pH on the toxicity of ammonia to a murine hybridoma. J Biotechnol 15(1-2):91–100
Essers R, Kewes H, Schiedner G (2011) Improving volumetric productivity of a stable human CAP cell line by bioprocess optimization. BMC Proc 5(Suppl 8):–P66
Franek F (2010) Oligopeptides as External Molecular Signals Affecting Growth and Death in Animal Cell Cultures. In: Pasupuleti VK, Demain AL (eds) Protein Hydrolysates in Biotechnology. Springer Netherlands, Dordrecht, pp 79–89
Fu T, Zhang C, Jing Y, Jiang C, Li Z, Wang S, Ma K, Zhang D, Hou S, Dai J, Kou G, Wang H (2016) Regulation of cell growth and apoptosis through lactate dehydrogenase C over-expression in Chinese hamster ovary cells. Appl Microbiol Biotechnol 100(11):5007–5016
Fujisawa S, Romin Y, Barlas A, Petrovic LM, Turkekul M, Fan N, Xu K, Garcia AR, Monette S, Klimstra DS, Erinjeri JP, Solomon SB, Manova-Todorova K, Sofocleous CT (2014) Evaluation of YO-PRO-1 as an early marker of apoptosis following radiofrequency ablation of colon cancer liver metastases. Cytotechnology 66(2):259–273
Gagnon M, Hille G, Luan YT, Kittredge A, DeFelice J, Drapeau D (2011) High-end pH-controlled delivery of glucose effectively suppresses lactate accumulation in CHO fed-batch cultures. Biotechnol Bioeng 108(6):1328–1337
Galanakis C M (2019) Proteins: Sustainable source, processing and applications: Academic Press.
Ganglberger P, Obermüller B, Kainer M, Hinterleitner P, Doblhoff O, Landauer K (2007) Optimization of culture medium with the use of protein hydrolysates Cell technology for cell products (pp. 553-557): Springer.
Girón-Calle J, Vioque J, Pedroche J, Alaiz M, Yust MM, Megías C, Millán F (2008) Chickpea protein hydrolysate as a substitute for serum in cell culture. Cytotechnology 57(3):263–272
Griffiths S, Baraniak PR, Copland IB, Nerem RM, McDevitt TC (2013) Human platelet lysate stimulates high-passage and senescent human multipotent mesenchymal stromal cell growth and rejuvenation in vitro. Cytotherapy 15(12):1469–1483
Gstraunthaler G, Lindl T, van der Valk J (2013) A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology 65(5):791–793
Gu X, Xie L, Harmon BJ, Wang DI (1997) Influence of Primatone RL supplementation on sialylation of recombinant human interferon-γ produced by Chinese hamster ovary cell culture using serum-free media. Biotechnol Bioeng 56(4):353–360
Gupta AJ, Gruppen H, Maes D, Boots JW, Wierenga PA (2013) Factors causing compositional changes in soy protein hydrolysates and effects on cell culture functionality. J Agric Food Chem 61(45):10613–10625
Hartley F, Walker T, Chung V, Morten K (2018) Mechanisms driving the lactate switch in Chinese hamster ovary cells. Biotechnol Bioeng 115(8):1890–1903
Heidemann R, Zhang C, Qi H, Rule JL, Rozales C, Park S, Chuppa S, Ray M, Michaels J, Konstantinov K (2000) The use of peptones as medium additives for the production of a recombinant therapeutic protein in high density perfusion cultures of mammalian cells. Cytotechnology 32(2):157–167
Hou Y, Wu Z, Dai Z, Wang G, Wu G (2017) Protein hydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J Anim Sci Biotechnol 8:24–24
Jagschies G, Lindskog E, Lacki K, Galliher P M (2018) Biopharmaceutical Processing: Development, Design, and Implementation of Manufacturing Processes: Elsevier.
Jayme DW, Smith SR (2000) Media formulation options and manufacturing process controls to safeguard against introduction of animal origin contaminants in animal cell culture. Cytotechnology 33(1-3):27–36
Jiang H, Horwitz AA, Wright C, Tai A, Znameroski EA, Tsegaye Y, Warbington H, Bower BS, Alves C, Co C, Jonnalagadda K, Platt D, Walter JM, Natarajan V, Ubersax JA, Cherry JR, Love JC (2019) Challenging the workhorse: Comparative analysis of eukaryotic micro-organisms for expressing monoclonal antibodies. Biotechnol Bioeng 116(6):1449–1462
Kim NS, Lee GM (2002) Response of recombinant Chinese hamster ovary cells to hyperosmotic pressure: effect of Bcl-2 overexpression. J Biotechnol 95(3):237–248
Kim SH, Lee GM (2009) Development of serum-free medium supplemented with hydrolysates for the production of therapeutic antibodies in CHO cell cultures using design of experiments. Appl Microbiol Biotechnol 83(4):639–648
Landauer K (2014) Designing media for animal cell culture: CHO cells, the industrial standard. Methods Mol Biol 1104:89–103
Lewis NE, Liu X, Li Y, Nagarajan H, Yerganian G, O'Brien E, Bordbar A, Roth AM, Rosenbloom J, Bian C, Xie M, Chen W, Li N, Baycin-Hizal D, Latif H, Forster J, Betenbaugh MJ, Famili I, Xu X, Wang J, Palsson BO (2013) Genomic landscapes of Chinese hamster ovary cell lines as revealed by the Cricetulus griseus draft genome. Nat Biotechnol 31(8):759–765
Li F, Vijayasankaran N, Shen AY, Kiss R, Amanullah A (2010) Cell culture processes for monoclonal antibody production. mAbs 2(5):466–479
Lingg N, Zhang P, Song Z, Bardor M (2012) The sweet tooth of biopharmaceuticals: importance of recombinant protein glycosylation analysis. Biotechnol J 7(12):1462–1472
Liste-Calleja L, Lecina M, Lopez-Repullo J, Albiol J, Solà C, Cairó JJ (2015) Lactate and glucose concomitant consumption as a self-regulated pH detoxification mechanism in HEK293 cell cultures. Appl Microbiol Biotechnol 99(23):9951–9960
Liu B, Spearman M, Doering J, Lattova E, Perreault H, Butler M (2014) The availability of glucose to CHO cells affects the intracellular lipidlinked oligosaccharide distribution, site occupancy and the N-glycosylation profile of a monoclonal antibody. J Biotechnol 170:17–2
Luo J, Vijayasankaran N, Autsen J, Santuray R, Hudson T, Amanullah A, Li F (2012) Comparative metabolite analysis to understand lactate metabolism shift in Chinese hamster ovary cell culture process. Biotechnol Bioeng 109(1):146–156
Mosser M, Chevalot I, Olmos E, Blanchard F, Kapel R, Oriol E, Marc I, Marc A (2013) Combination of yeast hydrolysates to improve CHO cell growth and IgG production. Cytotechnology 65(4):629–641
Mullick A, Xu Y, Warren R, Koutroumanis M, Guilbault C, Broussau S, Malenfant F, Bourget L, Lamoureux L, Lo R, Caron AW, Pilotte A, Massie B (2006) The cumate gene-switch: a system for regulated expression in mammalian cells. BMC Biotechnol 6(1):43
Mulukutla BC, Gramer M, Hu WS (2012) On metabolic shift to lactate consumption in fed-batch culture of mammalian cells. Metab Eng 14(2):138–149
Nagodawithana TW, Nelles L, Trivedi NB (2008) Protein hydrolysates as hypoallergenic, flavors and palatants for companion animals Protein Hydrolysates in Biotechnology (pp. 191-207): Springer.
O’Flaherty R, Bergin A, Flampouri E, Mota LM, Obaidi I, Quigley A, Xie Y, Butler M (2020) Mammalian cell culture for production of recombinant proteins: A review of the critical steps in their biomanufacturing. Biotechnol Adv 43:107552
Obaidi I, Higgins M, Bahar B, Davis JL, McMorrow T (2018) Identification of the Multifaceted Chemopreventive Activity of Curcumin Against the Carcinogenic Potential of the Food Additive, KBrO3. Curr Pharm Des 24(5):595–614
Park SY, Lee J-S, Baek H-H, Lee HG (2010) Purification and characterization of antioxidant peptides from soy protein hydrolysate. J Food Biochem 34(s1):120–132
Patterson MK Jr (1979) Measurement of growth and viability of cells in culture. Methods Enzymol 58:141–152
Poulain A, Perret S, Malenfant F, Mullick A, Massie B, Durocher Y (2017) Rapid protein production from stable CHO cell pools using plasmid vector and the cumate gene-switch. J Biotechnol 255:16–27
Pristovšek N, Hansen HG, Sergeeva D, Borth N, Lee GM, Andersen MR, Kildegaard HF (2018) Using titer and titer normalized to confluence are complementary strategies for obtaining Chinese Hamster ovary cell lines with high volumetric productivity of etanercept. Biotechnol J 13(3):1700216
Renard JM, Spagnoli R, Mazier C, Salles MF, Mandine E (1988) Evidence that monoclonal antibody production kinetics is related to the integral of the viable cells curve in batch systems. Biotechnol Lett 10(2):91–96
Ritacco FV, Wu Y, Kheta A (2018) Cell culture media for recombinant protein expression in Chinese hamster ovary (CHO) cells: history, key components, and optimization strategies. Biotechnol Prog 34(6):1407–1426
Rodriguez J, Spearman M, Huzel N, Butler M (2005) Enhanced production of monomeric interferon-beta by CHO cells through the control of culture conditions. Biotechnol Prog 21(1):22–30
Rourou S, van der Ark A, van der Velden T, Kallel H (2009) Development of an animal-component free medium for vero cells culture. Biotechnol Prog 25(6):1752–1761
Ryu JS, Kim TK, Chung JY, Lee GM (2000) Osmoprotective effect of glycine betaine on foreign protein production in hyperosmotic recombinant chinese hamster ovary cell cultures differs among cell lines. Biotechnol Bioeng 70(2):167–175
Schlaeger EJ (1996) The protein hydrolysate, Primatone RL, is a cost-effective multiple growth promoter of mammalian cell culture in serum-containing and serum-free media and displays anti-apoptosis properties. J Immunol Methods 194(2):191–199
Siemensma A, Babcock J, Wilcox C, Huttinga H (2010) Towards an Understanding of How Protein Hydrolysates Stimulate More Efficient Biosynthesis in Cultured Cells. In: Pasupuleti VK, Demain AL (eds) Protein Hydrolysates in Biotechnology. Springer Netherlands, Dordrecht, pp 33–54
Slade PG, Caspary RG, Nargund S, Huang CJ (2016) Mannose metabolism in recombinant CHO cells and its effect on IgG glycosylation. Biotechnol Bioeng 113(7):1468–1480
Spearman M, Chan S, Jung V, Kowbel V, Mendoza M, Miranda V, Butler M (2016) Components of yeast (Sacchromyces cervisiae) extract as defined media additives that support the growth and productivity of CHO cells. J Biotechnol 233:129–142
Spearman M, Lodewyks C, Richmond M, Butler M (2014) The bioactivity and fractionation of peptide hydrolysates in cultures of CHO cells. Biotechnol Prog 30(3):584–593
Sung YH, Lim SW, Chung JY, Lee G-M (2004) Yeast hydrolysate as a low-cost additive to serum-free medium for the production of human thrombopoietin in suspension cultures of Chinese hamster ovary cells. Appl Microbiol Biotechnol 63(5):527–536
Toussaint C, Henry O, Durocher Y (2016) Metabolic engineering of CHO cells to alter lactate metabolism during fed-batch cultures. J Biotechnol 217:122–131
Urbano P, Urbano F (2007) Nanobacteria: facts or fancies? PLoS Pathog 3(5):e55
Usta SN, Scharer CD, Xu J, Frey TK, Nash RJ (2014) Chemically defined serum-free and xeno-free media for multiple cell lineages. Ann Transl Med 2(10):97
Villacrés C, Tayi VS, Lattová E, Perreault H, Butler M (2015) Low glucose depletes glycan precursors, reduces site occupancy and galactosylation of a monoclonal antibody in CHO cell culture. Biotechnol J 10(7):1051–1066
Wurm FM (2004) Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 22(11):1393–1398
Yao T, Asayama Y (2017) Animal-cell culture media: History, characteristics, and current issues. Reprod Med Biol 16(2):99–117
Zhang J, Liu X, Bell A, To R, Baral TN, Azizi A, Li J, Cass B, Durocher Y (2009) Transient expression and purification of chimeric heavy chain antibodies. Protein Expr Purif 65(1):77–82
Acknowledgements
The work was supported by an Enterprise Ireland, Innovation Partnership Project grant IP-2018-0717 in conjunction with the Kerry Group. We thank Kyle Liu and Hans Huttinga of Kerry for insightful comments on the manuscript.
Author information
Authors and Affiliations
Contributions
IO designed and conducted the experiments as well as writing the manuscript. AQ designed and conducted the experiments. LMM performed glycan analysis of the Mabs. MB conceived the research and edited and approved the manuscript.
Corresponding author
Ethics declarations
The article does not contain any studies with human participants or animals by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 443 kb)
Rights and permissions
About this article
Cite this article
Obaidi, I., Mota, L.M., Quigley, A. et al. The role of protein hydrolysates in prolonging viability and enhancing antibody production of CHO cells. Appl Microbiol Biotechnol 105, 3115–3129 (2021). https://doi.org/10.1007/s00253-021-11244-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11244-8