Abstract
Recent progress in synthetic and systems metabolic engineering technologies has explored the potential of microbial cell factories for the production of industrially relevant bulk and fine chemicals from renewable biomass resources in an eco-friendly manner. Corynebacterium glutamicum, a workhorse for industrial amino acid production, has currently evolved into a promising microbial platform for bioproduction of various natural and non-natural chemicals from renewable feedstocks. Notably, it has been recently demonstrated that metabolically engineered C. glutamicum can overproduce several commercially valuable aromatic and related chemicals such as shikimate, 4-hydroxybenzoate, and 4-aminobenzoate from sugars at remarkably high titer suitable to commercial application. On the other hand, overexpression and/or extension of its endogenous metabolic pathways by integrating heterologous metabolic pathways enabled production of structurally intricate and valuable natural chemicals like plant polyphenols, carotenoids, and fatty acids. In this review, we summarize recent advances in metabolic engineering of C. glutamicum for production of those value-added aromatics and other natural products, which highlights high potential and the versatility of this microbe for bioproduction of diverse chemicals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past few decades, along with growing concerns about environmental issues and establishing sustainable economy independent of fossil fuels, much efforts have been devoted in eco-friendly microbial production of a wide variety of fuels and commodity chemicals with industrial relevance from renewable resources (Atsumi and Liao 2008; Becker and Wittmann 2015; Choi et al. 2015a; Nielsen et al. 2013; Turner et al. 2018). An important class of diverse chemicals, biotechnological production of which has recently gained more and more attention is aromatic compounds. They serve a vast market in the chemical industry and have numerous industrial applications as the building blocks for the synthesis of polymer materials like functional plastics and fibers, food and feed additives, nutraceuticals, and pharmaceuticals (Averesch and Kromer 2018; Kromer et al. 2013). Currently, most of industrially important aromatic compounds are produced by chemical conversion from petroleum-based feedstocks (e.g., benzene, toluene, xylene), which is not sustainable and relies on the extensive use of energy and harmful solvents, resulting in large CO2 emissions. From the environmental point of view, biotechnological production of aromatic chemicals from renewable sugar feedstocks by eco-friendly manner has received much attention as a promising alternative (Averesch and Kromer 2018; Koma et al. 2012; Lee and Wendisch 2017; Noda and Kondo 2017; Wang et al. 2017). On the other hand, target compounds of bioproduction have also expanded to diverse and structurally intricate aromatic and other natural chemicals like plant polyphenols and terpenoids, which are produced as secondary metabolites by plants and microorganisms. They exhibit various human and animal health-promoting activities and thus have possible applications in pharmaceuticals, nutraceuticals, flavors, and cosmetic industries (Scalbert et al. 2005b; Suastegui and Shao 2016). They are currently produced by chemical synthesis or extraction from natural plant producers, but their productivities and yields are limited. In this context, fermentative production of those valuable natural chemicals directly from abundant and inexpensive sugar feedstocks also came into focus and has been extensively studied using model microorganisms like Escherichia coli and Saccharomyces cerevisiae (Jiang and Zhang 2016; Suastegui and Shao 2016).
Corynebacterium glutamicum, a Gram-positive, non-pathogenic soil bacterium, has been historically used in the large-scale industrial production of amino acids for more than 50 years since its discovery as a glutamate-producing bacterium from a Japanese soil (Eggeling and Bott 2005; Ikeda and Takeno 2013; Kinoshita 1985; Leuchtenberger et al. 2005). This bacterium has many physiological properties advantageous for fermetative production, which include (a) vigorous sugar consumption under either aerobic or anaerobic conditions regardless of cell growth by high-density cells (Inui et al. 2004; Okino et al. 2005), (b) an innate absence of carbon catabolite repression, allowing simultaneous utilization of various sugar mixtures such as hexoses and pentoses in engineered strains (Becker and Wittmann 2012; Blombach and Seibold 2010; Sasaki et al. 2009), and (c) high tolerance to toxic alcohols and aromatic compounds (Kitade et al. 2018; Kubota et al. 2016; Liu et al. 2013b), as well as to fermentation inhibitors derived from lignocellulosic biomass pretreatment (Sakai et al. 2007). In addition, the technological advancement of genetic engineering tools (Baritugo et al. 2018; Lee et al. 2016) and omics-based global analysis technologies along with the accompanying progress in molecular biological knowledge for its metabolism and physiology enabled rational metabolic engineering of this bacterium. Extensive research efforts have been focused on metabolic engineering of C. glutamicum that allowed this microbe to evolve into one of the most promising microbial platforms for bioproduction of diverse chemicals beyond classical amino acids from renewable resources (Baritugo et al. 2018; Becker et al. 2018; Becker and Wittmann 2015; Becker and Wittmann 2012; Jojima et al. 2013; Lee et al. 2016). These products include biofuels (e.g., ethanol, isobutanol, and higher alcohols) (Blombach et al. 2011; Jojima et al. 2015; Siebert and Wendisch 2015; Smith et al. 2010; Xiao et al. 2016; Yamamoto et al. 2013), organic acids, and diamines for biopolymer application [e.g., lactate, succinate, putrescine (1,4-diaminobutane), and cadaverine (1,5-diaminopentane)] (Baritugo et al. 2018; Becker et al. 2018; Becker and Wittmann 2012; Jojima et al. 2013; Tsuge et al. 2015; Tsuge et al. 2016). The access of C. glutamicum to non-native carbon sources such as pentoses (Kawaguchi et al. 2008; Kawaguchi et al. 2006; Sasaki et al. 2009; Schneider et al. 2011) and glycerol (Meiswinkel et al. 2013; Rittmann et al. 2008) has also been extensively engineered, enabling efficient utilization of lignocellulosic feedstocks as well as a waste material in the biodiesel industry (Becker and Wittmann 2012; Buschke et al. 2013; Wendisch et al. 2016; Zahoor et al. 2012).
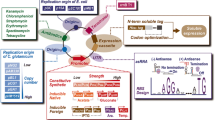
In addition to the above-mentioned commodity chemicals, product portfolio of C. glutamicum has also been recently extended to natural and non-natural value-added chemicals such as shikimate (Kogure et al. 2016), aromatic compounds like phenolic acids (Fig. 1 and Table 1) (Kallscheuer and Marienhagen 2018; Kitade et al. 2018; Kubota et al. 2016; Okai et al. 2016) as well as structurally more intricate plant polyphenols (e.g., stilbenoids and flavonoids) (Fig. 2 and Table 2) (Kallscheuer et al. 2016), carotenoids (Fig. 3 and Table 3) (Heider and Wendisch 2015), and fatty acids (Table 3) (Plassmeier et al. 2016; Takeno et al. 2013). These value-added chemicals have numerous applications in the field of high-performance biopolymers, pharmaceuticals, nutraceuticals, flavors, and cosmetics. In engineered C. glutamicum, these compounds can be formed in or derived from endogenous aromatics or terpenoid synthesis pathways by combining heterologous metabolic enzymes (Fig. 1, Fig. 2, and Fig. 3) (Pickens et al. 2011). In particular, recent findings that metabolically engineered C. glutamicum has ability to overproduce shikimate (Kogure et al. 2016), 4-aminobenzoate (4-ABA) (Kubota et al. 2016), and 4-hydroxybenzoate (4-HBA) (Kitade et al. 2018) at a markedly high titer highlighted great potential of this microbe as a platform for commercial production of value-added aromatic and related chemicals from renewable sugar feedstocks. In this review, we summarize recent advances in metabolic engineering of C. glutamicum focusing on the production of these recently explored groups of value-added chemicals.
Metabolic pathways for synthesis of shikimate pathway metabolites and derivative aromatic compounds in engineered C. glutamicum. Potential metabolic pathways that can be created by recruiting heterologous enzymes are also depicted. Genes encoding enzymes for corresponding catalytic steps are indicated in italics. G6P, glucose-6-phosphate; GAP, glyceraldehyde-3-phosphate; DHAP, 1,3-dihydroxyacetone phosphate; DHA, 1,3-dihydroxyacetone; PEP, phosphoenolpyruvate; PYR, pyruvate; AcCoA, acetyl-coenzyme A; OAA, oxaloacetate; Ru5P, ribulose-5-phosphate; R5P, ribose-5-phosphate; X5P, xylulose-5-phosphate; S7P, sedoheptulose-7-phosphate; E4P, erythrose 4-phosphate; DAHP, 3-deoxy-D-arabinoheptulosonate-7-phosphate; DHQ, 3-dehydroquinate; DHS, 3-dehydroshikimate; PCA, protocatechuate. Genes and coded enzymes: PTS, glucose-specific sugar:phosphoenolpyruvate phosphotransferase; iolT, myo-inositol permease; araE, pentose transporter; glk, glucokinase; ppgk, polyphosphate glucokinase; tkt, transketolase; tal, transaldolase; gapA, glyceraldehyde 3-phosphate dehydrogenase; hdpA, DHAP phosphatase; aroG, DAHP synthase; aroB, dehydroquinate synthase; aroD, dehydroquinate dehydratase; aroE, shikimate dehydrogenase; aroKAC, shikimate kinase, 5-enolpyruvyl shikimate-3-phosphate synthase, and chorismate synthase; qsuB, DHS dehydratase; pobA, 4-HBA 3-hydroxylase; pobA*, mutated pobA; catA, catechol 1,2-dioxygenase; ubiC, chorismiate pyruvate lyase; irp9, isochorismate synthase/isochorismate pyruvate lyase (salitylate synthase); hyg5, chorismatase (3-HBA synthase); trpEG, anthranilate synthase; pabABC, 4-amino 4-deoxychorismate (ADC) synthase and ADC lyase, COMT, catechol-O-methyltransferase; ACAR, aromatic carboxylic acid reductase; YBD, PCA decarboxylase
Metabolic pathways for synthesis of plant polyphenols and other aromatic compounds derived from aromatic amino acids in engineered C. glutamicum. Potential metabolic pathways that can be created by recruiting heterologous enzymes are also depicted. CSM, chorismate mutase; PDC, phenylpyruvate decarboxylase; ADH, alcohol dehydrogenase; CAH, cinnamic acid 4-hydroxylase; PAL, phenylalanine ammonia lyase; TAL, tyrosine ammonia lyase; CAH, cinnamic acid hydroxylase; 4CL, 4-coumarate: CoA ligase; STS, stilbene synthase; CHS, chalcone synthase; CHI, chalcone isomerase; ROMT, resveratrol-di-O-methyltransferase; F3H, flavanone 3-hydroxylase; FLS, flavonol synthase; Van, vanillate-O-demethylase; Vdh, vanillin dehydrogenase
Biosynthetic pathways for endogenous and heterologous carotenoids and other terpenoids in engineered C. glutamicum. Genes encoding enzymes for corresponding catalytic steps are indicated in italics. Heterologously expressed genes are illustrated in frames. IPP, isopentenyl pyrophosphate; DMAPP, dimethylallyl pyrophosphate; GPP, geranyl pyrophosphate; FPP, farnesyl pyrophosphate; GGPP, geranylgeranyl pyrophosphate; idi, isopentenyl-diphosphate isomerase; idsA, crtE, ispA, ERG20, and GPPS1, geranylgeranyl diphosphate synthase; PS, pinene synthase; VS and TPS1, (+)-valencene synthase; PcPS, plant patchoulol synthase; crtB and crtB2, phytoene synthase; crtI and crt2-1/2, phytoene desaturase; crtEb, lycopene elongase; crtYe/crtYf, carotenoid-ε-cyclase; crtY, lycopene β-cyclase; crtZ, β-carotene hydroxylase; crtW, β-carotene ketolase; lbtBC, lycopene elongase; lbtAB, carotenoid-ε-cyclase; crtNaNcM, dehydrosqualene synthase (crtNa), aldehyde dehydrogenase (crtNc), and aldehyde desaturase (crtM)
Suitability of C. glutamicum for production of aromatic compounds and derivatives
Whereas C. glutamicum is a well-known traditional aromatic amino acid producer and has been studied in this context for many years (Ikeda. 2006), this microbe’s potential to produce other aromatic compounds had not been explored until recently. This could be partly attributed to the intrinsic ability of this microbe to degrade and assimilate various aromatic compounds such as benzoate, 4-HBA, phenol, PCA, cinnamic acid, caffeic acid, and ferulic acid (Brinkrolf et al. 2006; Shen and Liu 2005; Shen et al. 2012). However, the presence of such multiple pathways for aromatics metabolism would be an advantage to create aromatic-producing strain since interception of these pathways may lead to the accumulation of aromatic intermediates such as PCA. Moreover, it has become evident that C. glutamicum exhibits higher resistance to various toxic aromatic compounds such as phenol, protocatechuate, 4-HBA, and 4-ABA, compared to other industrial microbes such as E. coli, Bacillus subtilis, or Pseudomonas putida (Kitade et al. 2018; Kubota et al. 2016). Such high tolerance would be attributed to the characteristic outer membrane-like structure (mycomembrane) mainly composed of coryno-mycolic acids that would function as permeability barrier against toxic aromatic compounds (Laneelle et al. 2013). High tolerance to toxic aromatic products would be a crucial factor for their production at high titer, which is required for the commercialization of bioprocess, and it is considered to contribute to the high productivity of engineered C. glutamicum strains overproducing shikimate, 4-HBA, and 4-ABA (Fig. 1 and Table 1).
Production of shikimate as a value-added hydroaromatic compound
In microbes and plants, most aromatic compounds are synthesized via the shikimate pathway as a common pathway for aromatic compound biosynthesis. This pathway serves an essential role in those organisms as the source of aromatic amino acids and other physiologically important secondary metabolites like vitamins (e.g., folate and quinones), siderophores, or antibiotics (Fig. 1). Since the shikimate pathway represents a source for a wide range of commercially relevant compounds with very diverse biological activities and industrial applications, its engineering has gained increasing attention for efficient production of pathway intermediates like shikimate as well as various compounds derived from this pathway (Jiang and Zhang 2016; Lee and Wendisch 2017; Rodriguez et al. 2014). The first and committed step in the shikimate pathway is the condensation reaction of phosphoenolpyruvate (PEP) and erythrose-4-phosphate (E4P) to generate 3-deoxy-d-arabinoheptulosonate-7-phosphate (DAHP), a reaction catalyzed by DAHP synthase. DAHP is then converted to chorismate, a branching point metabolite for aromatics synthesis, via six sequential reactions in this pathway. It is well-known that in both C. glutamicum and E. coli, carbon flow through the shikimate pathway is primarily controlled at the first reaction catalyzed by DAHP synthases, which are feedback inhibited by aromatic amino acids. For this reason, overexpression of feedback-resistant forms of DAHP synthases represents general approach to improve aromatic production derived from shikimate pathway. (Frost and Draths 1995; Ikeda 2006; Kramer et al. 2003).
Shikimate, a hydroaromatic compound formed as an intermediate of the shikimate pathway, is recently attracting attention as a building block chemical used crucially for the synthesis of an anti-influenza drug oseltamivir (Tamiflu®) (Martinez et al. 2015; Rawat et al. 2013; Tripathi et al. 2015). It also has a broad range of potential applications for pharmaceuticals and cosmetics such as a skin whitening agent and a hair-growing agent. Shikimate production currently depends on a low-yielding and costly extraction from the fruits of Illicium plants, which cannot meet the increasing demand for this compound (Bochkov et al. 2012; Ghosh et al. 2012; Rawat et al. 2013). This results in the large fluctuation of its market price from $40/kg to $1000/kg due to a huge demand of this compound for Tamiflu® synthesis along with outbreaks of human and avian influenza viruses (Rawat et al. 2013). In this context, microbial production of this compound from renewable sugars has been extensively studied as a promising alternative (Ghosh et al. 2012; Gu et al. 2017; Kramer et al. 2003; Martinez et al. 2015; Rawat et al. 2013; Rodriguez et al. 2013).
Microbial de novo production of shikimate from glucose was first achieved by engineering E. coli by the Frost group (Draths et al. 1999; Knop et al. 2001). In the engineered strain, aroK and aroL genes for shikimate kinase were disrupted to block shikimate pathway after shikimate synthesis and carbon flux to the same pathway was enhanced by introducing aroFfbr (feedback-resistant form of DAHP synthase), aroB (DHQ synthase), aroE (shikimate dehydrogenase), and tkt (transketolase). Further, availability of the precursor PEP was improved by employing non-PEP-consuming glucose uptake system consisting of the glf-encoded glucose facilitator and glk-encoded glucokinase from Zymomonas mobilis instead of endogenous phosphotransferase system (PTS) that consumes PEP along with glucose uptake (Chandran et al. 2003). The resulting strain produced 87 g/L of shikimate at the yield of 36% (mol/mol) from glucose in fermentor. Following this study, other E. coli and B. subtilis strains have been engineered to produce shikimate by similar metabolic engineering approaches (Cui et al. 2014; Escalante et al. 2010; Johansson et al. 2005; Licona-Cassani et al. 2013; Liu et al. 2014a; Liu et al. 2014b; Rodriguez et al. 2013). However, the shikimate titer attained by these recombinants remained at low level that is not suitable for commercial application. On the other hand, we recently succeeded in the construction of shikimate-overproducing strain of C. glutamicum (Kogure et al. 2016). In the constructed strain, carbon flux from consumed sugar was redirected toward shikimate synthesis via overexpression of aroGFBR encoding a feedback-resistant form of DAHP synthase from E. coli, as well as endogenous genes for aroB, aroD, aroE, tkt, and tal, whereas consumption of shikimate and DHS was blocked by the deletion of aroK, qsuB (encoding DHS dehydratase), and qsuD (encoding quinate dehydrogenase) genes (Fig. 1). To improve PEP availability, PEP-consuming PTS was inactivated by disrupting the ptsH gene and an endogenous myo-inositol permease (iolT1) and glucokinases (glk1 and glk2 and ppgk) consisting PEP-independent glucose uptake route were overexpressed. Notably, glucose consumption of engineered strains was significantly increased by cumulative overexpression of iolT1, glucokinases, a rate-limiting glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase, and the deletion of hdpA encoding 1,3-dihydroxyacetone phosphate phosphatase to inhibit loss to overflow byproducts 1,3-dihydroxyacetone (DHA) and glycerol. The enhanced glucose consumption concomitantly improved shikimate production. The resulting strain achieved production of 141 g/L shikimate from glucose with a yield of 51% (mol/mol) after 48 h in minimal medium in fermentor-controlled aerobic growth-arrested reaction (Table 1) (Kogure et al. 2016). Furthermore, similar productivity could be achieved by the simultaneous assimilation of glucose, xylose, and arabinose, allowing efficient shikimate production from lignocellulosic feedstocks (Table 1) (Kogure et al. 2016). The obtained titer and yield represented highest reported values not only for microbial production of shikimate but also for microbial aromatic production (Averesch and Kromer 2018). Considering the fact that the highest titer of aromatic compounds obtained by engineered yeast is only 3.1 g/L (Suastegui and Shao 2016), the above-mentioned results highlighted the superior potential of C. glutamicum to produce aromatic compounds derived from shikimate pathway.
Metabolically engineered C. glutamicum strains for shikimate production were also constructed recently by Zhang and colleagues. They utilized several genetic engineering techniques for strain construction such as CRISPRi system-mediated transcriptional regulation (Zhang et al. 2016), construction of genetic modules using ribosome binding site libraries (Zhang et al. 2015b), and introduction of a archaeal shikimate synthesis pathway (Zhang et al. 2015a). These approaches achieved shikimate production at 23.8, 11.3, and 4.7 g/L, respectively, in a fermenter or shake flask experiments (Table 1).
Production of protocatechuate and its derivatives
PCA (3, 4-dihydroxybenzoate) is a natural aromatic compound formed by several microbes and plants from a shikimate pathway intermediate 3-dehydroshikimate (DHS) and/or 4-HBA as direct precursors (Fig. 1). In the microbial aromatics metabolism, it represents a key precursor (a hub compound) for the biosynthesis of a wide range of value-added aromatic and related chemicals such as catechol, cis, cis-muconate (ccMA), adipate, gallate, pyrogallol, and vanillin (Fig. 1), as well as a common intermediate for degradation and assimilation of several aromatics (Shen et al. 2012). PCA itself is a valuable compound as a promising building block for synthesizing biopolymers. Moreover, it exhibits various pharmacological properties including antioxidant, anti-aging, anti-inflammatory, and anti-tumorigenic activities, and it has potential to be used in pharmaceuticals, functional foods, and cosmetic fields (Kakkar and Bais 2014; Krzysztoforska et al. 2017). While PCA is currently obtained by extraction from plant sources (Kakkar and Bais 2014; Krzysztoforska et al. 2017), more efficient fermentative production is desired. Microbial PCA production from glucose was first reported as an intermediate compound toward the production of catechol, which is a versatile organic building block for chemical industry (Li et al. 2005). In this study, PCA was produced via the shikimate pathway intermediate DHS by exploiting aroZ-encoded DHS dehydratase from Klebsiella pneumoniae in a strain where aroE-encoded shikimate dehydrogenase is inactivated to interrupt the conversion of DHS along the shikimate pathway (Fig. 1). To increase the carbon flow to the shikimate pathway and to improve precursor availability, aroFFBR-encoded feedback-resistant DAHP synthase, DHQ synthase (aroB), transketolase (tktA), and PEP synthase (ppsA) were overexpressed. The resultant engineered E. coli produced 41 g/L of PCA with a yield of 26% (mol/mol) from glucose, whereas PCA itself did not attract attention as a useful compound at that time (Li et al. 2005). On the other hand, Okai et al. recently reported PCA production in engineered C. glutamicum via an alternative production route. In this case, E. coli ubiC-encoded chorismate-pyruvate lyase (CPL) was overexpressed in a phenylalanine-producing C. glutamicum strain to convert chorismate into 4-HBA, which is further converted to PCA catalyzed by endogenous pobA-encoded 4-HBA hydroxylase. The resulting strain produced 1.1 g/L PCA from glucose after 96 h in the fermenter (Table 1) (Okai et al. 2016). They also developed an engineered C. glutamicum strain that could produce PCA from ferulic acid, an abundant lignin-derived phenolic compound in the plant biomass. They overexpressed vanillate O-demethylase genes vanAB from Corynebacterium efficiens in the PCA-producing host strain to convert ferulic acid into PCA via vanillate, which is formed via endogenous metabolic pathways. The resulting strain produced 1.1 g/L of PCA from 3.1 g/L of ferulic acid after 12 h in fed-batch fermenter biotransformation (Okai et al. 2017). Although the obtained PCA titer in these studies was far below the level applicable for practical use, the fact that engineered C. glutamicum can overproduce shikimate, and that PCA can be directly formed from the shikimate precursor DHS catalyzed by endogenous qsuB-encoded DHS dehydratase in the shikimate assimilation pathway strongly indicates that this bacterium has a potential to overproduce PCA as well.
Adipic acid and terephthalic acid are important platform chemicals as the building block of nylon-6,6 fiber and polyethylene terephthalate (PET), respectively (Sengupta et al. 2015). ccMA is a naturally occurring unsaturated dicarboxylic acid with extensive industrial applications as biological precursor for adipate and terephthalate (Polen et al. 2013; Xie et al. 2014). Bioproduction of ccMA from glucose via the shikimate pathway has also been pioneered in E. coli by the Frost group (Draths and Frost 1994; Niu et al. 2002). In this case, ccMA was synthesized by converting the shikimate pathway intermediate DHS via PCA and catechol by employing three heterologous enzymes: DHS dehydratase (AroZ) and PCA decarboxylase (AroY) from K. pneumoniae and a catechol 1,2-dioxygenase (CatA) from Acinetobacter calcoaceticus. As is the case of catechol production by engineered E. coli, shikimate dehydrogenase was inactivated to block the pathway below DHS and aroFFBR-encoded feedback-resistant DAHP synthase, DHQ synthase (aroB), and transketolase (tktA) were overexpressed. The resulting strain produced 36.8 g/L of ccMA with 22% (mol/mol) yield from glucose in fed-batch fermentations (Niu et al. 2002). Following this study, de novo ccMA production via different pathways starting from chorismate and proceeds via either anthranilate, salicylate, 2,3-dihydroxybenzoate, or 4-HBA has been engineered in E. coli, S. cerevisiae, P. putida, and K. pneumoniae (Jung et al. 2015; Lin et al. 2014; Sengupta et al. 2015; Sun et al. 2013; Sun et al. 2014; Weber et al. 2012; Xie et al. 2014). With C. glutamicum as a host, Shin et al. recently engineered a strain producing ccMA from glucose (Shin et al. 2018). They introduced heterologous PCA decarboxylase gene into the host C. glutamicum strain where catabolic pathways for DHS, PCA, and ccMA were inactivated by deletion of the aroE, pcaGH, and catB genes encoding shikimate dehydrogenase, PCA 3,4-dioxygenase, and chloromuconate cycloisomerase, respectively. Moreover, to improve PEP availability for the shikimate pathway, a non-PTS strain that does not consume PEP along with glucose uptake was generated by the deletion of ptsI and iolR (a transcriptional repressor of myo-inositol utilization genes including iolT1) to inactivate PTS and to induce expression of a non-PTS glucose permease IolT1, respectively (Ikeda et al. 2011). The overexpression of qsuB-encoded DHS dehydratase further improved ccMA production to 4.5 g/L at a yield of 22% (mol/mol) after 48 h by fed-batch fermentation (Table 1). Thus, whereas the titer was low, the yield was comparable to the highest reported one that had been achieved with an engineered E. coli ccMA producer (36.8 g/L at 22% yield (mol/mol)) (Niu et al. 2002).
Other industrially important aromatic compounds that are derived from PCA include gallate (Chandran and Frost 2001; Chen et al. 2017; Kambourakis et al. 2000), pyrogallol (Chamkha et al. 2002; Kambourakis et al. 2000; Meier et al. 2017), and vanillin (Brochado et al. 2010; Brochado and Patil 2013; Hansen et al. 2009) (Fig. 1). Their microbial production from glucose and other sugars has been widely studied especially in E. coli and S. cerevisiae (Averesch and Kromer 2018; Lee and Wendisch 2017). While production of these compounds has not yet been reported in C. glutamicum, it would be possible by extending the endogenous metabolic pathway from PCA with responsible heterologous enzymes (Fig. 1).
Production of chorismate-derived phenolic acids and related compounds
Most aromatic compounds including aromatic amino acids are synthesized from chorismate, a metabolite located at metabolic branch point from the shikimate pathway. Several industrially important aromatics can be biosynthesized from chorismate via a limited number of reaction steps, which include 4-HBA, 4-ABA, 2-hydroxybenzoate (salicylate), 3-hydroxybenzote, and anthranilate (Fig. 1). These phenolic acid aromatics represent typical bulk chemicals currently produced by chemical conversions from petroleum feedstocks. Therefore, their eco-friendly and sustainable bioproduction from renewable resources is needed. Microbial production of these phenolic acids has been widely researched by engineering model organisms such as E. coli, S. cerevisiae, and the solvent-tolerant bacterium P. putida (Gottardi et al. 2017; Lee and Wendisch 2017; Noda and Kondo 2017; Noda et al. 2016). However, to date, bioproduction of these phenolic acids has not been applied on a commercial basis because of low titers and yields. In contrast, recent studies revealed that C. glutamicum represents a promising microbial platform for their production at industrially applicable high productivity (Kitade et al. 2018; Kubota et al. 2016).
4-HBA is synthesized as a part of the ubiquinone biosynthesis pathway in several microbes and is a useful building block to produce liquid crystal polymers and paraben. While 4-HBA production systems have been developed in K. pneumoniae, E. coli, and P. putida (Barker and Frost 2001; Meijnen et al. 2011; Muller et al. 1995; Verhoef et al. 2007), their productivities were limited. Recently, Kitade et al. reported on 4-HBA overproduction by metabolically engineered C. glutamicum, which exhibited higher tolerance to 4-HBA toxicity than those previously reported microbes used for 4-HBA production (Kitade et al. 2018). In this study, all seven shikimate pathway genes and non-oxidative pentose phosphate pathway genes tkt and tal were overexpressed by their chromosomal integration to enhance the carbon flow into and through the shikimate pathway. To create the last step of 4-HBA biosynthesis from chorismate, which is not present in C. glutamicum, chorismate pyruvate-lyase (UbiC) encoded by Providencia rustigianii ubiC, which is shown to be highly resistant to feedback inhibition by 4-HBA, was overexpressed. Additionally, formation of major byproducts DHA and pyruvate was minimized by deletion of the hdpA and pyk genes encoding dihydroxyacetone phosphate phosphatase and pyruvate kinase, respectively. The resulting strain produced 36.6 g/L of 4-HBA from glucose with a yield of 41% (mol/mol) after 24 h in an aerobic growth-arrested bioprocess conducted in a fermenter with minimal medium (Table 1) (Kitade et al. 2018). This productivity represented the highest titer and yield of microbial 4-HBA production ever reported (Kitade et al. 2018), beyond those obtained in previous studies with E. coli (12 g/L at a yield of 13% (mol/mol)) (Barker and Frost 2001) or S. cerevisiae (90 mg/L at a yield of 0.8% (mol/mol))(Kromer et al. 2013).
Phenol is an important commodity chemical used as a raw material to produce phenolic plastics and pharmaceuticals and is currently produced from fossil resources. Microbial phenol production from glucose has been reported in E. coli, employing either tyrosine (Kim et al. 2014), 4-HBA (Thompson et al. 2016), or salicylate (Thompson et al. 2016) as a precursor that is converted to phenol via tyrosine phenol lyase, 4-HBA decarboxylase, or salicylate decarboxylase, respectively. However, microbial phenol production from renewable sugars has been limited due to its severe toxicity to host microorganisms and the maximal reported titer of phenol production is only 3.8 g/L (Kim et al. 2014). Since C. glutamicum exhibits high tolerance to many toxic aromatics (Kitade et al. 2018; Kubota et al. 2016) and engineered strain can overproduce 4-HBA, decarboxylation of 4-HBA by employing heterologous 4-HBA decarboxylase would be a favorable approach for bioproduction of phenol from sugars in this microbe.
4-ABA is a precursor compound for microbial folate biosynthesis in the pathway branched from chorismate. Biotechnologically, it has a great potential to serve as a building block of biopolymers including engineering plastics. Currently, 4-ABA is produced by chemical conversions from petroleum-derived toluene and its bioproduction from biomass resources is desired to address environmental issues. It is synthesized from chorismate via 4-amino-4-deoxychorismate (ADC) by consecutive reactions catalyzed by ADC synthase and ADC lyase encoded by the pabABC genes (Fig. 1). Kubota et al. recently reported on the metabolic engineering of C. glutamicum for 4-ABA overproduction. They screened heterologous pabABC genes that caused high 4-ABA production and found that overexpression of the pabAB from Corynebacterium callunae and the pabC from Xenorhabdus bovienii resulted in the highest 4-ABA production. Overexpression of these genes in a host strain overexpressing shikimate pathway genes resulted in production of 43 g/L of 4-ABA from glucose with a 20% yield (mol/mol) in fermentor, which represented the highest titer of 4-ABA produced by engineered microbes ever reported (Table 1) (Kubota et al. 2016). Intriguingly, it was shown that substantial part of the amino group of the product 4-ABA was unexpectedly converted to a N-glucosylated byproduct due to a non-enzymatical reaction between glucose used as a carbon source and the product 4-ABA during fermentation. Fortunately, this N-glucosylated byproduct could be easily reconverted to 4-ABA by acid treatment, preventing the loss of the product yield (Kubota et al. 2016).
In a recent study, Kallscheuer et al. engineered a C. glutamicum platform strain for the production of four industrially relevant hydroxybenzoates, i.e., PCA, salicylate, 3-hydroxybenzoate (3-HBA), and 4-HBA (Kallscheuer and Marienhagen 2018). Since C. glutamicum has a complex catabolic network for aromatic degradation and utilization that hamper aromatic production in this microbe, they constructed a platform C. glutamicum strain for aromatic production where 27 genes in five gene clusters comprising peripheral and central catabolic pathways of aromatics known in this microbe were deleted. This strain was subsequently engineered for the production of PCA, salicylate, 3-HBA, and 4-HBA by overexpression of heterologous genes coding for QsuB (DHS dehydratase), Irp9 (isochorismate synthase/isochorismate pyruvate lyase or salicylate synthase), Hyg5 (3-HBA synthase), and UbiC (chorismate-pyruvate lyase), respectively (Fig. 1). Production of these compounds was optimized by engineering the key enzymatic activities of the central carbon metabolism toward increased precursor availability. These included overexpression of feedback-resistant DAHP synthases (AroH or AroF from E. coli), PEP-independent glucose permease IolT1, and transketolase as well as reduction of the gltA-encoded citrate synthase activity by promoter replacement to lower the carbon flux into the tricarboxylic acid (TCA) cycle. By combining these modifications, constructed strains produced 2.0 g/L PCA, 0.01 g/L salicylate, 0.3 g/L 3-HBA, and 3.3 g/L 4-HBA in shaking flasks (Table 1) (Kallscheuer and Marienhagen 2018).
3-Amino-4-hydroxybenzoate (3,4-AHBA) is a natural aromatic compound that is synthesized as a metabolic intermediate of grixazone biosynthesis in Streptomyces griseus (Suzuki et al. 2006). It is a valuable compound serving as a precursor for the synthesis of polybenzoxazole, a thermostable bioplastic. The products of the griH and griI genes of S. griseus are involved in the biosynthesis of 3,4-AHBA, where GriI catalyzes an aldol condensation reaction between l-aspartate-4-semialdehyde and dihydroxyacetonephosphate, whereas GriH converts the resulting C7 metabolite into 3,4-AHBA. Thus, this pathway represents a simple and novel route for aromatic (benzene ring) formation from C4 and C3 metabolites that is independent of the shikimate pathway or chorismate, which is responsible for the synthesis of most aromatic compounds (Suzuki et al. 2006). Exploiting this unique enzyme system, Kawaguchi et al. constructed an engineered C. glutamicum producing 3,4-AHBA by expressing the griH and griI genes from S. griseus in lysine-producing strain C. glutamicum ATCC21799. The constructed strain produced 1.0 g/L of 3,4-AHBA from sweet sorghum juice, which was used as alternative renewable feedstock to sugar cane molasses (Table 1) (Kawaguchi et al. 2015).
Production of plant polyphenols
Plant polyphenols constitute a large group of aromatic compounds of the secondary plant metabolism (Scalbert et al. 2005a). They exhibit diverse human and animal health-promoting activities such as antioxidant, anti-inflammatory, anti-microbial, anti-cancer, or anti-diabetic activities and thus have potential applications as food supplements, pharmaceuticals, and cosmetic ingredients. These compounds are synthesized from phenylpropanoids and thus can be produced by extending microbial endogenous aromatic amino acids (l-phenylalanine or l-tyrosine) production pathways by integrating heterologous plant biosynthetic pathways (Fig. 2). This fact promoted extensive researches on biotechnological production of various plant polyphenols such as flavonoids, stilbenoids, and coumarins in the past decade, mainly focusing on engineering E. coli and S. cerevisiae as production hosts (Horinouchi. 2008; Katsuyama et al. 2007; Liu et al. 2013a; Marienhagen and Bott 2013; Suastegui and Shao 2016; van Summeren-Wesenhagen and Marienhagen 2013; Wang et al. 2017; Gottardi et al. 2017; Mei et al. 2015). More recently, Kallscheuer and colleagues proved that engineered C. glutamicum is also suitable as a platform to produce plant polyphenols such as stilbenes and (2S)-flavanones (Kallscheuer et al. 2016). In case of C. glutamicum, which can degrade and assimilate various aromatic compounds (Shen et al. 2012), it was initially found that it can grow on phenylpropanoids as a sole carbon and energy source following unknown catabolic pathway (Kallscheuer et al. 2016). Accordingly, to achieve plant polyphenol production in this bacterium, they constructed a host C. glutamicum strain in which 21 genes involved in the catabolism of aromatic compounds were deleted to block catabolic degradation of aromatics including phenylpropanoids in this microbe (Shen et al. 2012). Particularly, deletion of the phdBCDE genes responsible for CoA-dependent β-oxidative chain-shortening pathway for phenylpropanoid degradation turned out to be the key step toward enabling polyphenol production in C. glutamicum (Kallscheuer et al. 2016). Subsequent overexpression of plant-derived genes encoding a chalcone synthase (CHS) and a chalcone isomerase (CHI) enabled formation of (2S)-flavanones: 35 mg/L naringenin and 37 mg/L eriodictyol, from corresponding phenylpropanoids p-coumaric acid and caffeic acid, respectively (Fig. 2) (Table 2). On the other hand, overexpression of genes encoding a 4-coumarate:CoA-ligase (4CL) and a stilbene synthase (STS) led to the production of the stilbenes: 121 mg/L pinosylvin, 158 mg/L resveratrol, and 56 mg/L piceatannol from corresponding phenylpropanoids cinnamate, p-coumarate, and caffeic acid, respectively (Fig. 2) (Table 2). Direct production of 59 mg/L resveratrol and 32 mg/L naringenin from glucose was also achieved through engineering of the upstream pathways by overexpression of aroH gene encoding feedback-resistant DAHP synthase from E. coli and a gene encoding tyrosine ammonia lyase from Flavobacterium johnsoniae as well as deletion of qsuB gene encoding DHS dehydratase (Table 2) (Kallscheuer et al. 2016).
Following this study, Kallscheuer N. et al. further extended the heterologous metabolic pathway originating from polyphenol core structures like stilbene resveratrol, (2S)-flavanone naringenin, and eriodictyol toward production of modified products of these compounds with an even higher commercial value (Kallscheuer et al. 2017a). Expression of an O-methyltransferase in a resveratrol-producing strain allowed synthesis of 42 mg/L of the di-O-methylated pterostilbene from p-coumaric acid (Fig. 2) (Table 2). Notably, increasing the solubility of the O-methyltransferase by expressing it as a fusion protein with the maltose-binding protein of E. coli was required to obtain this titer. Similarly, expression of dioxygenase genes in (2S)-flavanone-producing strains enabled the production of the following flavonols: 23 mg/L of kaempferol and 10 mg/L of quercetin from p-coumaric acid and caffeic acid, respectively (Fig. 2) (Table 2) (Kallscheuer et al. 2017a). These results demonstrated that C. glutamicum is a favorable host organism for the production of complex plant polyphenols.
In a parallel study, Kallscheuer N. et al. constructed a novel biosynthetic pathway for resveratrol production from 4-HBA as a precursor based on the reverse reaction of a CoA-dependent, β-oxidative phenylpropanoid degradation pathway identified in the facultative denitrifying betaproteobacterium Azoarcus sp. EbN1 (Kallscheuer et al. 2017b). Engineered synthetic pathway produced p-coumaroyl-CoA from 4-HBA and acetyl-CoA by implemented β-oxidation pathway enzymes that is running in the non-natural direction, consisting of 4-HBA:CoA ligase, β-ketothiolase, 3-hydroxyacyl-CoA dehydrogenase, and enoyl-CoA hydratase from Azoarcus sp. EbN1. The overexpression of these genes alongside the heterologous stilbene synthase resulted in production of 5 mg/L of resveratrol from 4-HBA in engineered C. glutamicum. Thus, phenylpropanoid synthesis could be achieved without employing aromatic amino acids and a heterologous ammonia lyase, which represents a primary limiting step for microbial polyphenol synthesis (Table 2) (Kallscheuer et al. 2017b).
The biosynthetic pathway for aromatic amino acid has also been extended toward production of violacein, a tryptophan-derived bacterial purplish blue-colored indolocarbazole pigment with potential applications as a pharmaceutical due to its antibacterial, antitumoral, antiviral, and antioxidant activities (Choi et al. 2015b). Sun H. et al. recently engineered violacein-producing C. glutamicum strain by employing tryptophan-overproducing strain as a host and overexpressing violacein biosynthesis pathway genes vioABCDE from native violacein-producing bacterium Chromobacterium violaceum, which is involved in the condensation reaction of two tryptophan molecules to form violacein (Fig. 2). They overexpressed vioABCDE as a synthetic operon with an IPTG-inducible promoter and strong C. glutamicum ribosome-binding site (RBS) (Sun et al. 2016). The resulting strain produced 5.4 g/L of violacein in fed-batch fermentation in a bioreactor (Table 2), representing the highest titer and productivity to date. In contrast, the yield at 2.8% (mol/mol glucose) was lower than 6.0% (mol/mol glucose) previously achieved by engineered E. coli (Fang et al. 2015; Sun et al. 2016).
Derivatives of l-phenylalanine and l-tyrosine include other industrially important molecules, which include cinnamic acid, p-hydroxycinnamic acid, their decarboxylated metabolites styrene and p-hydroxystyrene, as well as caffeic acid, ferulic acid, and vanillin (Fig. 2). Metabolically engineered strains to produce these aromatic compounds have been constructed in E. coli, S. cerevisiae, and several other microbes by employing heterologous genes coding for appropriate enzymes, even though the productivity of most of these compounds remained at low level not applicable to practical use (Koma et al. 2012; Lee and Wendisch 2017; Noda and Kondo 2017; Vargas-Tah et al. 2015; Wang et al. 2017). While production of these compounds has not yet been reported in C. glutamicum, it would be possible by similarly extending aromatic amino acids biosynthetic pathways with corresponding heterologous catalytic steps (Fig. 2).
Production of carotenoids and other terpenoids
Carotenoids are important natural yellow- to red-colored pigments found ubiquitously in plants, fungi, algae, and bacteria, where they can have diverse functions such as photoprotection or light-harvesting molecules, membrane stabilizers, and precursors of hormones (Lee and Schmidt-Dannert 2002; Vershinin 1999). Carotenoids and other terpenoids are traditionally used in food, feed, and nutraceutical industries (Lee and Schmidt-Dannert 2002). To date, their diverse health-promoting activities due to their antioxidative properties have received more and more attention of the health care industry for possible applications as pharmaceuticals and nutraceuticals. All carotenoids are originating from the isoprenoid biosynthetic pathway and are derived from the universal isoprene units isopentenyl pyrophosphate (IPP) and its isomer dimethylallyl pyrophosphate (DMPP) (Fig. 3) (Rodriguez-Villalon et al. 2008; Tippmann et al. 2013). These isoprenoid precursors are derived from the methylerythritol phosphate (MEP) pathway in C. glutamicum like in most bacteria (Rodriguez-Concepcion and Boronat 2002). Whereas carotenoids are currently produced by chemical synthesis or by isolation from natural sources, such production methods harbor problems of low yield, high cost, and/or difficulty of synthesis or extraction (Misawa 2011). In this context, their efficient microbial production offers a promising alternative.
Carotenoids are classified according to the lengths of their carbon backbone with the majority consisting of C40 backbone, and only a rare number of C30 and C50 carotenoids have been described. C. glutamicum belongs to a rare group of carotenogenic bacteria that naturally produces a rare cyclic C50 carotenoid decaprenoxanthin and its glycosides (Krubasik et al. 2001b), which are responsible for its characteristic yellow pigmentation. Recently, respective genes comprising the entire carotenogenic pathway in this microbe have been elucidated (Fig. 3) (Heider et al. 2014a; Krubasik et al. 2001a; Krubasik et al. 2001b). Based on this knowledge, potential of this organism to produce native and non-native carotenoids has been explored through genetic engineering of the carotenoid biosynthesis pathway. Heider et al. engineered lycopene-producing C. glutamicum by deleting the crtEb gene-encoding lycopene elongase to prevent conversion of lycopene to decaprenoxanthin and by overexpression of crtE, crtB, and crtI genes encoding prenyl transferase, phytoene synthase, and phytoene desaturase, respectively, for conversion of geranylgeranyl pyrophosphate (GGPP) to lycopene (Fig. 3). The resulting strain showed intensely red-pigmented cells and produced 2.4 mg/g cell dry weight (CDW) lycopene (Table 3) (Heider et al. 2012). Based on the constructed lycopene-producing platform strain, C. glutamicum strains producing native and non-native C50 carotenoids decaprenoxanthin, C.p.450, and sarcinaxanthin, as well as non-native C40 carotenoids β-carotene and zeaxanthin, in the milligrams per CDW range have been engineered by overexpressing endogenous and/or heterologous carotenogenic genes (Fig. 3, Table 3) (Heider et al. 2014b). On the other hand, an endogenous gene, cg0730/crtX, responsible for glycosylation of carotenoids was identified, which enabled controlled glycosylation of formed carotenoids (Heider et al. 2014b).
C. glutamicum has also been engineered to produce several short-chain terpenoids with applications in perfume industry, including monoterpene pinene (Kang et al. 2014) and sesquiterpenes (+)-valencene (Binder et al. 2016; Frohwitter et al. 2014) and patchoulol (Fig. 3) (Henke et al. 2018). Pinene is a monoterpene (C10) naturally found in pine oil and has industrial applications in fragrances, flavors, and pharmaceuticals. Kang et al. constructed metabolically engineered C. glutamicum to produce pinene. They expressed geranyl diphosphate synthases (GPPS) and pinene synthases (PS) obtained from plants (Pinus taeda and Abies grandis) in a host strain overexpressing endogenous 1-deoxy-d-xylose-5-phosphate synthase (Dxs) and isopentenyl diphosphate isomerase (Idi), resulting in production of 27 μg/g CDW α-pinene (Fig. 3, Table 3) (Kang et al. 2014). Production of sesquiterpene, (+)-valencene, an aroma compound of citrus fruits used as flavor, was also achieved by engineering C. glutamicum (Binder et al. 2016; Frohwitter et al. 2014). Deletion of two endogenous prenyltransferase genes crtE and idsA, as well as overexpression of the heterologous farnesyl pyrophosphate (FPP) synthase gene ispA from E. coli and the (+)-valencene synthase gene from Nootka cypress allowed production of 2.4 mg/L (+)-valencene (Fig. 3, Table 3) (Frohwitter et al. 2014). More recently, sesquarterpenes as novel C35 carotenoids with antioxidant activity were synthesized by engineering C. glutamicum to express the crtNaNcM genes derived from the Planococcus sp. bacteria (Fig. 3) (Ravikumar et al. 2018). Various sesquarterpenes like 4-apolycopene (red-color) and 4-aponeurosporene (yellow-color) could be accumulated depending on the expression of the genetic elements of the crtNaNcM genes (Table 3) (Ravikumar et al. 2018).
Precursor availability is a key determinant to improve product yields, and in this regard, carotenogenesis was also engineered. Improved supply of the precursor IPP by overexpressing all of eight MEP pathway genes enhanced the production of lycopene, decaprenoxanthin, and astaxanthin by engineered C. glutamicum (Heider et al. 2014c). Improvement of the carotenogenesis is also examined via engineering of the regulation of gene expression. Henke et al. demonstrated that the MarR-type transcriptional regulator CrtR repressed carotenogenic genes in an isoprenoid pyrophosphate-dependent manner in C. glutamicum (Henke et al. 2017). They showed that deletion of the crtR gene represents a general strategy to increase production of native and non-native carotenoids. In addition to altering the regulation of a specific biosynthetic pathway, engineering of the global regulator has been shown to have a considerable impact on cellular metabolism (Taniguchi et al. 2017a; Taniguchi and Wendisch 2015). In this context, Taniguchi et al. demonstrated that overexpression of the primary sigma factor gene sigA increased production of native carotenoids lycopene and decaprenoxanthin as well as non-native carotenoids β-carotene and bisanhydrobacterioruberin (Taniguchi et al. 2017b). They also showed that deletion of the gene for the alternative sigma factor SigB also increased carotenoid production. On the other hand, Binder et al. recently developed a gene expression control system employing light-responsive photocaged IPTG to precisely control the timing of gene expression that triggers toxic product formation. This regulatory system was successfully employed to improve the production of (+)-valencene, which is a toxic compound inhibiting cell growth, via the control of the time point of light induction for its biosynthesis genes (Binder et al. 2016).
Production of fatty acids and lipids
Microbial production of fatty acids, lipids, and related compounds has attracted attention as a renewable source of biofuels as well as functional nutrients. Studies on microbial lipid production have been advanced in naturally oleaginous fungi (Sakuradani et al. 2013), yeasts (Beopoulos et al. 2011), and algae (Han et al. 2015), while naturally non-oleaginous bacteria like E. coli (Dellomonaco et al. 2011; Lennen and Pfleger 2012; Steen et al. 2010) and C. glutamicum have also been researched more recently for the potential of fatty acid and lipid production. Regarding fatty acid and lipid metabolism, C. glutamicum has several inherent properties that is distinct from that in most bacteria such as E. coli and B. subtilis, which include the presence of eukaryotic multifunctional type I fatty acid synthase (FAS-I) system comprising FAS IA and FAS IB, the absence of the fatty acids β-oxidation pathway involved in their degradation, as well as the presence of high cytoplasmic thioesterase (TES) activity that is involved in the formation of free fatty acids from acyl (ACP)-CoA (Takeno et al. 2013). Takeno and colleagues isolated an oleic acid-secreting mutant of C. glutamicum by isolating spontaneous mutants resistant to palmitic acid ester surfactant tween 40 and cerulenin, an inhibitor of fatty acid biosynthesis. The whole genome analysis of the resultant mutant specified responsible mutations for oleic acid-secreting phenotype in fasR encoding fatty acid biosynthesis repressor (Nickel et al. 2010) and in fasA encoding FAS IA. Reconstitution experiments in the wild-type strain demonstrated that only the fasR mutation could trigger oleic acid secretion. In this reconstituted fasR mutant, fatty acid biosynthetic pathway genes fasA, fasB (encoding FAS IB), and accD1 (encoding the β-subunit of acetyl-CoA carboxylase) were shown to be upregulated, indicating that fasR mutation caused functional impairment of its product (FasR repressor). These results revealed that derepression of fatty acid biosynthesis leads to an oversupply of acyl-CoAs, which would be converted into free fatty acids catalyzed by high endogenous acyl-CoA thioesterase activity and excreted without degradation due to the absence of fatty acid β-oxidation pathway in C. glutamicum (Takeno et al. 2013). The reconstructed strain harboring three causative mutations in the wild-type background produced 280 mg/L of fatty acids, which consisted mainly of oleic acid (208 mg/L) and palmitic acid (47 mg/L) showing the potential of C. glutamicum to produce fatty acids and related functional lipids (Table 1) (Takeno et al. 2013). Apart from fatty acid biosynthesis, fasA- and fasB-encoded FAS-I system was shown to have important physiological roles in the biosynthesis of cofactors biotin and alpha-lipoic acid in an engineered biotin-prototrophic C. glutamicum strain as the source of the precursors of these cofactors in this organism (Ikeda et al. 2017).
On the other hand, Plassmeier et al. engineered C. glutamicum to produce triacylglycerol (TAG) (Plassmeier et al. 2016). They completed a functional TAG biosynthesis pathway in C. glutamicum by complementing two missing enzymes, diacylglycerol acyltransferase (DGAT) and phosphatidic acid phosphatase (PAP), by expressing the atf1 and atf2 genes encoding DGAT from the native TAG producer Rhodococcus opacus and the pgpB gene encoding PAP from E. coli. In addition, to increase the fatty-acyl-CoA availability for lipid synthesis, tesA encoding thioesterase to form free fatty acid from fatty acyl-ACP and fadD encoding acyl-CoA synthetase, both derived from E. coli were expressed. TAGs could only be detected by the additional deletion of four cellular lipase genes and one diacylglycerol kinase gene, indicating that inactivation of lipase activity is crucial for lipid accumulation in C. glutamicum. Moreover, the deletion of fasR to derepress fatty acid biosynthetic genes and thereby to enhance fatty acid synthesis and the blockage of the acetate and lactate formation has a strong synergistic effect on TAG production (3.7-fold increase in the fatty acid content). The final strain achieved a 7.5% yield of total fatty acids from glucose (2.4 g/L intracellular fatty acids and 0.6 g/L extracellular fatty acids) in shake flasks, which corresponded to fatty acid content of 17.8% of the dry cell. This result demonstrated the potential of C. glutamicum as a lipid producer (Table 1) (Plassmeier et al. 2016).
Production of other value-added compounds
Recently, Tsuge et al. reported on production of shinorine, an ultraviolet-absorbing mycosporine-like amino acid useful in cosmetics and skin care to prevent UV-induced skin damage, by metabolically engineered C. glutamicum (Tsuge et al. 2018). They constructed a shinorine-producing strain by expressing a shinorine biosynthetic operon from the actinobacterium Actinosynnema mirum constituting four genes responsible for shinorine biosynthesis from sedoheptulose-7-phosphate (S7P), an intermediate of the pentose phosphate pathway. Thus, shinorine biosynthesis requires a distinct pentose phosphate pathway precursor from that for most aromatic compounds, namely, E4P formed in the same pathway. To allow for the accumulation of the precursor S7P, modification of the pentose phosphate pathway was performed, namely, deletion of tal gene encoding transaldolase and overexpression of gnd gene encoding 6-phosphogluconate dehydrogenase. This modification improved shinorine production from gluconic acid to 19.1 mg/L, that is 8.3-fold higher compared to the value exhibited by the parent strain without metabolic pathway modification (2.3 mg/L) (Table 1) (Tsuge et al. 2018). Such a strategy for increasing S7P would be applicable to other chemicals derived from this compound.
Conclusions and outlooks
Driven by the progress of metabolic engineering technologies, the portfolio of chemical products that can be produced by microbial fermentation from renewable resources has been extensively expanded. However, the productivity of most of bio-based value-added chemicals, e.g., aromatic compounds, isoprenoids, and advanced biofuels, remains at low level even at the stage confirming the proof of concept that is not applicable to commercial production. In this regard, engineered C. glutamicum, which achieved markedly high productivity of shikimate, 4-HBA, and 4-ABA at a commercially applicable level, can serve as a promising platform to produce such valuable chemicals from renewable sugars in an environmentally compatible bioprocess. However, further improvement of producer strains would be needed to enhance the cost-competitiveness of bio-derived aromatic chemicals to conventional petroleum- or plant-derived chemicals. One of key challenges would be improvement of the product yield. For microbial aromatic production, aerobic reaction condition is required for their efficient synthesis, because in their production, NADH formed via glycolysis must be reoxidized by respiration to keep redox balance. However, the aerobic reaction condition typically promotes carbon flux into the TCA cycle that accompanies formation of a large amount of CO2 as a major byproduct (at 30–50% as carbon yield) (Kogure et al. 2016). In this respect, cofactor engineering of key metabolic enzyme(s) (e.g., converting the cofactor preference of shikimate dehydrogenase from NADPH to NADH) would have potential to enable efficient aromatic production with high yield under microaerobic or even under anaerobic conditions like the case of organic acid production by C. glutamicum (Inui et al. 2004; Okino et al. 2005). This is based on the improvement of redox balance under anaerobic conditions as is demonstrated in l-valine production by engineered C. glutamicum (Hasegawa et al. 2012). Reduction of the carbon flux to the byproduct-forming pathways like TCA cycle by inactivating or reducing the activity of key enzymes, e.g., pyruvate kinase or citrate synthase, preferably in product-production phase-specific manner, represents an another possible approach to improve product yield (Kallscheuer and Marienhagen 2018; Noda et al. 2016).
Recent development of multi-omics analysis and advanced computer-assisted metabolic analysis technologies enabled rational and systematic metabolic engineering of microbial hosts to enhance productivity and yield of the target compound to the theoretical maximum, instead of the classical intuitive metabolic engineering. Such technologies include metabolic analysis based on the precise genome-scale metabolic model specialized for a particular organism, as well as molecular dynamics simulation of mRNA and protein structure, which enables optimization of pathway gene expression and improvement of the key enzyme function (Isa et al. 2018). These approaches in a synergistic way would contribute to remove metabolic bottlenecks and to design most appropriate metabolic pathways from genome-wide perspectives. Another important issue to address to realize industrial bioproduction is an enhancement of robustness and tolerance of a microbial host toward stressed conditions due to severe product toxicity and/or high osmotic pressure that would limit the productivity. In the case of shikimate production by engineered C. glutamicum, the tendency of the engineered strains to aggregate presumably due to impaired cell surface integrity represents another critical issue to address for large-scale production. To overcome these issues, evolutionary or mutational approaches to obtain strains with desired phenotype and following specification of responsible mutations would help rational engineering of robust strains with enhanced stress tolerance and/or improved cell integrity (Buschke et al. 2013; Lessmeier and Wendisch 2015; Mahr et al. 2015; Oide et al. 2015). Such efforts would further explore the full potential of C. glutamicum as a platform biocatalyst for production of various valuable chemicals from renewable feedstocks, opening the door to the sustainable and eco-friendly bio-industry that replaces the conventional petrochemical industry.
Availability of supporting data
No supporting data are provided.
References
Atsumi S, Liao JC (2008) Metabolic engineering for advanced biofuels production from Escherichia coli. Curr Opin Biotechnol 19(5):414–419
Averesch NJH, Kromer JO (2018) Metabolic engineering of the shikimate pathway for production of aromatics and derived compounds—present and future strain construction strategies. Front Bioeng Biotechnol 6:32
Baritugo KA, Kim HT, David Y, Choi JI, Hong SH, Jeong KJ, Choi JH, Joo JC, Park SJ (2018) Metabolic engineering of Corynebacterium glutamicum for fermentative production of chemicals in biorefinery. Appl Microbiol Biotechnol 102(9):3915–3937
Barker JL, Frost JW (2001) Microbial synthesis of p-hydroxybenzoic acid from glucose. Biotechnol Bioeng 76(4):376–390
Becker J, Wittmann C (2012) Bio-based production of chemicals, materials and fuels—Corynebacterium glutamicum as versatile cell factory. Curr Opin Biotechnol 23(4):631–640
Becker J, Wittmann C (2015) Advanced biotechnology: metabolically engineered cells for the bio-based production of chemicals and fuels, materials, and health-care products. Angew Chem Int Ed Engl 54(11):3328–3350
Becker J, Giesselmann G, Hoffmann SL, Wittmann C (2018) Corynebacterium glutamicum for sustainable bioproduction: from metabolic physiology to systems metabolic engineering. Adv Biochem Eng Biotechnol 162:217–263
Beopoulos A, Nicaud JM, Gaillardin C (2011) An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl Microbiol Biotechnol 90(4):1193–1206
Binder D, Frohwitter J, Mahr R, Bier C, Grunberger A, Loeschcke A, Peters-Wendisch P, Kohlheyer D, Pietruszka J, Frunzke J, Jaeger KE, Wendisch VF, Drepper T (2016) Light-controlled cell factories: employing photocaged isopropyl-beta-d-thiogalactopyranoside for light-mediated optimization of lac promoter-based gene expression and (+)-valencene biosynthesis in Corynebacterium glutamicum. Appl Environ Microbiol 82(20):6141–6149
Blombach B, Seibold GM (2010) Carbohydrate metabolism in Corynebacterium glutamicum and applications for the metabolic engineering of L-lysine production strains. Appl Microbiol Biotechnol 86(5):1313–1322
Blombach B, Riester T, Wieschalka S, Ziert C, Youn JW, Wendisch VF, Eikmanns BJ (2011) Corynebacterium glutamicum tailored for efficient isobutanol production. Appl Environ Microbiol 77(10):3300–3310
Bochkov DV, Sysolyatin SV, Kalashnikov AI, Surmacheva IA (2012) Shikimic acid: review of its analytical, isolation, and purification techniques from plant and microbial sources. J Chem Biol 5(1):5–17
Brinkrolf K, Brune I, Tauch A (2006) Transcriptional regulation of catabolic pathways for aromatic compounds in Corynebacterium glutamicum. Genet Mol Res 5(4):773–789
Brochado AR, Patil KR (2013) Overexpression of O-methyltransferase leads to improved vanillin production in baker's yeast only when complemented with model-guided network engineering. Biotechnol Bioeng 110(2):656–659
Brochado AR, Matos C, Moller BL, Hansen J, Mortensen UH, Patil KR (2010) Improved vanillin production in baker's yeast through in silico design. Microb Cell Factories 9:84
Buschke N, Schafer R, Becker J, Wittmann C (2013) Metabolic engineering of industrial platform microorganisms for biorefinery applications—optimization of substrate spectrum and process robustness by rational and evolutive strategies. Bioresour Technol 135:544–554
Chamkha M, Patel BK, Traore A, Garcia JL, Labat M (2002) Isolation from a shea cake digester of a tannin-degrading Streptococcus gallolyticus strain that decarboxylates protocatechuic and hydroxycinnamic acids, and emendation of the species. Int J Syst Evol Microbiol 52(Pt 3):939–944
Chandran SS, Frost JW (2001) Aromatic inhibitors of dehydroquinate synthase: synthesis, evaluation and implications for gallic acid biosynthesis. Bioorg Med Chem Lett 11(12):1493–1496
Chandran SS, Yi J, Draths KM, von Daeniken R, Weber W, Frost JW (2003) Phosphoenolpyruvate availability and the biosynthesis of shikimic acid. Biotechnol Prog 19(3):808–814
Chen Z, Shen X, Wang J, Yuan Q, Yan Y (2017) Rational engineering of p-hydroxybenzoate hydroxylase to enable efficient gallic acid synthesis via a novel artificial biosynthetic pathway. Biotechnol Bioeng 114(11):2571–2580
Choi S, Song CW, Shin JH, Lee SY (2015a) Biorefineries for the production of top building block chemicals and their derivatives. Metab Eng 28:223–239
Choi SY, Yoon KH, Lee JI, Mitchell RJ (2015b) Violacein: properties and production of a versatile bacterial pigment. Biomed Res Int 2015:465056
Cui YY, Ling C, Zhang YY, Huang J, Liu JZ (2014) Production of shikimic acid from Escherichia coli through chemically inducible chromosomal evolution and cofactor metabolic engineering. Microb Cell Factories 13:21
Dellomonaco C, Clomburg JM, Miller EN, Gonzalez R (2011) Engineered reversal of the beta-oxidation cycle for the synthesis of fuels and chemicals. Nature 476(7360):355–359
Draths KM, Frost JW (1994) Environmentally compatible synthesis of adipic acid from D-glucose. J Am Chem Soc 116(1):399–400
Draths KM, K DR, Frost JW (1999) Shikimic acid and quinic acid: replacing isolation from plant sources with recombinant microbial biocatalysis. J Am Chem Soc 121:1603–1604
Eggeling L, Bott M (2005) Handbook of Corynebacterium glutamicum. CRC Press LLC, Boca Raton, FL
Escalante A, Calderon R, Valdivia A, de Anda R, Hernandez G, Ramirez OT, Gosset G, Bolivar F (2010) Metabolic engineering for the production of shikimic acid in an evolved Escherichia coli strain lacking the phosphoenolpyruvate:carbohydrate phosphotransferase system. Microb Cell Factories 9:21
Fang MY, Zhang C, Yang S, Cui JY, Jiang PX, Lou K, Wachi M, Xing XH (2015) High crude violacein production from glucose by Escherichia coli engineered with interactive control of tryptophan pathway and violacein biosynthetic pathway. Microb Cell Factories 14:8
Frohwitter J, Heider SA, Peters-Wendisch P, Beekwilder J, Wendisch VF (2014) Production of the sesquiterpene (+)-valencene by metabolically engineered Corynebacterium glutamicum. J Biotechnol 191:205–213
Frost JW, Draths KM (1995) Biocatalytic syntheses of aromatics from D-glucose: renewable microbial sources of aromatic compounds. Annu Rev Microbiol 49:557–579
Ghosh S, Chisti Y, Banerjee UC (2012) Production of shikimic acid. Biotechnol Adv 30(6):1425–1431
Gottardi M, Reifenrath M, Boles E, Tripp J (2017) Pathway engineering for the production of heterologous aromatic chemicals and their derivatives in Saccharomyces cerevisiae: bioconversion from glucose. FEMS Yeast Res 17(4):fox035
Gu P, Fan X, Liang Q, Qi Q, Li Q (2017) Novel technologies combined with traditional metabolic engineering strategies facilitate the construction of shikimate-producing Escherichia coli. Microb Cell Factories 16(1):167
Han SF, Jin WB, Tu RJ, Wu WM (2015) Biofuel production from microalgae as feedstock: current status and potential. Crit Rev Biotechnol 35(2):255–268
Hansen EH, Moller BL, Kock GR, Bunner CM, Kristensen C, Jensen OR, Okkels FT, Olsen CE, Motawia MS, Hansen J (2009) De novo biosynthesis of vanillin in fission yeast (Schizosaccharomyces pombe) and baker's yeast (Saccharomyces cerevisiae). Appl Environ Microbiol 75(9):2765–2774
Hasegawa S, Uematsu K, Natsuma Y, Suda M, Hiraga K, Jojima T, Inui M, Yukawa H (2012) Improvement of the redox balance increases L-valine production by Corynebacterium glutamicum under oxygen deprivation conditions. Appl Environ Microbiol 78(3):865–875
Heider SA, Wendisch VF (2015) Engineering microbial cell factories: metabolic engineering of Corynebacterium glutamicum with a focus on non-natural products. Biotechnol J 10(8):1170–1184
Heider SA, Peters-Wendisch P, Wendisch VF (2012) Carotenoid biosynthesis and overproduction in Corynebacterium glutamicum. BMC Microbiol 12:198
Heider SA, Peters-Wendisch P, Beekwilder J, Wendisch VF (2014a) IdsA is the major geranylgeranyl pyrophosphate synthase involved in carotenogenesis in Corynebacterium glutamicum. FEBS J 281(21):4906–4920
Heider SA, Peters-Wendisch P, Netzer R, Stafnes M, Brautaset T, Wendisch VF (2014b) Production and glucosylation of C50 and C 40 carotenoids by metabolically engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol 98(3):1223–1235
Heider SA, Wolf N, Hofemeier A, Peters-Wendisch P, Wendisch VF (2014c) Optimization of the IPP precursor supply for the production of lycopene, decaprenoxanthin and astaxanthin by Corynebacterium glutamicum. Front Bioeng Biotechnol 2:28
Henke N, Heider S, Peters-Wendisch P, Wendisch V (2016) Production of the marine carotenoid astaxanthin by metabolically engineered Corynebacterium glutamicum. Mar Drugs 14(7):124
Henke NA, Heider SAE, Hannibal S, Wendisch VF, Peters-Wendisch P (2017) Isoprenoid pyrophosphate-dependent transcriptional regulation of carotenogenesis in Corynebacterium glutamicum. Front Microbiol 8:633
Henke NA, Wichmann J, Baier T, Frohwitter J, Lauersen KJ, Risse JM, Peters-Wendisch P, Kruse O, Wendisch VF (2018) Patchoulol production with metabolically engineered Corynebacterium glutamicum. Genes (Basel) 9(4):219
Horinouchi S (2008) Combinatorial biosynthesis of non-bacterial and unnatural flavonoids, stilbenoids and curcuminoids by microorganisms. J Antibiot (Tokyo) 61(12):709–728
Ikeda M (2006) Towards bacterial strains overproducing L-tryptophan and other aromatics by metabolic engineering. Appl Microbiol Biotechnol 69(6):615–626
Ikeda M, Takeno S (2013) Amino acid production by Corynebacterium glutamicum. In: Yukawa H, Inui M (eds) Corynebacterium glutamicum. Springer, Berlin Heidelberg, pp 107–147
Ikeda M, Mizuno Y, Awane S, Hayashi M, Mitsuhashi S, Takeno S (2011) Identification and application of a different glucose uptake system that functions as an alternative to the phosphotransferase system in Corynebacterium glutamicum. Appl Microbiol Biotechnol 90(4):1443–1451
Ikeda M, Nagashima T, Nakamura E, Kato R, Ohshita M, Hayashi M, Takeno S (2017) In vivo roles of fatty acid-biosynthetic enzymes in biosynthesis of biotin and alpha-lipoic acid in Corynebacterium glutamicum. Appl Environ Microbiol 83(19):e01322–e01317
Inui M, Murakami S, Okino S, Kawaguchi H, Vertes AA, Yukawa H (2004) Metabolic analysis of Corynebacterium glutamicum during lactate and succinate productions under oxygen deprivation conditions. J Mol Microbiol Biotechnol 7(4):182–196
Isa MA, Majumdhar RS, Haider S (2018) In silico docking and molecular dynamics simulation of 3-dehydroquinate synthase (DHQS) from Mycobacterium tuberculosis. J Mol Model 24(6):132
Jiang M, Zhang H (2016) Engineering the shikimate pathway for biosynthesis of molecules with pharmaceutical activities in E. coli. Curr Opin Biotechnol 42:1–6
Johansson L, Lindskog A, Silfversparre G, Cimander C, Nielsen KF, Liden G (2005) Shikimic acid production by a modified strain of E. coli (W3110.shik1) under phosphate-limited and carbon-limited conditions. Biotechnol Bioeng 92(5):541–552
Jojima T, Inui M, Yukawa H (2013) Biorefinery applications of Corynebacterium glutamicum. In: Yukawa H, Inui M (eds) Corynebacterium gluamicum biology and biotechnology. Springer-Verlag, Berlin Heidelberg, pp 149–172
Jojima T, Noburyu R, Sasaki M, Tajima T, Suda M, Yukawa H, Inui M (2015) Metabolic engineering for improved production of ethanol by Corynebacterium glutamicum. Appl Microbiol Biotechnol 99(3):1165–1172
Jung HM, Jung MY, Oh MK (2015) Metabolic engineering of Klebsiella pneumoniae for the production of cis,cis-muconic acid. Appl Microbiol Biotechnol 99(12):5217–5225
Kakkar S, Bais S (2014) A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol 2014:952943
Kallscheuer N, Marienhagen J (2018) Corynebacterium glutamicum as platform for the production of hydroxybenzoic acids. Microb Cell Factories 17(1):70
Kallscheuer N, Vogt M, Stenzel A, Gatgens J, Bott M, Marienhagen J (2016) Construction of a Corynebacterium glutamicum platform strain for the production of stilbenes and (2S)-flavanones. Metab Eng 38:47–55
Kallscheuer N, Vogt M, Bott M, Marienhagen J (2017a) Functional expression of plant-derived O-methyltransferase, flavanone 3-hydroxylase, and flavonol synthase in Corynebacterium glutamicum for production of pterostilbene, kaempferol, and quercetin. J Biotechnol 258:190–196
Kallscheuer N, Vogt M, Marienhagen J (2017b) A novel synthetic pathway enables microbial production of polyphenols independent from the endogenous aromatic amino acid metabolism. ACS Synth Biol 6(3):410–415
Kambourakis S, Draths KM, Frost JW (2000) Synthesis of gallic acid and pyrogallol from glucose: replacing natural product isolation with microbial catalysis. J Am Chem Soc 122(37):9042–9043
Kang MK, Eom JH, Kim Y, Um Y, Woo HM (2014) Biosynthesis of pinene from glucose using metabolically-engineered Corynebacterium glutamicum. Biotechnol Lett 36(10):2069–2077
Katsuyama Y, Funa N, Miyahisa I, Horinouchi S (2007) Synthesis of unnatural flavonoids and stilbenes by exploiting the plant biosynthetic pathway in Escherichia coli. Chem Biol 14(6):613–621
Kawaguchi H, Vertes AA, Okino S, Inui M, Yukawa H (2006) Engineering of a xylose metabolic pathway in Corynebacterium glutamicum. Appl Environ Microbiol 72(5):3418–3428
Kawaguchi H, Sasaki M, Vertes AA, Inui M, Yukawa H (2008) Engineering of an L-arabinose metabolic pathway in Corynebacterium glutamicum. Appl Microbiol Biotechnol 77(5):1053–1062
Kawaguchi H, Sasaki K, Uematsu K, Tsuge Y, Teramura H, Okai N, Nakamura-Tsuruta S, Katsuyama Y, Sugai Y, Ohnishi Y, Hirano K, Sazuka T, Ogino C, Kondo A (2015) 3-Amino-4-hydroxybenzoic acid production from sweet sorghum juice by recombinant Corynebacterium glutamicum. Bioresour Technol 198:410–417
Kim B, Park H, Na D, Lee SY (2014) Metabolic engineering of Escherichia coli for the production of phenol from glucose. Biotechnol J 9(5):621–629
Kinoshita S (1985) Glutamic acid bacteria. In: Demain AL, Solomon NA (eds) Biology of industrial microorganisms. Benjamin/Cummings, London, pp 115–146
Kitade Y, Hashimoto R, Suda M, Hiraga K, Inui M (2018) Production of 4-hydroxybenzoic acid by an aerobic growth-arrested bioprocess using metabolically engineered Corynebacterium glutamicum. Appl Environ Microbiol 84(6):e02587–e02517
Knop DR, Draths KM, Chandran SS, Barker JL, von Daeniken R, Weber W, Frost JW (2001) Hydroaromatic equilibration during biosynthesis of shikimic acid. J Am Chem Soc 123(42):10173–10182
Kogure T, Kubota T, Suda M, Hiraga K, Inui M (2016) Metabolic engineering of Corynebacterium glutamicum for shikimate overproduction by growth-arrested cell reaction. Metab Eng 38:204–216
Koma D, Yamanaka H, Moriyoshi K, Ohmoto T, Sakai K (2012) Production of aromatic compounds by metabolically engineered Escherichia coli with an expanded shikimate pathway. Appl Environ Microbiol 78(17):6203–6216
Kramer M, Bongaerts J, Bovenberg R, Kremer S, Muller U, Orf S, Wubbolts M, Raeven L (2003) Metabolic engineering for microbial production of shikimic acid. Metab Eng 5(4):277–283
Kromer JO, Nunez-Bernal D, Averesch NJ, Hampe J, Varela J, Varela C (2013) Production of aromatics in Saccharomyces cerevisiae—a feasibility study. J Biotechnol 163(2):184–193
Krubasik P, Kobayashi M, Sandmann G (2001a) Expression and functional analysis of a gene cluster involved in the synthesis of decaprenoxanthin reveals the mechanisms for C50 carotenoid formation. Eur J Biochem 268(13):3702–3708
Krubasik P, Takaichi S, Maoka T, Kobayashi M, Masamoto K, Sandmann G (2001b) Detailed biosynthetic pathway to decaprenoxanthin diglucoside in Corynebacterium glutamicum and identification of novel intermediates. Arch Microbiol 176(3):217–223
Krzysztoforska K, Mirowska-Guzel D, Widy-Tyszkiewicz E (2017) Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: review on the basis of in vitro and in vivo studies in rodents and humans. Nutr Neurosci Jul 26:1–11
Kubota T, Watanabe A, Suda M, Kogure T, Hiraga K, Inui M (2016) Production of para-aminobenzoate by genetically engineered Corynebacterium glutamicum and non-biological formation of an N-glucosyl byproduct. Metab Eng 38:322–330
Laneelle MA, Tropis M, Daffe M (2013) Current knowledge on mycolic acids in Corynebacterium glutamicum and their relevance for biotechnological processes. Appl Microbiol Biotechnol 97(23):9923–9930
Lee PC, Schmidt-Dannert C (2002) Metabolic engineering towards biotechnological production of carotenoids in microorganisms. Appl Microbiol Biotechnol 60(1–2):1–11
Lee JH, Wendisch VF (2017) Biotechnological production of aromatic compounds of the extended shikimate pathway from renewable biomass. J Biotechnol 257:211–221
Lee JY, Na YA, Kim E, Lee HS, Kim P (2016) The actinobacterium Corynebacterium glutamicum, an industrial workhorse. J Microbiol Biotechnol 26(5):807–822
Lennen RM, Pfleger BF (2012) Engineering Escherichia coli to synthesize free fatty acids. Trends Biotechnol 30(12):659–667
Lessmeier L, Wendisch VF (2015) Identification of two mutations increasing the methanol tolerance of Corynebacterium glutamicum. BMC Microbiol 15:216
Leuchtenberger W, Huthmacher K, Drauz K (2005) Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol 69(1):1–8
Li W, Xie D, Frost JW (2005) Benzene-free synthesis of catechol: interfacing microbial and chemical catalysis. J Am Chem Soc 127(9):2874–2882
Licona-Cassani C, Lara AR, Cabrera-Valladares N, Escalante A, Hernandez-Chavez G, Martinez A, Bolivar F, Gosset G (2013) Inactivation of pyruvate kinase or the phosphoenolpyruvate:sugar phosphotransferase system increases shikimic and dehydroshikimic acid yields from glucose in Bacillus subtilis. J Mol Microbiol Biotechnol 24(1):37–45
Lin Y, Sun X, Yuan Q, Yan Y (2014) Extending shikimate pathway for the production of muconic acid and its precursor salicylic acid in Escherichia coli. Metab Eng 23:62–69
Liu L, Redden H, Alper HS (2013a) Frontiers of yeast metabolic engineering: diversifying beyond ethanol and Saccharomyces. Curr Opin Biotechnol 24(6):1023–1030
Liu YB, Long MX, Yin YJ, Si MR, Zhang L, Lu ZQ, Wang Y, Shen XH (2013b) Physiological roles of mycothiol in detoxification and tolerance to multiple poisonous chemicals in Corynebacterium glutamicum. Arch Microbiol 195(6):419–429
Liu DF, Ai GM, Zheng QX, Liu C, Jiang CY, Liu LX, Zhang B, Liu YM, Yang C, Liu SJ (2014a) Metabolic flux responses to genetic modification for shikimic acid production by Bacillus subtilis strains. Microb Cell Factories 13(1):40
Liu X, Lin J, Hu H, Zhou B, Zhu B (2014b) Metabolic engineering of Escherichia coli to enhance shikimic acid production from sorbitol. World J Microbiol Biotechnol 30(9):2543–2550
Mahr R, Gatgens C, Gatgens J, Polen T, Kalinowski J, Frunzke J (2015) Biosensor-driven adaptive laboratory evolution of L-valine production in Corynebacterium glutamicum. Metab Eng 32:184–194
Marienhagen J, Bott M (2013) Metabolic engineering of microorganisms for the synthesis of plant natural products. J Biotechnol 163(2):166–178
Martinez JA, Bolivar F, Escalante A (2015) Shikimic acid production in Escherichia coli: from classical metabolic engineering strategies to omics applied to improve its production. Front Bioeng Biotechnol 3:145
Mei YZ, Liu RX, Wang DP, Wang X, Dai CC (2015) Biocatalysis and biotransformation of resveratrol in microorganisms. Biotechnol Lett 37(1):9–18
Meier AK, Worch S, Boer E, Hartmann A, Mascher M, Marzec M, Scholz U, Riechen J, Baronian K, Schauer F, Bode R, Kunze G (2017) Agdc1p—a gallic acid decarboxylase involved in the degradation of tannic acid in the yeast Blastobotrys (Arxula) adeninivorans. Front Microbiol 8:1777
Meijnen JP, Verhoef S, Briedjlal AA, de Winde JH, Ruijssenaars HJ (2011) Improved p-hydroxybenzoate production by engineered Pseudomonas putida S12 by using a mixed-substrate feeding strategy. Appl Microbiol Biotechnol 90(3):885–893
Meiswinkel TM, Rittmann D, Lindner SN, Wendisch VF (2013) Crude glycerol-based production of amino acids and putrescine by Corynebacterium glutamicum. Bioresour Technol 145:254–258
Misawa N (2011) Pathway engineering for functional isoprenoids. Curr Opin Biotechnol 22(5):627–633
Muller R, Wagener A, Schmidt K, Leistner E (1995) Microbial production of specifically ring-13C-labelled 4-hydroxybenzoic acid. Appl Microbiol Biotechnol 43(6):985–988
Nickel J, Irzik K, van Ooyen J, Eggeling L (2010) The TetR-type transcriptional regulator FasR of Corynebacterium glutamicum controls genes of lipid synthesis during growth on acetate. Mol Microbiol 78(1):253–265
Nielsen J, Larsson C, van Maris A, Pronk J (2013) Metabolic engineering of yeast for production of fuels and chemicals. Curr Opin Biotechnol 24(3):398–404
Niu W, Draths KM, Frost JW (2002) Benzene-free synthesis of adipic acid. Biotechnol Prog 18(2):201–211
Noda S, Kondo A (2017) Recent advances in microbial production of aromatic chemicals and derivatives. Trends Biotechnol 35(8):785–796
Noda S, Shirai T, Oyama S, Kondo A (2016) Metabolic design of a platform Escherichia coli strain producing various chorismate derivatives. Metab Eng 33:119–129
Oide S, Gunji W, Moteki Y, Yamamoto S, Suda M, Jojima T, Yukawa H, Inui M (2015) Thermal and solvent stress cross-tolerance conferred to Corynebacterium glutamicum by adaptive laboratory evolution. Appl Environ Microbiol 81(7):2284–2298
Okai N, Miyoshi T, Takeshima Y, Kuwahara H, Ogino C, Kondo A (2016) Production of protocatechuic acid by Corynebacterium glutamicum expressing chorismate-pyruvate lyase from Escherichia coli. Appl Microbiol Biotechnol 100(1):135–145
Okai N, Masuda T, Takeshima Y, Tanaka K, Yoshida KI, Miyamoto M, Ogino C, Kondo A (2017) Biotransformation of ferulic acid to protocatechuic acid by Corynebacterium glutamicum ATCC 21420 engineered to express vanillate O-demethylase. AMB Express 7(1):130
Okino S, Inui M, Yukawa H (2005) Production of organic acids by Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 68(4):475–480
Pickens LB, Tang Y, Chooi YH (2011) Metabolic engineering for the production of natural products. Annu Rev Chem Biomol Eng 2:211–236
Plassmeier J, Li Y, Rueckert C, Sinskey AJ (2016) Metabolic engineering Corynebacterium glutamicum to produce triacylglycerols. Metab Eng 33:86–97
Polen T, Spelberg M, Bott M (2013) Toward biotechnological production of adipic acid and precursors from biorenewables. J Biotechnol 167(2):75–84
Ravikumar S, Woo HM, Choi JI (2018) Analysis of novel antioxidant sesquarterpenes (C35 terpenes) produced in recombinant Corynebacterium glutamicum. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-018-2756-9
Rawat G, Tripathi P, Saxena RK (2013) Expanding horizons of shikimic acid. Recent progresses in production and its endless frontiers in application and market trends. Appl Microbiol Biotechnol 97(10):4277–4287
Rittmann D, Lindner SN, Wendisch VF (2008) Engineering of a glycerol utilization pathway for amino acid production by Corynebacterium glutamicum. Appl Environ Microbiol 74(20):6216–6222
Rodriguez A, Martinez JA, Baez-Viveros JL, Flores N, Hernandez-Chavez G, Ramirez OT, Gosset G, Bolivar F (2013) Constitutive expression of selected genes from the pentose phosphate and aromatic pathways increases the shikimic acid yield in high-glucose batch cultures of an Escherichia coli strain lacking PTS and pykF. Microb Cell Factories 12(1):86
Rodriguez A, Martinez JA, Flores N, Escalante A, Gosset G, Bolivar F (2014) Engineering Escherichia coli to overproduce aromatic amino acids and derived compounds. Microb Cell Factories 13(1):126
Rodriguez-Concepcion M, Boronat A (2002) Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol 130(3):1079–1089
Rodriguez-Villalon A, Perez-Gil J, Rodriguez-Concepcion M (2008) Carotenoid accumulation in bacteria with enhanced supply of isoprenoid precursors by upregulation of exogenous or endogenous pathways. J Biotechnol 135(1):78–84
Sakai S, Tsuchida Y, Nakamoto H, Okino S, Ichihashi O, Kawaguchi H, Watanabe T, Inui M, Yukawa H (2007) Effect of lignocellulose-derived inhibitors on growth of and ethanol production by growth-arrested Corynebacterium glutamicum R. Appl Environ Microbiol 73(7):2349–2353
Sakuradani E, Ando A, Shimizu S, Ogawa J (2013) Metabolic engineering for the production of polyunsaturated fatty acids by oleaginous fungus Mortierella alpina 1S-4. J Biosci Bioeng 116(4):417–422
Sasaki M, Jojima T, Kawaguchi H, Inui M, Yukawa H (2009) Engineering of pentose transport in Corynebacterium glutamicum to improve simultaneous utilization of mixed sugars. Appl Microbiol Biotechnol 85(1):105–115
Scalbert A, Johnson IT, Saltmarsh M (2005a) Polyphenols: antioxidants and beyond. Am J Clin Nutr 81(1 Suppl):215S–217S
Scalbert A, Manach C, Morand C, Remesy C, Jimenez L (2005b) Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr 45(4):287–306
Schneider J, Niermann K, Wendisch VF (2011) Production of the amino acids L-glutamate, L-lysine, L-ornithine and L-arginine from arabinose by recombinant Corynebacterium glutamicum. J Biotechnol 154(2–3):191–198
Sengupta S, Jonnalagadda S, Goonewardena L, Juturu V (2015) Metabolic engineering of a novel muconic acid biosynthesis pathway via 4-hydroxybenzoic acid in Escherichia coli. Appl Environ Microbiol 81(23):8037–8043
Shen X, Liu S (2005) Key enzymes of the protocatechuate branch of the beta-ketoadipate pathway for aromatic degradation in Corynebacterium glutamicum. Sci China C Life Sci 48(3):241–249
Shen XH, Zhou NY, Liu SJ (2012) Degradation and assimilation of aromatic compounds by Corynebacterium glutamicum: another potential for applications for this bacterium? Appl Microbiol Biotechnol 95(1):77–89
Shin WS, Lee D, Lee SJ, Chun GT, Choi SS, Kim ES, Kim S (2018) Characterization of a non-phosphotransferase system for cis,cis-muconic acid production in Corynebacterium glutamicum. Biochem Biophys Res Commun 499(2):279–284
Siebert D, Wendisch VF (2015) Metabolic pathway engineering for production of 1,2-propanediol and 1-propanol by Corynebacterium glutamicum. Biotechnol Biofuels 8:91
Smith KM, Cho KM, Liao JC (2010) Engineering Corynebacterium glutamicum for isobutanol production. Appl Microbiol Biotechnol 87(3):1045–1055
Steen EJ, Kang Y, Bokinsky G, Hu Z, Schirmer A, McClure A, Del Cardayre SB, Keasling JD (2010) Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463(7280):559–562
Suastegui M, Shao Z (2016) Yeast factories for the production of aromatic compounds: from building blocks to plant secondary metabolites. J Ind Microbiol Biotechnol 43(11):1611–1624
Sun X, Lin Y, Huang Q, Yuan Q, Yan Y (2013) A novel muconic acid biosynthesis approach by shunting tryptophan biosynthesis via anthranilate. Appl Environ Microbiol 79(13):4024–4030
Sun X, Lin Y, Yuan Q, Yan Y (2014) Biological production of muconic acid via a prokaryotic 2,3-dihydroxybenzoic acid decarboxylase. ChemSusChem 7(9):2478–2481
Sun H, Zhao D, Xiong B, Zhang C, Bi C (2016) Engineering Corynebacterium glutamicum for violacein hyper production. Microb Cell Factories 15(1):148
Suzuki H, Ohnishi Y, Furusho Y, Sakuda S, Horinouchi S (2006) Novel benzene ring biosynthesis from C(3) and C(4) primary metabolites by two enzymes. J Biol Chem 281(48):36944–36951
Takeno S, Takasaki M, Urabayashi A, Mimura A, Muramatsu T, Mitsuhashi S, Ikeda M (2013) Development of fatty acid-producing Corynebacterium glutamicum strains. Appl Environ Microbiol 79(21):6776–6783
Taniguchi H, Wendisch VF (2015) Exploring the role of sigma factor gene expression on production by Corynebacterium glutamicum: sigma factor H and FMN as example. Front Microbiol 6:740
Taniguchi H, Busche T, Patschkowski T, Niehaus K, Patek M, Kalinowski J, Wendisch VF (2017a) Physiological roles of sigma factor SigD in Corynebacterium glutamicum. BMC Microbiol 17(1):158
Taniguchi H, Henke NA, Heider SAE, Wendisch VF (2017b) Overexpression of the primary sigma factor gene sigA improved carotenoid production by Corynebacterium glutamicum: application to production of beta-carotene and the non-native linear C50 carotenoid bisanhydrobacterioruberin. Metab Eng Commun 4:1–11
Thompson B, Machas M, Nielsen DR (2016) Engineering and comparison of non-natural pathways for microbial phenol production. Biotechnol Bioeng 113(8):1745–1754
Tippmann S, Chen Y, Siewers V, Nielsen J (2013) From flavors and pharmaceuticals to advanced biofuels: production of isoprenoids in Saccharomyces cerevisiae. Biotechnol J 8(12):1435–1444
Tripathi P, Rawat G, Yadav S, Saxena RK (2015) Shikimic acid, a base compound for the formulation of swine/avian flu drug: statistical optimization, fed-batch and scale up studies along with its application as an antibacterial agent. Antonie Van Leeuwenhoek 107(2):419–431
Tsuge Y, Hasunuma T, Kondo A (2015) Recent advances in the metabolic engineering of Corynebacterium glutamicum for the production of lactate and succinate from renewable resources. J Ind Microbiol Biotechnol 42(3):375–389
Tsuge Y, Kawaguchi H, Sasaki K, Kondo A (2016) Engineering cell factories for producing building block chemicals for bio-polymer synthesis. Microb Cell Factories 15:19
Tsuge Y, Kawaguchi H, Yamamoto S, Nishigami Y, Sota M, Ogino C, Kondo A (2018) Metabolic engineering of Corynebacterium glutamicum for production of sunscreen shinorine. Biosci Biotechnol Biochem 82(7):1252–1259
Turner TL, Kim H, Kong II, Liu JJ, Zhang GC, Jin YS (2018) Engineering and evolution of Saccharomyces cerevisiae to produce biofuels and chemicals. Adv Biochem Eng Biotechnol 162:175–215
van Summeren-Wesenhagen PV, Marienhagen J (2013) Putting bugs to the blush: metabolic engineering for phenylpropanoid-derived products in microorganisms. Bioengineered 4(6):355–362
Vargas-Tah A, Martinez LM, Hernandez-Chavez G, Rocha M, Martinez A, Bolivar F, Gosset G (2015) Production of cinnamic and p-hydroxycinnamic acid from sugar mixtures with engineered Escherichia coli. Microb Cell Factories 14:6
Verhoef S, Ruijssenaars HJ, de Bont JA, Wery J (2007) Bioproduction of p-hydroxybenzoate from renewable feedstock by solvent-tolerant Pseudomonas putida S12. J Biotechnol 132(1):49–56
Vershinin A (1999) Biological functions of carotenoids—diversity and evolution. Biofactors 10(2–3):99–104
Wang J, Shen X, Rey J, Yuan Q, Yan Y (2017) Recent advances in microbial production of aromatic natural products and their derivatives. Appl Microbiol Biotechnol 102(1):47–61
Weber C, Bruckner C, Weinreb S, Lehr C, Essl C, Boles E (2012) Biosynthesis of cis,cis-muconic acid and its aromatic precursors, catechol and protocatechuic acid, from renewable feedstocks by Saccharomyces cerevisiae. Appl Environ Microbiol 78(23):8421–8430
Wendisch VF, Brito LF, Gil Lopez M, Hennig G, Pfeifenschneider J, Sgobba E, Veldmann KH (2016) The flexible feedstock concept in industrial biotechnology: metabolic engineering of Escherichia coli, Corynebacterium glutamicum, Pseudomonas, Bacillus and yeast strains for access to alternative carbon sources. J Biotechnol 234:139–157
Xiao S, Xu J, Chen X, Li X, Zhang Y, Yuan Z (2016) 3-Methyl-1-butanol biosynthesis in an engineered Corynebacterium glutamicum. Mol Biotechnol 58(5):311–318
Xie NZ, Liang H, Huang RB, Xu P (2014) Biotechnological production of muconic acid: current status and future prospects. Biotechnol Adv 32(3):615–622
Yamamoto S, Suda M, Niimi S, Inui M, Yukawa H (2013) Strain optimization for efficient isobutanol production using Corynebacterium glutamicum under oxygen deprivation. Biotechnol Bioeng 110(11):2938–2948
Zahoor A, Lindner SN, Wendisch VF (2012) Metabolic engineering of Corynebacterium glutamicum aimed at alternative carbon sources and new products. Comput Struct Biotechnol J 3:e201210004
Zhang B, Jiang CY, Liu YM, Liu C, Liu SJ (2015a) Engineering of a hybrid route to enhance shikimic acid production in Corynebacterium glutamicum. Biotechnol Lett 37:1861–1868
Zhang B, Zhou N, Liu YM, Liu C, Lou CB, Jiang CY, Liu SJ (2015b) Ribosome binding site libraries and pathway modules for shikimic acid synthesis with Corynebacterium glutamicum. Microb Cell Factories 14:71
Zhang B, Liu ZQ, Liu C, Zheng YG (2016) Application of CRISPRi in Corynebacterium glutamicum for shikimic acid production. Biotechnol Lett 38(12):2153–2161
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kogure, T., Inui, M. Recent advances in metabolic engineering of Corynebacterium glutamicum for bioproduction of value-added aromatic chemicals and natural products. Appl Microbiol Biotechnol 102, 8685–8705 (2018). https://doi.org/10.1007/s00253-018-9289-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9289-6