Abstract
Carbamoyl phosphate is an important precursor for L-arginine and pyrimidines biosynthesis. In view of this importance, the cell factory should enhance carbamoyl phosphate synthesis to improve related compound production. In this work, we verified that carbamoyl phosphate is essential for L-arginine production in Corynebacterium sp., followed by engineering of carbamoyl phosphate synthesis for further strain improvement. First, carAB encoding carbamoyl phosphate synthetase II was overexpressed to improve the synthesis of carbamoyl phosphate. Second, the regulation of glutamine synthetase increases the supply of L-glutamine, providing an effective substrate for carbamoyl phosphate synthetase II. Third, carbamate kinase, which catalyzes inorganic ammonia synthesis carbamoyl phosphate, was screened and selected to assist in carbamoyl phosphate supply. Finally, we disrupted ldh (encoding lactate dehydrogenase) to decrease by-production formation and save NADH to regenerate ATP through the electron transport chain. Subsequently, the resulting strain allowed a dramatically increased L-arginine production of 68.6 ± 1.2 g∙L−1, with an overall productivity of 0.71 ± 0.01 g∙L−1∙h−1 in 5-L bioreactor. Stepwise rational metabolic engineering based on an increase in the supply of carbamoyl phosphate resulted in a gradual increase in L-arginine production. The strategy described here can also be implemented to improve L-arginine and pyrimidine derivatives.
Key points
• The L-arginine production strongly depended on the supply of carbamoyl phosphate.
• The novel carbamoyl phosphate synthesis pathway for C. crenatum based on carbamate kinase was first applied to L-arginine synthesis.
• ATP was regenerated followed with the disruption of lactate formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
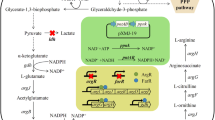
Carbamoyl phosphate (CP) is a high-energy phosphate compound (Eroglu and Powers-Lee 2002) and at the “intersection” of the arginine and pyrimidine synthesis pathways that provide a source of carbamoyl (Wang et al. 2008). In the arginine biosynthetic pathway, CP and ornithine are the substrates of ornithine carbamoyltransferase (OTCase) for the production of citrulline (Charlier et al. 2018), joining the biosynthesis of arginine (Fig. 1). In the pyrimidine biosynthetic pathway, the enzyme aspartate transcarbamoylase (ATCase) catalyzes the condensation of CP and L-aspartate to form N-carbamoyl-L-aspartate (CA) (Robin et al. 1989). The synthesis of CP is one of the effective pathways for cells to fix inorganic carbon sources. Meanwhile, as a high energy phosphate compound, CP has very active chemical properties (Wang et al. 2008). In view of the important position and instability of CP in the synthesis and metabolism, the synthesis of CP is of great significance for the production of arginine, pyrimidine, and their derivatives. In nearly all organisms, carbamoyl phosphate synthetase (CPS) is the most common enzyme that catalyzes the formation of CP (Shi et al. 2018). Three classes of CPSases are known. CPS-I is found in amphibians and mammals, and CPS-III is mainly present in invertebrates and fishes (Korte et al. 1997; Saeed-Kothe and Powers-Lee 2002). Both have an absolute requirement for the allosteric activator N-acetyl-L-glutamate (NAG) (Pekkala et al. 2009; Yefimenko et al. 2005). CPS-II (EC 6.3.5.5), which utilizes glutamine as the nitrogen-donating substrate present in fungi and bacteria, and does not require NAG for activity (Kaseman and Meister 1985). Fungi have two different forms of CPS-II, one for the pyrimidine pathway, and one for the arginine pathway, but bacteria usually only have one CPS-II for both pathways (Eroglu and Powers-Lee 2002; Nyunoya and Lusty 1983; Shi et al. 2018). In Corynebacterium sp., CP supplying both pathways is synthesized from HCO3-, ATP, and glutamine by glutamine-dependent CPS-II composed of a 43.91-kDa glutaminase subunit (CarA) and a 121.8 kDa synthase subunit (CarB), which catalyzes the hydrolysis of glutamine and the synthesis of CP, respectively (Shen et al. 2019). Another enzyme, carbamate kinase (CK, EC 2.7.2.2), which consists of a homodimer of a 33 kDa subunit, has been found in some anaerobes, which can produce CP in the presence of ATP, HCO3-, and NH3 (Marina et al. 1999; Uriarte and Marina 1999). It is worth noting that the true substrate of CK is carbamate generated chemically from HCO3- and ammonia, then Mg2+ and ATP are used to phosphorylate carbamate into CP, and the reaction is reversible (Marina et al. 1998; Ramon-Maiques et al. 2010). Research has shown that CK could replace CPS in microbial cells for synthesizing CP (Alcantara et al. 2000).
The major L-arginine biosynthetic pathway in C. glutamicum and metabolic engineering approach applied to overproduce L-arginine. CPS-II, carbamoyl phosphate synthetase II; CK, carbamate kinase; LDH, lactate dehydrogenase; GS, glutamine synthetase; NAGS, N-acetylglutamate synthase;NAGK, N-acetylglutamate kinase; NAGPR, N-acetyl-gamma-glutamyl-phosphate reductase; NAT, N-acetylornithine transaminase; OAT, ornithine acetyltransferase; OTC, ornithine carbamoyltransferase; AGS, argininosuccinate synthase; AGL, argininosuccinate lyase
L-arginine is a semi-essential amino acid, which has numerous applications in food, pharmaceutical, chemical, and other industries (Moncada and Higgs 1993; Popolo et al. 2014). In recent years, with the increasing knowledge of metabolism and pathway regulation of industrial-related microorganisms, microbial fermentation has become the main method to produce L-arginine (Wieschalka et al. 2013). In our previous work, we obtained an L-arginine high-producing Corynebacterium crenatum SYPA5-5 (Corynebacterium glutamicum var. CCTCC AB 2021051) through screening and mutation breeding. Systematic metabolic engineering strategies, such as improvement of the intracellular environment, upregulation of nitrogen metabolism, and promotion of L-arginine excretion, alleviating feedback inhibition of key pathways, were applied to C. crenatum to increase the production of L-arginine (Man et al. 2016; Xu et al. 2013, 2019, 2020).
Lee et al. found that the carB gene carries a non-synonymous SNP in the L-arginine overproducer, compared with the wild strain, indicating the potential importance of this gene in enhancing L-arginine production, and that increased expression levels of the carAB genes allow L-arginine production to increase in C. glutamicum (Park et al. 2014). In the present study, we demonstrated that CP is essential for effective L-arginine production. For rapid CP supply, the host strain was engineered to activate the CP and L-glutamine synthesis pathway by overexpression of carAB encoding CPS-II and glnA encoding L-glutamine synthase (GS). In addition, a new synthesis approach was introduced via heterologous expression of CK using inorganic ammonia as a nitrogen-donating substrate. Finally, the by-product lactate was decreased by disrupting the ldh gene encoding lactate dehydrogenase (LDH), allowing the saved NADH to increase intracellular ATP levels. Fermentation of the resulting strain demonstrated that L-arginine production was significantly enhanced by the improvement of the intracellular supply of CP in C. crenatum.
Materials and methods
Strains and culture conditions
All bacterial strains and plasmids used in this study are listed in Table S1. Escherichia coli JM109 and BL21 (DE3) were used as hosts for plasmid construction and heterologous expression, respectively, and cultured at 37 °C and 180 rpm for 12 h in LB medium. LBG medium (LB medium supplemented with 12 g∙L−1 glucose) was used to culture C. crenatum and its recombinant derivatives at 30 °C and 180 rpm for growth and enzyme activity experiments. The minimal medium CGXII (containing 40 g∙L−1 glucose) was used to culture C. crenatum strains for growth experiments. The shake flask fermentation medium contained the following: 150 g∙L−1 glucose, 10 g∙L−1 yeast extract, 40 g∙L−1 (NH4)2SO4, 1.5 g∙L−1 KH2PO4, 1.0 g∙L−1 KCl, 1.0 g∙L−1 NaHCO3, 0.5 g∙L−1 MgSO4·7H2O, 0.02 g∙L−1 FeSO4·7H2O, 0.02 g∙L−1 MnSO4·H2O, and 2 g∙L−1 CaCO3. The 5-L fermentation medium contained the following: 100 g∙L−1 glucose, 20 g∙L−1 yeast extract, 1.5 g∙L−1 KH2PO4, 0.5 g∙L−1 MgSO4·7H2O, 1.0 g∙L−1 NaHCO3, 0.02 g∙L−1 FeSO4·7H2O, 0.02 g∙L−1 MnSO4, and 40 g∙L−1 (NH4)2SO4. Where appropriate, 50 μg∙mL−1 kanamycin and 10 μg∙mL−1 chloramphenicol were added to C. crenatum and E. coli cultures, respectively.
Construction of recombination plasmids and strains
The shuttle plasmids pXMJ-19 and pECXK-99E were used for gene overexpression in C. crenatum SYPA5-5. The suicide vector pK18mobsacB was used to delete DNA sequences and replace the promoter in C. crenatum SYPA5-5 via two-step homologous recombination.
The genes encoding CK from Enterococcus faecalis V583(GenBank: AE016830.1) and Lactococcus lactis subsp. lactis Il1403(GenBank: CP033607.1) were codon-optimized for expression in C. glutamicum and synthesized by Genewiz (Suzhou, China), and these were designated as efarcc (GenBank accession number: MW418530) and llarcc, respectively (Supplementary Note S1). Other genes were amplified via PCR from the corresponding genomic DNA using the primers listed in Table S2.
The yahI gene was amplified via PCR from the genomic DNA of Escherichia coli K-12 using the primer pair pECXK-99E-yahI-Xba I-F and pECXK-99E-yahI-Pst I-R. Then, DNA fragments and plasmid pECXK-99E double-digested by Xba I and Pst I were connected using the homologous recombination by ClonExpressR II One Step Cloning Kit, resulting in pECXK-99E-yahI which was transformed into E. coli BL21 (DE3) by heat shock. The plasmids pECXK-99E-efarcc, pECXK-99E-llarcc, and pECXK-99E-ccarcc were constructed in the same way using the primers listed in Table S2. For construction of pXMJ19-carA-carB, the DNA fragment containing the tac promoter and carB was amplified via PCR from pXMJ19-carB using the primer pair P19-carB-tac-F and P19-carB-tac-R. The recombinant plasmid pXMJ19-carA was linearized using the primer pair P19-carA-line-F and P19-carA-line-R. The two fragments were connected through homologous recombination, resulting in plasmid pXMJ19-carA-carB. The co-expressed plasmid pXMJ19-carB-carA was constructed in the same way using the primers listed in Table S2.
The gene carB in-frame deletions of DNA sequences in C. crenatum SYPA5-5 strains were performed via two-step homologous recombination using vector pK18mobsacB (Schäfer et al. 1994). For construction of pK18-carB, the upstream and downstream regions (approximately 1500 bp) of the carB gene were amplified using the primer combinations pK18-ΔcarB-1/pK18-ΔcarB-2 and pK18-ΔcarB-3/pK18-ΔcarB-4, respectively, from the genomic DNA of SYPA5-5. In the next step, the two amplified DNA fragments were fused in an overlap-extension PCR using primers pK18-ΔcarB-1 and pK18-ΔcarB-4. Subsequently, the fused fragments that contained an incomplete carB gene were inserted into the EcoR I/Hind III sites of the pK18mobsacB plasmid, generating the recombinant plasmid pK18-carB. This plasmid was electro-transformed into SYPA5-5, resulting in the first recombination. Colonies of successful first recombination were cultivated for 18 h in selective LBGS (12% w/v saccharose in LBG) medium and subjected to the second recombination. Subsequently, the culture broth in LBGS medium was separated on the LBG plate, and single colonies were selected for colony PCR verification to obtain the carB knockout strain. The recombinant plasmid pK18-Psod and pK18-ldh were constructed in the same way using the primers listed in Table S2.
Protein expression and purification
E. coli BL21 recombinant strains were cultured at 37 °C for 3 h in LB medium to an optical density at 600 nm (OD600) of 0.5–0.8, at which point 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) was added, and then they were incubated at 16 °C for 10 h to induce expression of the cloned gene. The only difference was that C. crenatum recombinant strains needed to be cultured and induced at 30 °C. Following, the strains were harvested by centrifugation at 8000 r∙min−1 for 10 min, and washed twice with 50 mM potassiubm phosphate buffer (pH 7.4). Subsequently, the cells were lysed by sonication on ice. After centrifugation at 4 °C and 12,000 r∙min−1 for 30 min, the resulting cell extract (supernatant) was retained for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and purification.
Ni2+-affinity chromatography and an AKTA purifier system (GE Healthcare, Sweden) were used to purify the crude enzyme. The crude enzyme was loaded onto a 1-mL HisTrapTM HP column with binding buffer (0.02 M Tris-HCl buffer and 0.5 M NaCl, pH 7.4) with a 0.5-mL∙min−1 loading rate. The enzyme was eluted at 1 mL∙min−1 with a linear gradient of imidazole concentrations ranging from 0 to 0.5 M. Subsequently, the proteins were analyzed by SDS-PAGE. The protein concentration was determined by the Bradford method (Bradford 1976) using bovine serum albumin as a standard.
Enzyme activity assays
The supernatant of the cell lysate or pure enzyme was used to determine the activities. GS activity was measured using a Glutamine Synthetase Assay Kit (Solarbio, Beijing, China) following the manufacturer’s instructions. One unit of the GS activity was defined as the amount of enzyme that resulted in a change in the absorbance of 0.01 (at 540 nm) per minute in a 1-mL reaction. The activity of CPS-II and CK was determined in the presence of ornithine by measuring citrulline formation in a coupled assay with OTC (Alcantara et al. 2000; Rubino et al. 1987). One unit of CPS-II or CK activity was defined as the amount of enzyme required to form 1 μmol citrulline per minute. The LDH activity assay was performed as previously described (Inui et al. 2004). One unit of LDH activity was defined as the amount of enzyme that oxidized 1 μmol of NADH per minute at 30 °C.
RT-qPCR
For real-time quantitative PCR (RT-qPCR) analysis, the cells cultured to the exponential phase in LBG medium were harvested for mRNA isolation. Total RNA was purified using the RNAprep Pure bacteria kit (Tiangen Biotech, Beijing, China). cDNA was synthesized using the HiScript II Q RT SuperMix for qPCR kit (Vazyme Biotech, Nanjing, China). RT-qPCR was performed using the StepOnePlus instrument (Applied Biosystems, Waltham, MA, USA) following the manufacturer’s instructions. Relative expression of the 16S rRNA gene was determined as the internal standard. The primer sequences used for RT-qPCR are shown in Table S2. Each assay was replicated three times.
Shake flask and fed-batch fermentation
For shake flask fermentation, the recombinant strain was cultured on an LBG plate at 30 °C for 24 h. Subsequently, a fresh colony was inoculated into the seed medium at 30 °C for 18 h. Next, 8–10% of the seed culture was transferred to 30 mL of the fermentation medium in a standard 250-mL shake flask with indentation and was cultured for 96 h at 30 °C, shaking at 180 rpm on a reciprocating shaker. To produce L-arginine via fermentation in 5-L bioreactors, the seed culture (150 mL) was transferred into 5-L fermenters (BIOTECH-5BG, Baoxing Co., Shanghai, China) containing 2.5 L of fermentation medium. The fermentation experiments were performed at 30 °C, and the pH was maintained at 7.0 by automatically adding 50% NH4OH solution (Schneider et al. 2011).
Analytical methods
In the fermentation process, 2-mL samples of the culture were harvested and centrifuged (8000 r∙min−1, 10 min, and 4 °C). A spectrophotometer (UNICOTM-UV2000, UNICO, Shanghai, China) was used to measure the OD562 (1 OD562 = 0.375 g∙L−1 dry cell weight, DCW) to monitor cell growth after dissolving CaCO3 with 0.125 M HCl. The glucose concentrations were detected using a SBA-40C bioanalyzer (developed by the Biology Institute of the Shandong Academy of Sciences, Jinan, China). The concentration of extracellular NH4+ in the fermentation broth was detected using a Thermo Scientific ICS-5000+ ion chromatograph (Thermo Fisher Scientific, New York, USA). To prepare the samples, the supernatant was centrifuged at 8000 r∙min−1 for 10 min. Before using the Thermo OnGuard II RP column, all supernatants were diluted to an NH4+ concentration of approximately 15 ppm and then filtered twice through 0.22-μm filters to remove organic substances in the sample. The intracellular ATP level was measured using an ATP Assay Kit (Beyotime, Shanghai, China) following the manufacturer’s instructions. The intracellular concentration of NADH was measured using an NAD+/NADH Quantification Kit (Sigma-Aldrich, Shanghai, China) according to the manufacturer’s instructions. L-arginine, lactate, and UMP were determined by high-pressure liquid chromatography (HPLC) (Agilent Technologies, Waldbronn, Germany) according to previous reports (Deporte et al. 2006; Zhang et al. 2014). All assays were performed in triplicate. GraphPad Prism 8 and Origin 2019b software were used for statistical analyses.
Results
Importance of CP for L-arginine synthesis in C. crenatum
CP is an important precursor of L-arginine, and some metabolic engineering strategies have begun to focus on it for constructing a high-yielding L-arginine strain. We first constructed a carB deletion strain 5-5/ΔcarB to confirm the essential role of CP in growth and metabolism. As shown in Fig. 2a, 5-5/ΔcarB did not grow. This is because C. crenatum has a single CPS-II that produces the CP needed for arginine and pyrimidine biosynthesis (Shi et al. 2018). The growth results showed that 5-5/ΔcarB, which lacks CPS-II, can restore growth similar to the SYPA 5-5 strain, only when 1 g∙L−1 L-arginine and 1 g∙L−1 UMP are simultaneously added into the CGXII minimal medium. This result indicates that 5-5/ΔcarB is arginine and pyrimidine auxotrophic. Furthermore, we did not detect L-arginine production by 5-5/ΔcarB in the fermentation medium, although the medium contained yeast extract and added 1 g∙L−1 UMP sufficient to sustain 5-5/ΔcarB growth. The above results demonstrate that CP is essential for cell growth and L-arginine production.
The effects of CPS-II on cell growth and L-arginine production. a Time course of DCW of SYPA 5-5 and 5-5/ΔcarB strains in CGXII minimal medium with 0, 1 g/L L-arginine, 1 g/L pyrimidines, 1 g/L L-arginine, and 1 g/L pyrimidines, respectively. b Structural arrangement of 5-5/CccarAB and 5-5/CccarBA. c Transcript levels of carA and carB investigated by RT-qPCR analysis and the activity of CPS-II in strains 5-5/CccarAB and 5-5/CccarBA, using SYPA 5-5 as a control. d Concentration of amino acids, UMP, and lactate in SYPA 5-5 and ARG-CP1 strains in the fermentation process. Experiments were conducted in triplicate. Error bars indicate ± SD
To increase the availability of CP, carA and carB were expressed simultaneously in SYPA 5-5. carA was upstream of carB, consistent with the order in the genome, resulting in strain 5-5/CccarAB (Fig. 2b). To analyze the gene expression levels by RT-qPCR, CPS-II activity was detected by coupling with OTC. As shown in Fig. 2c, the expression level of carA and carB in 5-5/CccarAB was significantly upregulated by 36.78- and 3.13-fold, respectively, compared with SYPA 5-5. Meanwhile, the activity of CPS-II increased 2.71-fold. carB encodes a large subunit of 118 kDa (1073 aa residues), which plays a key role in catalyzing the synthesis of CP from ammonia and carries all binding sites for effectors including activator IMP, ornithine, and inhibitor UMP (Charlier et al. 2018; Rubio et al. 1991). In order to assign better expression intensity to carB, we switched the order of carA and carB in pXMJ-19, and built strain 5-5/CccarBA (Fig. 2b). The transcription levels of carA and carB in 5-5/CccarBA were 28.38- and 10.18-fold higher than those in SYPA 5-5. Meanwhile, the CPS-II activity was 10.73-fold higher than that of SYPA 5-5 (Fig. 2c). This indicates that CarB is an important subunit for catalyzing CP formation and that the expression intensity of carA and carB is important for CPS-II activity.
To further analyze the importance of CP, 5-5/CccarBA was renamed ARG-CP1 and fermented for the production of basic amino acids and UMP. The results showed that ARG-CP1, grown similar to SYPA 5-5, yielded 29.9 ± 0.4 g∙L−1 L-arginine, which was 14.1% higher than the yield of SYPA 5-5 (Fig. 2d). Correspondingly, the UMP level in ARG-CP1 was 73.7 ± 4.1 mg∙L−1, representing an 18.1% increase, compared to that of the wild type. These results indicate that CP as an important donor of the carbamyl group in the cell is required for normal cell growth and plays a positive role in the biosynthesis of L-arginine and UMP in C. crenatum SYPA 5-5.
Overexpression of glnA for increasing L-Gln supply
From the analysis of amino acid production in the wild-type and ARG-CP1, it is worth noting that the L-glutamine concentration in the ARG-CP1 strain was 31.25% of that of SYPA 5-5 (Fig. 2d). The level of intracellular L-glutamine was detected at 48 h of fermentation, and the ARG-CP1 strain showed a lower L-glutamine concentration, which was decreased by 68.7% compared to SYPA 5-5. CPS-II utilizes L-glutamine as the nitrogen-donating substrate, and L-glutamine is then converted to L-glutamate. Therefore, intracellular L-glutamine concentration is necessary to ensure that CPS-II efficiently synthesizes CP.
To ensure intracellular L-glutamine for CP synthesis, the expression levels of glnA in ARG-CP1 genes were increased by replacing the native promoter with the strong promoter Psod, resulting in the ARG-CP2 strain. The RT-qPCR results showed that the level of glnA expression under the control of the Psod promoter in ARG-CP2 was 5.29-fold greater than that observed under the control of its native promoter in ARG-CP1. The increased glnA expression level resulted in the GS activity of ARG-CP2 reaching 2274.7 ± 14.8 mU∙mg−1, which was approximately 3.20-fold higher than that of ARG-CP1 (Table 1). To evaluate the effect of increased GS enzyme activity on CPS-II catalytic CP synthesis, the activity of CPS-II in ARG-CP2 was compared to that of ARG-CP1. We were surprised to find a significant increase (approximately 79.22%) in CPS-II activity in ARG-CP2 compared to ARG-CP1. These results indicate that GS plays an effective role in increasing L-glutamine synthesis to provide an adequate substrate for CPS-II.
The two recombinant strains, together with the control strain SYPA 5-5, were cultivated in shake flask fermentation medium. Dry cell weight, L-arginine yield, and L-glutamine content were determined to assess the effects of glnA overexpression in ARG-CP2. First, the growth of the constructed strains was similar to that of SYPA 5-5 throughout the fermentation period. After cultivation for 96 h, the ARG-CP2 strain showed a 9.70%, 2.76-fold increase, in L-arginine (32.8 ± 0.6 g∙L−1) and L-glutamine (0.69 ± 0.03 g∙L−1) concentrations compared to those of the ARG-CP1 strain, respectively. The results suggested that glnA overexpression could effectively increase the yield of L-arginine by increasing the substrate L-glutamine supply of CPS-II.
Introducing a novel CP synthesis pathway for C. crenatum
For the synthesis of CP, we introduced CK to C. crenatum. CK, which utilizes inorganic ammonia as a substrate, has the potential to assist CP synthesis, and can save many organic nitrogen sources for the production of L-arginine and other compounds.
We screened and cloned four genes encoding CK, efarcc, llarcc, ccarcc, and yahI, from Enterococcus faecalis V583, Lactococcus lactis Il1403, Clostridium carboxidivorans P7, and Escherichia coli BL21, respectively, and compared the capacities of the four encoded enzymes (Ef-CK, Ll-CK, CC-CK, and EC-CK, respectively) (Fig. S1). After comparison, Ef-CK was found to show better temperature and pH stability. Furthermore, its specific activity was dramatically higher than that of the others, reaching 14.761 U∙mg−1. The data are shown in Fig. S2 and Table S3.
To verify that four CK can replace CPS-II in SYPA 5-5 for synthesizing CP, the plasmid pECXK-99E carrying the CK was transformed into 5-5/ΔcarB, resulting in 5-5/ΔcarB/efarcc, 5-5/ΔcarB/llarcc, 5-5/ΔcarB/yahI, and 5-5/ΔcarB/ccarcc. To verify the function of CK as that of CPS-II in SYPA 5-5, the growth trend of recombinant bacteria was monitored. Similar to the wild strain, the growth of the constructed strains was normal in CGXII medium supplemented with 30 g∙L−1 (NH4)2SO4. CK enzyme activity was then measured to determine which CK is the most suitable for C. crenatum; the results are shown in Fig. S3. The large subunit of CPS-II has a slight ability to synthesize CP from ammonia (Rubino et al. 1987); therefore, SYPA 5-5 shows slight CK activity. The specific activity of 5-5/ΔcarB/efarcc reached 4.321 U∙mg−1, which was noticeably higher than that of other recombination strains. With the advantage of better stability and higher specific activity, and more importantly good suitability for SYPA 5-5, Ef-CK has the potential to provide a new CP synthesis pathway for SYPA 5-5 and use inorganic ammonia to assist CPS-II synthesis of CP. We transformed the plasmids pECXK-99E-efarcc carrying Ef-CK into ARG-CP1, resulting in ARG-CP1/efarcc, which has two pathways for synthesizing CP. The total synthesis rate of CP was determined using both L-glutamine and (NH4)2SO4 as substrates in accordance with the CK and CPS enzyme activity assay method described in the “Enzyme activity assays” section. As shown in Fig. 3a, to some extent, the strategy of overexpression of CPS-II alone could increase (2.4-fold) the synthesis rate of CP. However, in 5-5/ΔcarB/efarcc, Ef-CK, which can replace CPS-II, provided a more efficient CP synthesis pathway for SYPA 5-5. With the common action of CPS-II and CK, ARG-CP1/efarcc presented a higher CP synthesis rate, which is 10.35-fold higher than that in the wild-type strain. This indicates that Ef-CK has great potential for application in improving the CP supply for L-arginine production.
Changes in CP synthesis rate and L-arginine yield due to the introduction of CK. a Total synthesis rate of CP in SYPA 5-5, ARG-CP1, 5-5/ΔcarB/efarcc, and ARG-CP1/efarcc. b Time course of L-arginine production, DCW, and extracellular NH4+ levels in shake flask fermentation of the ARG-CP2, ARG-CP3, and SYPA 5-5 strains. Experiments were conducted in triplicate. Error bars indicate ± SD
In order to further explore the influence of CK on L-arginine synthesis, the ARG-CP3 strain (ARG-CP2 harboring pECXK-99E-efarcc) was constructed. Subsequently, the ARG-CP3, ARG-CP2, and SYPA 5-5 strains were cultivated for 96 h in the fermentation medium, and their corresponding characteristics were compared. The fermentation curves, including dry cell weight, extracellular NH4+ consumption, and L-arginine productivity, are presented in Fig. 3b. The growth rates of the three strains were similar during the fermentation period while the synthesis of L-arginine increased with the cell growth. It indicated that heterologous expression of the efarcc gene had no significant effect on cell growth. The L-arginine yield of ARG-CP3 reached 38.1 ± 0.9 g∙L−1 after 96 h in 250-mL shake flask fermentation, which was 16.16% and 45.26% greater than the amount secreted by ARG-CP2 and SYPA 5-5, respectively. The carbon utilization ratio of AGR-CP3 was 26.2%, while that of SYPA5-5 is only 17.2%. Meanwhile, it is notable that the extracellular NH4+ levels in the ARG-CP3 strain were more quickly consumed than was observed in the ARG-CP2 and SYPA 5-5 strains. Taken together, these results indicate that the L-arginine yield can be increased by integrating Ef-CK, which contributes to the supply of CP by utilizing inorganic ammonia. This provides a new strategy for improving the ability of microbial cell factories to synthesize CP and promote L-arginine production in subsequent studies.
Optimizing carbon flux and increasing the ATP supply by knocking out the ldh gene
During L-arginine production by the C. crenatum strain, due to the rapid growth and high cellular metabolism requirements of the bacteria, the oxygen supply is frequently insufficient, leading to the accumulation of lactate as a by-product (Zhang et al. 2014). As shown in Fig. 2d, the engineered strain ARG-CP1 accumulated 8.25 ± 0.64 g∙L−1 lactate, which will lead to carbon loss and energy dissipation carried by NADH (Inui et al. 2004).
The reduction in lactate accumulation was expected to further improve L-arginine production in C. crenatum. The ldh gene (NCgl2810), which encodes lactate dehydrogenase, was inactivated in the ARG-CP2 strain, resulting in ARG-CP2/Δldh. The RT-qPCR results confirmed that the transcript levels of ldh in ARG-CP2/Δldh were significantly decreased (approximately 6.13-fold) after ldh was knocked out. To validate whether NADH and ATP were indeed increased as a result of the knockdown of ldh, the intracellular NADH and ATP levels in ARG-CP2/Δldh and ARG-CP2 strains during their exponential growth phase were measured. In response to ldh modification, the NADH concentration of ARG-CP2/Δldh was improved by 67.94% (Fig. 4c). Similarly, we also observed a 52.75% increase in intracellular ATP concentration in the ARG-CP2/Δldh strain at the stationary phase. From these data, it was clear that disrupting lactate synthesis has a significant effect on reducing the consumption of NADH, and adequate NADH contributes H+ and electrons to the electron transport chain (ETC), making it possible to drive ATP synthesis. Subsequently, in ARG-CP2/Δldh, the levels of L-arginine and lactate in shake flask fermentation were measured, compared with ARG-CP2. Both strains yielded approximately the same biomass. The ARG-CP2 strain accumulated 8.30 ± 1.1 g∙L−1 lactate, whereas the ldh-knockout strain significantly decreased the lactate titer to 0.67 ± 0.08 g∙L−1 (Fig. 4a). L-arginine production reached 39.5 ± 1.5 g∙L−1 by ARG-CP2/Δldh, 20.43% more than that attained by ARG-CP2 at 96 h. The data showed that ldh gene deletion markedly improved L-arginine production and decreased by-product lactate formation.
Concentration of lactate and intracellular ATP and NADH level after the ldh gene was disrupted. a Concentration of lactate and L-arginine in the ARG-CP2 and ARG-CP2/Δldh strains after 96h shake flask fermentation. b Structural arrangement of ARG-CP4. c Concentration of intracellular ATP and NADH in SYPA 5-5 and relevant recombination strain. Exponentially growing cells cultured in shake fermentation medium containing 40 g/L glucose were used for analysis. All values are derived from three independent experiments
For the production of L-arginine, the recombinant plasmids and 99E-efarcc were transformed into ARG-CP2/Δldh, resulting in a novel strain, ARG-CP4. Then, the activities of enzymes involved in CP synthesis pathways were investigated (Table 1). Here, we found that deletion of ldh had a positive effect on the enzyme activities of CPS-II and GS. Evidence indicates that the enzyme activities of CPS-II and GS were 54.60% and 39.20% higher in the ARG-CP4 strain than in the ARG-CP3 strain. Therefore, deletion of ldh genes can also be a strategy that activates ATP-dependent enzymes by increasing ATP supply. These increased enzyme activities would be beneficial to promote CP synthesis and L-arginine production. For shake flask fermentation, this allowed enhanced L-arginine production in ARG-CP4 to 45.09 ± 2.1 g∙L−1, which is 71.91 % higher than the yield obtained in SYPA 5-5. Meanwhile, in the process of L-arginine overproduction, intracellular ATP levels could be maintained at levels similar to those in the wild-type strain (Fig. 4c). Taken together, our results indicate that deletion of the ldh gene is effective in improving L-arginine production in C. crenatum.
Fed-batch fermentation of ARG-CP4 and SYPA5-5
The L-arginine production performance of the final strain, ARG-CP4, was examined in fed-batch fermentation in 5-L bioreactors. The time courses of L-arginine fed-batch fermentations of SYPA 5-5 and ARG-CP4 are shown in Fig. 5a, b. The growth rate curves of the two strains indicated that a series of molecular manipulations had no effect on cell growth in ARG-CP4.
It can be observed that the glucose consumption and L-arginine yield of ARG-CP4 remained fairly consistent at the beginning of fermentation, compared with the initial strain. After 24 h, the glucose consumption of ARG-CP4 accelerated quickly, which could have resulted from the increased biosynthetic pathway of CP, and showed superior L-arginine synthesis. At the end of fermentation, SYPA 5-5 could produce 44.4 ± 1.5 g∙L−1 L-arginine with a yield of 0.28 ± 0.02 g∙g−1 and productivity of 0.46 ± 0.02 g∙L−1∙h−1 after 96 h. In comparison, the ARG-CP4 strain resulted in 68.6 ± 1.2 g∙l−1 of L-arginine with a yield of 0.34 ± 0.02 g∙g−1 glucose and achieved a productivity of 0.71 ± 0.01 g∙l−1∙h−1. The data showed that the L-arginine production of ARG-CP4 was increased by 54.5% compared to SYPA 5-5. The metabolically engineered ARG-CP4 was proven to be an efficient L-arginine-producing strain, which could become a promising industrial L-arginine microbial producer. Furthermore, increasing the supply of CP might be a useful strategy for improving production of L-arginine or other products in C. crenatum.
Discussion
Carbamoyl phosphate (CP), a labile energy-rich molecule with multiple facets, is at the crossroad of L-arginine and pyrimidine synthesis pathway that provide a source of carbamoyl (Shen et al. 2019; Shi et al. 2018). The CPS-II encoded by the carAB operon converts HCO3-, ATP, and glutamine to CP (Charlier et al. 2018). In our study, the deletion of the gene coding for CPS-II not only interrupts the synthesis of amino acids, but also causes cells to stop growing. The same phenomenon was also found in E. coli (Alcantara et al. 2000). Only when L-arginine and pyrimidine were present in medium did the 5-5/ΔcarB resume growth. Our research could be considered as a validation that CPS-II is the only pathway that synthesizes CP in Corynebacterium sp. The ARG-CP1 with overexpressed carAB shown increased L-arginine production. CPS-II provides a source of carbamoyl which is subsequently converted to L-arginine by a three-step enzymatic reaction (Charlier et al. 2018; Tuchman et al. 1997). Moreover, CP synthesis by CPS-II is also one of the pathways by which cells fix CO2 used to synthesize biomass. Therefore, the increased enzyme activity of CPS-II can significantly promote the production of L-arginine.
In our previous study, we overexpressed glnA in C. crenatum to enhance L-arginine synthesis in cells for the biosynthesis of cell material (Guo et al. 2017). L-arginine has the highest N:C ratio among the natural amino acids, corresponding to a nitrogen content of 32.1% (Xu et al. 2019). L-Glutamine is the primary products of ammonia assimilation and the major intracellular organic nitrogen donors (Reitzer 2003; Silberbach et al. 2005). Since there is a direct link between L-arginine and L-glutamine, the rational regulation of L-glutamine metabolism is essential to increase nitrogen source assimilation and enhance L-arginine derivatives production. The nitrogen metabolism in Corynebacterium is regulated by nitrogen regulatory factor AmtR. When L-glutamine and L-arginine are synthesized, the intracellular NH4+ concentration decrease, in this case, the transcription level of genes which repressed by AmtR is increased. These genes involved in the ammonia assimilation pathway, for example, glnA, gltBD, and amtB. The activation of the ammonia assimilation pathway will inevitably increase the flux of the whole L-arginine synthesis pathway (Xu et al. 2019). We overexpressed that glnA in ARG-CP1 can effectively increase the activity of CPS-II. The smaller subunit of CPS-II catalyzes the hydrolysis of L-glutamine to ammonia and L-glutamate, then ammonia subsequently traverses the molecular tunnel leading to larger subunit where it reacts to generate the final product, carbamoy phosphate (Hart and Powers-Lee 2009; Thoden et al. 2002). Accordingly, as the only nitrogen-donating substrate of CPS-II, the concentration of L-glutamine will directly affect the activity of CPS-II. It increases the supply of CPS-II substrates, and may induce the regulation of the expression level of CPS-II encoded genes. The increased activity of CPS-II had promoted the synthesis of L-arginine, as indicated by more L-arginine accumulation in fermentation process.
It is notable that CP was synthesized by CK from ATP, HCO3-, and NH3 rather than L-glutamine in the hyperthermophilic archaea (Alcantara et al. 2000; Uriarte and Marina 1999). It was discovered, purified, and characterized in Enterococcus faecalis; most studies and the majority of the practical applications of CK have used the enzyme from E. faecalis; it is classic and best characterized CK. CK, a homodimer of a 33-kDa subunit, can also phosphorylate bicarbonate that was believed to resemble the larger subunit of CPS-II (Marina et al. 1999). It is an important finding in the understanding of the CP synthesis method. We expressed, purified, and compared four CK from Enterococcus faecalis V583, Lactococcus lactis Il1403, Clostridium carboxidivorans P7, and Escherichia coli BL21, respectively. The optimum temperature of Ef-CK is measured to be 45°C, the optimum pH is 8.0, and it had good stability in a certain temperature and pH range than others. Although it had higher activity than other CK, further optimization aimed at reducing the higher Km value by molecular modification. Our work provides a basis for the research about properties and applications of CK. The Ef-CK can restore the growth of 5-5/ΔcarB when inorganic nitrogen was present in the medium. The results confirmed that the CK could replace CPS-II to supply CP for cell growth and metabolism in C. crenatum. In ARG-CP2, the introduction of Ef-CK increased the overall rate of CP synthesis and L-arginine productivity. L-Glutamine provides nitrogen for purines, pyrimidines, tryptophan, histidine, glucosamine, p-aminobenzoate, arginine, and so on (Reitzer 2003). The introduction of Ef-CK not only provides a new CP synthesis pathway for C. crenatum, but also saves valuable L-glutamine for growth and other biomass synthesis. This is the first example of CK applied to L-arginine production, which should serve as a guide for further strain development for other applications.
Lactate is by-product produced by the C. crenatum strain when the oxygen supply is frequently insufficient (Kondoh and Hirasawa 2019; Zhang et al. 2014). By blocking the lactate synthesis pathway, many high-yielding industrial strains have been successfully constructed. In an ldh-deficient host, the C. glutamicum intracellular chondroitin titer increased from 0.25 to 0.88 g∙L−1 compared with that in a wild-type host (Cheng et al. 2019). In our study, the ldh-knockout strain ARG-CP2/Δldh shown significantly decreased the lactate titer and 20.43% increased L-arginine production than wild strain. Pyruvate is a branch point of glucose distribution, as it is the portal for several metabolic pathways, and lactate synthesis from pyruvate by LDH leads to carbon loss (Zhan et al. 2019; Zhang et al. 2014). The blocking of lactate synthesis pathway increases the metabolic flux of glucose to L-arginine synthesis. Furthermore, a 52.75% increase of intracellular ATP concentration was observed in ARG-CP2/Δldh. The reduction of lactate synthesis can save a large amount of NADH, which is an important carrier of protons and electrons (Anraku 1988; Sawada et al. 2012). The protons and electrons are transferred along the ETC to oxygen; coupled with this process, the energy released is phosphorylated to form ATP (Bott and Niebisch 2003; Wang et al. 2018). The increased intracellular ATP level effectively facilitated L-arginine biosynthesis by activating ATP-dependent enzymes that are related to CP and L-arginine synthesis pathways. Among them, the enzyme activities of CPS-II and GS were 54.60% and 39.20% higher in the ARG-CP4 strain than in the ARG-CP3 strain. On the one hand, the increase of intracellular ATP level can provide the power for these enzyme-catalyzed reactions, and on the other hand, it may regulate the transcription level of coding genes by activating intracellular regulatory networks. Adequate intracellular ATP levels are essential in maintaining the intracellular environment, central metabolic pathways, cell growth, and production of target metabolites (Zhou et al. 2009).
In recent years, L-arginine demand has continued to increase for its wide applications in industry. With the development of genetic engineering technology, an L-arginine high-yielding strain based on metabolic engineering has become a research focus. In this study, a series of genetic manipulation strategies were applied to the C. crenatum SYPA 5-5 strain to enhance the CP supply to improve L-arginine production, including activating the synthesis of CP (overexpression of carAB and glnA), introducing a novel heterogeneous CP synthesis pathway (carbamate kinase from Enterococcus faecalis V583), increasing ATP supply, and reducing lactate formation through deletion of ldh. The final C. crenatum strain developed by systems metabolic engineering was able to efficiently produce L-arginine. In addition, the metabolic studies on CP conducted herein are useful for a better understanding of L-arginine production in microorganisms. The present work provides a strong foundation for further strain development towards the improvement of L-arginine productivity, supply of energy, and conversion efficiency of carbon sources.
References
Alcantara C, Cervera J, Rubio V (2000) Carbamate kinase can replace in vivo carbamoyl phosphate synthetase. Implications for the evolution of carbamoyl phosphate biosynthesis. FEBS Lett 484(3):261–264. https://doi.org/10.1016/S0014-5793(00)02168-2
Anraku Y (1988) Bacterial electron transport chains. Annu Rev Biochem 57:101–132. https://doi.org/10.1146/annurev.bi.57.070188.000533
Bott M, Niebisch A (2003) The respiratory chain of Corynebacterium glutamicum. J Biotechnol 104(1-3):129–153. https://doi.org/10.1016/s0168-1656(03)00144-5
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Charlier D, Nguyen Le Minh P, Roovers M (2018) Regulation of carbamoylphosphate synthesis in Escherichia coli: an amazing metabolite at the crossroad of arginine and pyrimidine biosynthesis. Amino Acids 50(12):1647–1661. https://doi.org/10.1007/s00726-018-2654-z
Cheng F, Luozhong S, Yu H, Guo Z (2019) Biosynthesis of chondroitin in engineered Corynebacterium glutamicum. J Microbiol Biotechnol 29(3):392–400. https://doi.org/10.4014/jmb.1810.10062
Deporte R, Amiand M, Moreau A, Charbonnel C, Campion L (2006) High-performance liquid chromatographic assay with UV detection for measurement of dihydrouracil/uracil ratio in plasma. J Chromatogr B Anal Technol Biomed Life Sci 834(1-2):170–177. https://doi.org/10.1016/j.jchromb.2006.02.046
Eroglu B, Powers-Lee SG (2002) Unmasking a functional allosteric domain in an allosterically nonresponsive carbamoyl-phosphate synthetase. J Biol Chem 277(47):45466–45472. https://doi.org/10.1074/jbc.M208185200
Guo J, Man Z, Rao Z, Xu M, Yang T, Zhang X, Xu Z (2017) Improvement of the ammonia assimilation for enhancing L-arginine production of Corynebacterium crenatum. J Ind Microbiol Biotechnol 44(3):443–451. https://doi.org/10.1007/s10295-017-1900-9
Hart EJ, Powers-Lee SG (2009) Role of Cys-1327 and Cys-1337 in redox sensitivity and allosteric monitoring in human carbamoyl phosphate synthetase. J Biol Chem 284(9):5977–5985. https://doi.org/10.1074/jbc.M808702200
Inui M, Murakami S, Okino S, Kawaguchi H, Vertès AA, Yukawa H (2004) Metabolic analysis of Corynebacterium glutamicum during lactate and succinate productions under oxygen deprivation conditions. J Mol Microbiol Biotechnol 7(4):182–196. https://doi.org/10.1159/000079827
Kaseman DS, Meister A (1985) Carbamyl phosphate synthetase (glutamine-utilizing) from Escherichia coli. Methods Enzymol 113:305–326. https://doi.org/10.1016/s0076-6879(85)13044-2
Kondoh M, Hirasawa T (2019) L-Cysteine production by metabolically engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol 103(6):2609–2619. https://doi.org/10.1007/s00253-019-09663-9
Korte JJ, Salo WL, Cabrera VM, Wright PA, Felskie AK, Anderson PM (1997) Expression of carbamoyl-phosphate synthetase III mRNA during the early stages of development and in muscle of adult rainbow trout (Oncorhynchus mykiss). J Biol Chem 272(10):6270–6277. https://doi.org/10.1074/jbc.272.10.6270
Man Z, Rao Z, Xu M, Guo J, Yang T, Zhang X, Xu Z (2016) Improvement of the intracellular environment for enhancing L-arginine production of Corynebacterium glutamicum by inactivation of H2O2-forming flavin reductases and optimization of ATP supply. Metab Eng 38:310–321. https://doi.org/10.1016/j.ymben.2016.07.009
Marina A, Uriarte M, Barcelona B, Fresquet V, Cervera J, Rubio V (1998) Carbamate kinase from Enterococcus faecalis and Enterococcus faecium--cloning of the genes, studies on the enzyme expressed in Escherichia coli, and sequence similarity with N-acetyl-L-glutamate kinase. Eur J Biochem 253(1):280–291. https://doi.org/10.1046/j.1432-1327.1998.2530280.x
Marina A, Alzari PM, Bravo J, Uriarte M, Barcelona B, Fita I, Rubio V (1999) Carbamate kinase: new structural machinery for making carbamoyl phosphate, the common precursor of pyrimidines and arginine. Protein Sci 8(4):934–940. https://doi.org/10.1110/ps.8.4.934
Moncada S, Higgs A (1993) The L-arginine-nitric oxide pathway. N Engl J Med 329(27):2002–2012. https://doi.org/10.1056/NEJM199312303292706
Nyunoya H, Lusty CJ (1983) The carB gene of Escherichia coli: a duplicated gene coding for the large subunit of carbamoyl-phosphate synthetase. Proc Natl Acad Sci U S A 80(15):4629–4633. https://doi.org/10.1073/pnas.80.15.4629
Park SH, Kim HU, Kim TY, Park JS, Kim SS, Lee SY (2014) Metabolic engineering of Corynebacterium glutamicum for L-arginine production. Nat Commun 5:4618. https://doi.org/10.1038/ncomms5618
Pekkala S, Martinez AI, Barcelona B, Gallego J, Bendala E, Yefimenko I, Rubio V, Cervera J (2009) Structural insight on the control of urea synthesis: identification of the binding site for N-acetyl-L-glutamate, the essential allosteric activator of mitochondrial carbamoyl phosphate synthetase. Biochem J 424(2):211–220. https://doi.org/10.1042/BJ20090888
Popolo A, Adesso S, Pinto A, Autore G, Marzocco S (2014) L-Arginine and its metabolites in kidney and cardiovascular disease. Amino Acids 46(10):2271–2286. https://doi.org/10.1007/s00726-014-1825-9
Ramon-Maiques S, Marina A, Guinot A, Gil-Ortiz F, Uriarte M, Fita I, Rubio V (2010) Substrate binding and catalysis in carbamate kinase ascertained by crystallographic and site-directed mutagenesis studies: movements and significance of a unique globular subdomain of this key enzyme for fermentative ATP production in bacteria. J Mol Biol 397(5):1261–1275. https://doi.org/10.1016/j.jmb.2010.02.038
Reitzer L (2003) Nitrogen assimilation and global regulation in Escherichia coli. Annu Rev Microbiol 57:155–176. https://doi.org/10.1146/annurev.micro.57.030502.090820
Robin J-P, Penverne B, Herve G (1989) Carbamoyl phosphate biosynthesis and partition in pyrimidine and arginine pathways of Escherichia coli. In situ properties of carbamoyl-phosphate synthase, ornithine transcarbamylase and aspartate transcarbamylase in permeabilized cells. Eur J Biochem 183(3):519–528
Rubino SD, Nyunoya H, Lusty CJ (1987) In vivo synthesis of carbamyl phosphate from NH3 by the large subunit of Escherichia coli carbamyl phosphate synthetase. J Biol Chem 262(9):4382–4386
Rubio V, Cervera J, Lusty CJ, Bendala E, Britton HG (1991) Domain structure of the large subunit of Escherichia coli carbamoyl phosphate synthetase. Location of the binding site for the allosteric inhibitor UMP in the COOH-terminal domain. Biochemistry 30(4):1068–1075. https://doi.org/10.1021/bi00218a027
Saeed-Kothe A, Powers-Lee SG (2002) Specificity determining residues in ammonia- and glutamine-dependent carbamoyl phosphate synthetases. J Biol Chem 277(9):7231–7238. https://doi.org/10.1074/jbc.M110926200
Sawada K, Kato Y, Imai K, Li L, Wada M, Matsushita K, Yokota A (2012) Mechanism of increased respiration in an H+-ATPase-defective mutant of Corynebacterium glutamicum. J Biosci Bioeng 113(4):467–473. https://doi.org/10.1016/j.jbiosc.2011.11.021
Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145(1):69–73. https://doi.org/10.1016/0378-1119(94)90324-7
Schneider Jens, Niermann K, Wendisch VF (2011) Production of the amino acids L-glutamate, L-lysine, L-ornithine and L-arginine from arabinose by recombinant Corynebacterium glutamicum. J Biotechnol 154(2–3):191–198
Shen S, Zhang X, Li Z (2019) Development of an engineered carbamoyl phosphate synthetase with released sensitivity to feedback inhibition by site-directed mutation and casting error-prone PCR. Enzym Microb Technol 129:109354. https://doi.org/10.1016/j.enzmictec.2019.05.011
Shi D, Caldovic L, Tuchman M (2018) Sources and fates of carbamyl phosphate: a labile energy-rich molecule with multiple facets. Biology (Basel) 7(2). https://doi.org/10.3390/biology7020034
Silberbach M, Schafer M, Huser AT, Kalinowski J, Puhler A, Kramer R, Burkovski A (2005) Adaptation of Corynebacterium glutamicum to ammonium limitation: a global analysis using transcriptome and proteome techniques. Appl Environ Microbiol 71(5):2391–2402. https://doi.org/10.1128/AEM.71.5.2391-2402.2005
Thoden JB, Huang X, Raushel FM, Holden HM (2002) Carbamoyl-phosphate synthetase. Creation of an escape route for ammonia. J Biol Chem 277(42):39722–39727. https://doi.org/10.1074/jbc.M206915200
Tuchman M, Rajagopal BS, McCann MT, Malamy MH (1997) Enhanced production of arginine and urea by genetically engineered Escherichia coli K-12 strains. Appl Environ Microbiol 63(1):33–38. https://doi.org/10.1128/AEM.63.1.33-38.1997
Uriarte M, Marina A (1999) The carbamoyl-phosphate synthetase of Pyrococcus furiosus is enzymologically and structurally a carbamate kinase. J Biol Chem 274(23):16295–16303. https://doi.org/10.1074/jbc.274.23.16295
Wang Q, Xia J, Guallar V, Krilov G, Kantrowitz ER (2008) Mechanism of thermal decomposition of carbamoyl phosphate and its stabilization by aspartate and ornithine transcarbamoylases. Proc Natl Acad Sci U S A 105(44):16918–16923. https://doi.org/10.1073/pnas.0809631105
Wang S, Qiu L, Liu X, Xu G, Siegert M, Lu Q, Juneau P, Yu L, Liang D, He Z, Qiu R (2018) Electron transport chains in organohalide-respiring bacteria and bioremediation implications. Biotechnol Adv 36(4):1194–1206. https://doi.org/10.1016/j.biotechadv.2018.03.018
Wieschalka S, Blombach B, Bott M, Eikmanns BJ (2013) Bio-based production of organic acids with Corynebacterium glutamicum. Microb Biotechnol 6(2):87–102. https://doi.org/10.1111/1751-7915.12013
Xu M, Rao Z, Yang J, Dou W, Xu Z (2013) The effect of a LYSE exporter overexpression on L-arginine production in Corynebacterium crenatum. Curr Microbiol 67(3):271–278. https://doi.org/10.1007/s00284-013-0358-x
Xu M, Li J, Shu Q, Tang M, Zhang X, Yang T, Xu Z, Rao Z (2019) Enhancement of L-arginine production by increasing ammonium uptake in an AmtR-deficient Corynebacterium crenatum mutant. J Ind Microbiol Biotechnol 46(8):1155–1166. https://doi.org/10.1007/s10295-019-02204-3
Xu M, Tang M, Chen J, Yang T, Zhang X, Shao M, Xu Z, Rao Z (2020) PII signal transduction protein GlnK alleviates feedback inhibition of N-acetyl-L-glutamate kinase by L-arginine in Corynebacterium glutamicum. Appl Environ Microbiol 86(8). https://doi.org/10.1128/AEM.00039-20
Yefimenko I, Fresquet V, Marco-Marin C, Rubio V, Cervera J (2005) Understanding carbamoyl phosphate synthetase deficiency: impact of clinical mutations on enzyme functionality. J Mol Biol 349(1):127–141. https://doi.org/10.1016/j.jmb.2005.03.078
Zhan M, Kan B, Dong J, Xu G, Han R, Ni Y (2019) Metabolic engineering of Corynebacterium glutamicum for improved L-arginine synthesis by enhancing NADPH supply. J Ind Microbiol Biotechnol 46(1):45–54. https://doi.org/10.1007/s10295-018-2103-8
Zhang D, Guan D, Liang J, Guo C, Xie X, Zhang C, Xu Q, Chen N (2014) Reducing lactate secretion by ldhA Deletion in L-glutamate- producing strain Corynebacterium glutamicum GDK-9. Braz J Microbiol 45(4):1477–1483. https://doi.org/10.1590/s1517-83822014000400044
Zhou J, Liu L, Shi Z, Du G, Chen J (2009) ATP in current biotechnology: regulation, applications and perspectives. Biotechnol Adv 27(1):94–101. https://doi.org/10.1016/j.biotechadv.2008.10.005
Availability of data and material
All data related to this study has been included in the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (31770058, 32070035), the National Key Research and Development Program of China (2018YFA0900300), Natural Science Foundation of Jiangsu Province (BK20181205), the Key Research and Development Program of Ningxia Hui Autonomous Region (No. 2019BCH01002), the national first-class discipline program of Light Industry Technology and Engineering (LITE2018-06), and the 111 Project (111-2-06).
Author information
Authors and Affiliations
Contributions
ZR and MX conceived and designed the study and critically revised the manuscript. QW carried out the experiments, analyzed the data, and drafted the manuscript. AJ, JT, and HG carried out the experiments. XZ, TY, and ZX contributed to the revision of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with human participants performed by any of the authors.
Consent for publication
All authors revised and consented to publication of the findings.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 685 kb)
Rights and permissions
About this article
Cite this article
Wang, Q., Jiang, A., Tang, J. et al. Enhanced production of L-arginine by improving carbamoyl phosphate supply in metabolically engineered Corynebacterium crenatum. Appl Microbiol Biotechnol 105, 3265–3276 (2021). https://doi.org/10.1007/s00253-021-11242-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11242-w