Abstract
During high gravity fermentation, a set of hexose transporters in yeasts plays an important role in efficient sugar transport. However, hexose transporters have been studied mainly in the Saccharomyces cerevisiae model and at low or moderate sugar concentrations. The hexose transporters are still poorly understood in the industrial glycerol producer Candida glycerinogenes, which assimilates sugar efficiently at high glucose concentration. To explore these hexose transporters, 14 candidates were identified using a hidden Markov model and characterized. Five of these functioned as hexose transporters when expressed in S. cerevisiae. In particular, CgHxt4 showed the highest efficiency of glucose transport at elevated glucose concentration among a group of transporters including Hxt1 and Hxt7 from S. cerevisiae. qRT-PCR in C. glycerinogenes revealed that transcription of CgHXT4 was induced by high glucose concentrations while fluorescence localization analysis indicated that CgHxt4 remained relatively stable on the membrane under these conditions. In addition, site-directed mutagenesis revealed that the asparagine 329 from CgHxt4, located in the YYX(T/P) conserved motif of hexose transporters, promoted an increased glucose transport. Overexpressing CgHXT4 in S. cerevisiae enhanced glucose consumption and ethanol production more effectively at high glucose concentrations than ScHXT1, the most significant native transporter from S. cerevisiae. These results indicate that CgHxt4 plays an important role in the fermentation process as a hexose transporter with strong transport activity and efficient expression regulation at high glucose concentrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High gravity fermentation (HGF) is an economical and environmentally friendly technology using high concentrations of substrate which allows increases in product titer and prevents bacterial contamination. It also reduces energy consumption at the industrial scale (Chao et al. 2017). However, at high sugar concentrations, the activity of Saccharomyces cerevisiae is severely inhibited, which limits the efficiency of sugar conversion and the ultimate yield of products (Arshad et al. 2017). Thus, fermentation is usually restricted to sugar concentrations < 200 g/L. Candida glycerinogenes, an osmotolerant yeast, quickly adapts to HGF conditions (> 500 g/L glucose) and then rapidly and efficiently converts glucose to ethanol and glycerol (Hairu et al. 2003; Zhuge et al. 2001). A prerequisite for effective glucose utilization under these conditions is the activity of glucose transporters in the presence of high substrate concentrations (Dimarco and Romano 1985; Tanino et al. 2012; Zhang et al. 2017).
Many studies have characterized S. cerevisiae hexose transporters (HXT), which are responsible for sugar uptake in this yeast (Özcan and Johnston 1999). S. cerevisiae contains 18 hexose transporter genes (HXT1–17 and GAL2) that belong to the sugar porter family-TCDB 2.A.1.1, which with certain conserved structures, such as 12 hydrophobic transmembrane domains, five conserved sequence motifs, and the cytosolic amino- and carboxyl-terminal regions (Leandro et al. 2009). However, only eight of them are transcribed as major sugar transporters under moderate or low concentrations of sugar (Özcan and Johnston 1999). These HXTs show variant activities and specificities compared to other glucose transporters (Wieczorke et al. 1999). Further modifications of the conserved motifs by genetic engineering improved the activities and altered the specificities of the HXTs, leading to better fermentation performance (Wang et al. 2015; Wang et al. 2016; Zhang et al. 2015b). In addition, many studies have indicated that the transcription of HXT genes is regulated by different substrate concentrations and physiological states through transcription factors (Kim et al. 2015; Tomas-Cobos et al. 2004). The ubiquitin-mediated endocytosis of HXTs is another important regulation mechanism that also affects transport persistence (Nijland et al. 2016). The expression levels and stabilities of HXTs were significantly strengthened through engineering the transcription factors and ubiquitination of the sugar transport system, leading to more efficient cell growth and product titer (Nijland et al. 2017; Sen et al. 2016).
Although hexose transporters have been intensively studied in the S. cerevisiae model, information for industrial strains is still scarce (Leandro et al. 2009), especially on C. glycerinogenes, which has otherwise been subject to extensive research on its physiological mechanisms (Xie et al. 2009; Yongguang et al. 2007; Zong et al. 2016). Moreover, previous research on sugar transporters has generally focused on moderate glucose concentrations (Leandro et al. 2009). The characteristics and the regulatory mechanisms of hexose transport under HGF conditions are poorly understood (Zhang et al. 2015a). Because of the efficient carbohydrate assimilation of C. glycerinogenes during HGF, it is useful to study hexose transport in this yeast to gain insight into the factors influencing rapid utilization of glucose when present at high concentration. In this study, hexose transporters from C. glycerinogenes were identified by homology detection using a hidden Markov model and characterized in a hxt-null S. cerevisiae (Wieczorke et al. 1999). Transcriptional regulation, membrane stability regulation, and the important residues of the identified hexose transporters were then analyzed.
Materials and methods
Molecular biology techniques and chemicals
DNA polymerase, restriction enzymes, and T4 DNA ligase were purchased from Takara (Dalian, China). TRIzon Reagent and SYBR Green were acquired from CWBIO (Beijing, China). The reverse transcription reagent (HiScript II Q RT SuperMix for qPCR) was obtained from Vazyme (Nanjing, China). The plasmid extraction kit and PCR product purification kit were purchased from Sangon Biotech (Shanghai, China), which also carried out sequencing and synthesis of primers. Yeast extract and peptone were acquired from OXOID (Basingstoke, England). Yeast nitrogen base was obtained from BBI (Toronto, Canada).
Strains and culture conditions
The strains used in this study are listed in Table S1. Escherichia coli JM109 was routinely used as the host for plasmid construction. S. cerevisiae EBY.VW4000 (CEN.PK2-1C Δhxt1–17 Δgal2 Δstl1 Δagt1 Δmph2 Δmph3), a gift generously provided by Prof. E. Boles, is unable to transport hexose and was used for complementary growth tests and fluorescence localization analysis to characterize the performance of hexose transporters (Wieczorke et al. 1999). C. glycerinogenes WL2002-5 (Saccharomycetales, Saccharomycetales incertae sedis, Candida in NCBI database, CCTCC M 93018) was used for gene cloning and transcriptional analysis (Zhuge et al. 2001).
For maintenance, C. glycerinogenes WL2002-5, S. cerevisiae CEN.PK2-1C, and the derivatives of CEN.PK2-1C were grown aerobically (30 °C, 100 rpm in reciprocating shake flasks) on YPD medium formulated with 20 or 250 g/L glucose. S. cerevisiae EBY.VW4000 and its derivatives were cultured in minimal medium (YNB), which consisted of 6.7 g/L yeast nitrogen base (without amino acids and ammonium sulfate), 20 or 200 g/L individual sugars (maltose, glucose, fructose, mannose, or galactose), and supplemented with uracil (20 mg/L), leucine (60 mg/L), tryptophan (20 mg/L), or histidine (20 mg/L). E. coli JM109 was grown in Luria-Bertani (LB) medium at 37 °C with 100 μg/mL ampicillin for selection of recombinant plasmids.
Screening and cloning of sugar transporter genes from C. glycerinogenes
The sugar transporter hidden Markov model (PF00083), built from the sugar transporter sequences of several organisms, was obtained from the Pfam database (Finn et al. 2016). The candidate hexose transporters were screened by the HMMER v3.0 tool (Eddy 2009) with this model using the genomic information of Pichia kudriavzevii (teleomorph of C. glycerinogenes). The screened candidate genes were then amplified from the genomic DNA of C. glycerinogenes with the appropriate primers (Table S2). The obtained DNA fragments were digested and inserted into the 2μ-based plasmid pYX212 using the constitutive triosephosphate isomerase (TPI) promoter to generate recombinant plasmids (Table S3, Fig. S1). The green fluorescent protein gene (GFP) fragment was cloned and inserted into the SalI and XhoI sites of pYX212, generating the recombinant plasmid pYX212-GFP. Then, the HXT gene fragments without stop codon were inserted into the BamHI and SalI sites of the plasmid pYX212-GFP for fluorescence localization analysis (Fig. S1b). For site-specific mutagenesis, CgHXT4 was separated into two fragments with a 25-bp overlapping region and amplified using designed primers to introduce the required mutations (Table S2), respectively. Subsequently, the two fragments were amplified into a complete sequence through overlap PCR (Shin et al. 2017; Xiao and Pei 2011).
Growth tests for recombinant S. cerevisiae
The recombinant plasmids containing the sugar transporter candidate genes were transformed into the hxt-null S. cerevisiae EBY.VW4000 and selected on YNB medium containing 20 g/L maltose as the sole carbon source to isolate the different recombinant S. cerevisiae (Table S1). The obtained recombinant yeasts were incubated in liquid YNB medium formulated with 20 g/L maltose. The cells were collected by centrifugation and washed twice in sterile distilled water. For drop-test assays, cell suspensions standardized to the OD600 of 1- and 10-fold serial dilutions (from 100 to 10−3) were spotted onto YNB solid medium with 20 g/L sole carbon source (glucose, fructose, mannose, or galactose) for 2.5 days at 30 °C. For liquid culture tests, the washed recombinant strains were diluted to OD600 that was 1. And then the recombinant strains were cultivated aerobically (30 °C, 100 rpm in reciprocating shake flasks) with 1% inoculation for 2 days in liquid YNB medium with 20 or 200 g/L glucose as carbon source.
Glucose and ethanol quantitation
Residual glucose and ethanol in the culture supernatants were determined by high-pressure liquid chromatography (HPLC) analysis. The samples after filtration were analyzed using an HPX-87H Aminex ion exchange column (300 × 7.8 mm; Bio-Rad) at 60 °C, eluted with 5 mM H2SO4 at a flow rate of 0.6 mL/min. A differential refractive index detector was employed (Shodex RI-101, Dionex, Japan).
Transcription analysis
C. glycerinogenes samples cultured up to the mid-exponential growth phase in YNB medium with 2, 20, and 200 g/L glucose concentration were collected. In addition, parts of the cells obtained from the medium containing 20 g/L glucose were then incubated with 20 g/L glucose YNB medium with and without supplementation of 0.5 M NaCl.
After collection of cells by centrifugation, the total RNA was extracted according to the previous protocols (Ji et al. 2014). HiScript II Q RT SuperMix for qPCR (Vazyme) was then used to synthesize cDNA from the RNA following the manufacturer’s instructions. The expression levels of CgHXT4 were determined using a Bio-Rad CFX96 Real-Time PCR system with the UltraSYBR Mixture (CWBIO), according to the kit manual. The relative transcription levels were calculated and analyzed via the 2−ΔΔCt method (Livak and Schmittgen 2001).
Fluorescence microscopy
S. cerevisiae EBY.VW400 strains harboring the HXT-GFP fusion proteins were grown in liquid YNB medium supplemented with 200 g/L glucose at 30 °C. Cells were taken at set intervals, washed with PBS, and viewed with a Leica TCS SP8 Confocal System microscope (Wetzlar, Germany).
3D structural modeling and protein sequence alignment
All the 3D models of hexose transporters were predicted and constructed using the SWISS-MODEL (http://www.swissmodel.expasy.org). The multiple protein sequence alignments were implemented with ClustalX2 (Larkin et al. 2007).
The accession numbers of nucleotide sequences
Identified hexose transporter genes from C. glycerinogenes were sequenced by Sangon Biotech (Shanghai, China) and deposited in the National Center for Biotechnology Information (NCBI). CgHXT1–12 have the GenBank accession numbers MG548725 to MG548736.
Results
Glucose consumption performance of C. glycerinogenes at high glucose concentration
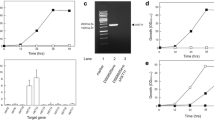
The glucose consumption, cell growth, and ethanol production of C. glycerinogenes and S. cerevisiae CEN.PK2-1C were analyzed in YPD medium formulated with 250 g/L glucose. As shown in Table S4, the maximum specific growth rate of C. glycerinogenes was about 1.3 times greater than that of S. cerevisiae CEN.PK2-1C, leading to a biomass > 46% higher than for S. cerevisiae CEN.PK2-1C at 33 h (Fig. 1). In addition, the ethanol titer and the maximum specific ethanol production rate of C. glycerinogenes were 23 and 30% higher, respectively, than those of S. cerevisiae CEN.PK2-1C (Fig. 1, Table S4). Moreover, the maximum specific glucose consumption rate of S. cerevisiae CEN.PK2-1C was only 60% that of C. glycerinogenes, resulting in 4.9-fold higher residual glucose in the former at the end of fermentation (Fig. 1, Table S4). As a result of its superior specific glucose consumption rate, C. glycerinogenes exhibited better growth and ethanol production, suggesting the existence of an efficient sugar transport system in C. glycerinogenes under HGF conditions.
Aerobic fermentation performance of C. glycerinogenes and S. cerevisiae at high glucose concentration. Residual glucose (squares), biomass (circles), and ethanol yield (triangles) of C. glycerinogenes (open) and S. cerevisiae (closed) were determined. The error bars are the standard error of the mean from three independent experiments each with triplicate samples
Identification of sugar transporters in C. glycerinogenes
Sugar transporter-like candidate genes in C. glycerinogenes were screened by the hidden Markov model (PF00083) from the genomic information of Pichia kudriavzevii (Finn et al. 2016; Sloothaak et al. 2016). Fourteen sugar transporter-like (SP) candidates with scores > 200 were selected for further investigation. Phylogenetic analysis of these 14 SP candidates, which may possess sugar transport function, is shown in Fig. S2.
To confirm and characterize the sugar transport activities and specificities of the selected SP candidates, they were complemented into S. cerevisiae EBY.VW4000, an hxt-null strain unable to grow on a variety of monosaccharides (Wieczorke et al. 1999). All the recombinant strains grew on maltose with similar growth states (Fig. 2). Five of these SP candidate proteins were identified as hexose transporters CgHxts 1, 2, and 4–6. They were able to restore the growth of S. cerevisiae EBY.VW4000 on YNB medium containing 20 g/L of the single saccharides, glucose, fructose, mannose, or galactose (Fig. 2). Complementation of S. cerevisiae EBY.VW4000 with CgHxt1 marginally recovered cell growth on all of sugars tested. CgHxt5 also showed limited ability to rescue cell growth of the recombinants on glucose and fructose, but not on mannose and galactose. In contrast, CgHxt2 and CgHxt6 largely recovered the activities on glucose, fructose, and mannose, with slightly weaker growth of the strains on galactose. Notably, S. cerevisiae EBY.VW4000 complemented with CgHxt4 that grew vigorously on glucose, fructose, and mannose, but normally on galactose.
Growth test of S. cerevisiae hxt-null mutants complemented with various sugar transporter candidate genes. The recombinants were grown on YNB plates with 20 g/L of glucose (a), fructose (b), mannose (c), galactose (d), or maltose (e). The hxt-null S. cerevisiae complemented with pYX212 was used as a control; maltose was positive control
CgHXT4 encoded a sugar transporter with greater efficiency at high glucose concentrations
We further investigated CgHxt4 and CgHxt6, which showed better abilities to rescue the growths of the S. cerevisiae mutants. Biomass and residual glucose were monitored in shake flasks containing YNB medium with 20 and 200 g/L glucose. ScHxt1 and ScHxt7, the major glucose transporters with the greatest effect in S. cerevisiae, were chosen as the controls (Kim et al. 2015; Reifenberger et al. 1997; Rossi et al. 2010). All the recombinants harboring the HXTs improved sugar transport at both glucose concentrations (Fig. 3). Glucose consumption and cell growth of recombinant S. cerevisiae EBY/CgHXT6 were lower than those of the hxt-null mutants complemented with ScHXTs (Fig. 3). In contrast, although the cell growth and glucose consumption of EBY/CgHXT4, EBY/ScHXT1, and EBY/ScHXT7 were similar at 20 g/L glucose concentration (Fig. 3a, b), S. cerevisiae EBY/CgHXT4 adapted to the high glucose (200 g/L) environment more quickly, with biomass > 38 and > 25% higher than those of EBY/ScHXT1 and EBY/ScHXT7 at 24 h (Fig. 3c). In addition, the maximum specific glucose consumption rate of S. cerevisiae EBY/CgHXT4 at 200 g/L glucose concentration was 2.22- and 1.42-fold greater than those of EBY/ScHXT1 and EBY/ScHXT7, respectively (Table S5). Accordingly, the glucose consumption of both ScHXTs complementing strains was only 80% that of EBY/CgHXT4 at the end of high glucose fermentation (Fig. 3d). It has been reported that the specific rates of growth and glucose consumption may be employed as surrogates for glucose transport capacities of cells (Wang et al. 2016; Young et al. 2011). Thus, CgHxt4 had a better ability to improve glucose consumption and cell growth than other HXTs, indicating that it was an active sugar transporter contributing to more efficient carbohydrate assimilation at high glucose concentration.
Inducible expression of CgHXT4 by osmotic pressure
To investigate inducible expression of CgHXT4 by osmotic pressure, mRNA levels of CgHXT4 in C. glycerinogenes were analyzed after growth under difference concentrations of glucose and NaCl. As shown in Fig. 4a, transcription of CgHXT4 was significantly induced with increasing glucose concentration. Notably, at high glucose (200 g/L glucose), there was much greater induction of transcription of CgHXT4 than at 20 g/L. Moreover, after incubation for 20 min with 20 g/L glucose plus 0.5 M NaCl, the expression level of CgHXT4 greatly increased relative to incubation with 20 g/L glucose alone (Fig. 4b). These results suggest that expression of CgHXT4 is induced by osmotic pressure.
Transcriptional levels of CgHXT4 under different conditions. a Transcriptional levels of CgHXT4 under different concentrations of glucose. b Transcriptional levels of CgHXT4 with or without NaCl incubation. The treatment of samples was carried out according to “Materials and methods.” mRNA expression levels were measured by qRT-PCR and normalized to 18S rDNA. The transcriptional levels relative to those of wild-type control are indicated with standard deviations of three independent experiments
The membrane stability of CgHxt4 at high extracellular glucose concentration
Hexose transporter adaptation to complex and changing environments is regulated by mRNA expression levels and also by ubiquitin-mediated endocytosis. This causes hexose transporters to be removed from the membrane under certain fermentation conditions (Kruckeberg et al. 1999). To visualize membrane stability of CgHxt4, we compared subcellular localization of CgHxt4 and ScHXTs tagged with fluorescence protein during culture with 200 g/L glucose (Malinska et al. 2003). All the HXT-GFP fusion proteins were expressed and localized in the cell membrane during the whole course of culture (Fig. 5). Obvious endocytosis of ScHxt7 was observed at an early stage of fermentation (3 h). The results showed that ScHxt1 and CgHxt4 were both much more stable on the membrane than ScHxt7 at up to 12 h. Further, fluorescence in the cytoplasm of EBY/ScHXT1-GFP was relatively more intensive than that of EBY/CgHXT4-GFP at the same stage of fermentation. During the late phase of fermentation, all three transporters showed significant degradation under high glucose concentration, although CgHxt4 was the least affected. In addition, a prediction of protein ubiquitination sites through UbPred (http://www.ubpred.org) revealed that the numbers and associated confidence of N-terminal ubiquitinated residues of CgHxt4 were relatively lower than those of ScHxt1 and ScHxt7 (Table S6). These results suggest that membrane stability of CgHxt4 is significantly better than for ScHxt1 and ScHxt7.
Site-specific mutagenesis at the N329 of CgHxt4
The YYX(T/P) motif has been identified as a “gate” controlling the activity and specificity of sugar transporters (Wang et al. 2016). Amino acid sequence alignment of HXTs from C. glycerinogenes and S. cerevisiae showed that asparagine N329 from CgHxt4 occupied the fourth amino acid of the YYX(T/P) conserved motif, unlike other transporters (Fig. S3). Several CgHxt4 variants with site-specific mutagenesis replacing N329 were created to explore the relationship between amino acid properties and sugar transport efficiency. As shown in Fig. 6a, b, at 200 g/L glucose concentration, cell growth and glucose consumption of strains that complemented with the mutant CgHxt4s were all weaker than for the wild type. The N329F and N329W single mutations of CgHxt4 removed the sugar transport function. Relative to other CgHxt4 mutants, those containing N329A, N329G, and N329K showed a longer lag period, although they still supported transport of glucose. Threonine (T) and proline (P), the usual amino acids at position 4 of the YYX(T/P) motif, both supported relatively high glucose transport capacity when replacing N329 in CgHxt4, but cell growth and glucose consumption of the recombinants containing N329T and N329P were < 80% of the native strain. In addition, CgHxt4-N329D and CgHxt4-N329Q showed efficiencies of sugar transport similar to CgHxt4-N329T and CgHxt4-N329P. None of the site-directed mutations had much effect on membrane localization and stability of transporters (Fig. 6c). These results suggest that the N329 in CgHxt4 is vital to high glucose transport efficiency and a factor in the dominant sugar transport capacity of CgHxt4.
Improvement of ethanol production by overexpressing CgHXT4
Since CgHxt4 exhibited better performance under high glucose concentration, CgHXT4 was overexpressed in S. cerevisiae CEN.PK2-1C using the vector pYX212. ScHxt1, a high efficiency glucose transporter in S. cerevisiae, was overexpressed as a control. As shown in Fig. 7, from the beginning of fermentation, CEN/CgHXT4 showed higher cell growth, glucose consumption, and ethanol production rates. The glucose consumption of both strains was correlated with ethanol titer. After further cultivation to 32 h, cell growth and glucose consumption of CEN/CgHXT4 were respectively about 5 and 16% higher than those of the control. Ethanol titer was increased by over 17%. These results further indicate that CgHxt4 is a high-activity glucose transporter and shows potential application for improving cell growth and product synthesis under HGF conditions.
Discussion
As the first step in the biological fermentation process, sugar transport in yeast depends on the expression and activity of hexose transporters (Boles and Hollenberg 1997; Özcan and Johnston 1999). The 14 SP candidates we identified in the osmophilic yeast C. glycerinogenes shared some conserved structures of the sugar porter family (Leandro et al. 2009). However, only five SP candidates showed the ability to recover sugar uptake in a knock-out strain. The other proteins might be a sugar transport cooperator (Reis et al. 2013) or transporters for glycerol, gluconate, and inositol, which have structures similar to hexose transporters (Duskova et al. 2015; Heiland et al. 2000; Pereira et al. 2014).
Cell growth and glucose consumption analyses indicated that CgHxt4 is a more efficient hexose transporter than other HXTs from C. glycerinogenes and S. cerevisiae. The activity of sugar transporters is closely related to certain important sequences, including G-G/F-XXX-G, YFFYY, and YYX(T/P) (Li et al. 2016; Wang et al. 2017; Young et al. 2014). The highly conserved YYX(T/P) motif located in the extracellular end of transmembrane helix 7 (TM7) has been identified as a “gate” for the solvent-accessible channel which plays an important role in controlling the activity and specificity of sugar transporters (Wang et al. 2016). Notably, the fourth amino acid of the YYX(T/P) motif in CgHxt4 is asparagine (Fig. S3) and wild-type CgHxt4 showed the highest efficiency of glucose transport relative to all the tested variants. It is possible that large and nonpolar residues (Fig. S4 a), such as phenylalanine and tryptophan, may create steric exclusion and obstruct entry of glucose to the solvent-accessible channel (Wang et al. 2016). In contrast, the hydrophilic side chains of some of other amino acids can interact with glucose. Most CgHxt4 mutants with N329 replaced by a hydrophilic amino acid showed relatively high glucose transport efficiencies. Nevertheless, hydrophilic residues with too-long or too-short side chains at N329 of CgHxt4 permitted only limited glucose transport (Fig. S4 b and c). A hydrophilic amino acid residue of appropriate size may avoid any steric hindrance and facilitate better hydrophilic interactions between the residue and sugar molecules. The slightly longer hydrophilic group of asparagine in CgHxt4 is in particular more likely to form hydrophilic interaction with the hydroxyls on glucose molecules, thus promoting their entry into the solvent-accessible channel (Fig. S4 e). Although asparagine and glutamine have very similar properties, there is an extra carbon in the backbone of glutamine. This extra carbon seems to cause a steric hindrance, which may be responsible for decreasing glucose uptake (Fig. S4 d).
In addition, membrane stability of hexose transporters can greatly influence the constancy of sugar transport in the fermentation process (Shin et al. 2017). Ubiquitin-mediated endocytosis is the most important recognized mechanism regulating membrane stability of hexose transporters. Ubiquitin is typically linked to the target protein by covalent bonding to a lysine residue (Finley et al. 2012). Studies have shown that for many hexose transporters, the target lysine residues for ubiquitination are located near the N-terminus (Nijland et al. 2016; Roy et al. 2014; Shin et al. 2017). Fluorescence localization analysis confirmed that CgHxt4 was more stable than ScHxt1 or ScHxt7 in the plasma membrane. This is consistent with predictions that CgHxt4 has fewer ubiquitination sites (and those are of lower confidence) than the S. cerevisiae transporters (Table S6).
In addition to characterization of hexose transporters, transcriptional studies were used to elucidate the relationship between CgHXT4 and the efficient sugar assimilation in C. glycerinogenes at high glucose concentrations. The expression of most hexose transporters is tightly regulated by conditions including glucose concentration/starvation, osmotic pressure, and the physiological state of the cells (Greatrix and van Vuuren 2006). Similar to ScHXT1 in S. cerevisiae, transcription of CgHXT4 is highly induced by elevated concentrations of glucose or NaCl (Greatrix and van Vuuren 2006). Previous studies have shown that expression of ScHXT1 is also activated by sugar-induced osmotic stress, which required the coordinated activities of HOG1 (Tomas-Cobos et al. 2004). Moreover, CgHOG1 plays an important role in cell tolerance and excessive glycerol synthesis in C. glycerinogenes (Ji et al. 2016). The absence of CgHOG1 significantly inhibited cell growth and glycerol overproduction in C. glycerinogenes at high glucose concentrations. Hence, the expression of CgHXT4 is also likely to be regulated by CgHOG1.
Taken together, a set of hexose transporters from C. glycerinogenes were characterized and five candidates identified as active substrate transporters, of which CgHxt4 was the most effective glucose transporter. Further, the expression of CgHXT4 was significantly induced by sugar-induced osmotic stress. The slightly longer hydrophilic side chain of N329 in the conserved motif and the better membrane stability might be responsible for its high transport efficiency. At high glucose concentrations, overexpression of CgHXT4 also led to a significant increase in glucose consumption, cell growth, and ethanol production compared with ScHXT1. The results presented here provide new information about the excellent substrate transport and ethanol synthesis abilities of C. glycerinogenes under high glucose conditions. Identification and characterization of five CgHxts provide a deeper understanding of the potential mechanisms of hexose transport and suggest further applications of genetic engineering to improve substrate transport and fermentation performance under HGF conditions.
References
Arshad M, Hussain T, Iqbal M, Abbas M (2017) Enhanced ethanol production at commercial scale from molasses using high gravity technology by mutant S. cerevisiae. Braz J Microbiol 48(3):403–409. https://doi.org/10.1016/j.bjm.2017.02.003
Boles E, Hollenberg CP (1997) The molecular genetics of hexose transport in yeasts. FEMS Microbiol Rev 21(1):85–111. https://doi.org/10.1111/j.1574-6976.1997.tb00346.x
Chao B, Liu R, Zhang X, Zhang X, Tan T (2017) Tannin extraction pretreatment and very high gravity fermentation of acorn starch for bioethanol production. Bioresour Technol 241:900–907. https://doi.org/10.1016/j.biortech.2017.06.026
Dimarco AA, Romano AH (1985) D-glucose transport system of Zymomonas mobilis. Appl Environ Microbiol 49(1):151–157
Duskova M, Ferreira C, Lucas C, Sychrova H (2015) Two glycerol uptake systems contribute to the high osmotolerance of Zygosaccharomyces rouxii. Mol Microbiol 97(3):541–559. https://doi.org/10.1111/mmi.13048
Eddy SR (2009) A new generation of homology search tools based on probabilistic inference. Genome Inform 23(1):205–211
Finley D, Ulrich HD, Sommer T, Kaiser P (2012) The ubiquitin-proteasome system of Saccharomyces cerevisiae. Genetics 192(2):319–360. https://doi.org/10.1534/genetics.112.140467
Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, Potter SC, Punta M, Qureshi M, Sangrador-Vegas A, Salazar GA, Tate J, Bateman A (2016) The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res 44(D1):D279–D285. https://doi.org/10.1093/nar/gkv1344
Greatrix BW, van Vuuren HJ (2006) Expression of the HXT13, HXT15 and HXT17 genes in Saccharomyces cerevisiae and stabilization of the HXT1 gene transcript by sugar-induced osmotic stress. Curr Genet 49(4):205–217. https://doi.org/10.1007/s00294-005-0046-x
Hairu J, Huiying F, Jian Z (2003) By-product formation by a novel glycerol-producing yeast, Candida glycerinogenes, with different O2 supplies. Biotechnol Lett 25:311–314. https://doi.org/10.1023/A:1022349401575
Heiland S, Radovanovic N, Höfer M, Winderickx J, Lichtenberg H (2000) Multiple hexose transporters of Schizosaccharomyces pombe. J Bacteriol 182(8):2153–2162. https://doi.org/10.1128/JB.182.8.2153-2162.2000
Ji H, Lu X, Wang C, Zong H, Fang H, Sun J, Zhuge J, Zhuge B (2014) Identification of a novel HOG1 homologue from an industrial glycerol producer Candida glycerinogenes. Curr Microbiol 69(6):909–914. https://doi.org/10.1007/s00284-014-0670-0
Ji H, Zhuge B, Zong H, Lu X, Fang H, Zhuge J (2016) Role of CgHOG1 in stress responses and glycerol overproduction of Candida glycerinogenes. Curr Microbiol 73(6):827–833. https://doi.org/10.1007/s00284-016-1132-7
Kim D, Song JY, Hahn JS (2015) Improvement of glucose uptake rate and production of target chemicals by overexpressing hexose transporters and transcriptional activator Gcr1 in Saccharomyces cerevisiae. Appl Environ Microbiol 81(24):8392–8401. https://doi.org/10.1128/AEM.02056-15
Kruckeberg AL, Ye L, Berden JA, Van DK (1999) Functional expression, quantification and cellular localization of the Hxt2 hexose transporter of Saccharomyces cerevisiae tagged with the green fluorescent protein. Biochem J 339(2):299–307. https://doi.org/10.1042/0264-6021:3390299
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23(21):2947–2948. https://doi.org/10.1093/bioinformatics/btm404
Leandro MJ, Fonseca C, Goncalves P (2009) Hexose and pentose transport in ascomycetous yeasts: an overview. FEMS Yeast Res 9(4):511–525. https://doi.org/10.1111/j.1567-1364.2009.00509.x
Li H, Schmitz O, Alper HS (2016) Enabling glucose/xylose co-transport in yeast through the directed evolution of a sugar transporter. Appl Microbiol Biotechnol 100(23):10215–10223. https://doi.org/10.1007/s00253-016-7879-8
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC T method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Malinska K, Malinsky J, Opekarova M, Tanner W (2003) Visualization of protein compartmentation within the plasma membrane of living yeast cells. Mol Biol Cell 14(11):4427–4436. https://doi.org/10.1091/mbc.E03-04-0221
Nijland JG, Shin HY, Boender LGM, de Waal PP, Klaassen P, Driessen AJM (2017) Improved xylose metabolism by a CYC8 mutant of Saccharomyces cerevisiae. Appl Environ Microbiol 83(11):e00095–e00017. https://doi.org/10.1128/AEM.00095-17
Nijland JG, Vos E, Shin HY, de Waal PP, Klaassen P, Driessen AJ (2016) Improving pentose fermentation by preventing ubiquitination of hexose transporters in Saccharomyces cerevisiae. Biotechnol Biofuels 9:158. https://doi.org/10.1186/s13068-016-0573-3
Özcan S, Johnston M (1999) Function and regulation of yeast hexose transporters. Microbiol Mol Biol Rev 63(3):554–569
Pereira I, Madeira A, Prista C, Loureirodias MC, Leandro MJ (2014) Characterization of new polyol/H+ symporters in Debaryomyces hansenii. PLoS One 9(2):e88180. https://doi.org/10.1371/journal.pone.0088180
Reifenberger E, Boles E, Ciriacy M (1997) Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur J Biochem 245(2):324–333. https://doi.org/10.1111/j.1432-1033.1997.00324.x
Reis TFD, Menino JF, Bom VLP, Brown NA, Colabardini AC, Savoldi M, Goldman MHS, Rodrigues F, Goldman GH (2013) Identification of glucose transporters in Aspergillus nidulans. PLoS One 8(11):e81412. https://doi.org/10.1371/journal.pone.0081412
Rossi G, Sauer M, Porro D, Branduardi P (2010) Effect of HXT1 and HXT7 hexose transporter overexpression on wild-type and lactic acid producing Saccharomyces cerevisiae cells. Microb Cell Factories 9:15. https://doi.org/10.1186/1475-2859-9-15
Roy A, Kim YB, Cho KH, Kim JH (2014) Glucose starvation-induced turnover of the yeast glucose transporter Hxt1. Biochim Biophys Acta 1840(9):2878–2885. https://doi.org/10.1016/j.bbagen.2014.05.004
Sen A, Acosta-Sampson L, Alvaro CG, Ahn JS, Cate JH, Thorner J (2016) Internalization of heterologous sugar transporters by endogenous α-arrestins in the yeast Saccharomyces cerevisiae. Appl Environ Microbiol 82(24):7074–7085. https://doi.org/10.1128/AEM.02148-16
Shin HY, Nijland JG, Waal PP, Driessen AJM (2017) The amino-terminal tail of Hxt11 confers membrane stability to the Hxt2 sugar transporter and improves xylose fermentation in the presence of acetic acid. Biotechnol Bioeng 114(9):1937–1945. https://doi.org/10.1002/bit.26322
Sloothaak J, Tamayo-Ramos JA, Odoni DI, Laothanachareon T, Derntl C, Mach-Aigner AR, Martins Dos Santos VA, Schaap PJ (2016) Identification and functional characterization of novel xylose transporters from the cell factories Aspergillus niger and Trichoderma reesei. Biotechnol Biofuels 9:148. https://doi.org/10.1186/s13068-016-0564-4
Tanino T, Ito T, Ogino C, Ohmura N, Ohshima T, Kondo A (2012) Sugar consumption and ethanol fermentation by transporter-overexpressed xylose-metabolizing Saccharomyces cerevisiae harboring a xyloseisomerase pathway. J Biosci Bioeng 114(2):209–211. https://doi.org/10.1016/j.jbiosc.2012.03.004
Tomas-Cobos L, Casadome L, Mas G, Sanz P, Posas F (2004) Expression of the HXT1 low affinity glucose transporter requires the coordinated activities of the HOG and glucose signalling pathways. J Biol Chem 279(21):22010–22019. https://doi.org/10.1074/jbc.M400609200
Wang C, Bao X, Li Y, Jiao C, Hou J, Zhang Q, Zhang W, Liu W, Shen Y (2015) Cloning and characterization of heterologous transporters in Saccharomyces cerevisiae and identification of important amino acids for xylose utilization. Metab Eng 30:79–88. https://doi.org/10.1016/j.ymben.2015.04.007
Wang C, Li Y, Qiu C, Wang S, Ma J, Shen Y, Zhang Q, Du B, Ding Y, Bao X (2017) Identification of important amino acids in Gal2p for improving the L-arabinose transport and metabolism in Saccharomyces cerevisiae. Front Microbiol 8:1391. https://doi.org/10.3389/fmicb.2017.01391
Wang M, Yu C, Zhao H (2016) Identification of an important motif that controls the activity and specificity of sugar transporters. Biotechnol Bioeng 113(7):1460–1467. https://doi.org/10.1002/bit.25926
Wieczorke R, Krampe S, Weierstall T, Freidel K, Hollenberg CP, Boles E (1999) Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett 464(3):123–128. https://doi.org/10.1016/S0014-5793(99)01698-1
Xiao YH, Pei Y (2011) Asymmetric overlap extension PCR method for site-directed mutagenesis. Methods Mol Biol 687:277–282. https://doi.org/10.1007/978-1-60761-944-4_20
Xie T, Fang HY, Zhuge B, Zhuge J (2009) Promotional mechanism of high glycerol productivity in the aerobic batch fermentation of Candida glycerinogenes after feeding several amino acids. Appl Biochem Microbiol 45(3):303–308. https://doi.org/10.1134/S0003683809030119
Yongguang Z, Wei S, Zhiming R, Huiying F, Jian Z (2007) Deletion of the CgTPI gene encoding triose phosphate isomerase of Candida glycerinogenes inhibits the biosynthesis of glycerol. Curr Microbiol 55(2):147–151. https://doi.org/10.1007/s00284-007-0070-9
Young E, Poucher A, Comer A, Bailey A, Alper H (2011) Functional survey for heterologous sugar transport proteins, using Saccharomyces cerevisiae as a host. Appl Environ Microbiol 77(10):3311–3319. https://doi.org/10.1128/AEM.02651-10
Young EM, Tong A, Bui H, Spofford C, Alper HS (2014) Rewiring yeast sugar transporter preference through modifying a conserved protein motif. Proc Natl Acad Sci U S A 111(1):131–136. https://doi.org/10.1073/pnas.1311970111
Zhang Q, Nurhayati CC-L, Lo Y-C, Nagarajan D, Hu J, Chang J-S, Lee D-J (2017) Ethanol production by modified polyvinyl alcohol-immobilized Zymomonas mobilis and in situ membrane distillation under very high gravity condition. Appl Energy 202:1–5. https://doi.org/10.1016/j.apenergy.2017.05.105
Zhang Q, Wu D, Lin Y, Wang X, Kong H, Tanaka S (2015a) Substrate and product inhibition on yeast performance in ethanol fermentation. Energy Fuel 29(2):1019–1027. https://doi.org/10.1021/ef502349v
Zhang W, Cao Y, Gong J, Bao X, Chen G, Liu W (2015b) Identification of residues important for substrate uptake in a glucose transporter from the filamentous fungus Trichoderma reesei. Sci Rep 5:13829. https://doi.org/10.1038/srep13829
Zhuge J, Fang HY, Wang ZX, Chen DZ, Jin HR, Gu HL (2001) Glycerol production by a novel osmotolerant yeast Candida glycerinogenes. Appl Microbiol Biotechnol 55(6):686–692. https://doi.org/10.1007/s002530100596
Zong H, Zhang C, Zhuge B, Lu X, Fang H, Sun J (2016) Effects of xylitol dehydrogenase (XYL2) on xylose fermentation by engineered Candida glycerinogenes. Biotechnol Appl Biochem 64(4):590–599. https://doi.org/10.1002/bab.1514
Acknowledgements
We thank Prof. Eckhard Boles for providing us the S. cerevisiae strain EBY.VW4000. This work was supported by the Collaborative Innovation Center of Jiangsu Modern Industrial Fermentation.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 31570052, 31601456, 21708016). This work was supported by national first-class discipline program of Light Industry Technology and Engineering (LITE2018-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(DOCX 1700 kb)
Rights and permissions
About this article
Cite this article
Liang, Z., Liu, D., Lu, X. et al. Identification and characterization from Candida glycerinogenes of hexose transporters having high efficiency at high glucose concentrations. Appl Microbiol Biotechnol 102, 5557–5567 (2018). https://doi.org/10.1007/s00253-018-9027-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9027-0