Abstract
The present study addresses the synthesis and properties of polyhydroxyalkanoates (PHA) of different composition synthesized by Cupriavidus eutrophus B-10646 using glycerol as a carbon substrate. Poly(3-hydroxybutyrate) [P(3HB)] was effectively synthesized in fed-batch culture in a 30-L fermenter on glycerol of various purification degrees, with 99.5, 99.7, and 82.1% content of the main component. Purified glycerol (99.7%) was used for 150-L pilot scale fermentation. The total biomass and P(3HB) concentration reached 110 and 85.8 g/L, respectively, after 45 h of fed-batch fermentation. An average volumetric productivity of P(3HB) was 1.83 g/(L h). The degree of crystallinity and molecular weight of P(3HB) synthesized on glycerol were lower than and temperature characteristics were the same as those of P(3HB) synthesized on sugars.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
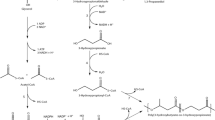
The development of new technologies aimed at integrated waste processing and production of target products, including the production of environmentally friendly energy carriers and materials, is consistent with the concept of environmentally sound sustainable industrial development. Microorganisms are a source for producing a variety of food, fodder, medical, and technical products. Polyhydroxyalkanoates (PHAs) are a valuable product of biotechnology; they have a wide range of useful properties, including biocompatibility and biodegradability in biological media, and are promising for use in various fields (Laycock et al. 2013; Volova et al. 2013a; Rebouillat and Pla 2016; Koller et al. 2017; Kourmentza et al. 2017). To a large extent (up to 45–48%), the cost of PHAs is determined by the cost of high purity carbon substrates (Hermann-Krauss et al. 2013; Kourmentza et al. 2017); therefore, one of the most high-priority areas of research is the development of technologies using low-cost substrates. Potential raw materials for PHA synthesis are various substrates with different degrees of reduction, energy content, and cost, including individual compounds (carbon dioxide and hydrogen, sugars, alcohols, organic acids) and by-products of alcohol and sugar industries, chemical processing of plant raw materials, production of olive, soybean, and palm oils (Du et al. 2012; Mozejko-Ciesielska and Kiewisz 2016; Sabbagha and Muhamada 2017; Jost 2018). The choice of the raw material for PHA production is based on the physiological and biochemical properties of the producers and the economic feasibility of the chosen strategy, taking into account the field of application of the end product. One of the promising substrates for large-scale production of PHAs is glycerol, the scale of production of which is currently increasing. This is due to the growing production of biodiesel as an alternative renewable energy source (Posada et al. 2011; Fernández-Dacosta et al. 2015). Biodiesel production increased dramatically from 500,000 gal in 1999 to 450 million gallons in 2007, and it has been growing steadily over the past few years. In 2010, the global production of biodiesel reached 19 billion liters (Global Renewable Fuels Alliance 2012), rising to more than 28 billion liters in 2017 (Statista 2018). Glycerol is a by-product, amounting to about 10%, of biodiesel production by transesterification of animal and vegetable fats and oils (rapeseed, mustard, soybean, and palm oils) (Du et al. 2012). In industrial glycerol grades, the water content varies between 5.3 and 14.2%; methanol content, 0.001 to 1.7%; NaCl—traces—5.5%; and K2SO4, 0.8 to 6.6% (Mothes et al. 2007). Glycerol has been explored as a possible carbon source for the fermentative production of hydrogen, succinic acid, glycolipid biosurfactants, citric acid, and single cell oils (Papanikolaou et al. 2007; Khanna et al. 2012; Mattam et al. 2013), as well as for PHA synthesis (da Silva et al. 2009). The analysis of publications (Table 1) showed the prospective viability of glycerol and the ability of representatives of various taxa to utilize it for PHA synthesis. Glycerol could be the ideal source for industrial production of PHAs. However, if glucose is used as carbon source, it is metabolized to pyruvate via the Entner-Doudoroff pathway (2-keto-3-deoxy-6-phosphogluconate pathway), and pyruvate can be converted by a dehydrogenase to acetyl-CoA, the central intermediate of the cellular metabolism, and the starting compound for the P(3HB) synthesis; then, glycerol can be metabolized to pyruvate as well, but via the intermediate compound glyceraldehyde-3-phosphate (Khanna et al. 2012; Rodríguez-Contreras et al. 2015).
Representatives of the genus Cupriavidus (formerly Ralstonia) are characterized by the highest production of PHAs on various substrates. Studies were conducted to implement the synthesis of PHAs of various chemical compositions under autotrophic and heterotrophic conditions on gas mixtures of hydrogen and CO2, synthesis gas, sugars, and other organic substrates (Volova et al. 2013a, b, 2014; Zhila et al. 2015). The possibility of synthesizing poly(3-hydroxybutyrate) on glycerol was shown in 1990 in the culture of Alcaligenes eutrophus Z-1 (later renamed to Ralstonia eutropha) (Volova and Kalacheva 1990). Systematic studies of this substrate were deployed in the late 1990s—early 2000s and became more active in the last decade. To date, the synthesis of PHAs (mainly P(3HB) but also copolymers P(3HB/3HV), P(3HB/4HB)) has been studied under different cultivation conditions on mineral salt medium containing glycerol by wild-type strains of various taxa: Methylobacterium rhodesianum (Bormann and Roth 1999), Methylobacterium extorquens (Taidi et al. 1994), Cupriavidus necator (Mothes et al. 2007), Paracoccus denitrificans (Mothes et al. 2007), Pseudomonas oleovorans (Ashby et al. 2005), Pseudomonas corrugate (Ashby et al. 2005), Burkholderia cepacia (Zhu et al. 2010), Haloferax mediterranei (Hermann-Krauss et al. 2013), Caldimonas manganoxidans (Hsiao et al. 2018), as well as mutant microorganisms, for example, Cupriavidus necator DSM 545 (Cavalheiro et al. 2009, 2012), Pandoraea sp. prp25 (de Paula et al. 2017) and recombinant strains Ralstonia eutropha KNK-DCD1 (Tsuge et al. 2013), E.coli CT106 (Pablo et al. 2008) (Table 1). Processes were described that were implemented in shake flask сultures and fed-batch cultures in 2.0- to 10–15-L fermenters, as well as a process of P(3HB) synthesis by Burkholderia cepacia ATCC 17759 in a culture volume of 200 L (Zhu et al. 2010). The achieved concentrations of bacterial biomass and polymer contents in cells vary considerably, from 2–10 to 40–65 g/L and from 20–40 to 60–70% of CDM, respectively. The production parameters of the processes are significantly influenced by the type of glycerol used and the contents of the main component and impurities (chlorides, sulfates, methanol), which inhibit bacterial growth and PHA synthesis and reduce molecular weight of the PHA (Mothes et al. 2007; Cavalheiro et al. 2009; Ashby et al. 2011; García et al. 2013; Tsuge et al. 2013). The analysis of publications generally indicates that glycerol has the potential to be used as a substrate for PHA production. It is obvious that involving new strains and upgrading the technological stages of the process will contribute to the improvement of PHA production.
The present work demonstrates the kinetic and production parameters of Cupriavidus eutrophus B-10646 culture on purified and crude glycerol, the properties of PHAs synthesized, and the results of the technology scale-up in pilot production.
Material and methods
Bacterial strains
The strains used in this study were Ralstonia eutropha В 5786, R.eutropha В 8562, and Cupriavidus eutrophus B-10646, registered in the Russian Collection of Industrial Microorganisms (RCIM). Chemolithoorganotrophic bacteria of the genus Cupriavidus (formerly known as Ralstonia) are regarded as very promising PHA producers, as these bacteria are capable of synthesizing PHAs in very high yields (80–90% of cell dry mass, CDM) from various substrates (Volova et al. 2013a).
Media
Schlegel’s mineral medium was used as a basic solution for growing cells: Na2HPO4·H2O, 9.1; KH2PO4, 1.5; MgSO4·H2O, 0.2; Fe3C6H5O7·7H2O, 0.025; and urea, 1.0 (g/L). Nitrogen was provided in the form of urea, and, thus, no pH adjustment was needed. The pH level of the culture medium was 7.0 ± 0.1. A solution of iron citrate (5 g/L), which was used as a source of iron, was added to reach a concentration of 5 ml/L. Hoagland’s trace element solution was used: 3 ml of standard solution per 1 L of the medium. The standard solution contained H3BO3, 0.288; CoCl2·6H2O, 0.030; CuSO4·5H2O, 0.08; MnCl2·4H2O, 0.008; ZnSO4·7H2O, 0.176; NaMoO4·2H2O, 0.050; and NiCl2, 0.008 (g/L).
Synthesis of PHA copolymers [P(3HB/4HB) or P(3HB/3 HV)] was achieved as follows: after 10 h or 10 h and 40 h of cultivation, the culture medium was supplemented with precursor substrates (ɛ-caprolactone, and propionic or valeric acids in the form of potassium salts at a concentration of 1.0 g/L). The cells were grown in batch culture in flasks on Glycerol II.
The main carbon substrate was glycerol of various grades, which was sterilized by membrane filtration using Opticap XL300 Millipore Express SHC filters (USA):
Glycerol purified (Corporate Oleon, Sweden): glycerol, 99.3; chloride, 0.0001; salts (NH4), 0.005; Fe, 0.0005; Ar, 0.00004; moisture, 0.09; fatty acid and ester, 0.25 (% mass); and max heavy metal, 0.00005 μg/g (Glycerol I). Glycerol refinery B.V. (Dutch glycerol refinery, Netherlands): glycerol, 99.7; chloride, < 0.001; moisture, 0.09; fatty acid and ester, 1.0; sulfate, < 0.002; total organic impurities, 0.5–1.0; individual organic impurities, 0.1 (% mass); and heavy metal, < 5 (μg/g) (Glycerol II). Crude glycerol (Prisma Comercial Exportadora de Oleoquimicos LTDA, Brazil): glycerol, 82.1; chloride, 4.35; mong, 0.13; methanol, 0.13; ash, 6.59; moisture, 9.88 (% mass); and рН 5.8 (Glycerol III).
As biomass increased significantly during cultivation, substrate flows were supplied to the culture to provide the cells with the necessary substrates in accordance with the dynamics of the cell biomass increase in the culture. The feeding substrates were the solutions that were fed into the culture using a multichannel dosing pump: glycerol, urea (60 g/L), MgSO4 (30 g/L) + trace element solution. Substrate feeding rates were controlled by varying the concentrations of the medium components (glycerol 5–10 g/L, phosphorus 20–40 mg/L; sulfur, potassium, magnesium 10 mg/L of each).
Cultivation of bacteria
To cultivate bacteria in shake flask culture, an Innova 44 constant temperature incubator shaker (New Brunswick Scientific, USA) was used. Inoculum was prepared by resuspending the reference bacterial culture maintained on agar medium. The reference culture was grown in 1.0–2.0-L glass flasks half-filled with liquid saline medium, with the initial concentration of glycerol from 5 to 10 g/L.
Growth kinetics of bacterial cells was studied in automated laboratory fermenters (Bioengineering AG, Switzerland), with 30-L and 150 L fermentation vessels and the working volume of the culture from 18 to 100 L, under strictly aseptic conditions. Cultivation of C. eutrophus B-10646 cells was carried out in a 30-L fermenter with a starting cell concentration in the inoculate of 1.0–1.5 g/L, 25 g/L glycerol, and 18 L working volume of the culture.
Pilot production (PP) consisted of units for media and inoculum preparation; a unit for fermentation; a unit for polymer extraction and purification. The PP fermentation unit included a steam generator (Biotron, South Korea) for sterilizing fermenters and service lines, a compressor (Remeza, Belarus) for air supply, a 30-L seed culture fermenter, a 150-L production fermenter, an ultrafiltration unit (Vladisart, Russia) to concentrate the culture, and a unit for cool dehumidification of the condensed cell suspension (LP10R ILSHIN C, South Korea) (Fig. S1).
Fermenters were equipped with systems for monitoring pH level, foam level, temperature, pressure, and dissolved oxygen. The fermenters were controlled by BioScadaLab software in automatic mode. To supply the feeding substrates, the fermenters were equipped with Bioengineering Peripex peristaltic pumps. The concentration of dissolved oxygen was maintained at DO 30%. The air supply control was carried out in a cascade mode (DO-air flow-mixer revolutions). During cultivation in a 30-L fermenter, the amount of air supplied per cultivation process varied from 0 to 5.5 Nl/min, the speed varied from 500 to 1000 rpm. During cultivation in a 150-L fermenter, the amount of air supplied per cultivation process varied from 10 to 5.5 Nl/min, the speed varied from 300 to 750 rpm.
A two-stage process was used. In the first stage (30–32 h), cells were grown under nitrogen deficiency: the amount of nitrogen supplied in this stage was 60 mg/g cell biomass synthesized (i.e., 50% of the cell’s physiological requirements—120 mg/g); the cells were cultured in complete mineral medium and with glycerol flux regulated in accordance with the requirements of the cells. Glycerol was fed into the culture with a peristaltic pump dispenser. In the second stage (24–30 h), cells were cultured in nitrogen-free medium; the other parameters were the same as in the first stage. The temperature of the culture medium was 30 ± 0.5 °C and pH was 7.0 ± 0.1.
Monitoring process parameters
During the cultivation, samples of culture medium were taken for analysis every 4–5 h (from fermenters) or every 8–10 h (from flasks); cell concentration in the culture medium was determined based on the weight of the cell samples dried at 105 °С for 24 h (CDM); the total biomass (Xtotal) and catalytically active biomass (Xc) (Xc = Xtotal − PHA), g/L, were distinguished. Cell concentration in the culture medium was determined every hour by converting the optical density of culture broth at 440 nm to cell dry mass by using a standard curve prepared previously.
Glycerol concentration was determined using the method based on the oxidation of glycerol by sodium periodate in a sulfuric acid solution to formaldehyde and determination by a colorimetric method with chromotropic acid (Nakamura et al. 2016). Nitrogen concentration in the culture medium was analyzed at different time points, using a photometric method, with Nessler’s reagent (Standard methods for the examination of water and wastewater 1989).
The criteria for evaluating the process of PHA biosynthesis were as follows: concentration of cell biomass in culture, polymer content in cells, consumption of the main growth substrate, duration and productivity of the process. The kinetic and production parameters of the culture were determined by conventional methods. The biomass concentration (X, g/L), the catalytic biomass concentration (Xc = X − PHA, g/L), the yield coefficient of the polymer (Y, g PHA/g substrate), the specific growth rate (μ, h−1), and the volumetric productivity (P, g/(L h)) were calculated.
Specific growth rate of the culture (µ, h−1) was determined using the following equation:
where Xc is catalytic biomass, g/L and t is the duration of cultivation, h.
Specific rate of polymer synthesis (qp, g/(g h)) was determined using the following formula:
where PHA are initial and final intracellular polymer concentrations, g/L.
The yield coefficient of the polymer, Y, g/g, was calculated using the following formula:
where P is initial and final polymer content, g, and ΔS is consumed substrate, g.
PHA samples were extracted from bacterial biomass with chloroform and precipitated in hexane. The optimized extraction procedure enabled the production of medically pure specimens that contained no organic impurities (proteins, carbohydrates or lipids, including fatty acids).
Analysis of PHAs
Intracellular polymer content at different time points was determined by analyzing samples of dry cell biomass. Intracellular PHA content and composition of extracted polymer samples were analyzed using a GC-MS (6890/5975C, Agilent Technologies, USA). Both lyophilized cells and extracted polymer were subjected to methanolysis in the presence of sulfuric acid, and polymer was extracted and methyl esterified at 100 °C for 3 h. Benzoic acid was used as an internal standard to determine total intracellular PHA.
1H NMR spectra of the polymer were taken at room temperature in CDCl3 on a Bruker AVANCE III 600 spectrometer (Germany) operating at 600.13 MHz.
Molecular weights and molecular weight distributions of PHA were examined using a gel permeation chromatograph (Agilent Technologies 1260 Infinity, USA) with a refractive index detector, using an Agilent PLgel Mixed-C column. Chloroform was the eluent. Calibration was made using polystyrene standards (Fluka, Switzerland, Germany). Molecular weights (weight average, Mw, and number average, Mn) and polydispersity (Ð = Mw/Mn) were determined.
Thermal analysis of PHA specimens was performed using a DSC-1 differential scanning calorimeter (METTLER TOLEDO, Switzerland). The specimens were heated at a rate of 5 °C/min to 200 °C, then cooled to − 20 °C, held for 20 min and re-heated to 320 °C. Glass transition temperature (Tg), crystallization temperature (Tc), melting point (Tmelt), and thermal degradation temperature (Tdegr) were determined from peaks in thermograms using the “StarE” software.
In order to determine the crystallinity of the PHAs, three film samples 2 cm in diameter and 0.15 mm thick were prepared from a 2% polymer solution in chloroform. The samples had a circular shape because during measurement the sample spun in a direction perpendicular to the surface. X-ray structure analysis and determination of crystallinity of PHAs were performed employing a D8ADVANCE X-ray powder diffractometer equipped with a VANTEC fast linear detector, using CuKa radiation (“Bruker, AXS”, Germany). The scan step was 0.016°, measurement time in each step 114 s, and scanning range from 5° to 60° (from 48° to 60° there only was a uniformly decreasing background); the registered parameter was intensity of X-rays scattered by the sample; 55°/0.016° = 3438 times. The degree of crystallinity (Cx) was calculated as a ratio of the total area of crystalline peaks to the total area of the radiograph (the crystalline + amorphous components). Measurement accuracy: point measurement accuracy ±0.4 PPS, with the lowest intensity 1.5 PPS and the highest intensity 32 PPS; the error in determination of the degree of crystallinity, which was calculated based on multiple measurements, was 2% or less.
Statistics
Statistical analysis of the results was performed by conventional methods, using the standard software package of Microsoft Excel. Arithmetic means and standard deviations were calculated. The statistical significance of results was determined using Student’s t test (significance level: P ≤ 0.05).
Results
Selection of a productive strain in shake flask culture
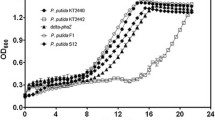
Glycerol II was used to select the most productive strain capable of synthesizing PHA on glycerol and to study bacterial growth and polymer accumulation. Cells were grown in a batch culture in 1.0 L flasks using reduced urea content (0.5 g/L) and initial glycerol concentrations of 5 and 10 g/L. The utilization of glycerol began after the lag phase that lasted from 20 to 30–40 h, regardless of the initial concentration of glycerol in the medium. A higher biomass concentration after 80 h was obtained in the culture of C. eutrophus B-10646 (2.0 g/L) compared to R. eutropha B 5786 (0.8 g/L) and R. eutropha B 8562 (1.1 g/L). The polymer contents also differed and reached 57, 45 and 38% of CDM, respectively (Fig. 1). To adapt the culture to glycerol, successive replatings were performed. As the bacteria adapted to glycerol, the lag-phase became shorter until its complete elimination, while the volumetric productivity increased with regard to the cell biomass concentration and polymer content. The cell concentrations after 60 h varied between 5.1 and 7.2 g/L depending on strains, and that was comparable with the cell concentrations on sugars. The polymer content in the cells ranged from 57 to 71% of CDM (Fig. 1). Gas chromatography showed that the synthesized polymer was poly-3-hydroxybutyrate [P(3HB)]. The strain Cupriavidus eutrophus B-10646 was selected for further research and scale-up of the process as the most productive strain.
Synthesis of PHA copolymers by C. eutrophus B-10646 on glycerol as the main C-substrate
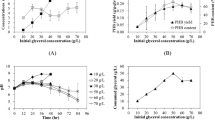
The accumulation of PHA copolymers was studied in the culture of С. eutrophus В-10646 grown on glycerol and potassium propionate, potassium valerate, and ε-caprolactone (precursors of 3-hydroxyvalerate and 4-hydroxybutyrate monomers, respectively). The use of these substrates had different effects on the cell concentration, PHA content, and the ratio of monomers. By varying the amount of the added potassium valerate, potassium propionate or ε-caprolactone, we managed to synthesize a series of PHA copolymers of different compositions (Fig. 2).
Ten hours after adding 1.0 g/L of potassium valerate to the culture, the fraction of 3-hydroxyvalerate (3 HV) reached 23.4 mol% (Fig. 2), and polymer content was about 40% of CDM. By the end of the process (60 h), the intracellular content of the polymer had increased to 70% of CDM, the 3 HV monomer content remaining practically unchanged. Ten hours after adding 1.0 g/L of potassium propionate as a precursor of 3 HV monomers, the inclusion of 3 HV monomers in the polymer was about 14 mol%, and the polymer content was 57.9% (Fig. 2). At the end of the experiment, the polymer content reached 81.9%, and the content of 3 HV monomers was 23.1 mol%. One addition of 1.0 g/L of ε-caprolactone as a precursor of 4-hydroxybutyrate (4HB) led to the incorporation of 5 mol% 4HB into the polymer in 10 h of the process, and this fraction remained unchanged until the end of cultivation (Fig. 2). The cell concentration and polymer content were about 4.8 g/L and 71.2% of CDM. Two additions of 1.0 g/L of potassium valerate or potassium propionate to the bacterial culture (Fig. 2) inhibited bacterial growth and caused a decrease in cell concentration to 3.1 g/L and 3.8 g/L and an increase in the inclusion of 3 HV monomers to 35.7 and 28.5 mol%, respectively. The subsequent addition of ε-caprolactone resulted in an increase in 4HB content to 9.8 mol%.
The effect of glycerol concentration on bacterial growth and PHA synthesis
C. eutrophus B-10646 cells were cultivated in the media with concentrations of purified glycerol varied within a wide range to determine the limits of physiological action of this substrate for the strain and the kinetic constants. The limits of physiological action of glycerol for this strain are very wide, varying between 0.5 and 60.0 g/L. There was a wide plateau (from 1 to 30 g/L), and the zones of limitation and inhibition of bacterial growth by glycerol were 0.1–3.0 and 30–60 g/L, respectively. The dependence of the specific growth rate (μ) on the substrate concentration (S) was described by the Andrews equation, which is a modified Monod equation. Using the graphical analysis method of Lineweaver-Burke (1/μ:1/S) and Dickson’s method (1/μ:S), we calculated kinetic constants for this strain (saturation constant (Ks) and inhibition constant (Ki), μmax). For the strain C. eutrophus B-10646, the limits of the physiological action of glycerol were found to be 1–30 g/L; Ks and Ki were 0.36 g/L (0.004 mol/L) and 62.0 g/L (0.673 mol/L), respectively; μmax = 0.085 h−1.
A study of the growth and synthesis of PHA by C. eutrophus B-10646 on purified and crude glycerol
The content of the main component (glycerol) in purified glycerol is more than 95–99%. In unpurified (crude) glycerol, depending on the raw material and the technology used, the content of glycerol is 80–85%; the other part comprises impurities, including free fatty acids (FFA) and methyl esters of FFA, alcohols, as well as water and salts, which, as a rule, inhibit the microorganisms responsible for PHA production.
Two grades of purified glycerol, with a 99.3 (Glycerol I) and 99.7% (Glycerol II) content of the basic material, and unpurified, crude glycerol (82.1%) were used in this study. Cultivation of C. eutrophus B-10646 cells was carried out in a 30-L fermenter. The results of PHA synthesis in a fermenter on three glycerol sources are shown in Fig. 3.
The fed-batch culture parameters of C. eutrophus B-10646 in a 30-L fermenter on various sources of glycerol: cell biomass concentrations (total and catalytic) and polymer content in cells and dynamics of the specific growth rate of total (μ) and catalytic (μc) cell biomass and specific rate of polymer synthesis (qp)
Comparable cell concentrations and polymer contents were achieved in the experiments with C.eutrophus B-10646 cultivated on purified glycerol (Glycerol I and Glycerol II). When Glycerol I and Glycerol II were used, the maximum of total biomass concentration was 70 g/L, and the polymer content in cells was 72–75% of CDM. The analysis of the results revealed some differences in the kinetic parameters during the cultivation of bacterial cells. As shown in Fig. 3, the specific growth rate of the total and active cell biomass and consumption of purified Glycerol I varied during the experiment. The specific growth rate of cells cultivated on glycerol purified was the highest (estimated from total and active biomass) in the initial period of the first stage of cultivation on complete nutrient medium with a limited supply of nitrogen (50% of the physiological requirement of bacteria), reaching 0.15 h−1 and 0.14 h−1, respectively (Fig. 3). This period corresponded to the most active consumption of glycerol by the culture, at an average rate of 4.0 ± 0.2 g/(g h). The specific rate of polymer synthesis at this stage was 0.18 g/(g h), and tended to decrease. After 30–32 h, the total cell biomass concentration was 42.1 ± 1.7 g/L, and the polymer content in the cells reached 47.8 ± 2.3% of CDM. During the second stage, the supply of nitrogen to the culture was stopped; the controlled supply of glycerol and mineral elements precluded the deficiency of these substrates in the culture. In the second stage, the specific growth rates of bacterial cells and polymer synthesis decreased gradually, as the consumption of glycerol dropped. At the end of the fermentation period (60 h), the total cell concentration was 69.3 ± 3.5 g/L, and the polymer content in the cells was 72.4 ± 3.6% of CDM. The consumption of glycerol for the whole period was 3.5 ± 0.2 kg.
On the second type of purified glycerol (Glycerol II) (Fig. 3), the maximum values of the specific growth rate of bacteria (in terms of total and active biomass) in the first stage of the process were 0.15 h−1 and 0.13 h−1, and they were comparable to the results for glycerol purified during this period. The consumption of glycerol in this period was between 3.8 and 4.0 g/(g h) while the specific polymer synthesis rate was 0.18 ± 0.02 g/(g h), and tended to decrease. After 30 h, the total cell biomass concentration was 45.6 ± 2.2 g/L, and the polymer content in the cells reached 56.1 ± 2.7% of CDM. At the end of the fermentation period, the total cell biomass concentration was 69.4 ± 3.5 g/L, and the polymer content in the cells was 73.3 ± 3.6% of CDM. The consumption of glycerol for the whole period was 3.4 ± 0.2 kg.
On glycerol III, the maximum values of bacteria specific growth rate (in terms of total and active biomass) in the first stage of the process were 0.14 h−1 and 0.13 h−1, respectively, when glycerol was consumed by the culture at a rate of 4.2 ± 0.2 g/(g·h). The specific average rate of polymer synthesis in this stage was 0.17 ± 0.02 g/(g h). After 30 h, the total cell biomass concentration was 46.2 ± 1.9 g/L, and the polymer content in the cells reached 52.2 ± 2.1% of CDM. At the end of the fermentation period, the total cell biomass concentration was 69.3 ± 2.9 g/L, and the polymer content was 78.1 ± 3.2% of CDM. The consumption of raw glycerol for the whole period amounted to 3.8 ± 0.2 kg, which corresponded to the yield coefficient for the polymer—0.26 ± 0.01—taking into account that the concentration of raw glycerol was 82.07%.
Pilot production of PHA on glycerol
The process of PHA synthesis by C. eutrophus B-10646 on Glycerol II was scaled-up and studied under PP conditions. Inoculum (4 L) with a cell concentration of 14–17 g/L was inoculated into a 30-L fermenter containing phosphate buffer. The process of building up the seed material was carried out on complete nutrient medium. For this purpose, dosing pumps were used to feed continuously separate flows of the solutions of glycerol, urea, magnesium sulfate, and ferric citrate with trace elements into the fermenter.
The inoculum produced in a seed fermenter, was pumped into a 150-L production fermenter containing a sterile buffer solution of potassium and sodium phosphates in a sterile seeding line; thus, a starting cell concentration in the inoculate was 7.0 g/L. The process was carried out in two stages with continuous supply of sterile air and feeding solutions. At the end of fermentation (45 h), the cell biomass concentration was 110 ± 5.5 g/L, and the polymer content 78 ± 3.1% of CDM. Thus, the average volumetric productivities of the polymer and cell biomass were 1.83 and 2.29 g/(L h), respectively.
Properties of PHA synthesized by C.eutrophus B-10646 on glycerol
The results of the study of the chemical composition and physicochemical properties of PHA samples synthesized on glycerol of different purification degrees are presented in Table 2. The properties of PHA samples synthesized by C. eutrophus B-10646 on three types of glycerol did not differ dramatically. The polymers synthesized by the glycerol-adapted productive culture C. eutrophus B-10646 on two types of purified glycerol had similar values of Mn 104 and 115 kDa, Mw 355 and 416 kDa, and polydispersity 3.42 and 3.63, respectively. The Mn and Mw values of the PHAs produced on crude glycerol were somewhat lower, 87 and 304 kDa. These values were generally lower than those obtained earlier on other substrates. The 1H-NMR spectra of three PHA samples synthesized on three glycerol grades were similar (Fig. S2) and showed the expected resonances for P(3HB) as demonstrated by the methyl group at 1.25 ppm, the methylene group between 2.45 and 2.65 ppm, and the methine group at 5.25 ppm. The 1H-NMR-obtained spectra of PHA samples synthesized on glycerol were similar to those obtained by other researchers (Ashby et al. 2005; Zhu et al. 2010; Rodríguez-Contreras et al. 2015). The P(3HB) samples synthesized on glycerol had a reduced Cx (50–55%), i.e. the amorphous and crystalline regions had become nearly equal to each other. No deviations in the temperature properties were found in the samples of P(3HB). Tm and Td values were within the previously identified value limits, 172–176 and 295–296 °С, respectively.
Discussion
In the present study, we investigated the possibility of growing C. eutrophus B-10646 cells and their ability to synthesize PHAs from glycerol of different purity grades. The amounts of cell biomass and polymer produced in the culture of this strain were superior to the corresponding parameters obtained under similar conditions in cultures of many wild-type strains such as C. necator IPT 026, Ps. oleovorans NRRLB-14682, Ps. corrugate 388, and Paracoccus sp. LL1 (Ashby et al. 2005; Campos et al. 2014; Kumar et al. 2018) and somewhat lower than in cultures of individual mutant and recombinant producers (Cavalheiro et al. 2009; Tsuge et al. 2013) (Table 1).
The most valuable PHA producers are strains capable of synthesizing, in addition to highly crystalline P(3HB), copolymers containing monomers other than 3HB. The inclusion of different monomers depends on many factors: the physiological and biochemical specificity of the strains, the substrate specificity of PHA synthase, the resistance to the inhibitory effect of the substrates that are precursors of the desired monomers, the conditions of PHA accumulation on the mixed carbon substrates. The strain C. eutrophus B-10646 is characterized by the ability to synthesize PHA copolymers with a different set of monomers when sugars, acetate, fatty acids, mixtures of Н2 and СО2 and precursor substrates (valeric acid, hexanoic acid, γ-butyrolactone, etc.) are used as the main C-substrate (Volova et al. 2013a). The present study shows that by using glycerol as the main C-substrate in the culture of C. eutrophus B-10646, it is possible to achieve productive synthesis of not only homopolymer P(3HB) but also PHA copolymers with different monomer fractions. These results are comparable with the data reported in the literature (Cavalheiro et al. 2012; García et al. 2013; Ramachandran and Amirul 2014).
The present study showed the ability of the strain C. eutrophus B-10646 to synthesize high polymer yields in a 30-L fermenter both from high-purity glycerol (with 99.7 or 99.3% glycerol content) and from crude glycerol (with 82.1% glycerol content). Comparison of the results with the literature data suggests that the use of crude glycerol is usually accompanied by inhibition of cell growth and polymer synthesis. In the cultures of Cupriavidus necator JMP 134 and Paracoccus denitrificans DSMZ 4134 on purified glycerol with yeast extract additions, the cell concentration and polymer content were 70 g/L and 70% of CDM, respectively; on crude glycerol these parameters were lower: 50 g/L and 48% of CDM, respectively (Mothes et al. 2007). The authors showed a stronger negative effect of the NaCl impurities compared with K2SO4. A similar inhibitory effect of the impurities of crude glycerol on the cell concentration and PHA content was detected in the culture of C. necator DSM 545 (Cavalheiro et al. 2009). The mutant strain Cupriavidus necator DSM 545 was also inhibited by the NaCl impurities (2–6 g/L) of crude glycerol (García et al. 2013). A similar negative effect of crude glycerol on the synthesis of PHA was obtained when glucose was used as co-substrate in cultures of the natural strains Cupriavidus necator DSM 545 and Burkholderia sacchari DSM 17165 (Rodríguez-Contreras et al. 2015). In a study by Ashby et al. (2011), the authors compared the synthesis of PHA on purified and crude glycerol (waste of biodiesel production) and showed that the cell concentration and polymer content were 1.5 times lower on crude glycerol. Thus, the synthesis of PHA was found to be less productive on crude glycerol than on purified glycerol. Nevertheless, comparison of the results of PHA production on crude and purified glycerol obtained in the process of biodiesel production from plant raw materials showed that it was preferable to use purified glycerol for the effective synthesis of the polymer, despite the costs associated with its purification. The consumption of carbon substrate for polymer synthesis on purified glycerol is 8% while in the cultures with sugars and other substrates it reaches 40–45% (Posada et al. 2011, 2012).
An important parameter of microbiological processes is the yield coefficient: the amount of the substrate consumed for the formation of the product. The consumption of various substrates for PHA synthesis and the yield coefficient vary considerably, amounting, for example, to 0.3–04 g/g of glucose; 1 g/g of palm oil (Tsuge et al. 2013), and 1.0 g/g of hydrogen (Volova et al. 2013a). The yield coefficient for glycerol obtained in this study was YP(3HB) 0.29 g/g. This is consistent with the data reported in the study by Mothes et al. (2007). However, in the works of different authors, for different cultures, this parameter varies between 0.05 and 0.37 g/g (Bormann and Roth 1999; Rodríguez-Contreras et al. 2015; Hsiao et al. 2018). The scale-up of P(3HB) synthesis using glycerol was described in a study by Zhu et al. (2010). In a 400-L fermenter containing 200 l of the culture of the wild-type strain Burkholderia cepacia ATCC 17759 on crude glycerol (85%), after 120 h of cultivation, the cell concentration and the content of polymer, reached 23.6 g/L and 31% of CDM, respectively. With an increase in the concentration of glycerol from 3 to 9%, the Mw decreased from 300 to 170 kDa. These values were considerably inferior to the results achieved in the present study.
The conditions for cultivation of microorganisms, especially the carbon substrate used, determine the composition and properties of PHAs. A number of studies addressed the properties of PHAs synthesized on glycerol. Special attention was given to the molecular weight properties. Glycerol, a multi-hydroxy component of triacylglycerides of different origin, has been reported to function as a catalytic chain transfer (CT) agent in PHA polymerization, resulting in the formation of low molecular weight PHAs. In case of a CT reaction, the number of carbon atoms in PHA chain increases in inverse proportion to the PHA molecular weight (Tomozawa et al. 2010). However, the data on Mw and Mn are rather contradictory. A series of studies showed a decrease in the Mw of P(3HB) synthesized on glycerol, to 260–400 kDa (Tsuge et al. 2013) and lower (Ashby et al. 2005, 2011), but other publications reported very high Mw values on glycerol, reaching 620 and 750 kDa (Mothes et al. 2007) and even 790–960 kDa (Cavalheiro et al. 2009). Molecular weight is an important parameter of polymers, PHAs in particular. Mw and Mn of PHAs are highly variable: the Mn of bacterially synthesized PHA usually varies in the range of (10–100) × 104. PHAs having higher molecular weight are mechanically stronger polymers (Laycock et al. 2013).
This study showed that molecular weight of the polymer synthesized from glycerol was lower than the molecular weight obtained previously on other substrates. The decrease in molecular weight was previously recorded in polymer samples synthesized by a non-glycerol-adapted strain C. eutrophus B-10646 in shake flask culture (Mn and Mw of 59 and 210 kDa, respectively), while on sugars these values reached 130–150 and 495–640 kDa, and on СО2 + Н2, they were even higher – 250 and 830 kDa (Zhila et al. 2015).
P(3HB) is a highly crystalline polymer, in which the crystalline phase prevails over the amorphous phase, and its Cx is 65–80% (Laycock et al. 2013). The Cx of P(3HB) produced in this study was considerably lower. A similar effect was noted in other studies. The Сх of P(3HB) synthesized on glycerol by Cupriavidus sр. USMAHM13 was 49% (Ramachandran and Amirul 2014); polymer samples synthesized by Cupriavidus necator IPT 026 had reduced Cx, which varied between 52 and 62% (Campos et al. 2014).
The results of the present study showed that the use of the wild-type strain C. eutrophus B-10646 enabled effective synthesis of PHAs on glycerol, comparable to synthesis on sugars. An important finding was that productive process of PHA synthesis could be achieved on not only purified glycerol, but also crude glycerol containing impurities, without drastically reducing the productivity of the bacterial culture. In scaled-up pilot production, a highly efficient process, with the high total biomass concentration (110 g/L) and polymer content in the cells (78% of CDM), was implemented using purified glycerol.
References
Ashby RD, Solaiman DKY, Foglia TA (2005) Synthesis of short-/medium-chain-length poly(hydroxyalkanoate) blends by mixed culture fermentation of glycerol. Biomacromolecules 6:2106–2112. https://doi.org/10.1021/bm058005h
Ashby RD, Solaiman DKY, Strahan GD (2011) Efficient utilization of crude glycerol as fermentation substrate in the synthesis of poly(3-hydroxybutyrate) biopolymers. J Am Oil Chem Soc 88:949–959. https://doi.org/10.1007/s11746-011-1755-6
Bormann EJ, Roth M (1999) The production of polyhydroxybutyrate by Methylobacterium rhodesianum and Ralstonia eutropha in media containing glycerol and casein hydrolysates. Biotechnol Lett 21:1059–1063. https://doi.org/10.1023/A:1005640712329
Campos MI, Figueiredo TVB, Sousa LS, Druzian JI (2014) The influence of crude glycerin and nitrogen concentrations on the production of PHA by Cupriavidus necator using a response surface methodology and its characterizations. Ind Crop Prod 52:338–346. https://doi.org/10.1016/j.indcrop.2013.11.008
Cavalheiro JMBT, de Almeida MCMD, Grandfils C, da Fonseca MMR (2009) Poly(3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process Biochem 44:509–515. https://doi.org/10.1016/j.procbio.2009.01.008
Cavalheiro JMBT, Raposo RSM, de Almeida MCMD, Cesário MT, Sevrin C, Grandfils C, da Fonseca MMR (2012) Effect of cultivation parameters on the production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) and poly(3-hydroxybutyrate-4-hydroxybutyrate-3-hydroxyvalerate) by Cupriavidus necator using waste glycerol. Bioresour Technol 111:391–397. https://doi.org/10.1016/j.biortech.2012.01.176
da Silva GP, Mack M, Contiero J (2009) Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv 27:30–39. https://doi.org/10.1016/j.biotechadv.2008.07.006
de Paula FC, de Paula CBC, Gomez JGC, Steinbüchel A, Contiero J (2017) Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production from biodiesel by-product and propionic acid by mutant strains of Pandoraea sp. Biotechnol Prog 33:1077–1084. https://doi.org/10.1002/btpr.2481
Du C, Sabirova J, Soetaert W, Lin SKC (2012) Polyhydroxyalkanoates production from low-cost sustainable raw materials. Curr Chem Biol 6:14–25. https://doi.org/10.2174/2212796811206010014
Fernández-Dacosta C, Posada JA, Kleerebezem R, Cuellar MC, Ramirez A (2015) Microbial community-based polyhydroxyalkanoates (PHAs) production from wastewater: techno-economic analysis and ex-ante environmental assessment. Bioresour Technol 185:368–377. https://doi.org/10.1016/j.biortech.2015.03.025
García IL, López JA, Dorado MP, Kopsahelis N, Alexandri M, Papanikolaou S, Villar MA, Koutinas AA (2013) Evaluation of by-products from the biodiesel industry as fermentation feedstock for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production by Cupriavidus necator. Bioresour Technol 130:16–22. https://doi.org/10.1016/j.biortech.2012.11.088
Global Renewable Fuels Alliance. World ethanol production playing an increasing role in energy security. http://www.globalrfa.org/pr_021111.php. Accessed 04 Dec 2012
Hermann-Krauss C, Koller M, Muhr A, Fasl H, Stelzer F, Braunegg G (2013) Archaeal production of polyhydroxyalkanoates (PHA) co- and terpolyesters from biodiesel industry-derived by-products. Archaea Article ID 129268, 10 pages. https://doi.org/10.1155/2013/129268
Hsiao L-J, Lee M-C, Chuang P-J, Kuo Y-Y, Lin J-H, Wu T-M, Li S-Y (2018) The production of poly(3-hydroxybutyrate) by thermophilic Caldimonas manganoxidans from glycerol. J Polym Res 25:85–92. https://doi.org/10.1007/s10965-018-1486-6
Jost V (2018) Packaging related properties of commercially available biopolymers – an overview of the status quo. Express Polym Lett 12:429–435. https://doi.org/10.3144/expresspolymlett.2018.36
Khanna S, Goyal A, Moholkar VS (2012) Microbial conversion of glycerol: present status and future prospects. Crit Rev Biotechnol 32:235–262. https://doi.org/10.3109/07388551.2011.604839
Koller M, Maršálek L, de Sousa Dias MM, Braunegg G (2017) Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol 37:24–38. https://doi.org/10.1016/j.nbt.2016.05.001
Kourmentza C, Plácido J, Venetsaneas N, Burniol-Figols A, Varrone C, Gavala HN, Reis MAM (2017) Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 4:e55. https://doi.org/10.3390/bioengineering4020055.
Kumar P, Jun H-B, Kim BS (2018) Co-production of polyhydroxyalkanoates and carotenoids through bioconversion of glycerol by Paracoccus sp. strain. Int J Biol Macromol 107:2552–2558. https://doi.org/10.1016/j.ijbiomac.2017.10.147
Laycock B, Peter H, Pratt S, Werker A, Lant P (2013) The chemomechanical properties of microbial polyhydroxyalkanoate. Prog Polym Sci 38:536–583. https://doi.org/10.1016/j.progpolymsci.2012.06.003
Mattam AJ, Clomburg JM, Gonzalez R, Yazdani SS (2013) Fermentation of glycerol and production of valuable chemical and biofuel molecules. Biotechnol Lett 35:831–842. https://doi.org/10.1007/s10529-013-1240-4
Mothes G, Schnorpfeil C, Ackermann J-U (2007) Production of PHB from crude glycerol. Eng Life Sci 7:475–479. https://doi.org/10.1002/elsc.200620210
Mozejko-Ciesielska J, Kiewisz R (2016) Bacterial polyhydroxyalkanoates: still fabulous? Microbiol Res 192:271–282. https://doi.org/10.1016/j.micres.2016.07.010
Nakamura M, Iso H, Kitamura A, Imano H, Noda H, Kiyama M, Sato S, Yamagishi K, Nishimura K, Nakai M, Vesper HW, Teramoto T, Miyamoto Y (2016) Comparison between the triglycerides standardization of routine methods used in Japan and the chromotropic acid reference measurement procedure used by the CDC lipid standardization Programme. Ann Clinic Biochem 53:632–639. https://doi.org/10.1177/0004563215624461
Pablo N, Pettinary MJ, Calvagno M, Mendwez B (2008) Poly(3-hydroxybutyrate) synthesis from glycerol by a recombinant Escherichia coli arsA mutant in fed-batch microaerobic cultures. Appl Microbiol Biotechnol 77:1337–1343. https://doi.org/10.1007/s00253-007-1255-7
Papanikolaou S, Fakas S, Fick M, Chevalot I, Galiotou-Panayotou M, Komaitis M, Marc I, Aggelis G (2007) Biotechnological valorization of raw glycerol discharged after bio-diesel (fatty acid methyl esters) manufacturing process: production of 1,3-propanediol, citric acid and single cell oil. Biomass Bioenergy 32:60–71. https://doi.org/10.1016/j.biombioe.2007.06.007
Posada JA, Naranjo JM, Lуpez JA, Higuita JC, Cardona CA (2011) Design and analysis of poly-3-hydroxybutyrate production processes from crude glycerol. Process Biochem 46:310–317. https://doi.org/10.1016/j.procbio.2010.09.003
Posada JA, Rincón LE, Cardona CA (2012) Design and analysis of biorefineries based on raw glycerol: addressing the glycerol problem. Bioresour Technol 111:282–293. https://doi.org/10.1016/j.biortech.2012.01.151
Ramachandran H, Amirul AA (2014) Bioconversion of glycerine pitch into a novel yellow-pigmented P(3HB-co-4HB) copolymer: synergistic effect of ammonium acetate and polymer characteristics. Appl Biochem Biotechnol 172:891–909. https://doi.org/10.1007/s12010-013-0552-0
Rebouillat S, Pla F (2016) Recent strategies for the development of biosourced-monomers, oligomers and polymers-based materials: a review with an innovation and a bigger data focus. J Biomater Nanobiotechnol 7:167–213. https://doi.org/10.4236/jbnb.2016.74017
Rodríguez-Contreras A, Koller M, de Sousa Dias MM, Calafell-Monfort M, Braunegg G, Marqués-Calvo MS (2015) Influence of glycerol on poly(3-hydroxybutyrate) production by Cupriavidus necator and Burkholderias acchari. Biochem Eng J 94:50–57. https://doi.org/10.1016/j.bej.2014.11.007
Sabbagha F, Muhamada II (2017) Production of poly-hydroxyalkanoate as secondary metabolite with main focus on sustainable energy. Renew Sust Energ Rev 72:95–104. https://doi.org/10.1016/j.rser.2016.11.012
Standard methods for the examination of water and wastewater (1989) American Publication of Health Association, Washington
Statista (2018) Leading biodiesel producers worldwide in 2017, by country (in billion liters). https://www.statista.com/statistics/271472/biodiesel-production-in-selected-countries
Taidi B, Anderson A, Dawes EA, Byrom D (1994) Effect of carbon sources and concentration on the molecular mass of poly(3-hydroxybutyrate) produced by Methylobacterium extorquens and Alcaligenes eutrophus. Appl Microbiol Biotechnol 40:786–790. https://doi.org/10.1007/BF00173975
Tomozawa S, Saito Y, Nakamura Y, Abe H, Tsuge T (2010) Chain transfer reaction catalyzed by various PHA synthases with poly(ethylene glycol) as an exogenous chain transfer agent. Appl Microbiol Biotechnol 87:1427–1435. https://doi.org/10.1007/s00253-010-2601-8
Tsuge T, Ko T, Tago M, Abe H (2013) Effect of glycerol and its analogs on polyhydroxyalkanoate biosynthesis by recombinant Ralstonia eutropha: a quantitative structure-activity relationship study of chain transfer agents. Polym Degrad Stab 98:1586–1590. https://doi.org/10.1016/j.polymdegradstab.2013.06.026
Volova TG, Kalacheva GS (1990) Polyhydroxybutyrate – thermoplastic biodegradable plymer (obtaining, properties, application). Preprint 131B, Krasnoyarsk. 47 p (in Russian)
Volova TG, Shishatskaya EI, Sinskey AJ (2013a) Degradable polymers: Production, properties, applications. Nova Science Pub. Inc., New York
Volova T, Kiselev E, Shishatskaya E, Zhila N, Boyandin A, Syrvacheva D, Vinogradova O, Kalacheva G, Vasiliev A, Peterson I (2013b) Cell growth and PHA accumulation from CO2 and H2 of a hydrogen-oxidizing bacterium, Cupriavidus eutrophus В-10646. Bioresour Technol 146:215–222. https://doi.org/10.1016/j.biortech.2013.07.070
Volova TG, Zhila NO, Shishatskaya EI, Mironov PV, Vasil’ev AD, Sukovatyi AG, Sinskey AJ (2013c) The physicochemical properties of polyhydroxyalkanoates with different chemical structures. Polym Sci Ser A 55:427–437. https://doi.org/10.1134/S0965545X13070080
Volova TG, Kiselev EG, Vinogradova ON, Nikolaeva ED, Chistyakov AA, Sukovatyi AG, Shishatskaya EI (2014) A glucose-utilizing strain, Сupriavidus eutrophus В-10646: growth kinetics, characterization and synthesis of multicomponent PHAs. PLoS One 9:e87551. https://doi.org/10.1371/journal.pone.0087551
Zhila NO, Kalacheva GS, Volova TG (2015) Fatty acid composition and polyhydroxyalkanoates production by Cupriavidus eutrophus B-10646 cells grown on different carbon sources. Process Biochem 50:69–78. https://doi.org/10.1016/j.procbio.2014.10.018
Zhu C, Nomura CT, Perrotta JA, Stipanovic AJ, Nakas JP (2010) Production and characterization of poly-3-hydroxybutyrate from biodiesel-glycerol by Burkholderia cepacia ATCC 17759. Biotechnol Prog 26:424–430. https://doi.org/10.1002/btpr.355
Funding
This study was financially supported by the project “Agropreparations of the new generation: a strategy of construction and realization” (Agreement No. 074-02-2018-328) in accordance with Resolution No 220 of the Government of the Russian Federation of April 9, 2010, “On measures designed to attract leading scientists to the Russian institutions of higher learning”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals, performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 395 kb)
Rights and permissions
About this article
Cite this article
Volova, T., Demidenko, A., Kiselev, E. et al. Polyhydroxyalkanoate synthesis based on glycerol and implementation of the process under conditions of pilot production. Appl Microbiol Biotechnol 103, 225–237 (2019). https://doi.org/10.1007/s00253-018-9460-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9460-0