Abstract
Light management strategy can be used to improve algal biomass and nutrient production. However, the response of algal metabolism to different light qualities, especially their interaction with other environmental factors, is not well understood. This study focuses on the interactive effects of light quality and culturing temperature on algal protein content and carbohydrate content of C. reinhardtii. Three LED light sources (blue light, red-orange light, and white-yellow light) were applied to grow algae in batch cultures with a light intensity of 105 μmol/m2s under the temperatures of 24 °C to 32 °C. The protein and carbohydrate content were measured in both the late exponential growth phase and the late stationary growth phase. The results revealed that there was an interactive effect of light quality and culturing temperature on the protein and carbohydrate content. The combined conditions of blue light and a temperature of 24 °C or 28 °C, which induced a larger algal cell size with a prolonged cell cycle and a low division rate, resulted in the highest protein content; the protein mass fraction and concentration were 32% and 52% higher than that under white-yellow light at 32 °C. The combined conditions of red-orange light and a temperature of 24 °C, which promoted both the cell division and size growth, enhanced the carbohydrate content; the carbohydrate mass fraction and concentration were 161% and 155% higher than that under white-yellow light at 24 °C. When there was temperature stress (32 °C) or nutrient stress, the effect of light quality reduced, and the difference of protein and carbohydrate content among the three light qualities decreased.
Key points
• Studied light quality-temperature interactive effect on protein, carbohydrate synthesis.
• Protein content was high under low cell division rate.
• Carbohydrate content was high under high cell division and cell size growth rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Algae have recently become a topic of research as an alternative source for the increasing demand for food and energy, and even as a part of life support system in long-range space travel (Matula and Nabity 2016; Niederwieser et al. 2018; Svetoslav 2016). They have a short harvest cycle, high growth rate, multiple nutrient compositions, and the ability to thrive under extreme environments (Daniela et al. 2019; Trainer et al. 2019). They have been studied for environmental control and life support (ECLS) in space travel, including air revitalization, wastewater treatment, and most importantly, food supply for astronauts (Matula and Nabity 2016, 2019; Niederwieser et al. 2018; Svetoslav 2016). Because of the physiological effects of microgravity, nutrient or energy balance becomes essential for astronauts’ health (Laurens et al. 2019). Improving the algal biomass production and nutrient composition would make algae an even more promising food source.
In space, harmful radiation from cosmic rays, such as X-rays and UV light, can cause cellular damage to algae (Matula and Nabity 2019). As a result, the application of artificial light or tailored sunlight (Matula and Nabity 2019; Michael 2015; Sforza et al. 2015; Sung et al. 2018; Vadiveloo et al. 2015) in a well-controlled photobioreactor (PBR) could be the best option. To improve algal biomass and nutrient production, culturing conditions, such as the light source, culturing temperature, and other environmental factors must be optimized for enhancement of the cultural yield of food and other valued products (Wells et al. 2017).
Light provides energy for photosynthesis and transfers environmental signals for metabolic regulation (Jones 2018). Photoreceptors in algae are rich in structure and function. They perceive photons with different spectra and signal with different pathways, and they also interact with each other in controlling the algal physiology and biochemistry (Casal 2000; Hegemann 2008; Huang and Beck 2003; Jones 2018).
Due to the emission wavelength matching with the absorption peak wavelength of chlorophylls, red light and blue light are generally considered favorable for plant growth and are widely applied for indoor agriculture (Gobble and Headrick 2019; Pennisi et al. 2019; del Pilar and Voltolina 2006) and algae culture (Ajayan et al. 2019; Alego et al. 2018; Kim et al. 2019), but the absorption rate peak and the photon yield peak may not be at the same wavelength (Schulze et al. 2014). Algae can adapt to different spectral qualities through changing the proportions of photosynthetic pigments, i.e., chromatic adaption (Rochet et al. 1986; Rockwell et al. 2014). When the light quality changes, algae can produce a specific pigment that has a complementary color to absorb the light that induces it (Rochet et al. 1986).
The effect of light also interacts with other factors, such as temperature, nutrient supply, and biomass concentration (Juneja et al. 2013). It has been reported that activated photoreceptors contribute to temperature perception (Jones 2018), and temperature change can cause a shift of algal light saturation or inhibition (Jensen and Knutsen 1993). Additionally, biomass concentration affects the quantity of light accepted by each algae cell and the penetration depth of light (Janssen et al. 2018). Weakly absorbed green light or yellow light can penetrate deeper into the dense culture than either red or blue light (Kubín et al. 1983), and has been reported to result in a high biomass production and protein content (de Mooij et al. 2016; Kubín et al. 1983).
It is crucial to understand how algae respond to different light conditions, and how to optimize light regime for manipulating signaling pathways and enhance the algal biomass and nutrient production (Satthong et al. 2019; Schulze et al. 2014, 2016; Wu 2016). In previous work, authors have studied the light quality and temperature on lipid synthesis of C. reinhardtii (Li et al. 2020). This work focuses on (a) the study of the interactive effects of light quality and culturing temperature on the protein and carbohydrate synthesis and accumulation in green alga C. reinhardtii; (b) measuring the variation patterns of protein and carbohydrate content under different light quality with variable temperatures; (c) determining the optimal combination of light quality and temperature on protein and carbohydrate production to aid in the PBR design with artificial light or spectral conversion (or filtration) of natural sunlight. An LED light with three options of blue light, red-orange mixing light, and white-yellow mixing light was applied as the light source. The temperature range of 24 °C to 32 °C was tested. Protein content and carbohydrate content were checked in both the late exponential growth phase and the late stationary growth phase.
Material and methods
Strain, medium, and culturing method
The green alga strain C. reinhardtii WT CC-125 was purchased from the Chlamydomonas Resource Center of the University of Minnesota. This alga has a high content of protein and carbohydrates and has been widely used as a model organism. Heterotrophic growth of algae in TAP medium (Tris-Acetate-Phosphate) was carried out with batch culture in 250 mL baffled flasks. The TAP medium consisted of (mM): Tris base, 20; acetic acid, 17; NH4Cl (Fisher A 649-500), 7.5; CaCl2•2H2O (Sigma C-3881), 0.35; MgSO4•7H2O (Sigma 230391), 0.4; Na2HPO4 (Sigma S5136), 0.69; KH2PO4 (Sigma P 0662), 0.45; Na2EDTA•2H2O (Sigma ED255), 0.134; ZnSO4•7H2O (Sigma Z 0251), 0.077; H3BO3 (Baker 0084), 0.184; MnCl2•4H2O (Baker 2540), 0.026; FeSO4•7H2O, 0.018; CoCl2•6H2O (Sigma C-3169), 0.007; CuSO4•5H2O (MCIB3M11), 0.005; (NH4)6Mo7O24•4H2O (Mallinckrodt 3420), 0.0008.
Before inoculation, algae culture was acclimated under each condition for 1 week; more specifically, algae cells from the solid medium were inoculated in 250 mL baffled flasks in 50 mL TAP medium under each of the three different LED light sources at different temperature. After 4–5 days of acclimation, the culture was saturated, and a total of 1 mL culture was pipetted into each flask containing 50 mL of medium. Flasks with culture were placed in a constant-temperature incubator-shaker (Lab-line instruments, Inc. 3525-3CC) with a shaking speed of 120 rpm and under continuous lighting of the LED light, as shown in Fig. S1. The light intensity was controlled constant to 105 ± 3 μmol/m2s for all the three light qualities tested, which is lower than the photo-saturation point (120 μmol/m2s) reported in the literature for C. reinhardtii (Goold et al. 2016; Kirst et al. 2012).
The optical density (OD) of the cultures was checked twice per day for the growth kinetics analysis from the beginning of the growth process to the late exponential growth phase when the effect of nutrient starvation is limited, and the effect of light quality is highlighted. The OD was checked daily during the stationary growth phase when the impact of nutrient starvation is predominant. The protein and carbohydrate content were measured twice a week in the late exponential phase and late stationary phase, respectively. The growth cycle was set to 144 h. A total of twelve cultures were grown and analyzed for each culturing condition.
Light source
To study the effect of light quality, colored LED lights are the best to use because of their monochrome and small peak width. Nevertheless, since they do not provide light in a broad spectrum, it is better to use them in combination with other light colors (Kommareddy and Anderson 2004; Mitchell 2012; Olle and Viršile 2013; Schulze et al. 2016). An LED light panel (VIPARSPECTRA Dimmable Reflector-Series 450 W LED Grow Light) with 3 Dimmers 12-Band Full Spectrum was applied as the light source. The emission spectra were measured with a spectrometer (Flame Ocean Optics Miniature Spectrometer, Model: FLAME-S-VIS-NIR-ES), as shown in Fig. 1. The light intensity was calibrated at a height close to the flask top with a light meter (LightScout Solar/Electric Quantum Meter 3415FSE).
Emission light spectrum of the LEDs. a White-yellow light, b blue light, and c red-orange light. Blue lights peak at 433 nm to 447 nm (10 LED lights) and 458 nm to 470 nm (16 LED lights). Red lights peak at 630 nm (8 LED lights), 656 nm (8 LED lights), and 735 nm in IR (4 LED lights). The orange light peak at 580–594 nm (8 LED lights) and 604 nm (9 LED lights). White and yellow light (14 LED white lights, and 13 LED yellow lights) have two peaks in blue (peaking at 456–458 nm) and green to yellow (peaking at 545–570 nm)
We can see the white-yellow light is the mixing spectra of blue-green-yellow light. The red-orange light has a negligible blue light fraction and is used to compare with the blue light or white-yellow light.
Cell size determination and doubling time
The cell morphology under different culturing conditions was checked in the early stationary growth phase (fifth day of cultivation) when the culture has become mature but has not been impacted by nutrient starvation. The culture was concentrated through a centrifuge at a speed of 10,000 rpm for about 10 s. The algae paste was observed and imaged under a microscope (Olympus BH2). Image analysis is conducted with the open-source platform Image J (Rasband 2020). The cell area is obtained through the Image J analysis, and the cell size in diameter (μm) is calculated with Microsoft Excel.
The doubling time can be a reference to the cell cycle (or division rate); the longer the doubling time, the longer the cell cycle and the lower the division rate. The doubling time of algae was determined by:
where td is the doubling time of culture optical density, and μ is the maximum specific growth rate.
Bradford protein assay
Bradford protein assay is a dye-binding assay in which a differential color change of a dye occurs in response to various concentrations of protein. It is simple, accurate, and stable for solubilized protein checking (Guerlava et al. 1998). Sonication was proved to be an efficient method for algal cell disruption among the French press, Mutanolysin treatment, and NaOH-SDS solubilization (Guerlava et al. 1998).
The procedure of the Bradford protein assay applied is as follows: (a) centrifuge the algae slurry (Eppendorf 5810R Centrifuge) at 4000 rpm for 7 min and dump the supernatant; (b) wash the algae pellets with distilled and deionized water (DDW) and resuspended with DDW to the same volume as before centrifuge; (c) sonicate the algae slurry and turn on the temperature monitor to control the algae broth temperature below 60 °C, avoiding the degradation of protein; (d) dilute the algae broth after sonication (dilution factor = 5) and pipette a volume of 0.2 mL dilution to a 4.5 mL cuvette (VWR Standard Spectrophotometer Cuvettes), and add with 2 mL Bradford dye reagent (AMRESCO Biotechnology Grade) directly, and mix well (the difference checked between the samples with or without centrifuge after sonication was as small as 0.92%, so the algae broth was diluted directly and used for protein checking); (e) incubate the mixture at room temperature for 5 min, and check the absorbance at 595 nm.
The absorbance of samples after adding the Bradford dye will vary over time, so the algae slurry was measured with the standard solution each time. The standard solution was prepared by dissolving the bovine serum albumin (BSA) (AMRESCO Biotechnology Grade) in DDW. The standard curve was obtained by checking the OD (595 nm) of a serially diluted standard solution after adding dye reagent. Shaking moderately (not vigorously) to avoid foaming because foaming will lead to poor reproducibility (Kruger 2009). Samples were blanked with a mixture of 0.2 mL DDW and 2 mL Bradford dye and measured at 595 nm with a spectrometer (Thermo Scientific NANODROP 2000c).
Phenol-sulfuric acid carbohydrate assay
Phenol-sulfuric acid carbohydrate assay (Chaplin and Kennedy 1994) was applied to check the presence of carbohydrates in algae. In a hot acidic medium, glucose is dehydrated to hydroxymethylfurfural, which forms a colored product with phenol and has an absorption maximum at 490 nm.
The first two steps of the procedure are the same as the protein assay (a) and (b); (c) dilute the algae slurry (dilution factor = 2); (d) pipette 0.2 mL dilution to a 10 mL-glass tube; (e) add 0.4 mL of 5% w/v phenol solution (AMRESCO Ultrapure) and 2 mL of sulfuric acid (Acros Organics 96 ± 0.15%) to each tube and leave the solutions undisturbed for 10 min then shake the mixture vigorously; (f) after another 30 min, pipette the solution to a cuvette and check the absorbance at 490 nm.
The standard curve was obtained by measuring the OD490 of serially diluted glucose (Sigma-Aldrich 99.5%) solution after adding phenol and sulfuric acid. It was measured that within the glucose concentration range of 25–128 μg/mL, the standard line is linear, which was not shown here. Algae slurry and standard solutions were measured together.
The protein content and carbohydrate content were measured both in mass fraction (% w/w dry weight of biomass) and concentration (mg/L culture).
Statistical analysis
Statistical analysis methods, including one-way analysis of variance (ANOVA) and Tukey test, were used to study the significant difference for all the experimental data. Two-way ANOVA with replications and two-way ANOVA using regression (Unbalanced Factorial ANOVA) (Zaiontz 2020) were applied to analyze the interaction of light quality and culturing temperature for the results data with the same size and unequal size, respectively. A value of P = 0.05 was set as a threshold for the significant difference.
Results
Cell size and doubling time
The cell morphology was imaged under a microscope (Olympus BH2) at × 100 magnification. Cell images were analyzed with the Image J, and the cell size in diameter (μm) was calculated. Figure 2 shows the cell image and size of C. reinhardtii under four culturing conditions. The average size under blue light is 15.1 ± 2.3 μm (mean ± SD, n = 99) and 11.6 ± 3.2 μm (mean ± SD, n = 154) 28 °C and 32 °C, respectively; under red-orange light is 11.6 ± 2.4 μm (mean ± SD, n = 110) and 10.0 ± 1.3 μm (mean ± SD, n = 102) 28 °C and 32 °C, respectively. The cells were bigger in size under the blue light than that under the red-orange light (P < 0.01), and bigger at 28 °C than that at 32 °C (P < 0.01).
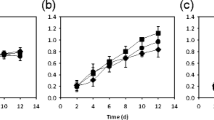
The doubling time can determine the cell cycle or division rate and be calculated from the maximum specific growth rate obtained by the OD measurement, as shown in Fig. 3. As the temperature decreased from 32 °C to 24 °C, the doubling time increased from 8.3 h to 10.2 h under the white-yellow light, 8.9 h to 10.2 h under the red-orange light, and 8.7 h to 13.5 h under blue light (Fig. 3). Under blue light, the doubling time was much higher than that under the red-orange and white-yellow light at temperatures 24 °C and 28 °C. When the temperature was increased to 30 °C and 32 °C, the doubling time became closer to the same value for the three lights.
Effect of light quality on protein content
According to a two-way ANOVA test, there was an interactive effect between light quality and culturing temperature on the protein content (protein mass fraction P = 9.3 × 10-5 and concentration P = 1.5 × 10-6). The combination of blue light and a temperature of 24 °C or 28 °C performed the best.
In this work, as shown in Fig. 4, algae grown under blue light showed higher protein mass fraction and concentration at 24 °C compared with that under white-yellow light or red-orange light (P < 0.05) in both the late exponential phase and late stationary phase. At 28 °C, algae grown under blue light showed higher protein mass fraction and concentration compared with that under white-yellow light (P < 0.05) but was not significantly greater than algae grown under red-orange light. At 30 °C and 32 °C, the protein content under different light qualities was close to each other (P > 0.05). This indicates a strengthened effect of temperature and an undermined impact of light quality on protein synthesis, i.e., at high temperatures, the influence of temperature overtook that of light quality on protein synthesis and accumulation.
Variation of protein content with light quality under different temperature, a protein mass fraction, and b protein mass concentration (mean ± SD, n = 6). Statistical differences (p < 0.05) within the contents of light quality (lowercase), temperature (uppercase), and growth phase are indicated by different letters
The protein content declined under most conditions from the exponential phase to the stationary phase. For instance, at 28 °C, the protein mass fraction decreased 11% (P = 0.003), 7% (P = 0.02), and 17% (P = 0.002) and protein mass concentration decreased 17% (P = 0.006), 20% (P = 3.37 × 10-9), and 21% (P = 8.26 × 10-5) respectively under the white-yellow light, blue light, and red-orange light.
Effect of culturing temperature on protein content
Four temperatures were tested: 24 °C, 28 °C, 30 °C, and 32 °C, as shown in Fig. 4. In the late exponential growth phase, the protein content reached the maximum at 28 °C almost under all light qualities except that the protein mass fraction under blue light reached the peak at 24 °C. With further increase of temperature, both protein mass fraction and concentration declined.
Protein content generally reaches the maximum at the optimal temperature then declines under either higher or lower temperatures (Sayegh and Montagnes 2011). In this study, both protein mass fraction and mass concentration peaked at 28 °C under the red-orange light or the white-yellow light in the late exponential phase. Under blue light, the protein mass fraction was the highest at 24 °C, but the protein concentration peaked at 28 °C. A significant difference can be observed when compared with those at 30 °C and/or 32 °C. In the late stationary phase, the protein mass fraction and concentration were still high at temperature 24 °C and 28 °C and decreased with the temperature under the three light qualities.
Effect of light quality on carbohydrate content
According to the two-way ANOVA, there was a significant interactive effect of light quality and culturing temperature on the carbohydrate content in C. reinhardtii (carbohydrate mass fraction P = 0.02 and concentration P = 7.3 × 10-14). The variation of carbohydrate content with light quality is shown in Fig. 5. We can see that the red-orange light performed better than the other two light qualities, especially in the late exponential phase.
Variation of carbohydrate content with light quality under different temperature, a carbohydrate mass fraction, and b carbohydrate mass concentration (mean ± SD, n = 6). Statistical differences (P < 0.05) within the contents of light quality (lowercase), temperature (uppercase), and growth phase are indicated by different letters
From Fig. 5, we can see the red-orange light enhanced the carbohydrate content in C. reinhardtii. In the late exponential phase, both carbohydrate mass fraction and concentration under the red-orange light were the highest for the four temperatures tested (P < 0.05). In the late stationary phase, the red-orange light still performed the best, but the difference of carbohydrate content among three light qualities was not significant as that in the late exponential phase.
Effect of culturing temperature on carbohydrate content
As shown in Fig. 5, the variation pattern was different for the carbohydrate mass fraction and concentration under different light qualities in each growth phase.
Under white-yellow light, the carbohydrate mass fraction increased from 24 °C to 28 °C (P = 0.0005) and then kept stable to 32 °C in the late exponential growth phase. In the late stationary growth phase, it increased from 24 °C to 32 °C, but the difference was not significant (P = 0.2). Under the blue light, the mass fraction increased from 24 °C to 32 °C (P > 0.05) in both the late exponential phase and the late stationary phase. While under red-orange light, the carbohydrate mass fraction was detected the highest 22.5% w/w at 24 °C and decreased with temperature to 32 °C (P = 0.0002) in the late exponential phase. In the late stationary phase, it was found the highest at 28 °C and the lowest at 24 °C (P = 0.009). It decreased from 28 °C to 32 °C (P = 0.2). Compared with the carbohydrate mass fraction, the carbohydrate mass concentration varied inversely with temperature under blue light or white-yellow light. Under both lights, in the late exponential phase, the carbohydrate concentration increased first with temperature from 24 °C to 28 °C or 30 °C (P < 0.01) and then declined with temperature to 32 °C. In the late stationary phase, it decreased with temperature (P < 0.05). Under red-orange light, it decreased with temperature in the late exponential phase; in the late stationary phase, it was found the highest at 28 °C and decreased with temperature (P < 0.05).
Discussion
Variation of environmental conditions will change the metabolism balance of algae and result in a change of growth kinetics and nutrient composition. Temperature or light quality affects both the cell cycle and size growth. It has been reported that the algal cell size and the cell cycle increased significantly with the decrease of temperature from 30 to 15 °C or from 25 to 9 °C (Bišová and Zachleder 2014; Sayegh and Montagnes 2011). The blue light has been reported to have a division inhibitory effect and promoted the algae growth to a bigger size than that under the red light (Oldenhof et al. 2006). Similarly, in this study, when the temperature was decreased, both the cell size and the biomass doubling time of C. reinhardtii increased under either blue light or red-orange light (Fig. 2 and Fig. 3). Under the blue light, C. reinhardtii had a long doubling time (low cell division rate) and a big cell size, and under the red-orange light, algae cells had a short doubling time (high cell division rate) and a small cell size.
For protein synthesis, a promotive effect of blue light on the protein synthesis has been reported for terrestrial plants, such as soybean plants (Barro et al. 1989). For algae, according to some of the published results shown in Table S1, blue light or light with a high percentage of blue light emission has been reported enhanced the protein content (del Pilar Sánchez-Saavedra and Voltolina 1996, 2006; Korbee et al. 2005; Rivkin 1989). Red light and other light qualities were also reported favorable for protein synthesis in some cases (Abiusi et al. 2014; Rivkin 1989). In this work, high protein content was detected under the red-orange light only at temperatures 24 °C and 28 °C. Though the optimal light quality varied with species and light intensity, the blue light was proved the optimum in most cases.
Algae in the exponential phase with a rapid growth rate are generally featured by high protein content and low carbohydrate content (Zhu et al. 1997). It has been reported that the protein content increased gradually within the first five cell-cycles (Cookson et al. 2010). In the late stationary phase, nutrient starvation can inhibit protein synthesis (Gameiro and Struhl 2018). It might have induced the diversion of protein synthesis to carbohydrate and/or lipid synthesis for energy accumulation.
Both algal cell division and size affect protein synthesis. It has been reported that the algal protein content declined sharply after cell division (Cookson et al. 2010), which indicates the high division rate could result in low protein content. As shown in this work and the results reported in the literature (Bišová and Zachleder 2014; Sayegh and Montagnes 2011), the algal cell size and the cell cycle increased remarkably as the temperature was decreased. Additionally, at temperatures of 24 °C or 28 °C, C. reinhardtii accumulated more protein than at temperature 30 °C or 32 °C. This interestingly coincides with the effect of light quality. Blue light inhibits cell division (Oldenhof et al. 2006) and promotes the algae cells growing to larger sizes. Blue light has also been reported to enhance the protein content of many algae species (Table S1). In this study, under the blue light, C. reinhardtii had a bigger cell size than that under the red-orange light (Fig. 2), and the longest doubling time at 24 °C or 28 °C (Fig. 3), and the highest protein content (Fig. 4). The most significant difference (mass fraction 32% and concentration 52%) appeared between the two combinations of blue light with temperature 24 °C or 28 °C and white-yellow light with temperature 32 °C. Therefore, we reasoned that under certain light intensity, a relatively slow growth rate with delayed division and big cell size can induce more protein synthesis and accumulation.
When the temperature is too low, the algae growth and protein synthesis will be hindered (Sayegh and Montagnes 2011; Uslu et al. 2009). As shown in the present study (Fig. 4), compared to 28 °C, the protein mass fraction and concentration at 24 °C declined under the white-yellow light and red-orange light, and the protein mass concentration was lower under blue light. Therefore, 28 °C could be the optimum for protein synthesis of C. reinhardtii.
For carbohydrate synthesis, it is already known that red (and amber) light can stimulate the carbohydrate synthesis and accumulation (Barro et al. 1989; Soeder and Stengel 1974) as a feedback regulation of photosynthesis (Hogewoning et al. 2010; Paul and Foyer 2001), but the effect is species-dependent, as shown in some of the published results listed in Table S2. Like that of protein, the optimal light quality/qualities for carbohydrate synthesis are temperature and light intensity dependent.
In the late stationary phase, the difference in carbohydrate content among the three light qualities reduced. This is possibly due to nutrient starvation, which induced carbohydrate accumulation even under the blue or white-yellow light (Cheng et al. 2017; Gibson 1978). It indicates that under nutrient stress, the effect of light quality was repressed. It also explained why in most cases, the carbohydrate content increased from the exponential phase to the stationary phase except that under red-orange light at 24 °C.
Algal carbohydrate content fluctuates with temperature (Renaud et al. 2002; Sayegh and Montagnes 2011). It has been reported that, when a white light is used as the light source, the carbohydrate mass fraction increases with temperature before an optimal temperature. The strains Rhodomonas sp., Cryptomonas sp., Prymnesiophyte, and Isochrysis sp. obtained increasing carbohydrate mass fraction until the optimal temperature when being inoculated under 18 W cool-white fluorescent lights within a temperature range of 25–35 °C (Renaud et al. 2002). An increased carbohydrate mass fraction (14.3 to 20.7%) has also been reported for Spirulina sp. as the temperature increases from 25 °C to 40 °C under white fluorescent light (Ogbonda et al. 2007). When grown between 15 and 30 °C, I. galbana and Nannochloropsis sp. showed an increased carbohydrate mass fraction (content per cell) with temperature (the light source was not given) (Sayegh and Montagnes 2011).
The optimal temperature for carbohydrate content is species-dependent, and which could vary within the range of 20–33 °C as reported for different algae strains (de Castro Araújo and Garcia 2005; Renaud et al. 2002; Zhu et al. 1997). In the present study, it is found that under different light quality, the optimal temperature for carbohydrate synthesis varied. From 24 °C to 32 °C, a significant increase of carbohydrate mass fraction under the white-yellow light and a small increase under the blue light were observed in C. reinhardtii, but due to the declination of the total biomass concentration with temperature, the final carbohydrate mass concentration declined with temperature. For carbohydrate mass fraction, a temperature of 32 °C would be the optimum under blue light or white-yellow light, while for carbohydrate concentration (production), temperature 24 °C or 28 °C would be the best for the three light qualities tested.
Unlike the results obtained under the blue light and white-yellow light, the red-orange light experiments showed a decreasing trend of carbohydrate mass fraction with temperature. This demonstrates the effect of light-quality on the carbohydrate synthesis.
Under the red-orange light, both the carbohydrate mass fraction and concentration of C. reinhardtii peaked in the late exponential phase at 24 °C. The maximum difference (mass fraction 161% and concentration 155%) appeared between red-orange light at 24 °C and white-yellow light at 24 °C. It has been reported that red light stimulates carbohydrate synthesis and as a feedback down-regulation of photosynthesis to minimize the damage of excess light (Hogewoning et al. 2010; Paul and Foyer 2001; Soeder and Stengel 1974). When the temperature lowers down from the optimal temperature, light saturation/inhibition increases (Jensen and Knutsen 1993); thus, more carbohydrates tend to accumulate (high mass fraction) as a protective means to the inner cellular structure. Meanwhile, the red-orange light at 24 °C promoted the cell division and cell size growth at the same time, enhancing the total biomass concentration. The high carbohydrate mass fraction and total biomass concentration together resulted in a high carbohydrate concentration. Similarly, as the temperature was increased to 30 °C and 32 °C (promoted the cell division) under the blue light (stimulated the cells growing to bigger size), algae obtained high carbohydrate mass fraction (Fig. 5a), but the carbohydrate mass concentration was observed decreased, which is due to the declination of total biomass concentration with the temperature.
In conclusion, with a light intensity of 105 μmol/m2s, the combination of blue light and temperature of 24 °C or 28 °C, which induced a prolonged cell cycle, a low division rate, and a bigger cell size, enhanced both the protein mass fraction and concentration. The combination of the red-orange light and temperature of 24 °C, which promoted both the cell division and cell size growth, enhanced the carbohydrate mass fraction and concentration. The combination of temperatures 30 °C or 32 °C and blue light promoted the carbohydrate mass fraction, but the carbohydrate concentration declined due to the decrease of total biomass concentration.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abiusi F, Sampietro G, Marturano G, Biondi N, Rodolfi L, D'Ottavio M, Tredici MR (2014) Growth, photosynthetic efficiency, and biochemical composition of T. suecica F&M-M33 grown with LEDs of different colors. Biotechnol Bioeng 111(5):956–964. https://doi.org/10.1002/bit.25014
Ajayan KV, Harilal CC, Gani P (2019) Performance of reflector coated LED Bio-box on the augmentation of growth and lipid production in aerophytic trebouxiophyceaen algae Coccomyxa sp. Algal Res 38:101401. https://doi.org/10.1016/j.algal.2018.101401
Alego G, Bentley C, Smith R, Kelley D, Synder R (2018) LEDs to replace fluorescent tubes for growth of cultured algae VIMS Eastern Shore Laboratory Technical Bulletin No1. Virginia Institute of Marine Science, College of William and Mary. https://doi.org/10.25773/emkh-d241
Barro F, De La Haba P, Maldonado JM, Fontes AG (1989) Effect of light quality on growth, contents of carbohydrates, protein and pigments, and nitrate reductase activity in soybean plants. J Plant Physiol 134(5):586–591. https://doi.org/10.1016/S0176-1617(89)80152-X
Bišová K, Zachleder V (2014) Cell-cycle regulation in green algae dividing by multiple fission. J Exp Bot 65(10):2585–2602
Casal JJ (2000) Phytochromes, cryptochromes, phototropin: photoreceptor interactions in plants. Photochem Photobiol 71(1):1–11. https://doi.org/10.1562/0031-8655(2000)0710001pcppii2.0.co2
Chaplin MF, Kennedy JF (1994) Carbohydrate analysis: a practical approach. IRL Press, New York
Cheng D, Li D, Yuan Y, Zhou L, Li X, Wu T, Wang L, Zhao Q, Wei W, Sun Y (2017) Improving carbohydrate and starch accumulation in Chlorella sp. AE10 by a novel two-stage process with cell dilution. Biotechnol Biofuels 10(1):75. https://doi.org/10.1186/s13068-017-0753-9
Cookson NA, Cookson SW, Tsimring LS, Hasty J (2010) Cell cycle-dependent variations in protein concentration. Nucleic Acids Res 38(8):2676–2681. https://doi.org/10.1093/nar/gkp1069
Daniela B, Clelia S, Cyprien V, Claudia F, Claudia M, Mickael B, Elke R, Petra R (2019) Dried biofilms of desert strains of Chroococcidiopsis survived prolonged exposure to space and Mars-like conditions in low earth orbit. Astrobiology 19:1008–1017. https://doi.org/10.1089/ast.2018.1900
de Castro Araújo S, Garcia VMT (2005) Growth and biochemical composition of the diatom C. cf. wighamii brightwell under different temperature, salinity and carbon dioxide levels. I. Protein, carbohydrates and lipids. Aquaculture 246(1):405–412. https://doi.org/10.1016/j.aquaculture.2005.02.051
de Mooij T, de Vries G, Latsos C, Wijffels RH, Janssen M (2016) Impact of light color on photobioreactor productivity. Algal Res 15:32–42. https://doi.org/10.1016/j.algal.2016.01.015
del Pilar Sánchez-Saavedra M, Voltolina D (1996) Effect of blue-green light on growth rate and chemical composition of three diatoms. J Appl Phycol 8(2):131–137. https://doi.org/10.1007/bf02186316
del Pilar Sánchez-Saavedra M, Voltolina D (2006) The growth rate, biomass production and composition of Chaetoceros sp. grown with different light sources. Aquac Eng 35(2):161–165. https://doi.org/10.1016/j.aquaeng.2005.12.001
Gameiro PA, Struhl K (2018) Nutrient deprivation elicits a transcriptional and translational inflammatory response coupled to decreased protein synthesis. Cell Rep 24(6):1415–1424. https://doi.org/10.1016/j.celrep.2018.07.021
Gibson CE (1978) Field and laboratory observations on the temporal and spatial variation of carbohydrate content in planktonic blue-green algae in Lough Neagh, Northern Ireland. J Ecol 66(1):97–115. https://doi.org/10.2307/2259183
Gobble MM, Headrick D (2019) Converging innovations nourish growth of indoor farming. Res Technol Manag 62(2):7
Goold HD, Cuiné S, Légeret B, Liang Y, Brugière S, Auroy P, Javot H, Tardif M, Jones B, Beisson F, Peltier G, Li-Beisson Y (2016) Saturating light induces sustained accumulation of oil in plastidal lipid droplets in C. reinhardtii. Plant Physiol 171(4):2406–2417. https://doi.org/10.1104/pp.16.00718
Guerlava P, Izac V, Tholozan JL (1998) Comparison of different methods of cell lysis and protein measurements in C. perfringens: Application to the Cell Volume Determination. Curr Microbiol 36(3):131–135. https://doi.org/10.1007/pl00006756
Hegemann P (2008) Algal sensory photoreceptors. Annu Rev Plant Biol 59(1):167–189. https://doi.org/10.1146/annurev.arplant.59.032607.092847
Hogewoning SW, Trouwborst G, Maljaars H, Poorter H, van Ieperen W, Harbinson J (2010) Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of C. sativus grown under different combinations of red and blue light. J Exp Bot 61(11):3107–3117. https://doi.org/10.1093/jxb/erq132
Huang K, Beck CF (2003) Phototropin is the blue-light receptor that controls multiple steps in the sexual life cycle of the green alga C. reinhardtii. Proc Natl Acad Sci U S A 100(10):6269–6274. https://doi.org/10.1073/pnas.0931459100
Janssen JH, Driessen JLSP, Lamers PP, Wijffels RH, Barbosa MJ (2018) Effect of initial biomass-specific photon supply rate on fatty acid accumulation in nitrogen depleted N. gaditana under simulated outdoor light conditions. Algal Res 35:595–601. https://doi.org/10.1016/j.algal.2018.10.002
Jensen S, Knutsen G (1993) Influence of light and temperature on photoinhibition of photosynthesis in S. platensis. J Appl Phycol 5(5):495–504. https://doi.org/10.1007/bf02182508
Jones MA (2018) Using light to improve commercial value. Hortic Res 5(1):47. https://doi.org/10.1038/s41438-018-0049-7
Juneja A, Ceballos RM, Murthy GS (2013) Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: a review. Energies 6(9):4607–4638. https://doi.org/10.3390/en6094607
Kim SH, Sunwoo IY, Hong HJ, Awah CC, Jeong G-T, Kim S-K (2019) Lipid and unsaturated fatty acid productions from three microalgae using nitrate and light-emitting diodes with complementary LED wavelength in a two-phase culture system. Bioprocess Biosyst Eng 42:1–10. https://doi.org/10.1007/s00449-019-02149-y
Kirst H, García-Cerdán JG, Zurbriggen A, Melis A (2012) Assembly of the light-harvesting chlorophyll antenna in the green alga C. reinhardtii requires expression of the TLA2-CpFTSY gene. Plant Physiol 158(2):930–945
Kommareddy A, Anderson G (2004) Study of light requirements of a photobioreactor. North Central ASAE/CSAE Conference Presentation MB04-111, Winnipeg, USA
Korbee N, Figueroa FL, Aguilera J (2005) Effect of light quality on the accumulation of photosynthetic pigments, proteins and mycosporine-like amino acids in the red alga P. leucosticta (Bangiales, Rhodophyta). J Photochem Photobiol B Biol 80(2):71–78
Kruger NJ (2009) The bradford method for protein quantitation. In: Walker JM (ed) The Protein Protocols Handbook. Humana Press, Totowa, pp 17–24
Kubín Š, Borns E, Doucha J, Seiss U (1983) Light absorption and production rate of C. vulgaris in light of different spectral composition. Biochem Physiol Pflanz 178(2):193–205. https://doi.org/10.1016/S0015-3796(83)80032-8
Laurens C, Simon C, Vernikos J, Gauquelin-Koch G, Blanc S, Bergouignan A (2019) Revisiting the role of exercise countermeasure on the regulation of energy balance during space flight. Front Physiol 10:321–321. https://doi.org/10.3389/fphys.2019.00321
Li X, Slavens S, Crunkleton DW, Johannes TW (2020) Interactive effect of light quality and temperature on C. reinhardtii growth kinetics and lipid synthesis. Algal Res in press:102127. https://doi.org/10.1016/j.algal.2020.102127
Matula E, Nabity J (2016) Feasibility of photobioreactor systems for use in multifunctional environmental control and life support system for spacecraft and habitat environments. In: 46th International Conference on Environmental Systems, Vienna, Austria. 46th International Conference on Environmental Systems
Matula EE, Nabity JA (2019) Failure modes, causes, and effects of algal photobioreactors used to control a spacecraft environment. Life Sci Space Res 20:35–52. https://doi.org/10.1016/j.lssr.2018.12.001
Michael CI (2015) Utilizing optical light filters and biofilm based cultivating to enhance microalgal growth. Iowa State University, Ames, IA
Mitchell CA (2012) Plant lighting in controlled environments for space and earth applications. Acta Hortic 956:23–36. https://doi.org/10.17660/ActaHortic.2012.956.1
Niederwieser T, Kociolek P, Klaus D (2018) A review of algal research in space. Acta Astronaut 146:359–367. https://doi.org/10.1016/j.actaastro.2018.03.026
Ogbonda KH, Aminigo RE, Abu GO (2007) Influence of temperature and pH on biomass production and protein biosynthesis in a putative Spirulina sp. Bioresour Technol 98(11):2207–2211. https://doi.org/10.1016/j.biortech.2006.08.028
Oldenhof H, Zachleder V, Van Den Ende H (2006) Blue- and red-light regulation of the cell cycle in C. reinhardtii (Chlorophyta). Eur J Phycol 41(3):313–320. https://doi.org/10.1080/09670260600699920
Olle M, Viršile A (2013) The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric Food Sci 22(2):223–234. https://doi.org/10.23986/afsci.7897
Paul MJ, Foyer CH (2001) Sink regulation of photosynthesis. J Exp Bot 52(360):1383–1400. https://doi.org/10.1093/jexbot/52.360.1383
Pennisi G, Blasioli S, Cellini A, Maia L, Crepaldi A, Braschi I, Spinelli F, Nicola S, Fernandez JA, Stanghellini C, Marcelis LFM, Orsini F, Gianquinto G (2019) Unraveling the role of red:blue led lights on resource use efficiency and nutritional properties of indoor grown sweet Basil. Front Plant Sci 10(305). https://doi.org/10.3389/fpls.2019.00305
Rasband W (2020) Image processing and analysis in Java. PUblisher. https://imagej.nih.gov/. Accessed 4 Dec 2020
Renaud SM, Thinh L-V, Lambrinidis G, Parry DL (2002) Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 211(1):195–214. https://doi.org/10.1016/S0044-8486(01)00875-4
Rivkin RB (1989) Influence of irradiance and spectral quality on the carbon metabolism of hytoplankton. I. Photosynthesis, chemical composition and growth. Mar Ecol Prog Ser 55:291–304
Rochet M, Legendre L, Demers S (1986) Photosynthetic and pigment responses of sea-ice microalgae to changes in light intensity and quality. J Exp Mar Biol Ecol 101(3):211–226. https://doi.org/10.1016/0022-0981(86)90264-9
Rockwell NC, Duanmu D, Martin SS, Bachy C, Price DC, Bhattacharya D, Worden AZ, Lagarias JC (2014) Eukaryotic algal phytochromes span the visible spectrum. PNAS 111(10):3871–3876. https://doi.org/10.1073/pnas.1401871111
Satthong S, Saego K, Kitrungloadjanaporn P, Nuttavut N, Amornsamankul S, Triampo W (2019) Modeling the effects of light sources on the growth of algae. Adv Differ Equ 2019(1):170. https://doi.org/10.1186/s13662-019-2112-6
Sayegh FAQ, Montagnes DJS (2011) Temperature shifts induce intraspecific variation in microalgal production and biochemical composition. Bioresour Technol 102(3):3007–3013. https://doi.org/10.1016/j.biortech.2010.10.011
Schulze PSC, Barreira LA, Pereira HGC, Perales JA, Varela JCS (2014) Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol 32(8):422–430. https://doi.org/10.1016/j.tibtech.2014.06.001
Schulze PS, Pereira HG, Santos TF, Schueler L, Guerra R, Barreira LA, Perales JA, Varela JC (2016) Effect of light quality supplied by light emitting diodes (LEDs) on growth and biochemical profiles of N. oculata and T. chuii. Algal Res 16:387–398
Sforza E, Barbera E, Bertucco A (2015) Improving the photoconversion efficiency: an integrated photovoltaic-photobioreactor system for microalgal cultivation. Algal Res 10:202–209. https://doi.org/10.1016/j.algal.2015.05.005
Soeder CJ, Stengel E (1974) Physico-chemical factors affecting metabolism and growth rate. In: Stewart WDP (ed) Algal Physiology and Biochemistry. Univ of California Press, Oakland, CA
Sung MG, Han JI, Lee B, Chang YK (2018) Wavelength shift strategy to enhance lipid productivity of N. gaditana. Biotechnol Biofuels 11:70–70. https://doi.org/10.1186/s13068-018-1067-2
Svetoslav A (2016) Algal research in space: history, current status and future prospects. Innovare J Life Sci 4(4):1–4
Trainer VL, Moore SK, Hallegraeff G, Kudela RM, Clement A, Mardones JI, Cochlan WP (2019) Pelagic harmful algal blooms and climate change: lessons from nature’s experiments with extremes. Harmful Algae 91:101591. https://doi.org/10.1016/j.hal.2019.03.009
Uslu LH, Işık O, Sayın S, Durmaz Y, Göksan T, Gökpınar Ş (2009) The effect of temperature on protein and amino acid composition of S. platensis. J Fish Aquat Sci 26:139–142
Vadiveloo A, Moheimani NR, Cosgrove JJ, Bahri PA, Parlevliet D (2015) Effect of different light spectra on the growth and productivity of acclimated Nannochloropsis sp.(Eustigmatophyceae). Algal Res 8:121–127
Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE, Smith AG, Camire ME, Brawley SH (2017) Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol 29(2):949–982. https://doi.org/10.1007/s10811-016-0974-5
Wu H (2016) Effect of different light qualities on growth, pigment content, chlorophyll fluorescence, and antioxidant enzyme activity in the red alga P. haitanensis (Bangiales, Rhodophyta). Biomed Res Int 2016:1–8
Zaiontz C (2020) Unbalanced Factorial ANOVA. PUblisher. http://www.real-statistics.com/multiple-regression/unbalanced-factorial-anova/
Zhu CJ, Lee YK, Chao TM (1997) Effects of temperature and growth phase on lipid and biochemical composition of I. galbana TK1. J Appl Phycol 9(5):451–457. https://doi.org/10.1023/a:1007973319348
Funding
This work was supported by the NASA (grant number 15-EPSCoR2015-0026).
Author information
Authors and Affiliations
Contributions
XL conceived the research and designed the experiments. XL performed the experiments, analyzed, and interpreted the data. XL wrote the original draft. TWJ and DC: funding acquisition, supervised and edited the manuscript. SS analyzed and interpreted the data, and edited the manuscript. JM assisted the experimented and edited the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 195 kb).
Rights and permissions
About this article
Cite this article
Li, X., Manuel, J., Slavens, S. et al. Interactive effects of light quality and culturing temperature on algal cell size, biomass doubling time, protein content, and carbohydrate content. Appl Microbiol Biotechnol 105, 587–597 (2021). https://doi.org/10.1007/s00253-020-11068-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-11068-y