Abstract
Saccharomyces cerevisiae contains a family of 17 hexose transporter (HXT) genes; only nine have assigned functions, some of which are still poorly defined. Despite extensive efforts to characterize the hexose transporters, the expression of HXT6 and HXT8-17 remains an enigma. In nature, S. cerevisiae finds itself under extreme nutritional conditions including sugars in excess of 40% (w/v), depletion of nutrients and extremes of both temperature and pH. Using HXT promoter–lacZ fusions, we have identified novel conditions under which the HXT17 gene is expressed; HXT17 promoter activity is up-regulated in media containing raffinose and galactose at pH 7.7 versus pH 4.7. We demonstrated that HXT5, HXT13 and, to a lesser extent, HXT15 were all induced in the presence of non-fermentable carbon sources. HXT1 encodes a low-affinity transporter and in short-term osmotic shock experiments, HXT1 promoter activity was reduced when cells were exposed to media containing 40% glucose. However, we found that the HXT1 mRNA transcript was stabilized under conditions of osmotic stress. Furthermore, the stabilization of HXT1 mRNA does not appear to be gene specific because 30 min after transcriptional arrest there is a fourfold more mRNA in osmotically stressed versus non-stressed yeast cells. A large portion of S. cerevisiae mRNA molecules may, therefore, have a decreased rate of turnover during exposure to osmotic stress indicating that post-transcriptional regulation plays an important role in the adaptation of S. cerevisiae to osmotic stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucose import into the yeast cell is facilitated by a group of membrane-spanning proteins, termed hexose transporters (HXT). There are at least 20 members of the yeast hexose transporter family (HXT1 to HXT17, SNF3, RGT2 and GAL2), as identified by genetic studies and/or sequence homology (for reviews, see Bisson et al. 1993; Kruckeberg 1996; Boles and Hollenberg 1997; Ozcan and Johnston 1999). All the HXT gene products, with the exception of Hxt12p, are able to support growth on glucose when expressed individually in a strain deleted for all 20 transporter genes (Wieczorke et al. 1999), indicating all the HXT genes encode functional glucose transporters. HXT1, HXT2, HXT3 and HXT4 are the best characterized members of the HXT family. The presence of multiple hexose transporters with differing affinities for glucose is reasonable given that Saccharomyces cerevisiae is able to grow in an extensive range of sugar concentrations (0.1 to >40% w/v).
HXT1 encodes a low-affinity transporter that is maximally expressed in the presence of high levels of extracellular glucose (>1% w/v or 56 mM) (Ozcan and Johnston 1995). HXT1 was originally isolated as a multicopy suppressor of a high-affinity glucose transport defect in snf3Δ cells (Lewis and Bisson 1991) and later as a suppressor of a potassium transport defect in trk1Δtrk2Δ cells (Ko et al. 1993). Early studies revealed that HXT1 expression was maximal during lag and early-exponential phases of growth (Lewis and Bisson 1991). HXT1 expression increased during exposure to osmotic stress caused by salt (1.0 M NaCl), sorbitol (1.5 M) (Hirayama et al. 1995) or high sugar (40% w/v) (Erasmus et al. 2003). Induction of HXT1 by osmotic stress is dependent on the high osmolarity glycerol (HOG) pathway (Rep et al. 2000). It has been proposed that HOG1-dependent HXT1 expression provides additional glucose for the synthesis of glycerol, a compatible solute that accumulates during conditions of osmotic stress (Hirayama et al. 1995).
HXT2 and HXT4 are high-affinity transporters. Expression of HXT2 and HXT4 is increased approximately 5 to 20-fold in cells growing in the presence of low glucose [~0.1% (w/v) or 5.6 mM] versus cells grown either in the absence of glucose or in the presence of high glucose (Ozcan and Johnston 1995). Like HXT1, HXT2 and HXT4 were cloned as multicopy suppressors of the high-affinity glucose uptake defect in snf3Δ mutants (Kruckeberg and Bisson 1990). HXT2 and HXT4 are maximally expressed at low concentrations [~0.1% (w/v) or 5.6 mM] of glucose (Ozcan and Johnston 1995). Rgt1p represses the expression of HXT2 and HXT4 in the absence of glucose. However, these genes have an additional level of regulation by Mig1p and Snf1p that limits their expression to low concentrations of glucose. Indeed HXT4 has a K m for glucose of 6.2–9.0 mM, whereas the corresponding K m values reported for HXT2 are 1.5–2.9 mM (Reifenberger et al. 1997; Maier et al. 2002).
HXT3 is a low-affinity glucose transporter that was originally identified along with HXT1 as a suppressor of a potassium transport defect in trk1Δtrk2Δ cells (Ko et al. 1993). HXT3 is also a multicopy suppressor of the snf3Δ growth defect on raffinose (Ko et al. 1993; Theodoris et al. 1994). Raffinose is a trisaccharide composed of galactose–glucose–fructose. Raffinose serves as a low source of fermentable carbon as the glucose–fructose bond can be gradually hydrolyzed by invertase. HXT3 promoter activity is constitutive in the presence of glucose but is independent of sugar concentration (Ozcan and Johnston 1995). In the absence of glucose, HXT3 is repressed by Rgt1p (Ozcan and Johnston 1995). HXT3 expression reaches maximal levels upon entry into stationary phase (Ko et al. 1993).
HXT5 encodes a functional hexose transporter with moderate affinity for glucose (K m=10 mM) that is maximally expressed under conditions that cause slow growth (Diderich et al. 2001; Verwaal et al. 2002). For example, in batch cultures increases in temperature or osmolarity, as well as growth in the presence of ethanol or glycerol or a depletion of glucose, all induce the expression of HXT5 (Verwaal et al. 2002). Microarray data have also identified HXT5 as inducible by increased temperature (Gasch et al. 2000) or osmolarity (Gasch et al. 2000; Posas et al. 2000; Rep et al. 2000; Yale and Bohnert 2001; Erasmus et al. 2003). The induction of HXT5 when glucose is depleted is a function of growth rate and is independent of glucose derepression because in exponentially growing hxk2Δ cells in the presence of glucose, HXT2 and HXT4 are derepressed, while HXT5 expression is not detected.
HXT6 and HXT7 exist in tandem on chromosome IV, separated by approximately 3.5 kb, and are 1.5 kb downstream of HXT3. Hxt6p and Hxt7p are highly related, differing by only two amino acids over the entire 570 amino acid sequence (Reifenberger et al. 1995). Neither of the differing amino acids appears conserved within the hexose transporter family (Boles and Hollenberg 1997). Of the characterized transporters from this family (HXT1-7), HXT6 and HXT7 have the highest affinity for glucose, with a K m value of approximately 1.0 mM (Reifenberger et al. 1997; Maier et al. 2002). In wild-type strains, the expression of HXT7 is repressed in the presence of high concentrations of glucose, but increases as glucose is depleted (Ye et al. 2001). Interestingly, despite their high sequence similarity, HXT6 and HXT7 appear to be regulated independently. The expression of HXT7 is much higher than that of HXT6 in wild-type strains under similar growth conditions (Reifenberger et al. 1997; Diderich et al. 1999). Furthermore, HXT6, in addition to being regulated by the general glucose repression pathway, responds to a novel signal transduction pathway involving Snf3p (Liang and Gaber 1996). More specifically, the maintenance of HXT6 glucose repression is dependent on SNF3 expression even when glucose is abundant.
To date, little is known about the regulation of HXT8-16. HXT11 is capable of restoring glucose uptake in a rag1Δ strain of K. lactis (Nourani et al. 1997). HXT11 and HXT9 were also identified as targets for the transcriptional activator Pdr3p (for pleiotropic drug resistance) (Nourani et al. 1997). Deletion of HXT11 and/or HXT9 confers resistance to cycloheximide (protein synthesis inhibitor), sulfomethuron methyl (aceto-lactate synthase inhibitor) or 4-nitroquinoline-N-oxide (mutagen). This is interesting given that when expressed individually, Hxt9p and Hxt11p are functional glucose carriers (Wieczorke et al. 1999), and yet their expression is entirely independent of extracellular glucose. Rather, their expression is linked to a transcription activator that also regulates proteins that confer drug resistance.
HXT17 was identified by a microarray experiment as a target of a constitutively active form of the Mac1p transcription factor (Gross et al. 2000). Mac1p regulates the expression of high-affinity copper uptake genes under copper-deficient conditions (Jungmann et al. 1993). However, when cells were treated with a copper-specific chelator to mimic copper limitation, HXT17 was not induced, indicating the effect may have been due to a property of the specific MAC1 mutant allele.
Regulatory components of HXT gene expression: Rgt1p, Grr1p, Mth1p and Std1p
RGT1 encodes a DNA-binding protein that serves as both an activator and a repressor of HXT gene expression. In the absence of glucose, Rgt1p represses HXT1–HXT4; addition of glucose to the media causes inhibition of Rgt1p activity and subsequent derepression of various HXT genes (Ozcan and Johnston 1995). Repression of transcription by Rgt1p requires the general transcriptional repressors Ssn6p and Tup1p (Ozcan et al. 1996). Glucose-mediated inhibition of Rgt1p activity requires Grr1p. Interestingly, Rgt1p is required for both repression and activation of HXT1 gene expression. In rgt1Δ cells, HXT1 is expressed in the absence of glucose, but does not reach maximum expression levels in the presence of high amounts of glucose (2% w/v) (Ozcan and Johnston 1995). Recently it was shown that Rgt1p becomes hyperphosphorylated in response to high concentrations of glucose (4% w/v) (Mosley et al. 2003). Hyperphosphorylation is required for converting the protein to an activator because phosphorylation was abolished in snf3Δ, rgt2Δ and grr1Δ mutants, and induction of HXT1 expression is also lacking in these strains.
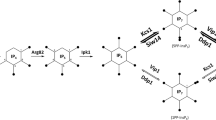
GRR1 (glucose repression resistant) encodes an F-box protein associated with the ubiquitin proteolysis machinery (Li and Johnston 1997). GRR1 expression is required for HXT gene expression, and this is due to the requirement of Grr1p for Rgt1p inactivation. The glucose repression defect of grr1Δ strains is therefore indirect, as cells are unable to transport any amount of glucose (Vallier et al. 1994; Ozcan and Johnston 1995). It has been proposed that Grr1p regulates Rgt1p activity by targeting the protein for degradation (Ozcan and Johnston 1999). However, a recent report suggests an intermediate, Mth1p, links these two proteins (Flick et al. 2003). MTH1 and the closely related STD1 are two genes that are important for the proper regulation of HXT gene expression (Schmidt et al. 1999; Schulte et al. 2000); mth1Δstd1Δ cells express HXT1-4 even in the absence of glucose (Schmidt et al. 1999). Both Mth1p and Std1p are able to interact with the membrane-bound glucose sensors Rgt2p and Snf3p (Schmidt et al. 1999; Lafuente et al. 2000), and both proteins localize to the membrane and the nucleus, making them good candidates as transducers of the glucose signal that activates or derepresses transcription (Schmidt et al. 1999). Indeed, it was recently established that the phosphorylation and dissociation of Rgt1p from HXT promoters is mediated by Grr1p-dependent degradation of Mth1p (Flick et al. 2003). Three modes of transcriptional regulation of HXT transporters by different levels of glucose are summarized in Fig. 1.
Three models of transcriptional regulation of the yeast hexose transporters by different concentrations of glucose. Arrows represent positive regulation and blunt-ended lines represent negative regulation. (Adapted from Ozcan and Johnston 1999). LGS low glucose, HGS high glucose
In this study we examined the transcriptional regulation of yeast hexose transporter genes, with a particular emphasis on the response of HXT genes to conditions that may be encountered during wine fermentations rather than laboratory conditions. Our primary focus was on HXT transcription in the presence of 0.2–40% (w/v) glucose or fructose, as well as the effect of anaerobiosis, changing pH, osmotic pressure and glucose starvation on HXT mRNA levels. We identified novel conditions under which the HXT17 gene is expressed; HXT17 promoter activity is up-regulated in media containing raffinose and galactose at pH 7.7 versus pH 4.7. We demonstrated that HXT5, HXT13 and, to a lesser extent, HXT15 were all induced in the presence of non-fermentable carbon sources. Furthermore, HXT1 mRNA transcripts were stabilized in a 40% glucose-containing medium. Global mRNA decay was reduced in osmotically stressed yeast cells and a large portion of S. cerevisiae mRNA molecules has a decreased rate of turnover during exposure to osmotic stress. Yeast cells therefore seem to adapt to osmotic stress by regulating transcription as well as mRNA turnover.
Materials and methods
Strains, plasmids and media
The yeast strains used in this study are listed in Table 1. Yeast cells were grown on standard media: YPD [1% (w/v) yeast extract (Difco), 2% (w/v) Bacto peptone (Difco), 2% (w/v) dextrose] or YNB [0.67% (w/v) yeast nitrogen base without amino acids or ammonium sulfate (Difco) supplemented with the appropriate amino acids, 0.5% (w/v) ammonium sulfate and containing raffinose/galactose (1.5:0.5% w/v) or glucose or fructose (0.2–40% (w/v) as indicated]. HXT promoters (0.6–1.8 kb DNA fragments generated by restriction enzyme digests) were fused to lacZ in the vector YEp357R (Ozcan and Johnston 1995, 1999). Plasmids are shown in Table 2.
Yeast transformations and β-galactosidase assays
Individual HXT–lacZ plasmids (Table 2) were transformed into S. cerevisiae TCY1 cells using the high-efficiency lithium acetate method (Gietz and Schiestl 1995). Transformed cells were inoculated into lacZ medium (final density A 600 nm 0.05) and grown with shaking at 30°C until an OD of A 600 nm of 0.6–0.8 was reached. β-Galactosidase (β-gal) assays were performed as described previously (Ausubel 1995), except that 2 or 10 ml of cell culture was analyzed instead of 25 ml. Each sample was analyzed in duplicate within the experiment, and the entire experiment was performed in triplicate. β-gal activity values are reported in Miller units (Miller 1972).
RNA isolation
Total RNA was isolated using the hot phenol method (Ausubel 1995). For real-time PCR analyses, an additional step was required during isolation to remove contamination by genomic DNA. This was performed using Qiagen RNeasy kits (catalogue # 74104), with the Rnase-free DNase kit (catalogue # 79254) using the manufacturer’s recommended protocols (available at http://www.qiagen.com).
Real-time PCR
cDNA synthesis from 2.0 μg of total RNA was performed using the Omniscript RT Kit (Qiagen) according to the manufacturer’s suggested protocol. The reverse transcription reaction was primed using random hexamer oligonucleotides at a final concentration of 2.5 μM. The final cDNA product was dissolved in DEPC-treated H2O to a final volume of 500 μl.
Real-time PCR was performed using the SYBR Green PCR Master Mix (Applied Biosystems) according to the manufacturer’s standard protocol, except that the final reaction volume was reduced to 20 μl. Gene-specific oligonucleotide primers (Table 3) were used at a final concentration of 0.5 μM. PCR reactions were performed in an ABI Prism® 7000 Sequence Detector (Applied Biosystems) with the following conditions: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. All samples were assayed in triplicate for each gene, and differences in cDNA synthesis efficiency were corrected for by normalizing all expression values to constitutively expressed 18S rRNA.
Determination of water activity (a w)
Water activity measurements were carried out in triplicate, using an Aqualab Series 3 water activity meter (Decagon Devices, Pullman, WA, USA). The values reported represent the mean of three assays.
Osmotic shock assays
TCY1 cells transformed with HXT1–lacZ were grown in 300 ml YNB (galactose 2% w/v) to mid-log phase and then centrifuged at 9,000g for 10 min at room temperature. The supernatant was discarded, and the cells were resuspended in 6 ml sterile dH2O. Flasks containing 50 ml of YNB media plus the indicated carbon or salt were inoculated with 1 ml (approximately 1.6×107 cells) of the transformants and then incubated at 30°C. After 4 h, 10 ml of each culture was harvested in duplicate and analyzed for β-galactosidase expression. The experiment was performed at least three times using independent cultures.
RNA stability assays
For these studies the yeast strain Y260 was employed. Y260 cells bear a temperature-sensitive mutation in an essential RNA polymerase II protein, Rpb1p, and rapidly arrest mRNA transcription upon shifting to the non-permissive temperature (Nonet et al. 1987). A 1 l volume of YPD was inoculated with Y260 cells at an initial cell density of A 600 nm=0.2 and grown at 25°C until an A 600 nm 1.0 was reached (~11 h). The cells were harvested at 9,000g for 5 min at room temperature. The supernatant was discarded, and the cell pellet was resuspended in 4 ml sterile YPD media, pre-warmed to 37°C (the non-permissive temperature). Seven 50 ml volumes of YPD, containing either 2% glucose (w/v) or 40% glucose (w/v) as the carbon source, were warmed to 37°C and then added to Y260 cell pellets to yield a final concentration of approximately 3×107 cells/ml. All flasks were immediately placed in a water bath at 37°C with shaking. In addition, one flask was immediately centrifuged after inoculation, the supernatant was discarded and the cells were quickly frozen by placing the tube in ethanol at −80°C. This marked the zero-minute time point. For each time point (15, 30 and 60 min) the entire 50 ml culture was harvested by centrifugation, and the cell pellet was frozen in the same manner and stored at −80°C until RNA extractions were performed.
For quantifying mRNA as a percentage of total RNA, poly-adenylated molecules were purified from 1 mg DNase-treated total RNA using the Qiagen Oligotex mRNA Midi kit (catalogue # 70042). Total RNA and mRNA were quantified by measuring the absorbance at 260 nm. Experiments were repeated three times.
Statistical analyses
A two-way analysis of variance (ANOVA) was used to evaluate the effect of carbon source and sugar concentration on HXT promoter activities and for comparing mRNA levels after transcriptional arrest in low and high-sugar media. Differences in promoter activity were analyzed using a Fisher’s least significant difference (LSD) test (P=0.05). Statistics were calculated using Minitab software (release 14, Minitab Inc., USA).
Results
Effect of glucose or fructose on HXT promoter activity at concentrations ranging between 0.2 and 40% (w/v)
To advance our understanding of the regulation of yeast hexose transporter gene expression, we tested whether increased sugar concentrations (up to 40% w/v) or specific carbon sources (fructose vs. glucose) affected HXT expression. As an initial screen, we prepared X-gal plates containing either glucose or fructose from 0.2 to 40% (w/v) glucose or fructose as carbon source. We incubated TCY1 cells transformed with one of HXT1-5 and HXT10-17 promoter–lacZ fusions on these plates for several days but observed no significant difference between glucose and fructose-grown cells. In addition, the only HXT promoters that exhibited activity under these conditions were HXT1-5 and HXT13 (data not shown). To examine the promoter activity of these genes more closely, we assayed liquid-grown cultures of TCY1 transformed with HXT1-5, HXT13–lacZ for β-gal activity. HXT1–lacZ expression increased almost linearly with increasing sugar concentrations up to 30% (w/v) (R 2=0.8662, Fig. 2a). There was, however, no statistical difference in HXT1 promoter activity in cells grown in 30 versus 40% glucose or fructose. HXT2 promoter activity was increased in cells exposed to sugar concentrations at or below 2% (w/v). Interestingly, induction of HXT2 was twofold higher with 2% fructose compared to 2% glucose (Fig. 2b). HXT3 was expressed across all sugar concentrations (Fig. 2c), while HXT4 activity was found to be highest at 0.2% (w/v) glucose and fructose (Fig. 2d), in agreement with previous observations (Ozcan and Johnston 1995). Surprisingly both HXT3 and HXT4 had a spike of promoter activity in cells grown in 40% glucose or fructose (w/v). HXT5 promoter activity remained relatively unchanged in both glucose and fructose-grown cultures until the sugar concentration exceeded 20% (w/v). Increasing the sugar concentration from 20 to 40% induced the HXT5 promoter approximately fivefold (Fig. 2e). HXT13–lacZ was moderately induced in response to low (<2%) glucose and fructose (Fig. 2f). Similar to HXT2, there appeared to be a differential induction of HXT13 between glucose and fructose at very low sugar concentrations (0.2%). It is important to note that the cells grown in the 0.2% glucose or fructose media had likely reached carbon starvation conditions at the time of harvest. Indeed these cells entered stationary phase at a lower cell density (not shown).
Promoter activity of HXT1 (a), HXT2 (b), HXT3 (c), HXT4 (d), HXT5 (e) and HXT13 (f) in response to various concentrations of extracellular glucose or fructose. Strain TCY1 was grown in YNB media with the carbon source as indicated. At mid-log phase, cells were harvested and assayed for β-gal activity. Results shown represent the mean of three experiments. Error bars represent one standard deviation. Means with the same letter are not significantly different (Fisher’s LSD a=871.8, b=57.5, c=267.7, d=22.4, e=55.2, f=43.9, P=0.05)
Effect of pH on HXT17 promoter activity
During growth on X-gal plates containing raffinose and galactose as the carbon source (1.5:0.5% w/v), there appeared to be an increase in HXT17 promoter activity when compared to cells grown on 2% glucose (w/v) (data not shown). Initial attempts to quantify this induction using β-gal assays in liquid-grown cultures were unsuccessful. However, one key difference between plate and liquid β-gal assays is that the X-gal plates are buffered to have a neutral pH, whereas liquid YNB media has a pH of 4.5–4.7. After adjusting the pH of the YNB media with KOH, we observed that HXT17 promoter activity in cultures grown in pH 7.7 versus 4.7 (using raffinose and galactose as the carbon source) was approximately 12-fold higher (Fig. 3).
HXT17 is expressed in cells grown with raffinose and galactose (1.5:0.5% w/v) as the carbon source at pH 7.7 versus 4.7. Strain TCY1 was grown in YNB media containing raffinose and galactose with the pH adjusted to 4.7 or 7.7. At mid-log phase, cells were harvested and assayed for β-gal activity. Results shown are the mean of three experiments. Error bars represent one standard deviation
HXT5, HXT13 and HXT15 are expressed during growth on ethanol or glycerol and ethanol together
After prolonged incubation of X-gal plates containing low amounts of glucose or fructose we observed slight induction of HXT5, HXT13 and HXT15–lacZ (data not shown). We hypothesized that these genes may be induced in response to glucose starvation and hence tested the expression level of these genes in cells grown on the non-fermentable carbon source ethanol alone (2% v/v) or in combination with glycerol (2% v/v each). As seen in Fig. 4, HXT5, HXT13 and to a lesser extent HXT15 are up-regulated during growth on ethanol and glycerol versus 2% glucose (ethanol data is similar to that of the ethanol/glycerol combination and is therefore not shown). We also tested glycerol alone, but TCY1 cells were unable to grow under this condition. Because HXT15 was expressed much lower than the other two transporters, the physiological significance of this observation is uncertain.
HXT5, HXT13 and HXT15 are expressed in cells grown in YNB media containing glucose (2% w/v) or ethanol and glycerol (2% v/v each). TCY1 cells were grown in YNB media containing the indicated carbon source (ethanol EtOH, glycerol Gly). At mid-log phase, cells were harvested and assayed for β-gal activity. Results shown are the mean of three experiments. Error bars represent one standard deviation
Reduction of HXT1 promoter activity by high glucose (40% w/v) and 1.4 M NaCl
HXT1 promoter activity is up-regulated by osmotic shock in media containing NaCl (0.7–1.0 M) or sorbitol (0.95–1.5 M) as osmolyte (Hirayama et al. 1995; Rep et al. 2000). The water activity of media containing 1.4 M NaCl is approximately 0.952 (Erasmus et al. 2003). This is similar to the water activity of 40% glucose (w/v) which we found to be approximately 0.939. To compare the effects of NaCl and 40% glucose (w/v) on HXT1 promoter activity, we designed an experiment to test the effect of a short-term osmotic shock on HXT1–lacZ induction. Short-term stress exposure reflects the immediate response, rather than long-term adaptation to a stressor, and is therefore distinct from experiments like those shown in Fig. 2. As seen in Fig. 5a, HXT1 was highly induced by 2% glucose (3,500 Miller units); 1.4 M NaCl (8% w/v) in the presence of glucose activated the HXT1 promoter to the same extent as 40% glucose (250 Miller units). Unexpectedly, the level of promoter activation was ~14-fold higher in 2% glucose compared to 40% glucose (Fig. 5a). These data indicated that osmotic shock actually reduces the HXT1 promoter activity. Galactose (2% w/v) and 2% galactose plus 1.4 M NaCl failed to activate the HXT1 promoter (Fig. 5b).
Short-term osmotic shock-induced HXT1 expression requires glucose but decreases HXT1 promoter activity relative to non-stressed cells. a Osmotic shock reduced the HXT1 promoter activity. TCY1 cells were transformed with an HXT1–lacZ construct and grown in YNB media containing 2% galactose (w/v) as the carbon source. At mid-log phase cells were harvested and inoculated into media containing either 2% glucose (with or without 8% NaCl) or 40% glucose (w/v). After 4 h cells were harvested and analyzed for β-gal activity. b Glucose is required for HXT1 promoter activation in response to osmotic shock. TCY1 cells were prepared as described in a, but inoculated into YNB media containing glucose (2% w/v) or galactose (2% w/v), in the presence or absence of 8% NaCl (w/v). The results shown are the mean of three experiments. Error bars represent one standard deviation
Effect of high glucose concentrations on HXT1 mRNA stability
Previous studies (northern blotting and/or DNA microarrays) have indicated that HXT1 mRNA levels are increased in response to NaCl or sorbitol (Hirayama et al. 1995; Rep et al. 2000). However, our results indicated that the HXT1 promoter activity was reduced by osmotic shock. To reconcile these observations, we hypothesized that for HXT1 mRNA to accumulate under osmotic stress despite the promoter being down-regulated, the turnover of the molecule must be decreased. To address this possibility, the yeast strain Y260, bearing the temperature-sensitive rpb1-1 allele was used. This conditional RNA polymerase mutant rapidly ceases mRNA synthesis at the non-permissive temperature (Nonet et al. 1987). S. cerevisiae Y260 was shifted to the non-permissive temperature in rich media containing either 2% glucose or 40% glucose (w/v) as the carbon source. Total RNA was harvested at various time points following cessation of transcription, and the level of HXT1 mRNA was assayed by real-time PCR. As confirmation of the functionality of the rpb1-1 allele, the cells that were shifted to 37°C failed to double and did not appear to arrest at any particular stage of the cell cycle (data not shown), both of which are characteristics of the Y260 strain (Nonet et al. 1987). As expected, in 2% glucose (w/v) the level of HXT1 mRNA declined immediately upon shifting to the non-permissive temperature and had diminished approximately 16-fold after 1 h at 37°C (Fig. 6a). However, in the presence of 40% (w/v) glucose there was only a threefold decline in HXT1 mRNA over the same time period. If transcription occurred at the non-permissive temperature, one would expect to see an increase in GPD1 mRNA levels since GPD1 is up-regulated within 10 min of exposure to osmotic stress (Rep et al. 1999a, b), and GPD1 expression increases greater than 30-fold in response to salt-induced osmotic stress (Rep et al. 2000). We included GPD1 as a positive control and did not observe a significant increase in GPD1 mRNA above the initial levels. Moreover, the level of GPD1 mRNA was maintained at a higher level in cells exposed to 40% glucose versus 2% glucose (Fig. 6b), indicating that this transcript also seems to be stabilized by osmotic stress. Finally, IPP1, which encodes the inorganic pyrophosphatase enzyme and is not transcriptionally regulated by osmotic stress (Rep et al. 1999a), was included as negative control; its mRNA decayed at a similar rate in both 2 and 40% glucose-treated cells as was expected (Fig. 6c).
Several distinct transcripts are stabilized by osmotic stress. Y260 cells were grown to mid-log phase and then shifted to the non-permissive temperature to inactivate the rpb1-1 allele. At the time intervals indicated after the temperature shift, the cells were harvested and mRNA levels were quantified by real-time PCR. a HXT1, b GPD1 and c IPP1. Error bars represent one standard deviation from three experiments
Osmotic stress enhances retention of mRNA molecules after termination of transcription in yeast cells
Due to the observation that HXT1 and GPD1 are both stabilized by osmotic stress, we investigated whether other transcripts may be stabilized by low water activity stress. As a reflection of mRNA levels, we purified poly-adenylated molecules from total RNA derived from Y260 cells after 30 min at the non-permissive temperature in either low (2% w/v) or high (40% w/v) glucose. As seen in Fig. 7, there was a fourfold reduction in cellular mRNA (as a percentage of total RNA) from cells grown at the permissive temperature to cells in 2% (w/v) glucose for 30 min at the non-permissive temperature. Remarkably, the decrease in mRNA was negligible for osmotically stressed cells at the non-permissive temperature.
Global mRNA decay is reduced in osmotically stressed yeast cells. Poly-adenylated mRNA molecules were purified from Dnase-treated total RNA derived from Y260 cells in mid-log phase at the permissive temperature or after 30 min at the non-permissive temperature with or without glucose-induced osmotic stress. mRNA levels are expressed as a percentage of total cellular RNA. The results represent the mean of three experiments and the error bars represent one standard deviation
Discussion
Effect of glucose or fructose on HXT promoter activity at concentrations of 0.2–40% (w/v)
For the HXT genes that have been characterized thus far the primary determinant of expression level is the extracellular glucose concentration. Each transporter protein has a unique affinity for glucose (and fructose), with higher affinity transporters being expressed at the lowest concentrations of glucose and vice versa. Despite the fact that S. cerevisiae can readily metabolize glucose at extracellular concentrations of less than 0.1% to greater than 40% (w/v), the transcriptional regulation of yeast HXT genes has only been tested up to 8% glucose (w/v). We hypothesized that the HXT genes with previously unknown function may be transcribed only at high (>10% w/v) sugar concentrations since S. cerevisiae is regularly exposed to such conditions in nature or during industrial fermentations. Furthermore previous studies have focused primarily on glucose-mediated regulation of HXT genes. Therefore, using HXT promoter–lacZ fusion constructs, we tested the promoter activity of HXT1-5 and HXT10-17 in glucose and fructose concentrations ranging from 0.2 to 40% (w/v). In agreement with previous observations (Ozcan and Johnston 1995), we showed that HXT2 and HXT4 are expressed when extracellular sugar concentrations are <2% (Fig. 2). Furthermore, HXT3 is expressed constitutively, and HXT1 is induced in the presence of >1% glucose or fructose. With the exception of HXT2, no differences in promoter activation were observed between glucose-grown and fructose-grown cells.
It has been reported that hxt2Δ strains of S. cerevisiae are prone to sluggish fermentations (cited in Bisson 1999). Because stuck fermentations typically arrest with 1.5–2.0% (w/v) fructose and only 0.1–0.5% glucose, it was interesting that the transcriptional activation of HXT2 at these levels was approximately twofold higher in fructose-grown cells than glucose-grown cells. However, two observations indicate that mis-regulation of HXT2 alone is unlikely to cause stuck fermentations. First, only one functional transporter is required to support growth on glucose (Wieczorke et al. 1999). Second, by deleting individual HXT genes in an industrial strain of S. cerevisiae, it was shown that HXT2 is involved in growth initiation during wine fermentations when the extracellular sugar concentration was 20% (Luyten et al. 2002). This observation implies that Hxt2p is not required at the end of fermentation when glucose and fructose concentrations are limited or depleted, and therefore mis-regulation of HXT2 is likely not associated with the residual fructose remaining in stuck fermentations.
Taken together, our results suggest that HXT1, HXT3 and HXT5 appear to be the major transporters at high sugar concentrations, making them most relevant during early stages of wine fermentations. Conversely, HXT2, HXT4 and HXT13 are expressed at low concentrations of extracellular glucose or fructose, suggesting these transporters could be important at the end of wine fermentations when only trace amounts of sugars remain. These observations do not entirely agree with the report of Luyten et al. (2002), which describes the role of individual transporters during the course of a wine fermentation, and further emphasizes the complexity of gene regulation of the HXT gene family. It could be that combinations of factors, including nitrogen availability, and/or ethanol concentration affect the in vivo performance of these transporter proteins. Alternatively, there may be protein–protein interactions among different transporters in vivo that are necessary for proper function and these interactions may affect the activity of these proteins when they are studied in isolation (Diderich et al. 1999; Ozcan and Johnston 1999).
Effect of pH on HXT17 promoter activity
S. cerevisiae is able to proliferate in a wide range of external pHs. Growth is optimal at pH 4–5; the typical pH during wine fermentations ranges from 3 to 4. Growth in more alkaline conditions elicit a stress response in S. cerevisiae, and the transcriptional response to alkaline pH is at least partially mediated by Rim101p (Lamb et al. 2001). We observed an induction of HXT17 promoter activity in response to a shift from pH 4.7 to 7.7 in a low-glucose environment (Fig. 3). The transcriptional response of S. cerevisiae to alkaline conditions has not been widely studied, although DNA microarray data have revealed that as many as 500 genes have altered expression in response to increasing extracellular pH (Causton et al. 2001; Lamb et al. 2001; Serrano et al. 2002). HXT4 was induced within 5 min of shifting from pH 6.4 to pH 7.6 in rich media containing 2% glucose (Serrano et al. 2002). Additionally the expression levels of HXT8, HXT9, HXT11 and HXT12 were repressed at least threefold by these conditions. The significance of pH-dependent HXT expression is unclear; however, it is possible that individual transporters have altered affinities for glucose depending on the local proton concentration. The predicted isoelectric point for the alkaline-induced genes HXT4 and HXT17 is 6.37 and 6.95, respectively, indicating a net-negative charge at pH 7.6 or 7.7. Conversely, the isoelectric points for alkaline-repressed HXT9, HXT11 and HXT12 are 8.17, 8.54, and 8.48, respectively, indicating these transporters would have a net-positive charge. However, HXT8, which was reported as alkaline repressed with a pI of 5.32 (the lowest of all HXT genes), fails to support the pattern. Still, considering that it is only the extracellular portions of these transporter proteins that are exposed to changing pH, an examination of the net charge of these domains may prove interesting.
Based on recent reports, there appears to be a link between alkaline pH tolerance and iron and copper uptake. More specifically, several mutants that displayed a severe sensitivity to alkali were shown to be defective in gene products that are required for efficient copper and iron uptake (Serrano et al. 2004). Interestingly, the expression of high-affinity copper uptake genes is regulated by Mac1p (Jungmann et al. 1993), and a constitutively active allele of this protein MAC1 up1 was shown to increase the expression of HXT17 more than twofold (Gross et al. 2000). However, it should be pointed out that when the same group used a copper chelator to mimic copper starvation (when MAC1-dependent genes are transcriptionally active), HXT17 was only marginally induced (1.1–1.4 fold increased). Whether copper (or iron) uptake is involved in the transcriptional activation of HXT17 remains to be seen. An interesting hypothesis would be that HXT17 serves as a symporter for particular minerals (presumably copper and iron) along with glucose. Given that HXT9 and HXT11 were identified in a screen for multidrug resistance (Nourani et al. 1997) and HXT1 and HXT3 are suppressors of a potassium transport defect (Ko et al. 1993), it is conceivable that many of the HXT genes have additional (non-hexose) substrates.
HXT5, HXT13 and HXT15 are expressed during growth on ethanol or glycerol and ethanol together
The expression of HXT5 is linked to growth rate in S. cerevisiae and previous studies have shown that cells growing on glycerol or ethanol exhibit increased HXT5 expression relative to glucose-grown cells (Diderich et al. 2001; Verwaal et al. 2002). We observed an induction of HXT5 when yeast cells were grown on non-fermentable carbon sources and also saw increased HXT13 and HXT15 expressions under the same conditions (Fig. 4). HXT5 does not appear to be regulated by the glucose sensors Snf3p/Rgt2p (Verwaal et al. 2002) which is expected, given that many of the conditions that induce HXT5 (low nitrogen, osmotic stress, heat stress) act independently of the extracellular glucose concentration. Our observations suggest that HXT13 may be regulated in a similar manner. Although HXT15 appeared to be induced on non-fermentable carbon sources, the low level of expression suggests this transporter may not be physiologically relevant under these conditions. Hxt5p has a moderate affinity for glucose (K m=10 mM) but no such data are available for Hxt13p. Our data show that HXT13 is induced by 0.2% glucose (w/v) indicating it may be a high-affinity transporter, similar to HXT2, HXT4 and HXT6/7. If this is the case, then Hxt5p and Hxt13p could serve as low- and high-affinity transporters, respectively, for the cell under conditions of glucose starvation. Further analysis of the promoters of HXT5 and HXT13 as well as a detailed biochemical study of the physical properties of these transporter proteins should help address this point. For example, a study of Hxt5p and Hxt13p protein turnover rates would be interesting because highly stable proteins would be ideally expressed under starvation conditions when transcriptional and translational activities are likely to be minimal.
HXT1 and GPD1 mRNA is stabilized by glucose-induced osmotic stress
Others and we have observed that HXT1 is up-regulated in response to osmotic stress (Hirayama et al. 1995; Rep et al. 1999b; Erasmus et al. 2003). Our observation that HXT1 transcription is increased more than threefold in cells grown in 40% glucose versus 10% glucose (Fig. 5) is counter-intuitive given that the growth rate of yeast is reduced by the osmotic stress of 40% glucose. Because the glycolytic flux decreases concomitantly with the growth rate, there should already be sufficient glucose to meet cellular requirements at the level of 10%. To better understand HXT1 promoter regulation, we compared the effect of osmotic shock caused by 40% glucose or 2% glucose with 8% NaCl. Both these media have similar water activity and therefore should inflict comparable osmotic stress upon yeast cells. The original intention was to delineate the extent of HXT1 induction that was attributable to glucose and to osmotic stress. Using the same HXT1–lacZ construct, Ozcan and Johnston (1995) reported that HXT1 reached maximal expression in cells grown in 4% glucose and remained constant up to 8%. We therefore suspected that the stress-dependent component of HXT1 expression would require at least 10% glucose. First we observed that salt-induced osmotic stress could only activate the HXT1 promoter in the presence of glucose (Fig. 5b). Rgt1p is a transcriptional repressor that restricts HXT1 expression to glucose-containing environments (Ozcan et al. 1996). Since Hog1p is required for HXT1 mRNA accumulation during osmotic stress (Rep et al. 1999b), our observation suggests that the HOG pathway is unable to overcome repression by Rgt1p. Furthermore, this result also indicates that the HOG pathway does not act directly on the HXT1 promoter. Alternatively, osmotic stress activates a second activator or repressor on the HXT1 promoter that acts in concert with Rgt1p (analogous to the dual repression of HXT2 and HXT4 by Rgt1p and Mig1p that restricts expression to low-glucose conditions). A recent report has confirmed this hypothesis, as the transcriptional repressor Sko1p was shown to bind and repress the HXT1 promoter in the absence of Hog1p kinase activity (Tomas-Cobos et al. 2004). As little as 4% glucose or 2% glucose plus NaCl (0.4 M) was able to induce Sko1p derepression. This explains why other groups observed maximal HXT1 promoter induction in cells grown in 4% glucose (Ozcan and Johnston 1995). Comparison of the HXT1 promoter activity during short-term osmotic shock in 2% glucose versus 40% glucose revealed that HXT1 was down-regulated by osmotic stress (Fig. 5a). These data are distinct from data shown in Fig. 2, since it reveals the immediate response of the promoter to the stress, rather than long-term adaptation of cells in the presence of 40% glucose as shown in Fig. 2. In order for mRNA molecules to accumulate despite lower promoter activity, the rate of turnover must be decreased. Indeed, when we tested the rate of decay of HXT1 mRNA we found that the transcript persisted at higher levels after transcription shut-off during osmotic stress by 40% glucose compared to 2% glucose (Fig. 6).
Previous studies have demonstrated both derepression and activation mechanisms lead to HXT1 expression (Ozcan and Johnston 1995; Tomas-Cobos et al. 2004). Here we show that there is a third component to the regulation—mRNA stabilization by osmotic stress. Given that HXT1 was not induced by osmotic stress with galactose as the carbon source, our data and data provided by others suggest two factors contributing to the high level of HXT1 mRNA accumulation during osmotic stress: (1) glucose-dependent promoter activation (Ozcan and Johnston 1999) and (2) osmotic stress-dependent mRNA stabilization. At this time we cannot predict what signal leads to mRNA stabilization under osmotic stress, but if there is a signal transduction pathway it does not require de novo transcription of a stabilizing factor, as HXT1 mRNA was stabilized in the absence of ongoing transcription.
The paradigm for the transcriptional response to osmotic stress is increased GPD1 expression (Rep et al. 1999a). In our study we found GPD1 mRNA to be stabilized by sugar-induced osmotic stress to a similar extent as HXT1 (Fig. 6b). An intriguing explanation for these observations is that the osmotic stress-mediated stabilization of HXT1 and GPD1 could occur to ensure an adequate supply of carbon for the production of glycerol, a compatible solute that is rapidly accumulated in osmotically stressed yeast cells (Blomberg and Adler 1992; Hohmann 2002).
As a reflection of cellular mRNA levels we isolated poly-adenylated molecules from yeast cells that had been without active transcription for 30 min in the presence of either 2 or 40% glucose. As a percentage of total cellular RNA, we observed a fourfold greater retention of poly-adenylated molecules in osmotically stressed yeast cells compared to non-stressed cells after transcription shut-off (Fig. 7). This implies that a large portion of S. cerevisiae mRNA molecules may have a decreased rate of turnover during exposure to osmotic stress. A similar observation has been made in glucose-starved yeast cells (Jona et al. 2000), indicating that it may be part of a general stress response. The same group also noticed that mRNA molecules were stabilized upon entry into stationary phase—and entry into stationary phase may be induced by stress exposure (Werner-Washburne et al. 1993; Rossignol et al. 2003). If this is true, then other stresses, to which yeast is exposed such as ethanol, should reduce mRNA turnover. Although this has not been demonstrated for S. cerevisiae, ethanol can stabilize TNFα mRNA in a p38-dependent manner in rat liver cells (Kishore et al. 2001, 2004).
References
Ausubel FM (1995) Short protocols in molecular biology: a compendium of methods from current protocols in molecular biology, 3rd edn. Wiley, New York, chapter 13
Bisson LF (1999) Stuck and sluggish fermentations. Am J Enol Vitic 50:107–119
Bisson LF, Coons DM, Kruckeberg AL, Lewis DA (1993) Yeast sugar transporters. Crit Rev Biochem Mol Biol 28:259–308
Blomberg A, Adler L (1992) Physiology of osmotolerance in fungi. Adv Microb Physiol 33:145–212
Boles E, Hollenberg CP (1997) The molecular genetics of hexose transport in yeasts. FEMS Microbiol Rev 21:85–111
Brachmann CB et al (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14:115–132
Causton HC et al (2001) Remodeling of yeast genome expression in response to environmental changes. Mol Biol Cell 12:323–337
Diderich JA et al (1999) Glucose uptake kinetics and transcription of HXT genes in chemostat cultures of Saccharomyces cerevisiae. J Biol Chem 274:15350–15359
Diderich JA, Schuurmans JM, Van Gaalen MC, Kruckeberg AL, Van Dam K (2001) Functional analysis of the hexose transporter homologue HXT5 in Saccharomyces cerevisiae. Yeast 18:1515–1524
Erasmus DJ, van der Merwe GK, van Vuuren HJJ (2003) Genome-wide expression analyses: metabolic adaptation of Saccharomyces cerevisiae to high sugar stress. FEMS Yeast Res 3:375–399
Flick KM et al (2003) Grr1-dependent inactivation of Mth1 mediates glucose-induced dissociation of Rgt1 from HXT gene promoters. Mol Biol Cell 14:3230–3241
Gasch AP et al (2000) Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell 11:4241–4257
Gietz RD, Schiestl RH (1995) Transforming yeast with DNA. Methods Mol Cell Biol 5:255–269
Gross C, Kelleher M, Iyer VR, Brown PO, Winge DR (2000) Identification of the copper regulon in Saccharomyces cerevisiae by DNA microarrays. J Biol Chem 275:32310–32316
Hirayama T, Maeda T, Saito H, Shinozaki K (1995) Cloning and characterization of seven cDNAs for hyperosmolarity-responsive (HOR) genes of Saccharomyces cerevisiae. Mol Gen Genet 249:127–138
Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev 66:300–372
Jona G, Choder M, Gileadi O (2000) Glucose starvation induces a drastic reduction in the rates of both transcription and degradation of mRNA in yeast. Biochim Biophys Acta 1491:37–48
Jungmann J et al (1993) Mac1, a nuclear regulatory protein related to Cu-dependent transcription factors is involved in Cu/Fe utilization and stress resistance in yeast. EMBO J 12:5051–5056
Kishore R, McMullen MR, Nagy LE (2001) Stabilization of tumor necrosis factor alpha mRNA by chronic ethanol: role of A + U-rich elements and p38 mitogen-activated protein kinase signaling pathway. J Biol Chem 276:41930–41937
Kishore R, McMullen MR, Cocuzzi E, Nagy LE (2004) Lipopolysaccharide-mediated signal transduction: stabilization of TNF-alpha mRNA contributes to increased lipopolysaccharide-stimulated TNF-alpha production by Kupffer cells after chronic ethanol feeding. Comp Hepatol 3(Suppl 1):S31
Ko CH, Liang H, Gaber RF (1993) Roles of multiple glucose transporters in Saccharomyces cerevisiae. Mol Cell Biol 13:638–648
Kruckeberg AL (1996) The hexose transporter family of Saccharomyces cerevisiae. Arch Microbiol 166:283–292
Kruckeberg AL, Bisson LF (1990) The HXT2 gene of Saccharomyces cerevisiae is required for high-affinity glucose transport. Mol Cell Biol 10:5903–5913
Lafuente MJ, Gancedo C, Jauniaux JC, Gancedo JM (2000) Mth1 receives the signal given by the glucose sensors Snf3 and Rgt2 in Saccharomyces cerevisiae. Mol Microbiol 35:161–172
Lamb TM, Xu W, Diamond A, Mitchell AP (2001) Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J Biol Chem 276:1850–1856
Lewis DA, Bisson LF (1991) The HXT1 gene product of Saccharomyces cerevisiae is a new member of the family of hexose transporters. Mol Cell Biol 11:3804–3813
Li FN, Johnston M (1997) Grr1 of Saccharomyces cerevisiae is connected to the ubiquitin proteolysis machinery through Skp1: coupling glucose sensing to gene expression and the cell cycle. EMBO J 16:5629–5638
Liang H, Gaber R (1996) A novel signal transduction pathway in Saccharomyces cerevisiae defined by Snf3-regulated expression of HXT6. Mol Biol Cell 7:1953–1966
Luyten K, Riou C, Blondin B (2002) The hexose transporters of Saccharomyces cerevisiae play different roles during enological fermentation. Yeast 19:713–726
Maier A, Volker B, Boles E, Fuhrmann GF (2002) Characterisation of glucose transport in Saccharomyces cerevisiae with plasma membrane vesicles (countertransport) and intact cells (initial uptake) with single Hxt1, Hxt2, Hxt3, Hxt4, Hxt6, Hxt7 or Gal2 transporters. FEMS Yeast Res 2:539–550
van der Merwe GK, van Vuuren HJJ, Cooper TG (2001) Cis-acting sites contributing to expression of divergently transcribed DAL1 and DAL4 genes in S. cerevisiae: a word of caution when correlating cis-acting sequences with genome-wide expression analyses. Curr Genet 39:156–165
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor
Mosley AL, Lakshmanan J, Aryal BK, Ozcan S (2003) Glucose-mediated phosphorylation converts the transcription factor Rgt1 from a repressor to an activator. J Biol Chem 278:10322–10327
Nonet M, Scafe C, Sexton J, Young R (1987) Eucaryotic RNA polymerase conditional mutant that rapidly ceases mRNA synthesis. Mol Cell Biol 7:1602–1611
Nourani A, Wesolowski-Louvel M, Delaveau T, Jacq C, Delahodde A (1997) Multiple-drug-resistance phenomenon in the yeast Saccharomyces cerevisiae: involvement of two hexose transporters. Mol Cell Biol 17:5453–5460
Ozcan S, Johnston M (1995) Three different regulatory mechanisms enable yeast hexose transporter (HXT) genes to be induced by different levels of glucose. Mol Cell Biol 15:1564–1572
Ozcan S, Johnston M (1999) Function and regulation of yeast hexose transporters. Microbiol Mol Biol Rev 63:554–569
Ozcan S, Leong T, Johnston M (1996) Rgt1p of Saccharomyces cerevisiae, a key regulator of glucose-induced genes, is both an activator and a repressor of transcription. Mol Cell Biol 16:6419–6426
Posas F, Chambers JR, Heyman JA, Hoeffler JP, de Nadal E, Arino J (2000) The transcriptional response of yeast to saline stress. J Biol Chem 275:17249–17255
Reifenberger E, Freidel K, Ciriacy M (1995) Identification of novel HXT genes in Saccharomyces cerevisiae reveals the impact of individual hexose transporters on glycolytic flux. Mol Microbiol 16:157–167
Reifenberger E, Boles E, Ciriacy M (1997) Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur J Biochem 245:324–333
Rep M, Albertyn J, Thevelein JM, Prior BA, Hohmann S (1999a) Different signalling pathways contribute to the control of GPD1 gene expression by osmotic stress in Saccharomyces cerevisiae. Microbiology 145:715–727
Rep M et al (1999b) Osmotic stress-induced gene expression in Saccharomyces cerevisiae requires Msn1p and the novel nuclear factor Hot1p. Mol Cell Biol 19:5474–5485
Rep M, Krantz M, Thevelein JM, Hohmann S (2000) The transcriptional response of Saccharomyces cerevisiae to osmotic shock. Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J Biol Chem 275:8290–8300
Rossignol T, Dulau L, Blondin B (2003) Genome-wide analysis of yeast gene expression during wine fermentation. Yeast 20:S314–S314
Schmidt MC et al (1999) Std1 and Mth1 proteins interact with the glucose sensors to control glucose-regulated gene expression in Saccharomyces cerevisiae. Mol Cell Biol 19:4561–4571
Schulte F, Wieczorke R, Hollenberg CP, Boles E (2000) The HTR1 gene is a dominant negative mutant allele of MTH1 and blocks Snf3- and Rgt2-dependent glucose signaling in yeast. J Bacteriol 182:540–542
Serrano R, Ruiz A, Bernal D, Chambers JR, Arino J (2002) The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Mol Microbiol 46:1319–1333
Serrano R, Bernal D, Simon E, Arino J (2004) Copper and iron are the limiting factors for growth of the yeast Saccharomyces cerevisiae in an alkaline environment. J Biol Chem 279:19698–19704
Theodoris G, Fong NM, Coons DM, Bisson LF (1994) High-copy suppression of glucose transport defects by HXT4 and regulatory elements in the promoters of the HXT genes in Saccharomyces cerevisiae. Genetics 137:957–966
Tomas-Cobos L, Casadome L, Mas G, Sanz P, Posas F (2004) Expression of the HXT1 low-affinity glucose transporter requires the coordinated activities of the HOG and glucose signalling pathways. J Biol Chem 279:22010–22019
Vallier LG, Coons D, Bisson LF, Carlson M (1994) Altered regulatory responses to glucose are associated with a glucose transport defect in grr1 mutants of Saccharomyces cerevisiae. Genetics 136:1279–1285
Verwaal R, Paalman JW, Hogenkamp A, Verkleij AJ, Verrips CT, Boonstra J (2002) HXT5 expression is determined by growth rates in Saccharomyces cerevisiae. Yeast 19:1029–1038
Werner-Washburne M, Braun E, Johnston GC, Singer RA (1993) Stationary-phase in the yeast Saccharomyces cerevisiae. Microbiol Rev 57:383–401
Wieczorke R, Krampe S, Weierstall T, Freidel K, Hollenberg CP, Boles E (1999) Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett 464:123–128
Yale J, Bohnert HJ (2001) Transcript expression in Saccharomyces cerevisiae at high salinity. J Biol Chem 276:15996–16007
Ye L, Berden JA, van Dam K, Kruckeberg AL (2001) Expression and activity of the Hxt7 high-affinity hexose transporter of Saccharomyces cerevisiae. Yeast 18:1257–1267
Acknowledgments
We thank Mark Johnston for providing the HXT–lacZ plasmids, Richard Young for donating the Y260 yeast strain and Russ Morris of the University of British Columbia Media group for preparing artwork. This research was supported by an NSERC grant 217271–99 to HJJVV.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Hohmann
Rights and permissions
About this article
Cite this article
Greatrix, B.W., van Vuuren, H.J.J. Expression of the HXT13, HXT15 and HXT17 genes in Saccharomyces cerevisiae and stabilization of the HXT1 gene transcript by sugar-induced osmotic stress. Curr Genet 49, 205–217 (2006). https://doi.org/10.1007/s00294-005-0046-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-005-0046-x