Abstract
In recent years, a variety of genetic tools have been developed and applied to various filamentous fungi, which are widely applied in agriculture and the food industry. However, the low efficiency of gene targeting has for many years hampered studies on functional genomics in this important group of microorganisms. The emergence of CRISPR/Cas9 genome-editing technology has sparked a revolution in genetic research due to its high efficiency, versatility, and easy operation and opened the door for the discovery and exploitation of many new natural products. Although the application of the CRISPR/Cas9 system in filamentous fungi is still in its infancy compared to its common use in E. coli, yeasts, and mammals, the deep development of this system will certainly drive the exploitation of fungal diversity. In this review, we summarize the research progress on CRISPR/Cas9 systems in filamentous fungi and finally highlight further prospects in this area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Filamentous fungi are a large and diverse group of eukaryotes which are ubiquitous in nature and closely associated with humans on many levels (Thrane et al. 2007). On one hand, filamentous fungi play important roles in the food, agricultural, and pharmaceutical industries. For instance, due to their high protein secretion ability and presence of complex post-translational processing, certain filamentous fungi such as Aspergillus niger, Aspergillus oryzae, and Trichoderma reesei, which are regarded as safe by the US Food and Drug Administration, are widely utilized to produce various enzymes and other useful proteins (Ward 2012). On the other hand, because of the considerable economic value of many of their metabolites, filamentous fungi also have been applied in the production of antibiotics, organic acids, pigments, polyunsaturated fatty acids (PUFAs), and so on (Xu et al. 2015; Dufossé et al. 2014; Ji et al. 2014). Conversely, some filamentous fungi such as Fusarium spp. and Aspergillus spp. can produce toxic substances and can thus contaminate food, or infect crops and even humans, which would result in incalculable economic losses (Yu et al. 2005; Lecellier et al. 2015; Harris et al. 2016; Table 1). Therefore, in order to control the adverse effects of filamentous fungi and make full use of their favorable aspects to create more economic value, it is necessary to fully understand their genetic and molecular-biological information and reconstruct them effectively.
Genetic engineering technologies opened the door for basic biological research in filamentous fungi. Since Mishra and Tatum (1973) first reported the successful DNA transformation of the filamentous fungus Neurospora crassa, the development of molecular-biological research in filamentous fungi has been so rapid. Up until now, a variety of technical methods, encompassing RNA interference, gene targeting, in vitro transposon tagging, heterologous expression, and gene knockout, have been developed to investigate and exploit the biosynthetic and regulatory mechanisms of filamentous fungi (Weld et al. 2006; Kück and Hoff 2010; Jiang et al. 2013). Moreover, with the advent of high-throughput sequencing technologies, the number of sequenced genomes of filamentous fungi has also been rapidly increasing, which further revealed the existence of a large number of uncharacterized and silent secondary metabolite gene clusters (Yang et al. 2009; Andersen et al. 2013). As a result, large-scale functional genomics of filamentous fungi has become a new research hot spot. Unfortunately, although the existing methods can be used to edit target genes at the genomic level, the low editing efficiency and the consequently large amount of necessary labor time gravely limit the further development of these technologies.
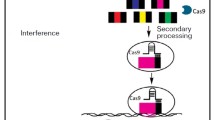
Clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated proteins (Cas) constitute the CRISPR/Cas system, which in recent years has emerged as a potential candidate to solve the problem of low gene editing frequency in filamentous fungi. Based on the different Cas proteins, the CRISPR/Cas systems have been divided into three types (I–III) (Haft et al. 2005; Makarova et al. 2011). Compared with types I and III, which possess more complex molecular mechanisms and need multiple Cas proteins working together to cleave the target DNA, the type II CRISPR system from Streptococcus pyogenes is much simpler and has consequently been applied widely (Makarova et al. 2013; Chylinski et al. 2013). This system consists of only two elements, a Cas9 nuclease and a single-guide RNA (sgRNA) composed of two small RNAs, a target-recognizing CRISPR RNA (crRNA) and auxiliary noncoding trans-activating crRNA (tracrRNA) (Jinek et al. 2012; Kuscu et al. 2014). The synthetic sgRNA binds to Cas9, and the resulting complex can catalyze a double-strand break (DSB) in the target DNA comprising a 20-bp sequence matching the protospacer of the sgRNA and a downstream protospacer adjacent motif (PAM) sequence (Cong et al. 2013; Mali et al. 2013; Liu et al. 2015; Fig. 1). Due to significant advantages, encompassing high efficiency, easy operation, the possibility of multi-gene editing, and so on, CRISPR/Cas9 has become one of the fastest-growing gene-editing technologies and is applied to various species including filamentous fungi. The CRISPR/Cas9 system therefore has become a further powerful genome-editing technology in addition to zinc finger nucleases (ZFN) and transcription activator-like effector nucleases (TALEN) (Mashimo 2014; Wu and Feng 2015; Lee et al. 2016; Estrela and Cate 2016).
CRISPR/Cas9 genome-editing platform. a A sgRNA will bind with Cas9 and catalyze a DNA double-strand breaks (DSBs) in the target DNA composed of a 20-bp sequence matching the protospacer of the sgRNA and an downstream protospacer adjacent motif (PAM) sequence. b The DNA repair mechanism can be divided into two types: the nonhomologous end-joining (NHEJ) as well as homologous repair (HR). When the DSB is created by CRISPR/Cas9 system, cells use these two pathways to repair the genome
In this review, we summarize the recent applications of the CRISPR/Cas9 system in filamentous fungi and briefly discuss further prospects of this technology. This review thus provides a useful quick-glance reference for anyone interested in state-of-the-art genome editing of filamentous fungi.
Development of the CRISPR/Cas9 system for filamentous fungi
Cas9 expression strategies
The efficient application of the CRISPR/Cas9 system requires the heterologous expression of the Cas9 gene fused to a nuclear localization signal (NLS), as well as simultaneous expression of the sgRNA (Schuster et al. 2016). Based on this principle, two main strategies have been established for the expression of Cas9 in vitro and in vivo. In the case of in vitro, the Cas9 and sgRNA are together transcribed in vitro and subsequently form the Cas9-sgRNA ribonucleoprotein complex to edit the target genes in Penicillium chrysogenum (Pohl et al. 2016). In the case of in vivo, human-optimized S. pyogenes Cas9 (hSpCas9) followed by a SV40 NLS is usually been utilized to meet the basic demands for gene targeting (Matsu-Ura et al. 2015; Fuller et al. 2015). Certainly, this method is not suitable for all cases, at least in T. reesei and Phytophthora sojae. In order to improve gene editing efficiency, researchers prefer to optimize the Cas9 and NLS genes based on the codon usage of the targeted filamentous fungi themselves, or alternatively to directly select a stronger NLS to efficiently target the fusion protein into cell nucleus (Liu et al. 2015; Fang and Tyler 2015). What is more, different promoter systems have also be taken into consideration. In general, the Cas9 gene was transcribed by strong constitutive promoters to improving the efficiency of genome editing; however, this way may lead to the uncontrollability of the CRISPR/Cas9 system in filamentous fungi and thus contribute to possible off-target effects. In order to realize the purpose of controllability of this system, some inducible promoters such as Pcbh1 and PniiA have been utilized to inhabit Cas9 expression under repressing conditions for minimal off-target effects, which further render the CRISPR/Cas9 system a spatiotemporal controller of genome editing of filamentous fungi (Liu et al. 2015; Pohl et al. 2016).
sgRNA expression strategies
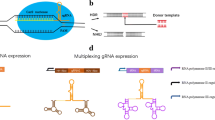
Apart from optimized expression of the Cas9 gene, the lack of optimal functional sgRNA modules also hampers the development of the CRISPR/Cas9 system in filamentous fungi (Schuster et al. 2016). Owing to the lack of a cap structure and poly A-tail, the synthetic sgRNA cannot be transcribed by RNA polymerase II and therefore typically needs to be transcribed using RNA polymerase III promoters (Nødvig et al. 2015). However, as these promoters are ill-defined in filamentous fungi, many efforts have been made to promote the release of sgRNA modules. Two main strategies have been established for the expression of sgRNAs in vitro and in vivo (Fig. 2).
Expression strategies of Cas9 and sgRNAs. a Functional sgRNA can been synthesized with RNA polymerase II (pPol II) and III (pPol III) promoters. In the case of pPol III, sgRNAs can be directly transcribed. In the case of pPol II, sgRNA will flank the HH and HDV ribozyme sequences, which can lead to self-processed RNA cleavage and subsequent release of the functional sgRNA. b The sgRNA is transcribed in vitro under the control of pPol III such as U6 and T7 promoters. c Both Cas9 and sgRNA will be transcribed in vitro and eventually form the ribonucleoproteins to cleave the target DNA
In some organisms, such as the T. reesei, A. niger, and Aspergillus fumigatus, sgRNAs were generated by in vitro transcription and subsequently used to co-transform the protoplasts together with a Cas9 vector or Cas9 protein (Kuivanen et al. 2016; Zhang et al. 2015). This strategy solves the problem that RNA polymerase III promoters cannot be identified by filamentous fungi and is suitable for almost all organisms. However, the stability of sgRNAs may still be an issue.
At the same time, attempts to develop sgRNAs in vivo have also been made in filamentous fungi. Previous studies have demonstrated that RNA polymerase III promoters such as SNR52 and some tRNA promoters can be applied to transcribe sgRNAs in A. fumigatus, N. crassa, and Penicillium chrysogenum (Fuller et al. 2015; Matsu-Ura et al. 2015). Furthermore, U6 promoters of small nuclear RNA (snRNA) genes are also often used for the expression of sgRNAs in various organisms (Ryan and Cate 2014). In order to investigate whether the U6 promoters can be used in filamentous fungi, researchers compared the snRNA sequences from different species based on available genome information and found ones with approximately 65% identity (Zhang et al. 2015). These identified U6 promoters were further confirmed to be able to transcribe sgRNAs and guide the Cas9 enzyme to the cleavage site for the formation of a DSB (Katayama et al. 2016; Schuster et al. 2016). However, due to the complexity of genetic background and uncertainty of genome information, some endogenous RNA polymerase III promoters from filamentous fungi are presently difficult to identify or are not suitable for sgRNA transcription. Thus, the most common method to express sgRNAs in vivo is to utilize two ribozyme sequences, 5′-end hammerhead (HH) and 3′-end hepatitis delta virus (HDV)—to flank the sgRNA (Nødvig et al. 2015). This way, the generation of functional sgRNAs for gene editing only needs a strong RNA polymerase II promoter, abrogating the need for the time-consuming investigation and verification of RNA polymerase III promoters. A similar strategy has previously been applied to other organisms, e.g., Arabidopsis (Gao et al. 2015), yeasts (Gao and Zhao 2015), and mammalian cells (Nissim et al. 2014). Interestingly, in wheat and sweet orange, but also in the filamentous fungus Pyricularia oryzae, the respective sgRNAs can be successfully transcribed using the 35S CAMV promoter as well as the Trpc promoter, albeit with low editing efficiency (Jia and Wang 2014; Upadhyay et al. 2013; Arazoe et al. 2015). Why these RNA-polymerase II promoters can directly be employed to express sgRNAs without two ribozyme sequences is still an interesting, unsolved question.
Target gene selection and vector construction
Compared with the application of the CRISPR/Cas9 system in model organisms such as E. coli, yeast, and zebrafish (Ng et al. 2016; Chung et al. 2017; Gao and Zhao 2015; Auer et al. 2014), the system is still in its infancy in filamentous fungi and it has been established in only a small number of species. In fact, only few studies have investigated the application of genome editing for fungal metabolic engineering, and most works mainly revolved around the feasibility of establishing the CRISPR/Cas9 system in filamentous fungi at all (Table 2). Therefore, in order to intuitively observe the presence of gene-editing events, as well as to reduce the workload need for the selection of positive clones, researchers preferred to target special functional genes that change the transformants’ phenotypic features for easier selection. For instance, Katayama et al. (2016) chose wA and yA as the target genes to investigate the expression of U6 snRNA promoters in the CRISPR/Cas9 system, since the loss of these two genes directly leads to the formation of different/colored conidia. Moreover, similar experimental setups have been reported in other filamentous fungi (Nødvig et al. 2015; Liu et al. 2015). This approach will be useful for early verification and establishment of the CRISPR/Cas9 system in filamentous fungi.
On the other hand, some studies have shown that the transient expression (TE) of Cas9 was sufficient for genome editing. However, in most cases, autonomously replicating plasmids based on AMA1 or ARS sequences have proven to be more effective for the construction of the CRISPR/Cas9 system. For one thing, the transformation efficiency of protoplasts is much higher, and for another, due to the instability of the replicating plasmids in nonselective media, resistance genes can be recycled and the chance of unexpected off-target events after gene editing is minimal. Based on these two strategies, Pohl et al. (2016) developed powerful CRISPR/Cas9 tools for marker-based and marker-free genome modifications in Penicillium chrysogenum. Additionally, the authors also comprehensively compared the influence of different Cas9 and sgRNA expression strategies in Penicillium chrysogenum, providing useful references for vector construction in filamentous fungi.
Applications of the CRISPR/Cas9 system in filamentous fungi
Cas9-mediated gene knockout
Compared with the traditional gene knockout technology applied in filamentous fungi, CRISPR/Cas9 promises to be time-saving and more efficient. Liu et al. (2015) were the first to establish the CRISPR/Cas9 system in the filamentous fungus T. reesei using specific codon optimization and in vitro RNA transcription. Cas9 was integrated into the genome to form a CRISPR expression chassis to further simplify the process of vector construction. In order to achieve controllability of the CRISPR/Cas9 system, an induced promoter was assembled to express the Cas9 protein and the predicted mutations were observed in the target gene, with a frequency approaching 100%. Nearly at the same time, Arazoe et al. (2015) selected the SDH and Srs2 loci as the target genes to verify the feasibility of CRISPR/Cas9 in Pyricularia oryzae. Two identified U6 promoters and a Trpc promoter were successfully used to transcribe sgRNAs. The on-target efficiency of co-transformation with the CRISPR/Cas9 cassettes and the targeting vector (TV) was much higher than the transformation with the TV cassette, which further demonstrated the enormous potential of this technology.
In the above studies, sgRNAs were transcribed by RNA polymerase III promoters or in vitro. By contrast, Nødvig et al. (2015) adopted a new strategy for the release of the sgRNA module. The authors fused the HH and HDV ribozymes on the 5′ and 3′ ends of sgRNA sequence. Through self-processed RNA cleavage and subsequent release of the gRNA sequence without modifications, CRISPR/Cas9 was successfully implemented in six different fungal species. This was the first time that this strategy has been demonstrated to be valid in filamentous fungi. Using similar approaches, this method was quickly extended to other filamentous fungi, such as Phytophthora sojae, A. fumigatus, Talaromyces atroroseus, and Aspergillus carbonarius (Fang and Xia 2015; Weber et al. 2016; Nielsen et al. 2017; Weyda et al. 2017). Although the gene editing efficiency varied greatly among different fungi (1–100%, Table 2), the advent of this approach appears to have solved the sgRNA construction problems, which should greatly accelerate the development of CRISPR/Cas9 in filamentous fungi.
In another study, Fuller et al. (2015) described the NHEJ-mediated integration of transforming DNA into the Cas9 cleavage site. Generally, filamentous fungi have a dominant nonhomologous end-joining (NHEJ) repair pathway, which results in random insertions or deletions of one or more nucleotides that can lead to gene disruption after the formation of a DSB (Zhang et al. 2015). However, in the A. fumigatus Cas9 knockout system, the result was really surprising. Based on the phenotypic analysis of colonies, CRISPR/Cas9 was clearly functional judging by the loss of the target gene. In order to test the results of this experiment, the researchers amplified target fragments from these phenotypically positive colonies, and finally found that the main reason for gene knockout was a premature translational stop and the larger insertions of the transforming DNA at the Cas9 cleavage site. Although the phenomenon of NHEJ-mediated integration has also been reported in mammalian cells, why such a mechanism might dominate in A. fumigatus rather than other fungi remains unclear (Bachu et al. 2015).
In addition, a news article was recently reported in Science to introduce the application of the CRISPR/Cas9 system in the higher fungus Agaricus bisporus. By deleting one of the six polyphenol oxidase (PPO) genes in the mushroom’s genome, the activity of PPO decreased by 30%, which effectively mitigated the browning of Agaricus bisporus. Although this news does not disclose the experimental process, it is the first time that the CRISPR/Cas9 system was proved to be effective in higher fungi (Waltz 2016; Yin et al. 2017). Furthermore, in latest research, the gene disruption in the higher fungus Ganoderma lucidum and G. lingzhi was also achieved by employing the CRISPR/Cas9 system with a codon-optimized Cas9 and in vitro transcribed sgRNA. These studies thus provide a widely applicable approach for gene disruption in higher fungi (Qin et al. 2017).
In summary, with the deepening of research, the Cas9-mediated gene knockout system is replacing traditional HR technology and is gradually established in more and more filamentous fungi. Although gene-editing events can be observed in all cases, different CRISPR/Cas9 expression setups greatly affect the on-target efficiency for the most part. Therefore, achieving highly efficient and precise gene editing is still a problem to be resolved.
Cas9-mediated gene knockin
One major obstacle for gene knockouts or integration in filamentous fungi is the fact that the HR frequency is extremely low—reportedly lower than 2% (Weld et al. 2006). As a consequence, high gene editing frequency mainly depends on the length of the homologous arms (> 1000 bp) and often requires ku70/80 knockout strains. What is more, the lack of multi-gene editing methods is unfavorable for the further study of filamentous fungi. The emergence of CRISPR/Cas9 genome-editing tools will hopefully contribute to the solution of these problems.
In the filamentous fungus T. reesei, researchers investigated the effect of the length of the homology arms on the gene integration frequency. The final result demonstrated that homology arms of 200 bp are sufficient to achieve quite high homologous integration efficiencies (> 93%) (Liu et al. 2015). Moreover, the simultaneous HR of multiple-gene using the CRISPR/Cas9 system has also been investigated in T. reesei. By optimizing the concentration ratio of sgRNA and dDNA, the frequency of single-recombination events approached 100%; for double recombination, it was ~ 45% and reached 4.2% for triple recombination. Although the triple-recombination frequency was still relatively low, the CRISPR/Cas9 system is a promising tool for multi-gene editing in filamentous fungi. In quick succession to these findings, the potential of multi-gene editing using CRISPR/Cas9 system was also confirmed in thermophilic filamentous fungi. Compared with the in vitro sgRNA synthesis used for T. reesei, the HR efficiency was much higher in thermophilic fungi Myceliophthora thermophila and M. heterothallica (~ 95, ~ 60, ~ 30, and ~ 20% for single, double, triple, and quadruple recombinations, respectively), in which the sgRNA was transcribed in vivo by the endogenous U6 promoter. This study thus indicates that endogenous RNA polymerase III promoters could offer a better performance for transcribing sgRNA modules than the widely used but unstable in vitro sgRNA (Liu et al. 2017).
Subsequently, CRISPR/Cas9 system was also established in N. crassa. Matsu-ura et al. (2015) replaced the endogenous clr-2 promoter with the tubulin promoter and integrated a codon-optimized firefly luciferase gene at the csr-1 locus. By comparing the HR efficiency in wild-type N. crassa and a mus-51 knockout strain, which was deficient in NHEJ, the researchers found that CRISPR/Cas9 technology did not require mus-51 or mus-52 mutant backgrounds for efficient HR. It can thus be expected that this technology will enable efficient gene editing of any natural isolate of Neurospora sp. and may even be extended to other filamentous fungi.
In addition, based on the phenomenon of NHEJ-mediated integration of transforming DNA in A. fumigatus CRISPR gene knockout system, Zhang et al. (2015) established a more efficient version of this system. The highly efficient CRISPR integration system referred to as microhomology-mediated end-joining (MMEJ) was developed to carry out precise in-frame integration with or without marker insertion via very short (only 35 bp) homology arms. Using this approach, simultaneous HR of gene pairs was investigated and all transformants displayed relative integration at these target loci, whereby the homologous integration efficiency was approximately 95–100%. Moreover, this system was proved to be independent of the ku80 pathway in A. fumigatus similarly to the mus-51 pathway mentioned above in N. crassa. These findings thus reconfirm the high efficiency of CRISPR/Cas9 in genome editing.
In summary, HR combined with CRISPR/Cas9 is an applicable and powerful tool to greatly mitigate the shortcomings of the traditional application of HR in filamentous fungi. It enables the development of marker-free, multi-gene knockout and integration systems, which opens the door for the research and exploitation of novel natural products in filamentous fungi and greatly facilitates the further study of fungal diversity.
Conclusion and perspectives
Filamentous fungi play an increasingly important role in various fields as microbial cell factories that provide a broad space for the development of a variety of economically important products. With the arrival of the post-genomic era, increasing numbers of uncharacterized or silent secondary metabolite gene clusters are being revealed. Thus, the elucidation of gene function in these metabolic pathways will become a major issue. Due to the complexity of metabolic regulation and genetic background in filamentous fungi, significant time and effort must be spent to overcome problems at different metabolic levels. All these require a powerful gene-editing tool for further research in filamentous fungi, which means that simple, efficient, and versatile CRISPR/Cas9 systems will undoubtedly bring great changes in the research to functional genomics.
Although the history of CRISPR/Cas9 is short and there still are many evolving factors affecting the editing frequency, this system already has invaluable prospects for application. With the deepening of research, innovative strategies such as CRISPR-interference (Kao and Ng 2017), Cas9n (Ran et al. 2013), and FokI-dCas9 (Guilinger et al. 2014) have been adopted to optimize the Cas9 system in order to carry out more precise gene-editing events. Such technologies will surely provide fresh wind for the fast development of genome editing in filamentous fungi and significantly advance the research of new natural products. Taken together, these developments are expected to be major drivers of the valorization of filamentous fungal diversity in the near and mid-term future.
References
Andersen MR, Nielsen JB, Klitgaard A, Petersen LM, Zachariasen M, Hansen TJ, Blicher LH, Gotfredsen CH, Larsen TO, Nielsen KF, Mortensen UH (2013) Accurate prediction of secondary metabolite gene clusters in filamentous fungi. Proc Natl Acad Sci U S A 110:E99–E107
Arazoe T, Ogawa T, Miyoshi K, Yamato T, Ohsato S, Sakuma T, Kuwata S (2015) Tailor-made talen system for highly efficient targeted gene replacement in the rice blast fungus. Biotechnol Bioeng 112:1335–1342
Auer TO, Duroure K, Concordet JP, Del BF (2014) CRISPR/Cas9-mediated conversion of Egfp-into Gal4-transgenic lines in zebrafish. Nat Protoc 9:2823–2840
Chung ME, Yeh I, Sung LY, Wu MY, Chao YP, Ng IS, Hu YC (2017) Enhanced integration of large DNA into E. coli chromosome by CRISPR/Cas9. Biotechnol Bioeng 114:172–183
Chylinski K, Le RA, Charpentier E (2013) The tracrRNA and Cas9 families of type II CRISPR-Cas immunity systems. RNA Biol 10:726–737
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N (2013) Multiplex genome engineering using CRISPR-Cas systems. Science 339:197–217
Dufossé L, Fouillaud M, Caro Y, Mapari SA, Sutthiwong N (2014) Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr Opin Biotechnol 26:56–61
Dwiarti L, Otsuka M, Miura S, Yaguchi M, Okabe M (2007) Itaconic acid production using sago starch hydrolysate by Aspergillus terreus Tn484-m1. Bioresour Technol 98:3329–3337
Dyal SD, Bouzidi L, Narine SS (2005) Maximizing the production of γ-linolenic acid in Mortierella ramanniana var. ramanniana as a function of ph, temperature and carbon source, nitrogen source, metal ions and oil supplementation. Food Res Int 38:815–829
Estrela R, Cate JHD (2016) Energy biotechnology in the CRISPR-Cas9 era. Curr Opin Biotechnol 38:79–84
Fakas S, Čertik M, Papanikolaou S, Aggelis G, Komaitis M, Galiotou-Panayotou M (2008) γ-Linolenic acid production by Cunninghamella echinulata, growing on complex organic nitrogen sources. Bioresour Technol 99:5986–5990
Fang H, Xia L (2015) Cellulase production by recombinant Trichoderma reesei, and its application in enzymatic hydrolysis of agricultural residues. Fuel 143:211–216
Fang YF, Tyler BM (2015) Efficient disruption and replacement of an effector gene in the Oomycete Phytophthora sojae using CRISPR/Cas9. Mol Plant Pathol 17:127–139
Francis F, Sabu A, Nampoothiri KM, Ramachandran S, Ghosh S, Szakacs G, Pandey A (2003) Use of response surface methodology for optimizing process parameters for the production of α-amylase by Aspergillus oryzae. Biochem Eng J 15:107–115
Fu YQ, Yin LF, Zhu HY, Jiang R (2016) High-efficiency l-lactic acid production by Rhizopus oryzae using a novel modified one-step fermentation strategy. Bioresour Technol 218:410–417
Fuller KK, Chen S, Loros JJ, Dunlap JC (2015) Development of the CRISPR/Cas9 system for targeted gene disruption in Aspergillus fumigatus. Eukaryot Cell 25:709–712
Gao YB, Zhao YD (2015) Self-processing of ribozyme-flanked RNAs into guide RNAs in vitro and in vivo for CRISPR-mediated genome editing. J Integr Plant Biol 56:343–349
Gao YB, Zhang Y, Zhang D, Dai XH, Estelle M, Zhao YD (2015) Auxin binding protein (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc Natl Acad Sci U S A 112:2275–2280
Guilinger JP, Thompson DB, Liu DR (2014) Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nat Biotechnol 32:577–582
Haft DH, Selengut J, Mongodin EF, Nelson KE (2005) A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR-Cas subtypes exist in prokaryotic genomes. PLoS Comput Biol 1:e60
Harris LJ, Margaret B, Anne J, Danielle S, Thérèse O (2016) Host-preferential Fusarium graminearum gene expression during infection of wheat, barley, and maize. Fungal Biol 120:111–123
Bachu R, Bergareche I, Chasin LA (2015) CRISPR-Cas targeted plasmid integration into mammalian cells via non-homologous end joining. Biotechnol Bioeng 112:2154–2162
Ji XJ, Reng LJ, Nie ZK, Huang H, Ouyang PK (2014) Fungal arachidonic acid-rich oil: research, development and industrialization. Crit Rev Biotechnol 34:197–214
Jia HG, Wang N (2014) Targeted genome editing of sweet orange using Cas9/sgRNA. PLoS One 9:e93806
Jiang D, Zhu W, Wang Y, Sun C, Zhang KQ, Yang J (2013) Molecular tools for functional genomics in filamentous fungi: recent advances and new strategies. Biotechnol Adv 31:1562–1574
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-Rna-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821
Katayama T, Tanaka Y, Okabe T, Nakamura H, Fujii W, Kitamoto K, Maruyama J (2016) Development of a genome editing technique using the CRISPR/Cas system in the industrial filamentous fungus Aspergillus oryzae. Biotechnol Lett 38:637–642
Kück U, Hoff B (2010) New tools for the genetic manipulation of filamentous fungi. Appl Microbiol Biotechnol 86:51–62
Kuivanen J, Wang YJ, Richard P (2016) Engineering Aspergillus niger for galactaric acid production: elimination of galactaric acid catabolism by using RNA sequencing and CRISPR/Cas9. Microb Cell Fact 15:210–219
Kuscu C, Arslan S, Singh R, Thorpe J, Adli M (2014) Genome-wide analysis reveals characteristics of off-target sites bound by the Cas9 endonuclease. Nat Biotechnol 32:677–683
Kao PH, Ng IS (2017) CRISPRi mediated phosphoenolpyruvate carboxylase regulation to enhance the production of lipid in Chlamydomonas reinhardtii. Bioresour Technol. https://doi.org/10.1016/j.biortech.2017.04.111
Lecellier A, Gaydou V, Mounier J, Hermet A, Castrec L, Barbier G, Ablain W, Manfait M, Toubas D, Sockalingum GD (2015) Implementation of an FTIR spectral library of 486 filamentous fungi strains for rapid identification of molds. Food Microbiol 45:126–134
Lee J, Chung J, Kim HM, Kim D, Kim H (2016) Designed nucleases for targeted genome editing. Plant Biotechnol J 14:448–462
Liu R, Chen L, Jiang YP, Zhou ZH, Zou G (2015) Efficient genome editing in filamentous fungus Trichoderma reesei using the CRISPR/Cas9 system. Cell Discov 1:15007
Liu Q, Gao R, Li J, Lin L, Zhao J, Sun W, Tian C (2017) Development of a genome-editing CRISPR/Cas9 system in thermophilic fungal Myceliophthora species and its application to hyper-cellulase production strain engineering. Biotechnol Biofuels 10:1. https://doi.org/10.1186/s13068-016-0693-9
Makarova KS, Wolf YI, Koonin EV (2013) The basic building blocks and evolution of CRISPR Cas systems. Biochem Soc Trans 41:1392–1400
Makarova KS, Haft DH, Barrangou R, Brouns SJ, Charpentier E, Horvath P, Moineau S, Mojica FJ, Wolf YI, Yakunin AF, Oost JVD, Koonin EV (2011) Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol 9:467–477
Mali P, Yang L, Esvelt KM, Aach J, Guell M, Dicarlo JE (2013) RNA-guided human genome engineering via Cas9. Science 339:823–826
Mantzouridou FT, Naziri E (2017) Scale translation from shaken to diffused bubble aerated systems for lycopene production by Blakeslea trispora under stimulated conditions. Appl Microbiol Biotechnol 101:1845–1856
Marumo S, Katayama M, Komori E, Ozaki Y, Natsume M, Kondo S (2014) Microbial production of abscisic acid by Botrytis cinerea. Agric Biol Chem 46:1967–1968
Mashimo T (2014) Gene targeting technologies in rats: zinc finger nucleases, transcription activator-like effector nucleases, and clustered regularly interspaced short palindromic repeats. Develop Growth Differ 56:46–52
Matsu-Ura T, Baek M, Kwon J, Hong C (2015) Efficient gene editing in Neurospora crassa with CRISPR technology. Fungal Biol Biotechnol 2:4. https://doi.org/10.1186/s40694-015-0015-1
Mishra NC, Tatum EL (1973) Non-mendelian inheritance of DNA-induced inositol independence in Neurospora. Proc Natl Acad Sci U S A 70:3875–3879
Mo X, Kang Y, Yan J, Liu J, Bi Y, Zhen K (2002) Production of linolenic acid by Mortierella isabellina grown on octadecanol. Curr Microbiol 44:141–144
Nanou K, Roukas T (2016) Waste cooking oil: a new substrate for carotene production by Blakeslea trispora in submerged fermentation. Bioresour Technol 203:198–203
Ng IS, Hung YH, Kao PH, Zhou Y, Zhang X (2016) CRISPR/Cas9 nuclease cleavage enables marker-free genome editing in Escherichia coli: a sequential study. J Taiwan Inst Chem Eng 68:31–39
Nielsen ML, Isbrandt T, Rasmussen KB, Thrane U, Hoof JB, Larsen TO, Mortensen UH (2017) Genes linked to production of secondary metabolites in Talaromyces atroroseus revealed using CRISPR/Cas9. PLoS One 12:e0169712
Nissim L, Perli SD, Fridkin A, Perezpinera P, Lu TK (2014) Multiplexed and programmable regulation of gene networks with an integrated RNA and CRISPR/Cas toolkit in human cells. Mol Cell 54:698–710
Nødvig CS, Nielsen JB, Kogle ME, Mortensen UH (2015) A CRISPR/Cas9 system for genetic engineering of filamentous fungi. PLoS One 10:e0133085
Pohl C, Kiel JA, Driessen AJ, Bovenberg RA, Nygard Y (2016) CRISPR/Cas9 based genome editing of Penicillium chrysogenum. ACS Synth Biol 5:754–764
Qin H, Xiao H, Zou G, Zhou Z, Zhong JJ (2017) CRISPR-Cas9 assisted gene disruption in the higher fungus Ganoderma species. Process Biochem 56:57–61
Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino A, Scott DA, Lnoue A, Matoba S, Zhang Y, Zhang F (2013) Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154:1380–1389
Ryan OW, Cate JH (2014) Multiplex engineering of industrial yeast genomes using CRISPRm. Methods Enzymol 546:473–489
Schuster M, Schweizer G, Reissmann S, Reissmannet S, Kahmann R (2016) Genome editing in Ustilago maydis using the CRISPR–Cas system. Fungal Genet Biol 89:3–9
Shi TQ, Peng H, Zeng SY, Ji RY, Shi K, Huang H, Ji XJ (2017) Microbial production of plant hormones: opportunities and challenges. Bioengineered 8:124–128
Tang WJ, Pan A, Lu HZ, Xia JY, Zhuang YP, Zhang SL, Chu J, Noorman H (2015) Improvement of glucoamylase production using axial impellers with low power consumption and homogeneous mass transfer. Biochem Eng J 99:167–176
Thrane U, Anderson B, Frisvad JC, Smedsgaard J (2007) The exo-metabolome in filamentous fungi. Top Curr Genet 18:235–252
Upadhyay SK, Kumar J, Alok A, Tuli R (2013) RNA-guided genome editing for target gene mutations in wheat. G3-Genes Genom Genet 3:2233–2238
Waltz E (2016) Gene-edited CRISPR mushroom escapes US regulation. Nature 532:293
Ward OP (2012) Production of recombinant proteins by filamentous fungi. Biotechnol Adv 30:1119–1139
Weber J, Valiante V, Nødvig CS, Mattern DJ, Slotkowski RA, Mortensen UH, Brakhage AA (2016) Functional reconstitution of a fungal natural product gene cluster by advanced genome editing. ACS Synth Biol 6:62–68
Weld RJ, Plummer KM, Carpenter MA, Ridgway HJ (2006) Approaches to functional genomics in filamentous fungi. Cell Res 16:31–44
Weyda I, Yang L, Vang J, Ahring BK, Lübeck M, Lübeck PS (2017) A comparison of Agrobacterium-mediated transformation and protoplast-mediated transformation with CRISPR/Cas9 and bipartite gene targeting substrates, as effective gene targeting tools for Aspergillus carbonarius. J Microbiol Methods 135:26–34
Wu Z, Feng G (2015) Progress of application and off-target effects of CRISPR/Cas9. Heredi 37:1003–1010
Xu Q, Li S, Huang H, Wen J (2012) Key technologies for the industrial production of fumaric acid by fermentation. Biotechnol Adv 30:1685–1696
Xu X, Zhang X, Wu Z, Wang Z (2015) Accumulation of yellow monascus, pigments by extractive fermentation in nonionic surfactant micelle aqueous solution. Appl Microbiol Biotechnol 99:1173–1180
Yang MQ, Athey BD, Arabnia HR, Sung AH, Liu Q, Yang JY, Mao JH, Deng YP (2009) High-throughput next-generation sequencing technologies foster new cutting-edge computing techniques in bioinformatics. BMC Genomics 10:1–3
Yin CM, Fan XZ, Shi DF, Gao H (2017) CRISPR/Cas genome editing technology and its application in fungi. Biotechnol Bull 33:58–65
Yu J, Cleveland TE, Nierman WC, Bennett JW (2005) Aspergillus flavus genomics: gateway to human and animal health, food safety, and crop resistance to diseases. Rev Iberoam Micol 22:194–202
Zhang C, Meng X, Wei X, Lu L (2015) Highly efficient CRISPR mutagenesis by microhomology-mediated end joining in Aspergillus fumigatus. Fungal Genet Biol 86:47–57
Zhang Y, Luan X, Zhang H, Garre V, Song Y, Ratledge C (2017) Improved γ-linolenic acid production in Mucor circinelloides by homologous overexpressing of delta-12 and delta-6 desaturases. Microb Cell Factories 16:113. https://doi.org/10.1186/s12934-017-0723-8
Zhou PP, Meng J, Bao J (2017) Fermentative production of high titer citric acid from corn stover feedstock after dry dilute acid pretreatment and biodetoxification. Bioresour Technol 224:563–572
Funding
This work was financially supported by the National Natural Science Foundation of China (Nos. 21376002, 21476111 and 21776131), the Jiangsu Province Natural Science Foundation of China (No. BK20131405), the Program for Innovative Research Team in University of Jiangsu Province, and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shi, TQ., Liu, GN., Ji, RY. et al. CRISPR/Cas9-based genome editing of the filamentous fungi: the state of the art. Appl Microbiol Biotechnol 101, 7435–7443 (2017). https://doi.org/10.1007/s00253-017-8497-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8497-9