Abstract
Pretreatment with white rot fungi is a promising method to enhance the digestibility of lignocelluloses; however, sterilization of feedstocks prior to inoculation is one of the costliest steps. To improve the colonizing ability of white rot fungi under non-sterile condition, Irpex lacteus, Pleurotus ostreatus, and Phanerochaete chrysosporium were inoculated in the wheat straw ensiled for 28 days and incubated for 56 days to determine the changes in microbe counts, organic acid content, chemical composition, and rumen and enzymatic digestibility. Results showed that ensiling produced abundant organic acids and suppressed most microbes in wheat straw. Significant growth of I. lacteus was observed after 3 days of incubation, and molds were only detectable at day 7 in the group. At the end of incubation, aerobic bacteria and lactic acid bacteria decreased by 18% and 38% in the wheat straw treated with I. lacteus, but molds, aerobic bacteria, and lactic acid bacteria thrived in those treated with P. ostreatus and P. chrysosporium. Even more, P. ostreatus and P. chrysosporium increased the lignin content of the ensiled wheat straw by 34% and 65%. However, I. lacteus selectively degraded lignin by 28% and improved the rumen and enzymatic digestibility by 18% and 34%. The finding indicates that ensiling prior to fermentation with I. lacteus is an effective method to control spoilage microbes and to enhance the rumen and enzymatic digestibility of wheat straw.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignocelluloses from agriculture and forestry residues are promising renewable resources for the production of biofuels and ruminant feeds. Because the complex and highly lignified cell walls of lignocelluloses are less accessible to microbial enzymes, effective pretreatment to disrupt such barriers is essential before further utilization (Ding et al. 2019; Thomsen et al. 2016). Thermal and chemical pretreatment methods have proved effective methods to improve enzymatic hydrolysis and rumen digestion of lignocellulose, but these techniques often involve side reactions and produce lignocellulose-derived by-products that can inhibit microbial and enzymatic biocatalysts (Jonsson and Martin 2016; Wan and Li 2012). As an alternative, fungal pretreatment with white rot fungi has received renewed interest for animal feed and cellulosic ethanol or methane production (Kainthola et al. 2019; Kumar et al. 2018; Niu et al. 2018a). However, most fungi do not colonize well under non-sterile conditions, which make sterilization of feedstocks prior to the fungal pretreatment one of the costliest steps and limit its commercialization (Vasco-Correa et al. 2016; Zhao et al. 2014).
In nature, degradation of lignin by white rot fungi often takes place in highly heterogeneous systems, in which they coexist and compete with other non-lignocellulolytic microbes (Johnston et al. 2016; Tejirian and Xu 2010). The symbiosis of white rot fungi and other microbes indicates that there may be some fungi degrading lignin efficiently under particular non-sterile conditions. Recently, multiple research groups have attempted to enhance the competitive advantage of some fungi by increasing the inoculation amount or disinfecting the raw materials in hydrated lime solution, but over 30% of inoculum ratio or washing with large amounts of water was necessary for these methods (Song et al. 2013; Vasco-Correa et al. 2016; Zhao et al. 2014). Therefore, developing more efficient strategies to control spoilage microbes and screening new species that is resistant to infection are crucial for the pretreatment of lignocelluloses under non-sterile conditions.
Generally, the spoilage microbes, primarily bacteria and yeasts, grow and propagate much faster than white rot fungi under most neutral and nutrient-rich conditions. Once these spoilage microbes have invaded the fermentation system, they can alter the pH, compete for nutrients with white rot fungi, and degrade the ligninolytic enzymes (Gao et al. 2008). Fortunately, bacteria do not grow well in low-pH and nitrogen-limited media (Yang et al. 2011); yeasts often require easily accessible carbon and nitrogen sources for rapid growth (Broach 2012). On the contrary, most white rot fungi prevail in lignocelluloses under acidic conditions, which is a less efficient carbon source for non-lignocellulolytic microbes (Johnston et al. 2016; Libra et al. 2003). Therefore, acidifying the substrates prior to inoculation, such as in ensiling conditions, might be an effective alternative to replace sterilization in the pretreatment of lignocellulosic biomass.
In the present study, three white rot fungi, Irpex lacteus, Pleurotus ostreatus, and Phanerochaete chrysosporium, were inoculated in the ensiled wheat straw and incubated at 28 °C for 56 days. Changes in pH, organic acid levels, microbial community, and chemical composition during the incubation were compared for assessing their growth and degradation characteristics. Enzymatic hydrolysis and in vitro rumen digestibility of the treated straw were also compared with that of the intact and ensiled straw.
Material and methods
Fungal cultures
The three white rot fungal species used in the present study were purchased from China General Microbiological Culture Collection Center (CGMCC) in Beijing, China. They were maintained on potato dextrose agar (PDA) slants at 4 °C. The fungal strains used were I. lacteus CGMCC 5.809, P. ostreatus CGMCC 5.374, and P. chrysosporium CGMCC 5.829. Before the experiments, the three fungi were grown on PDA plates at 28 °C until the mycelia covered the entire agar plates. Ten 5-mm agar plugs removed from each fungal plate were added to a separate flask containing 200 g of sterilized wheat grains and incubated at 28 °C until all the grains were colonized by mycelia. Thereafter, the three kinds of spawn were stored at 4 °C for further inoculation.
Preparation of substrate and method of incubation
Wheat straw, harvested from an experimental field of the National Experiment Station for Precision Agriculture (40.22° N, 116.20° E, Beijing, China), was chopped into 2–3 cm long pieces using a forage chopper. The prepared straw was adjusted to approximately 55% moisture content and filled into plastic film bags. All the bags were degassed and sealed with a vacuum-packing machine and stored at ambient temperature (26–32 °C). After 28 days of ensiling, the moisture content was adjusted to 70%. After thorough mixing, the samples were weighed into 25 × 17 cm polyethylene fresh-keeping bags (Miaojie, Wuxi, Jiangsu, China), which has a moderate air permeability and can reduce the water transfer process. Thereafter, 6 g of the wheat grains from each flask was inoculated in 150 g of the ensiled straw. The bags were sealed by tying a knot, and the moisture content was kept around 70% by spraying distilled water on the outer layers of substrates biweekly. All bags containing samples were incubated for 0, 3, 7, 14, 28, 42, or 56 days in an air-conditioned chamber at 28 °C with a relative humidity of 80%. Triplicate samples of each treatment were used for subsequent analyses.
Microbial and chemical analyses
Ten grams (fresh weight) of samples from each treatment was homogenized with 90 mL of sterilized distilled water. Serial dilutions of 10−1–10−9 were prepared for microbiological evaluation, and the remaining extracts were filtered for further evaluation. The numbers of lactic acid bacteria (LAB) were determined by plate counting on de Man, Rogosa, and Sharpe (MRS) agar (Difco Laboratories, Detroit, MI, USA) incubated at 37 °C for 48 h under anaerobic conditions. Molds and yeasts were counted on Rose Bengal medium (Aobox, Beijing, China) incubated at 30 °C for 48 h under aerobic conditions. Bacilli and aerobic bacteria were counted on nutrient agar (Nissui, Tokyo, Japan) incubated for 24 h at 37 °C under aerobic conditions. The pH was measured using a glass electrode pH meter (S20K, Mettler Toledo, Greifensee, Switzerland). The organic acid content of the straw was determined by high performance liquid chromatography (HPLC; column: Shodex RS Pak KC-811, Showa Denko K.K., Kawasaki, Japan; detector: DAD, 210 nm, SPD-20A, Shimadzu Co., Ltd., Kyoto, Japan; eluent: 3 m mol/L HClO4, 1.0 mL/min; temperature 50 °C). Ammonia nitrogen (NH3-N) was determined using the method of phenol–hypochlorite reaction (Broderick and Kang 1980).
Neutral detergent solute (NDS), neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) contents were determined according to the method described by Van Soest et al. (1991). The content of hemicellulose was calculated as the difference between NDF and ADF and that of cellulose as the difference between ADF and ADL. Water-soluble carbohydrate (WSC) content was determined by using the anthrone method (Murphy 1958). Ash content was determined by complete combustion of the samples in a muffle furnace at 550 °C until a constant weight was recorded.
In vitro rumen digestibility
The filter bag method developed by ANKOM Technology Corporation (Fairport, NY, USA) was used to determine the effect of fungal pretreatment on the digestibility of the wheat straw. The incubation media and procedure were same to that described by Zuo et al. (2018) except that rumen fluid was collected from three rumen-fistulated Angus bullocks fed a corn silage and wheat straw-based diet. At the end of incubation (48 h), the bags were rinsed four times with distilled water and dried at 60 °C for 24 h. The dry matter digestibility (DMD) of the samples was calculated and expressed as the percentage of dry matter (% DM).
Enzymatic hydrolysis
Enzymatic hydrolysis of raw, ensiled, and pretreated wheat straw was conducted according to the method of the National Renewable Energy Laboratory (NREL) (Selig et al. 2008). About 2 g (dry basis) of feedstock was supplemented with the enzyme Cellic CTec2 (Sigma-Aldrich, Saint Louis, MO, USA) at 10 FPU/g dry substrate and 0.08 g/L tetracycline hydrochloride (Adamas, Shanghai, China) to prevent microbial contamination. Enzymatic hydrolysis was carried out at pH 4.8 using 50 mM sodium citrate buffer and incubated at 50 °C, 180 rpm for 72 h. Hydrolysates were centrifuged at 12,000×g for 10 min and passed through a 0.2-μm nylon filter. The glucose concentrations were measured by a Dionex Ultimate 3000 ultra-HPLC system (Thermo Fisher Scientific, Waltham, MA, USA) with a Benson BP-100 H+ carbohydrates column (Benson Polymeric Inc., Reno, NV, USA) and RefractoMax 521 refractive index detector (Thermo Fisher Scientific, Waltham, MA, USA).
Statistical analysis
Data of pH, organic acids, microbial, chemical composition, and digestibility were analyzed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests and declared when P < 0.05 using IBM SPSS 21.0 for Windows (IBM SPSS Inc., Chicago, IL, USA). Regression analysis and graph processing were performed using Origin 2018 for Windows (OriginLab Corporation, Northampton, MA, USA).
Results
Compositions of the intact and ensiled wheat straw
The wheat straw is rich in hemicellulose, cellulose, and ADL contents, however, with low NDS and WSC content (Table 1). After 28 days of ensiling, the content of WSC and NDS of the wheat straw decreased by 89% and 13%, while that of hemicellulose, cellulose, and ADL contents increased by 4%, 7%, and 4%, respectively. Compared with chemical composition, ensiling had stronger effect on the microbial composition of wheat straw. At the end of ensiling, there was only a significant increase in the counts of LAB (P < 0.05). The numbers of aerobic bacteria decreased to 6.5 log10 cfu/g fresh matter (FM), while yeasts and molds decreased to a non-detectable level.
Microbial composition and pH
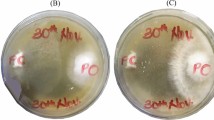
Morphology of the wheat straw treated with different white rot fungi for 56 days is shown in Fig. 1. The straw treated with I. lacteus was covered with white mycelium from day 7 to the end of incubation, but those treated with P. ostreatus and P. chrysosporium were occupied by molds. The experimental data of microbes revealed that the numbers of LAB and aerobic bacteria significantly increased (P < 0.001) after 3 days of incubation, with molds incapable of being detected (Fig. 2). The number of aerobic bacteria, LAB, and molds in the straw treated with I. lacteus, after 7 days of incubation, became significantly lower (P < 0.001) than those treated with P. ostreatus and P. chrysosporium. In the subsequent incubation, molds in the straw treated with I. lacteus decreased to non-detectable levels, with the number of aerobic bacteria and LAB decreasing over time. Yeasts were not detected in any of the samples. Correspondingly, the wheat straw treated with I. lacteus, after 7 days of incubation, showed a significantly lower pH (P < 0.001) than those treated with P. ostreatus and P. chrysosporium.
Organic acid and NH3-N content
The contents of lactic acid, acetic acid, butyric, and NH3-N during 56 days of incubation are shown in Fig. 3. Although the data of each organic acid in the wheat straws treated with I. lacteus, P. ostreatus, and P. chrysosporium had similar kinetic trends, the contents of the former decreased faster than those in the group of P. ostreatus and P. chrysosporium. After 3 days of incubation, the lactic acid content in the straw treated with I. lacteus and butyric acid content in all the treatments decreased significantly (P < 0.001), while the acetic acid content increased significantly (P < 0.05). The pH of straw in all the treatments showed a significant decrease (P < 0.01). However, the contents of lactic acid, acetic acid, and butyric acid in the straw treated with I. lacteus were significantly lower (P < 0.05) than those treated with P. ostreatus and P. chrysosporium, the contents of which in all of the treatments decreased significantly after 7 days of incubation (P < 0.01). Lactic acid and acetic acid could not be detected in the straw treated with I. lacteus, with the butyric acid content significantly lower (P < 0.05) than that of the straw treated with P. chrysosporium. Contrary to the contents of organic acids, pH of the straw treated with I. lacteus was significantly lower (P < 0.001) than that in the other treatments after 7 days of incubation. Then, it declined to be lower than 5 in the subsequent incubation, but organic acids could not be detected in all the samples. Similar to organic acids, NH3-N of the wheat straw treated with I. lacteus was significantly lower (P < 0.001) than that of others after 3 days of incubation.
Chemical composition and digestibility
The experimental data of NDS, cellulose, and ADL of each treatment are shown in Fig. 4. All the NDS of wheat straw treated with I. lacteus, P. ostreatus, and P. chrysosporium showed a rising tendency with time, while that of the former was significantly higher than the latter after 28, 42, and 56 days of incubation (P < 0.05). The content of both cellulose and hemicellulose could significantly be decreased through the treatment with white rot fungi (P < 0.05), but the change of cellulose slowed down after 28 days of incubation. The ADL content of wheat straw treated with I. lacteus, similar to NDS and cellulose, also had a different trend with P. ostreatus, and P. chrysosporium. After 14 days of incubation, the straw treated with I. lacteus showed the lowest ADL content (P < 0.05), indicating that more lignin was degraded by I. lacteus. The ADL content in the straw treated with P. ostreatus and P. chrysosporium, after 56 days of incubation, was approximately two times higher than that in the straw treated with I. lacteus. Corresponding to the high degree of lignification, the DMD and enzymatic hydrolysis of intact wheat straw were 49.3% and 19.3% DM, respectively (Fig. 5). After 28 days of ensiling, the enzymatic hydrolysis of ensiled wheat straw was 14.5% DM, which was significantly lower (P < 0.001) than that of the intact. Although incubation with P. ostreatus and P. chrysosporium significantly decreased the DMD and enzymatic hydrolysis (P < 0.001), those of wheat straw treated with I. lacteus were increased to 59.6% and 25.9% DM.
The in vitro rumen digestibility after 48 h (a) and enzymatic hydrolysis after 72 h (b) of the intact, ensiled, and treated wheat straw. IL Irpex lacteus, PO Pleurotus ostreatus, PC Phanerochaete chrysosporium. The data were expressed as the mean of three replicates ± SD. Means followed by different letters were significantly different at P < 0.05
Discussion
Interaction between microbes and environment
Ensiling is a common method used for preserving moist crops based on natural fermentation of sugars by lactic acid bacteria under anaerobic conditions (Muck 2010). After several weeks of ensiling, the substrates often have low pHs and microbe numbers (Dunière et al. 2013). In the present study, the ensiled wheat straw had a relatively higher pH than common silages. The poor fermentation quality was also observed by other researchers and attributed to the low WSC content of wheat straw (Yang et al. 2006; Ni et al. 2014). After air exposure, yeasts will be produced firstly in most silages because they grow faster than most other microbes under aerobic and acidic conditions (Muck 2010; Wang et al. 2018). However, natural lignocellulosic substrates serve as a less efficient carbon source for these microbes without lignocellulolytic enzymes (Šlosarčíková et al. 2017), and aerobic bacteria often begin to grow in silages when the pH is approximately 4.5 or higher (Muck 2010). This might help explain why aerobic bacteria dominated the substrates rather than yeasts during the first few days of incubation. In the subsequent incubation, lactic acid and butyric acid decreased to an undetectable level because of that the microbes metabolize organic acids for their growth under aerobic condition (Niu et al. 2018b). Contrary to the changes of organic acids, the pH of the straw treated with I. lacteus was kept at a low level and declined to be lower than 5 in the subsequent incubation. It is reported that white rot fungi can produce some organic acids to acidify their substrates and maintain their cultures at low pH, so the value of the pH is a good indicator of whether they gain the upper hand (Lang et al. 1997; Liaud et al. 2014). The low pH caused by I. lacteus reflected the fungus grew well in the ensiled wheat straw and secreted some acids for inhibiting the growth of other microbes. Even more, limited nitrogen also exerts strong inhibitory impacts on the growth of molds and plenty of bacteria (Gao et al. 2008; Jourdier et al. 2013; Yang et al. 2011). Thus, the acidified and nitrogen-limited condition might be the main abiotic reasons why I. lacteus colonized stably in the ensiled wheat straw.
Although the species of white rot fungi are diverse and the interaction between them and other microbes can be governed by an interplay of various factors, only extremely combative species possess the ability to determine the microflora in their substrates (Johnston et al. 2018). The lower pH and numbers of LAB, aerobic bacteria, and molds revealed that I. lacteus is strongly capable of colonizing the ensiled wheat straw compared to P. ostreatus and P. chrysosporium. In agriculture, antagonisms between white rot fungi and molds, especially Trichoderma, are of interest for mushroom cultivation. Molds can compete aggressively with white rot fungi, thus reducing the production of mushrooms (Savoie et al. 2001). It is reported that some white rot fungi can resist Trichoderma spp. attack by producing laccases with high quantities (Savoie et al. 2001). Interestingly, Svobodová et al. (2008) found activity of laccase bound to the mycelium of I. lacteus in mineral liquid medium began to increase after 7 days of incubation, and peaked after 15 days of incubation. Although the activity of laccase can be affected by many factors, it can be determined that laccase is a crucial enzyme of I. lacteus. So the decrease of molds after 14 days of incubation might be attributed to laccases or other antifungal substances produced by I. lacteus. Apart from molds, I. lacteus is also strongly capable of competing with soil bacteria, such as Pseudomonas and Sphingomonas (Borràs et al. 2010; Novotný et al. 2001). Above all, it can be concluded that I. lacteus is a highly competitive white rot fungus under non-sterile conditions.
Changes in chemical composition and digestibility
Among the common agricultural by-products, the lignified degree of wheat straw is higher than that of maize and rice straw, so the former often has lower nutritive value than the latter straws (Tuyen et al. 2012, 2013). Although ensiling can convert WSC to organic acids, and inhibit the growth of spoilage microbes, the process has little effects on the structure of cellulose and lignin (Ning et al. 2017). It is reported that most white rot fungi, such as P. ostreatus and P. chrysosporium, can degrade lignin in biomass effectively and selectively after a long incubation under sterile conditions (Wan and Li 2012). The higher ADL content in the present study also revealed that P. ostreatus and P. chrysosporium did not colonize well in the ensiled wheat straw. In contrast, the lignin of wheat straw treated with I. lacteus was degraded by 28%, but cellulose only decreased 6%. Similar degrading characteristics of I. lacteus were also observed in sterile wheat straw and hydrated lime treated corn stover (Niu et al. 2018a; Song et al. 2013). The different changes of cellulose and lignin reflected that I. lacteus has limited ability to consume cellulose but high capacity to degrade lignin. Apart from lignin, I. lacteus also had a significant effect on the contents of hemicellulose and NDS. Generally, NDS often consist of carbohydrates including starch, sugars, and pectic substances as well as non-carbohydrates, including protein, soluble phenolics, ash, and lipids, and white rot fungi that can liberate some monomers and oligomers from hemicellulose and cellulose (Niu et al. 2018a). The higher hemicellulose loss than that of cellulose in this study revealed that the increased NDS content can be mainly caused by the degradation of hemicellulose.
Typically, the energy content of feedstuff is highly correlated with their DMD (Yan and Agnew 2004), with the improved rumen digestibility of forage capable of increasing their potential as raw materials for biofuel production (Badhan et al. 2014). After 56 days of incubation, I. lacteus improved the DMD by 18%, but neither ensiling nor pretreatment with P. ostreatus and P. chrysosporium exerted positive effects on the digestibility of wheat straw. Similarly, the little or negative effects of ensiling on DMD were also observed in Italian ryegrass, lucerne, and orchardgrass (Yahaya et al. 2001; González et al. 2007). Although P. chrysosporium exerts negative influences on rumen digestibility of wheat straw under sterile condition, the DMD can often be slightly improved by P. ostreatus (Lynch et al. 2014; Niu et al. 2018a). Therefore, the lower DMD also indicates that P. ostreatus and P. chrysosporium cannot colonize well in the ensiled wheat straw.
Glucose, the dominant fermentable sugar from cellulose, serves as the major energy sources of life, which can potentially contribute to animal feeds and biofuel production. Enzymatic hydrolysis indicated the availability of cellulose and saccharification efficiency of the treated wheat straw. Specifically, the sugar yield of wheat straw treated with I. lacteus, compared with the intact straw, increased by 34%. Similarly, Salvachúa et al. (2013) reported that I. lacteus could improve the glucose yields of wheat straw from 33 to 68% under sterile condition, with the positive results revealing that I. lacteus is a promising species in the pretreatment of wheat straw under non-sterile conditions. The contaminating microbes and the poor nutrition might be responsible for the relative low improvement. Thus, future studies should pay more attention to controlling harmful microbes and screening positive additives.
In conclusion, most of the spoilage microbes in wheat straw could be suppressed or killed by the anaerobic and acidic condition during ensiling. The acidic environment and low microbe counts allowed I. lacteus to colonize well and degrade lignin efficiently under a favorable environment. Pretreatment with I. lacteus for 56 days can efficiently degrade the lignin of ensiled wheat straw and improve the in vitro rumen and enzymatic hydrolysis significantly. Therefore, ensiling is a novel strategy to improve the colonizing ability of I. lacteus in non-sterile wheat straw for enhanced rumen and enzymatic digestibility.
References
Badhan A, Jin L, Wang Y, Han S, Kowalczys K, Brown DC, Ayala CJ, Latoszek-Green M, Miki B, Tsang A, McAllister T (2014) Expression of a fungal ferulic acid esterase in alfalfa modifies cell wall digestibility. Biotechnol Biofuels 7:39. https://doi.org/10.1186/1754-6834-7-39
Borràs E, Caminal G, Sarrà M, Novotný Č (2010) Effect of soil bacteria on the ability of polycyclic aromatic hydrocarbons (PAHs) removal by Trametes versicolor and Irpex lacteus from contaminated soil. Soil Biol Biochem 42:2087–2093. https://doi.org/10.1016/j.soilbio.2010.08.003
Broach JR (2012) Nutritional control of growth and development in yeast. Genetics 192:73–105. https://doi.org/10.1534/genetics.111.135731
Broderick GA and Kang JH (1980) Automated simultaneous determination of ammonia and total amino acid in ruminal fluid and in vitro media. J Dairy Sci 63: 64–75. https://doi.org/10.3168/jds.S0022-0302(80)82888-8
Ding C, Wang X, Li M (2019) Evaluation of six white-rot fungal pretreatments on corn stover for the production of cellulolytic and ligninolytic enzymes, reducing sugars, and ethanol. Appl Microbiol Biotechnol 103:5641–5652. https://doi.org/10.1007/s00253-019-09884-y
Dunière L, Sindou J, Chaucheyras-Durand F, Chevallier I, Thévenot-Sergentet D (2013) Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim Feed Sci Technol 182:1–15. https://doi.org/10.1016/j.anifeedsci.2013.04.006
Gao D, Zeng Y, Wen X, Qian Y (2008) Competition strategies for the incubation of white rot fungi under non-sterile conditions. Process Biochem 43:937–944. https://doi.org/10.1016/j.procbio.2008.04.026
González J, Faríamármol J, Rodríguez CA, Martínez A (2007) Effects of ensiling on ruminal degradability and intestinal digestibility of Italian rye-grass. Anim Feed Sci Technol 136:38–50. https://doi.org/10.1016/j.anifeedsci.2006.08.022
Lang E, Kleeberg I, Zadrazil F (1997) Competition of Pleurotus sp. and Dichomitus squalens with soil microorganisms during lignocellulose decomposition. Bioresour Technol 60: 95–99. https://doi.org/10.1016/S0960-8524(97)00016-3
Johnston SR, Boddy L, Weightman AJ (2016) Bacteria in decomposing wood and their interactions with wood-decay fungi. FEMS Microbiol Ecol 92:fiw179. https://doi.org/10.1093/femsec/fiw179
Johnston SR, Hiscox J, Savoury M, Boddy L, Weightman AJ (2018) Highly competitive fungi manipulate bacterial communities in decomposing beech wood (Fagus sylvatica). FEMS Microbiol Ecol 95:fiy225. https://doi.org/10.1093/femsec/fiy225
Jonsson L, Martin C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112. https://doi.org/10.1016/j.biortech.2015.10.009
Jourdier E, Poughon L, Larroche C, Ben Chaabane F (2013) Comprehensive study and modeling of acetic acid effect on Trichoderma reesei growth. Ind Biotechnol 9:132–138. https://doi.org/10.1089/ind.2013.0002
Kainthola J, Kalamdhad AS, Goud VV, Goel R (2019) Fungal pretreatment and associated kinetics of rice straw hydrolysis to accelerate methane yield from anaerobic digestion. Bioresour Technol 286:121368. https://doi.org/10.1016/j.biortech.2019.121368
Kumar MN, Ravikumar R, Sankar MK, Thenmozhi S (2018) New insight into the effect of fungal mycelia present in the bio-pretreated paddy straw on their enzymatic saccharification and optimization of process parameters. Bioresour Technol 267:291–302. https://doi.org/10.1016/j.biortech.2018.07.003
Liaud N, Giniés C, Navarro D, Fabre N, Crapart S, Herpoël-Gimbert I, Levasseur A, Raouche S, Sigoillot JC (2014) Exploring fungal biodiversity: organic acid production by 66 strains of filamentous fungi. Fungal Biol Biotechnol 1:1–10. https://doi.org/10.1186/s40694-014-0001-z
Libra JA, Borchert M, Banit S (2003) Competition strategies for the decolorization of a textile-reactive dye with the white-rot fungi Trametes versicolor under non-sterile conditions. Biotechnol Bioeng 82:736–744. https://doi.org/10.1002/bit.10623
Lynch J, O'kiely P, Murphy R, Doyle E (2014) Changes in chemical composition and digestibility of three maize stover components digested by white-rot fungi. J Anim Physiol Anim Nutr 98:731–738. https://doi.org/10.1111/jpn.12131
Muck RE (2010) Silage microbiology and its control through additives. Rev Bras Zootec 39:183–191. https://doi.org/10.1590/S1516-35982010001300021
Murphy RP (1958) A method for the extraction of plant samples and the determination of total soluble carbohydrates. J Sci Food Agric 9:714–717. https://doi.org/10.1002/jsfa.2740091104
Ning T, Wang H, Zheng M, Niu D, Zuo S, Xu C (2017) Effects of microbial enzymes on starch and hemicellulose degradation in total mixed ration silages. Asian Australas J Anim Sci 30:171–180. https://doi.org/10.5713/ajas.16.0046
Ni K, Wang Y, Pang H, Cai Y (2014) Effect of cellulase and lactic acid bacteria on fermentation quality and chemical composition of wheat straw silage. Am J Plant Sci 5:1877–1884. https://doi.org/10.4236/ajps.2014.513201
Niu D, Zuo S, Jiang D, Tian P, Zheng M, Xu C (2018a) Treatment using white rot fungi changed the chemical composition of wheat straw and enhanced digestion by rumen microbiota in vitro. Anim Feed Sci Technol 237:46–54. https://doi.org/10.1016/j.anifeedsci.2018.01.005
Niu D, Zheng M, Zuo S, Jiang D, Xu C (2018b) Effects of maize meal and limestone on the fermentation profile and aerobic stability of smooth bromegrass (Bromus inermis Leyss) silage. Grass Forage Sci 73:622–629. https://doi.org/10.1111/gfs.12355
Novotný Č, Rawal B, Bhatt M, Patel M, Šašek V, Molitoris HP (2001) Capacity of Irpex lacteus and Pleurotus ostreatus for decolorization of chemically different dyes. J Biotechnol 89: 113–122. https://doi.org/10.1016/S0168-1656(01)00321-2
Salvachúa D, Prieto A, Vaquero ME, Martínez ÁT, Martínez MJ (2013) Sugar recoveries from wheat straw following treatments with the fungus Irpex lacteus. Bioresour Technol 131:218–225. https://doi.org/10.1016/j.biortech.2012.11.089
Savoie JM, Mata G, Mamoun M (2001) Variability in brown line formation and extracellular laccase production during interaction between white-rot basidiomycetes and Trichoderma harzianum biotype Th2. Mycologia 93:243–248. https://doi.org/10.2307/3761644
Selig M, Weiss N, Ji Y (2008) Enzymatic saccharification of lignocellulosic biomass: Laboratory Analytical Procedure (LAP) National Renewable Energy Laboratory. Technical Report: NREL/TP-510-42629; Golden, CO, 2008
Šlosarčíková P, Novotný Č, Malachová K, Válková H, Fojtík J (2017) Effect of yeasts on biodegradation potential of immobilized cultures of white rot fungi. Sci Total Environ 589:146–152. https://doi.org/10.1016/j.scitotenv.2017.02.079
Song L, Yu H, Ma F, Zhang X (2013) Biological pretreatment under non-sterile conditions for enzymatic hydrolysis of corn stover. BioResources 8:3802–3816. https://doi.org/10.15376/biores.8.3.3802-3816
Svobodová K, Majcherczyk A, Novotný Č, Kües U (2008) Implication of mycelium-associated laccase from Irpex lacteus in the decolorization of synthetic dyes. Bioresour Technol 99:463–471. https://doi.org/10.1016/j.biortech.2007.01.019
Tejirian A, Xu F (2010) Inhibition of cellulase-catalyzed lignocellulosic hydrolysis by iron and oxidative metal ions and complexes. Appl Environ Microbiol 76:7673–7682. https://doi.org/10.1128/AEM.01376-10
Thomsen ST, Londoño JEG, Ambyejensen M, Heiske S, Kádár Z, Meyer AS (2016) Combination of ensiling and fungal delignification as effective wheat straw pretreatment. Biotechnol Biofuels 9:16. https://doi.org/10.1186/s13068-016-0437-x
Tuyen VD, Cone JW, Baars JJ, Sonnenberg AS, Hendriks WH (2012) Fungal strain and incubation period affect chemical composition and nutrient availability of wheat straw for rumen fermentation. Bioresour Technol 111:336–342. https://doi.org/10.1016/j.biortech.2012.02.001
Tuyen DV, Phuong HN, Cone JW, Baars JJP, Sonnenberg ASM, Hendriks WH (2013) Effect of fungal treatments of fibrous agricultural by-products on chemical composition and in vitro rumen fermentation and methane production. Bioresour Technol 129:256–263. https://doi.org/10.1016/j.biortech.2012.10.128
Van Soest P, Robertson J, Lewis B (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74: 3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Vasco-Correa J, Ge X, Li Y (2016) Fungal pretreatment of non-sterile miscanthus for enhanced enzymatic hydrolysis. Bioresour Technol 203:118–123. https://doi.org/10.1016/j.biortech.2015.12.018
Wan C, Li Y (2012) Fungal pretreatment of lignocellulosic biomass. Biotechnol Adv 30:1447–1457. https://doi.org/10.1016/j.biotechadv.2012.03.003
Wang H, Hao W, Ning T, Zheng M, Xu C (2018) Characterization of culturable yeast species associating with whole crop corn and total mixed ration silage. Asian Australas J Anim Sci 31:198–207. https://doi.org/10.5713/ajas.17.0183
Yahaya MS, Kimura A, Harai J, Nguyen HV, Kawai M, Takahashi J, Matsuoka S (2001) Effect of length of ensiling on silo degradation and digestibility of structural carbohydrates of lucerne and orchardgrass. Anim Feed Sci Technol 92:141–148. https://doi.org/10.1016/S0377-8401(01)00265-6
Yan T, Agnew R (2004) Prediction of metabolisable energy concentrations from nutrient digestibility and chemical composition in grass silages offered to sheep at maintenance. Anim Feed Sci Technol 117:197–213. https://doi.org/10.1016/j.anifeedsci.2004.09.002
Yang H, Wang XF, Liu JB, Gao LJ, Ishii M, Igarashi Y, Cui Z (2006) Effects of water-soluble carbohydrate content on silage fermentation of wheat straw. J Biosci Bioeng 101:232–237. https://doi.org/10.1263/jbb.101.232
Yang Y, Zhou J, Lu H, Yuan Y, Zhao L (2011) Isolation and characterization of a fungus Aspergillus sp strain F-3 capable of degrading alkali lignin. Biodegradation 22:1017–1027. https://doi.org/10.1007/s10532-011-9460-6
Zhao J, Ge X, Vasco-Correa J, Li Y (2014) Fungal pretreatment of unsterilized yard trimmings for enhanced methane production by solid-state anaerobic digestion. Bioresour Technol 158:248–252. https://doi.org/10.1016/j.biortech.2014.02.029
Zuo S, Niu D, Zheng M, Jiang D, Tian P, Li R, Xu C (2018) Effect of Irpex lacteus, Pleurotus ostreatus and Pleurotus cystidiosus pretreatment of corn stover on its improvement of the in vitro rumen fermentation. J Sci Food Agric 98:4287–4295. https://doi.org/10.1002/jsfa.8951
Funding
This work was financially supported by Ministry of Science and Technology of the People’s Republic of China (No. 2015DFG32360), Chinese Academy of Engineering (No. 2019ZCQ04), and Shaanxi Xintiandi Grass Industry Co., Ltd., China (No. 2018K0947).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
This article does not contain any studies with human participants performed by any of the authors. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Niu, D., Zuo, S., Ren, J. et al. Novel strategy to improve the colonizing ability of Irpex lacteus in non-sterile wheat straw for enhanced rumen and enzymatic digestibility. Appl Microbiol Biotechnol 104, 1347–1355 (2020). https://doi.org/10.1007/s00253-019-10315-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10315-1