Abstract
Cyanobacteria are industrially robust photosynthetic microorganisms that can be genetically programmed to synthesize commodity products for domestic and industrial consumption. In the present work, Synechocystis was endowed with the synthesis of the plant secondary metabolite geranyllinalool, a diterpene alcohol of commercial interest. Total average yields of 360 μg of geranyllinalool per gram of dry cell weight were obtained in the course of a 48-h cultivation period. Geranyllinalool was primarily sequestered inside the transformant cells, corresponding to 60–70% of the total heterologous product, instead of being entirely exuded, as the case is with shorter heterologous terpene hydrocarbons. Extraction of geranyllinalool necessitated disruption of the cells in order to release and isolate this chemical product. Moreover, geranyllinalool accumulation in the cells caused a mild inhibitory effect on cell fitness and biomass growth rate, such that the duplication time of Synechocystis transformants was 1.4-fold longer than that of the control. The remaining 30–40% of the geranyllinalool product was found to float on the surface of sealed transformant cultures, where it was siphoned off by applying a hydrophobic overlayer, with no need to disrupt the cells in this case. Concluding, the work extended efforts to heterologously produce terpene and terpenol products in cyanobacteria, and addressed possibilities and constrains inherent to this production system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria can be metabolically engineered to drive photosynthetic carbon toward the synthesis of heterologous products for industrial and domestic consumption. Promising results were obtained for the synthesis of relatively small-sized molecules that can spontaneously diffuse away from the cells and the liquid culture. Such products of interest can be derived from intracellular metabolites, with relevant reactions catalyzed by heterologous enzymes (for a review see Angermayr et al. 2015). Terpenoids of plant origin are a diverse group of industrially relevant compounds (Gershenzon and Dudareva 2007; Caputi and Aprea 2011). Work from this laboratory pioneered heterologous production of the C-5 hemiterpene isoprene (Lindberg et al. 2010; Bentley and Melis 2012) and of the C-10 monoterpene β-phellandrene (Bentley et al. 2013; Formighieri and Melis 2014; Formighieri and Melis 2015) in cyanobacteria. Significant in these developments was the spontaneous and quantitative separation of the product from the biomass and the aqueous medium, alleviating potential inhibitory or toxic effects of the product molecule on cellular metabolism, and lowering the cost of product isolation and downstream processing.
In the present work, the cyanobacterium Synechocystis was employed for heterologous production of the longer chain terpenoid geranyllinalool. The latter is an acyclic C-20 diterpene alcohol, widely encountered in the plant kingdom. Many plant species evolved the ability to make further modified end-products from geranyllinalool. The latter serves as the precursor to 4,8,12-trimethyl-1,3,7,11-tridecatetraene (TMTT), a volatile C-16 homoterpene, emitted from species such as Arabidopsis thaliana, Solanum lycopersicum, and Phaseolus lunatus, among others (Falara et al. 2014). TMTT contributes to the floral scent of the plant and plays a role in the attraction of pollinators (Tholl et al. 2011), but it is also a component of volatile blends released from vegetative tissues in response to herbivore attack (Brillada et al. 2013). In tobacco, geranyllinalool is present in the form of 17-hydroxy-geranyllinalool diterpene glycosides (HGL-DTGs) that were found to be highly abundant (>2.5% dry mass) in at least 26 Nicotiana species (Snook et al. 1997; Heiling et al. 2010). HGL-DTGs were reported to accumulate in the tobacco mesophyll and to play a defense role against insect herbivores (Jassbi et al. 2008; Heiling et al. 2010).
Geranyllinalool has industrial value as fragrance in cosmetics, household cleaning supplies, and detergents (Lapczynski et al. 2008). It can also be used as precursor for the chemical synthesis of the drug teprenone (De Castro et al. 2009). This market potential encourages the development of heterologous microbial platforms for the production of geranyllinalool, which promises to overcome difficulties in the extraction of this compound from plant tissues or the chemical synthesis of precursor molecules (Wang et al. 2015).
In plants, geranyllinalool (GL) is synthesized from geranylgeranyl-diphosphate (GGPP) (Jassbi et al. 2008; Heiling et al. 2010), the C-20 intermediate of the terpenoid biosynthetic pathway. The reaction is catalyzed by the geranyllinalool synthase (GLS) that was identified in coyote tobacco (Nicotiana attenuata) (Falara et al. 2014). In vitro analysis of the NaGLS, a recombinant enzyme showed a slow K cat (7 s−1), while the affinity for the GGPP substrate was relatively high (K m = 31 μM) (Falara et al. 2014). The slow K cat, common to terpene synthases, is the main factor limiting rate and yield of product formation upon heterologous expression in cyanobacteria (Formighieri and Melis 2014). A strategy to overcome this limitation was to increase the amount of the terpene synthase enzyme in the transformant cells, by fusing it to the highly abundant in cyanobacteria phycocyanin β-subunit (Formighieri and Melis 2015). Accumulation of the fusion protein was associated with efficient translation and protein synthesis (Formighieri and Melis 2016).
In the present work, the DNA sequence encoding a Nicotiana attenuata GLS was heterologously expressed in Synechocystis as a fusion protein with the phycocyanin β-subunit. The recombinant fusion protein was active and sufficient to convert endogenous GGPP into the geranyllinalool product. In contrast to higher plants, where further processing of the geranyllinalool (GL) leads to glycosylated products, absence of the downstream hydroxylation/glycosylation machinery in cyanobacteria enabled accumulation of the GL diterpenol.
Materials and methods
Synechocystis strains, NaGLS recombinant construct, and culturing conditions
Synechocystis sp. PCC 6803 (Synechocystis) was used as the recipient strain and referred to as the wild type in this study. The sequence encoding the geranyllinalool synthase from Nicotiana attenuata (NaGLS, accession number KJ755868) was cloned, without codon optimization, at the 3′ end of the cpcB endogenous sequence in the recombinant plasmid for expression of the terpene synthase as a CpcB fusion (Formighieri and Melis 2015). The recombinant plasmid has been deposited and can be made available through Addgene (<https://www.addgene.org/Anastasios_Melis>) with accession number 74004. Moreover, a complete sequence of the resulting DNA nucleotides is given in Fig. S1.
The recombinant plasmid was then used to transform by homologous recombination the cpc genomic DNA locus of Synechocystis according to established molecular biology protocols (Eaton-Rye 2011). The resulting transformants expressed the cpcB•NaGLS construct under the endogenous cpc operon promoter, while replacing the native cpcB gene. Wild type and transformants were maintained on 1% agar BG11 media supplemented with 10 mM TES-NaOH (pH 8.2) and 0.3% sodium thiosulphate. Liquid cultures in BG11 were buffered with 25 mM phosphate (pH 7.5) and incubated under continuous low-stream bubbling with air at 28 °C. Transgenic DNA copy homoplasmy was achieved with cells incubated on agar in the presence of the chloramphenicol antibiotic selection. The resulting cpcB•NaGLS transformants looked more greenish than blue-green, as recently shown for similar lines by Kirst et al. (2014) and Formighieri et al. (2014).
Genomic DNA PCR analysis of Synechocystis transformants
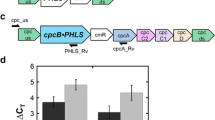
Genomic DNA templates were prepared with Chelex®100 Resin (BioRad), as described (Formighieri and Melis 2014). The following oligonucleotide primers were used to map transgene insertion in the Synechocystis genome, and to test for DNA copy homoplasmy ( Table S1 ): cpc_us and cpcA_Rv (Formighieri and Melis 2015), NaGLS_Rv (5′-gcagagggagattgaaacaaag-3′). The location of the primers on the genomic DNA is shown in Fig. 1a, b.
Construct for NaGLS expression in the cpc genomic locus of Synechocystis and PCR analysis results testing for DNA copy homoplasmy. a The cpc genomic locus in Synechocystis wild type, encoding the subunits of the phycobilisome peripheral phycocyanin light-harvesting antenna complex. b The recombinant construct used in this work for the Nicotiana attenuata geranyllinalool synthase (NaGLS) expression as a fusion with the cpcB gene, encoding the phycocyanin β-subunit, under the control of the cpc operon promoter. Arrows mark the position of the oligonucleotide primers used to genetically characterize the transformants. c Genomic DNA PCR analysis using primers cpc_us and cpcA_Rv, amplifying the cpc upstream-to-cpcA genomic region. The analysis was performed on three independent transformant lines (1, 2, 3). d Genomic DNA PCR analysis using primers cpc_us and NaGLS_Rv, amplifying the cpc upstream-to-NaGLS sequence in the transformants only
Protein analysis
Protein extraction from cell lysates was performed as described (Formighieri and Melis 2014; Formighieri and Melis 2015). Total cell proteins were resolved by SDS-PAGE, Coomassie-stained or transferred from the polyacrylamide gel to a nitrocellulose membrane, where they were probed with NaGLS-specific antibodies. In particular, the synthetic peptides SYNNQEKATNDHSFSGPMFES-C and YKNQMNNQNEYLSEISTLPER-C were used to raise antibodies in rabbit (Biomatik).
Geranyllinalool production assay
Cultures with an optical density at 730 nm (OD730nm) of 0.5 were bubbled with 100% CO2 to fill the gaseous headspace of the gaseous-aqueous two-phase reactor used in this lab (Bentley and Melis 2012), sealed and incubated for 48 h in the light. The GL product floating on the surface of the liquid cultures was siphoned off after dilution with a known volume of hexane overlayer. Mild agitation of the culture with the hexane overlayer was applied with a magnetic stirrer for 2 h, prior to siphoning off the hexane-GL solution. The GL product accumulated inside the cells was released upon cell disruption. Cells were precipitated by centrifugation and pellets were resuspended in a small volume of 50 mM Tris HCl pH 8, 50 mM NaCl, 10 mM CaCl2, 10 mM MgCl2, and disrupted by passing through a French Press at 20,000 psi. The cell lysates were then mixed with three volumes of hexane, vortexed vigorously for 1 min and additionally incubated at room temperature for 1 h, followed by centrifugation at 16,000 g for 10 min to separate the hexane from the aqueous phase. The hexane extracts were analyzed by GC-FID (Shimadzu GC-2014 equipped with a Rtx®-5 column). In this analysis, the temperature of the GC column was initially set at 40 °C for 2 min. The temperature was then increased to 140 °C at a rate of 20 °C/min, and held at 140 °C for 1 min. A second temperature gradient allowed attainment of 230 °C at a rate of 5 °C/min, then held at 230 °C for 1 min. Finally, the temperature was raised to 250 °C at a rate of 20 °C/min, and held at 250 °C for 2 min. The carrier gas (hydrogen) flow rate was 1.5 mL min−1. The retention profile of the GL product was compared with that of a GL standard (Santa Cruz Biotechnology, Inc., SC-228246). GL was quantified by the peak area, using the following integration parameters for GC peak identification: slope 100 μV/min, width 3 s. GL was further identified by GC-MS analysis, performed with an Agilent 6890GC/5973 MSD.
Dry cell weight, chlorophyll content, and optical density
For the gravimetric dry cell weight (dcw) measurements, 5 ml of culture aliquots were filtered through 0.22 μm millipore filters, the immobilized cells were dried in a convection oven at 80 °C overnight prior to weighing with a Sartorius CP124S analytical balance. Chlorophyll a was extracted from cell pellets in 90% methanol and quantified by its absorbance peak at 663 nm, according to Meeks and Castenholz (1971). The optical density (OD) was recorded as the absorbance of the culture at 730 nm.
Microscope imaging analysis
Imaging analysis of Synechocystis wild type and transformant cells was conducted with a Zeiss AxioImager M2 with Qimaging QiClick 12-bit camera, X-Cite 120Q light source, 100× objective 1.3NA, and the Chroma Texas Red filter set with excitation 560/40 (530–580 nm), 585 dichroic, and emission 630/75 (600–675 nm). The images were acquired and processed with iVision software from BioVision.
Results
Expression of Nicotiana attenuata geranyllinalool synthase in Synechocystis
The DNA sequence encoding the Nicotiana attenuata geranyllinalool synthase (NaGLS) was expressed in Synechocystis as a fusion with the endogenous cpcB gene, which encodes the highly abundant phycocyanin β-subunit of the phycobilisome antenna complex. The native NaGLS lacks a predicted plastid transit peptide (Falara et al. 2014), so that the entire encoding sequence was employed. Specifically, the endogenous cpcB gene within the cpc operon (Fig. 1a) was replaced via homologous recombination by the cpcB•NaGLS-cmR construct, while maintaining the other cpc operon genes in place (Fig. 1b). NaGLS was therefore expressed as a CpcB•NaGLS fusion under the control of the strong endogenous cpc operon promoter.
Transgenic DNA copy homoplasmy was tested by genomic PCR analysis (Fig. 1c, d). Primers cpc_us and cpcA_Rv were employed to amplify the cpc upstream-to-cpcA genomic region (Fig. 1a, b). A PCR product of 1289 bp was obtained in the wild type (Fig. 1c, wt), corresponding to the native sequence. A PCR product of 4707 bp was instead obtained in Synechocystis transformants (Fig. 1c, transformants 1, 2, 3). The higher molecular weight of the PCR product, as compared to the wild type, is due to the insertion of the cpcB•NaGLS-cmR construct (Fig. 1b). Moreover, absence of the wt PCR product in the Synechocystis transformants indicated attainment of transgenic DNA copy homoplasmy in the latter.
Primers cpc_us and NaGLS_Rv were used to further test for the correct transgene integration in the cpc genomic locus, by amplifying the cpc upstream-to-NaGLS sequence (Fig. 1b). While no PCR product was obtained in the wild type (Fig. 1d, wt), a PCR product of 1835 bp was obtained in the Synechocystis transformants with the aforementioned primers (Fig. 1d, transformants 1, 2, 3).
Analysis of NaGLS recombinant protein expression
Total proteins were extracted upon Synechocystis cell disruption, resolved by SDS-PAGE and Coomassie-stained (Fig. 2a). The CpcB•NaGLS fusion protein (expected molecular weight of 118 kD) was visible upon Coomassie-staining of total cell extracts slightly above the 100 kD marker (Fig. 2a, lanes 1–3, CpcB•NaGLS). This signature band was absent from the wild type (Fig. 2a, wt). As a consequence of the transformation event, the native CpcB subunit at 18 kD was absent from the NaGLS transformants, while it was highly abundant in the wild type protein extract (Fig. 2a, wt, CpcB). Fusion to CpcB enabled expression of the CpcB•NaGLS construct to levels that were visible upon Coomassie-staining of total cell protein extracts, even without prior optimization of the NaGLS encoding sequence for Synechocystis codon usage. This result indicates substantial enzyme accumulation, needed to overcome the slow K cat and to enable meaningful rate and yield of product formation. Noted also is the expression of the ~23 kD chloramphenicol resistance protein in the transformants (Fig. 2a, lanes 1–3, CmR).
Analysis of protein expression in the wild type and three independent NaGLS transformant lines (1, 2, 3) grown at 50 μmol photons m−2 s−1. a SDS-PAGE and Coomassie-staining of total cell protein extracts. Proteins of interest are labeled. Molecular weight markers are reported in kD. b Western blot analysis of (a) with specific NaGLS antibodies. Note the specific antibody cross-reaction with a protein band >100 kD in the transformants only
Identity and expression of the CpcB•NaGLS fusion was further tested by Western blot analysis (Fig. 2b). The polyclonal antibodies recognized the NaGLS protein in the CpcB•NaGLS fusion product, resulting in a specific cross-reaction with a band at ~118 kD (Fig. 2b, lanes 1–3, CpcB•NaGLS). No such cross-reaction could be observed in the wild type (Fig. 2b, wt).
Geranyllinalool production by Synechocystis transformants
Synechocystis transformant cultures were analyzed for photosynthetic production of GL, assayed by GC-FID of hexane extracts (Fig. 3). For comparison purposes, the GC-FID analysis of the GL standard in hexane showed a major peak with retention time of 22.7 min (Fig. 3a). Geranyllinalool produced by Synechocystis transformants was collected from the surface of the aqueous culture as a floating molecule. It was siphoned off following dilution with a known volume of hexane, which was applied as overlayer to the liquid culture. GC-FID analysis of the hexane overlayer from the Synechocystis transformants resulted in the detection of a peak with retention time of 22.6 min. This peak was absent from the hexane overlayer of wild type cultures (Fig. 3b vs. Fig. 3c).
Sensitive GC-FID analysis of Synechocystis transformant products, testing for the presence of geranyllinalool (GL). a GC-FID analysis of a GL standard, showing a major peak with retention time of 22.7 min. b GC-FID analysis of the hydrophobic products from Synechocystis transformants, showing a main peak (GL) with retention time of 22.6 min under our experimental conditions. Floating hydrophobic compounds were siphoned off from the surface of transformant cultures after application of a known volume of hexane overlayer. c GC-FID analysis of the hexane overlayer from wild type cultures, containing no detectable GL at the 22.6–22.7 retention time. d GC-FID analysis of hydrophobic products extracted with hexane after disruption of transformant cells. Notice the main GL product with a retention time of 22.6 min in these extracts. e GC-FID analysis of hexane extracts upon disruption of wild type cells, showing no detectable GL
The GL product was additionally detected from intracellular extracts of Synechocystis transformant cells. Intracellular GL was released upon cell disruption followed by application of a known volume of hexane to the surface of the lysate. GC-FID of such hexane extracts from Synechocystis transformants resulted in the detection of a peak with retention time of 22.6 min, which was absent from the wild-type intracellular extracts (Fig. 3d vs. Fig. 3e). Overall, these results clearly showed production of GL by the Synechocystis transformants.
Identity of the GL product was further assayed by MS analysis (Fig. 4). The MS signature of the GL standard (Fig. 4a) matched the MS signature of the molecule with retention time of 22.6 min from the Synechocystis transformant extracts, characterized by 69, 81, 93, 107, and 290 MS lines (Fig. 4b). These results confirmed the chemical identity of the molecule generated by the Synechocystis transformants to be geranyllinalool.
Mass spectrometry (MS) analysis of geranyllinalool. a MS signature of the GL standard, showing distinct 69, 81, 93, 107, and 290 lines. b MS signature of the hydrophobic molecule with retention time of 22.6 min, extracted from Synechocystis transformant cultures upon application of a known volume of hexane solvent
A systematic and quantitative analysis of GL production was performed with independent Synechocystis transformant lines upon growth for 48 h at either 50 or 180 μmol photons m−2 s−1 irradiance. GL yields, normalized on per gram of dried cell weight (dcw), are reported in Table 1. When growth and productivity were assayed at 50 μmol photons m−2 s−1, Synechocystis transformants produced an average of 120 μg of floating GL per gram of dcw that was siphoned off from the surface of the cultures (Table 1, 50 μmol photons m−2 s−1), plus an average of 233 μg of intracellular GL per gram of dcw that was released upon cell disruption (Table 1, 50 μmol photons m−2 s−1). When assayed at 180 μmol photons m−2 s−1, Synechocystis transformants generated an average of 54 μg of floating GL per gram of dcw that was siphoned off from the surface of the cultures (Table 1, 180 μmol photons m−2 s−1), plus an average of 98 μg of intracellular GL per gram of dcw that was released upon cell disruption (Table 1, 180 μmol photons m−2 s−1). Under both irradiance conditions, the GL that was released upon cell disruption accounted for 60–70% of the total GL product that was recovered.
Table 1 also reports the total product generated with an average of 360 μg of GL per gram of dcw under 50 μmol photons m−2 s−1 versus 150 μg of total GL per gram of dcw under 180 μmol photons m−2 s−1 conditions. A lower GL yield observed at 180 μmol photons m−2 s−1 as compared to 50 μmol photons m−2 s−1 is explained by the down-regulation in the activity of the endogenous cpc operon promoter, occurring upon increasing the light intensity (Formighieri and Melis 2014).
Photoautotrophic growth and cell fitness of the NaGLS transformants
In order to test if intracellular accumulation of the GL product negatively affects cell fitness, photoautotrophic growth of Synechocystis transformants was measured at 50 and 180 μmol photons m−2 s−1 over a 6-day growth period (Fig. 5). Growth rates of Synechocystis transformants expressing CpcB•NaGLS were compared to those of the wild type and to those of a strain expressing the Lavandula angustifolia β-phellandrene (C10H16) synthase fused to the phycocyanin β-subunit (CpcB•LaPHLS) (Formighieri and Melis 2015). We previously showed that expression of the CpcB•LaPHLS fusion and production of β-phellandrene do not affect cell fitness and growth, which are equivalent to strains carrying comparable genetic modifications but do not produce a heterologous product (Kirst et al. 2014; Formighieri and Melis 2014; Formighieri and Melis 2015). The strain expressing CpcB•LaPHLS was therefore employed as a control for the CpcB•NaGLS transformants, since both carry an equivalent modification of the cpc genomic locus.
Photoautotrophic growth and biomass accumulation of Synechocystis wild type and transformants. Cells were grown at either 50 μmol photons m−2 s−1 (a) or 180 μmol photons m−2 s−1 (b) of incident light intensity. Growth of wild type and transformant cultures was monitored from the dry cell weight (dcw) per milliliter culture as a function of cultivation time. Wild type, NaGLS, and LaPHLS transformants (Formighieri and Melis 2015) were compared. The latter were employed as a control, because they carry an equivalent modification of the cpc genomic locus leading to a ∆Cpc phenotype, albeit with the phellandrene synthase gene (LaPHLS). Growth rates of wild type cultures are also shown under the same experimental conditions. Averages and standard deviations were calculated from three independent lines
As a consequence of the ∆Cpc phenotype, rate of growth under low light intensities was slower for the CpcB•LaPHLS transformant than for the wild type because of the diminished light-harvesting capacity of the transformants (Fig. 5a, LaPHLS vs. wt). Such limitation was gradually alleviated, as the growth irradiance increased (Fig. 5b, LaPHLS vs. wt). This phenomenon and interpretation are consistent with the rates of growth that were previously reported for the wild type and a ΔcpcB-strain that does not make a heterologous product (Kirst et al. 2014; Formighieri and Melis 2014). The NaGLS transformants showed a slower rate of growth, when compared to the LaPHLS transformants, under both growth irradiance conditions (Fig. 5a, b, NaGLS vs. LaPHLS). This observation is consistent with the longer, by a factor of ~1.4-fold, cell duplication time of the NaGLS vs. the LaPHLS transformants (Table 2). Specifically, NaGLS transformants showed a cell duplication time of 61 and 38 h at 50 and 180 μmol photons m−2 s−1, respectively, as compared to 41 and 29 h for the LaPHLS transformants, and 27 and 22 h of the wild type (Table 2).
Microscope imaging analysis of wild type, LaPHLS, and NaGLS transformant cells. Differential interference contrast (DIC) is compared to the chlorophyll fluorescence image (F-Chl). Cells were collected from cultures grown photoautotrophically to an OD730nm of 0.9–1.4. Representative images are shown. Exposition time was 20 msec
Physiology characteristics of wild type, LaPHLS, and NaGLS transformants, as measured by the ratio of chlorophyll a content and optical density to cellular biomass (dcw). a Chlorophyll a-to-biomass ratio is expressed as μg of chlorophyll a divided by mg of dcw. b Optical density (OD)-to-biomass ratio is expressed as the absorbance at 730 nm, normalized per milligram of dcw per milliliter. Averages and standard deviations were calculated for the wild type, LaPHLS, and NaGLS transformants by systematically analyzing three independent lines
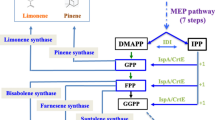
Schematic of the terpenoid biosynthetic pathway in cyanobacteria, as applied in this work. The endogenous methyl-erythritol-4-phosphate (MEP) pathway supplies carbon substrate for the synthesis of endogenous terpenoid molecules, such as carotenoids, phytol, sterols, and other prenyl molecules. Heterologous expression of the geranyllinalool synthase redirected part of the endogenous carbon flux from the geranylgeranyl-diphosphate (GGPP) pool toward synthesis of the geranyllinalool (GL) diterpene alcohol. The red arrow marks the heterologous reaction. G3P glyceraldehyde-3-phosphate, IPP isopentenyl-diphosphate, DMAPP dimethylallyl-diphosphate, GPP geranyl-diphosphate, FPP farnesyl diphosphate
Synechocystis wild type and transformant cells were further examined by microscopic imaging (Fig. 6). The objective here was to test for the presence of ghost cells among the transformants, i.e., cells that lysed with only the cell wall remaining in the suspension. Detailed comparison of differential interference contrast microscopy to chlorophyll fluorescence imaging of the cells, where only live cells can be detected, showed absence of ghost cells (Fig. 6), i.e., there was no significant difference in terms of cell viability between the NaGLS and the LaPHLS transformants, and/or the wild type.
A truncated phycobilisome antenna, resulting from the absence of the peripheral phycocyanin rods (Formighili and Melis 2015), and carbon flux toward the synthesis of a heterologous terpene did not affect synthesis and accumulation of chlorophyll a, evidenced in the case of the LaPHLS transformants (Fig. 7a, LaPHLS vs. wt). However, a lower chlorophyll a-to-biomass ratio, measured on a per dry cell weight, was noted in the NaGLS transformants (Fig. 7a, NaGLS). The NaGLS transformants also showed reduced light scattering, measured as a lower optical density (OD)-to-biomass ratio, as compared to the wild type and LaPHLS transformants (Fig. 7b, NaGLS).
Discussion
Synechocystis naturally synthesizes several terpenoid-type molecules essential for cell function, such as carotenoids, phytol, quinones, tocopherols, sterols, and other prenyl molecules, through the endogenous methyl-erythritol-4-phosphate (MEP) pathway (Fig. 8). In the Synechocystis transformants expressing the NaGLS gene, carbon flux was diverted from the endogenous pool of geranylgeranyl-diphosphate (GGPP) toward the synthesis of the heterologous geranyllinalool (GL) diterpenol. It is noteworthy that GGPP is also the terpenoid biosynthetic pathway C-20 intermediate leading to synthesis of the phytol tail of chlorophylls and C-40 carotenoids.
In contrast to hemiterpene and monoterpene hydrocarbons that spontaneously diffuse through the cell membrane and quantitatively separate from the biomass and aqueous media of Synechocystis transformants (Lindberg et al. 2010; Bentley et al. 2013; Formighieri and Melis 2014; Formighieri and Melis 2015), GL largely accumulated inside the cyanobacterial cells (Fig. 3 and Table 1). This result is attributed to the longer hydrocarbon chain of the GL and to the addition of a hydroxyl, hence a hydrophilic group, to the molecule. Both of these properties work to impede efficient diffusion through cellular membranes. Nevertheless, a fraction of GL, corresponding to 30–40% of the total product, was still able to escape from the transformant cells, and it was collected as a floating molecule on top of the aqueous culture, as the case was with monoterpene hydrocarbons.
Plants naturally produce GL to serve as defense against insects and even microbes (Lemaire et al. 1990; Chen and Viljoen 2010). The antimicrobial activity was attributed to the solubility of the molecule in the phospholipid bilayer of cell membranes, increasing the bilayer disorder (Chen and Viljoen 2010). It is, therefore, conceivable that intracellularly accumulated GL could exert some toxic effect on Synechocystis. Surprisingly, however, GL in the Synechocystis host did not preclude continuous cell growth and productivity, albeit at a slower rate. The cell duplication time was only 1.4-fold longer (Table 2), as compared to the control (Fig. 5). Otherwise, NaGLS transformants were stably cultivated over long periods of time, and the corresponding cultures consisted of viable cells, with no detectable differences in cell fitness as compared to the control (Fig. 6).
A lower chlorophyll a-to-biomass ratio was observed in the NaGLS transformants (Fig. 7a) that could be a consequence of the direct competition for GGPP substrate between GL and phytol synthesis enzymes (Fig. 8), affecting Chl distribution to the nascent photosystems (Masuda et al. 2002). However, this hypothesis seems unlikely since the terpenoid pathway in Synechocystis was shown to sustain both the synthesis of endogenous terpenoids and the generation of a heterologous terpene product, at least with rates and yields as those reported in this work (Formighieri and Melis 2014). Alternatively, the reduced chlorophyll content of the NaGLS transformants could be related to the perturbation of cellular membranes by the GL product. The latter is soluble in the phospholipid bilayer and could affect the biogenesis and assembly of thylakoid membranes, where the chlorophyll-binding photosystem complexes reside. Diminished density of thylakoid membranes in the cyanobacterial cell would also be consistent with the reduced light scattering properties of these cells, measured as lower OD-to-biomass ratio (Fig. 7b). In this respect, knowing that thylakoid membranes have a structural plasticity tied to the regulation of photosynthesis (Nagy et al. 2011; Liberton et al. 2013), it would be of interest to examine distances between and organization of the thylakoid membranes, as these might be affected by the absence of the phycobilisome and the lower chlorophyll a-to-biomass ratio in the transformants employed in this work.
In summary, the work described a biotechnology approach to exploit cyanobacteria for the production of plant-based diterpene-type molecules, such as GL. In particular, the work provided evidence for the stable transformation of Synechocystis with the Nicotiana attenuata GLS-encoding gene and the heterologous production of GL. The GL product was found to be primarily sequestered inside the cells, likely trapped in the cell-wall or phospholipid bilayer of cellular membranes. This had a negative effect on growth and cell duplication time, but the Synechocystis cells proved to be robust enough to sustain GL production up to 390 μg of GL per gram of dcw. This work informs on the possibility of generating terpenol products in cyanobacteria, and further points to the constrains of slower biomass growth rates and the necessity of cell disruption to collect the majority of the product. In contrast to NaGLS transformants, production of the monoterpene β-phellandrene, that is spontaneously exuded from the cells, does not entail adverse effects on biomass accumulation, enabling higher product yields (Formighieri and Melis 2014, 2015, 2016). This comparison points to the advantage of alleviating toxic effects that plant-based terpenoid products may exert upon their heterologous microbial hosts through efflux or detoxification mechanisms.
References
Angermayr SA, Rovira AG, Hellingwerf KJ (2015) Metabolic engineering of cyanobacteria for the synthesis of commodity products. Trends in Biotech 33:352–361
Bentley FK, Melis A (2012) Diffusion-based process for carbon dioxide uptake and isoprene emission in gaseous/aqueous two-phase photobioreactors by photosynthetic microorganisms. Biotechnol Bioeng 109:100–109
Bentley FK, García-Cerdán JG, Chen HC, Melis A (2013) Paradigm of monoterpene (β-phellandrene) hydrocarbons production via photosynthesis in cyanobacteria. BioEnergy Res 6:917–929
Brillada C, Nishihara M, Shimoda T, Garms S, Boland W, Maffei ME, Arimura G (2013) Metabolic engineering of the C16 homoterpene TMTT in Lotus japonicus through overexpression of (E,E)-geranyllinalool synthase attracts generalist and specialist predators in different manners. New Phytol 200:1200–1211
Caputi L, Aprea E (2011) Use of terpenoids as natural flavouring compounds in food industry. Recent Pat Food Nutr Agric 3:9–16
Chen W, Viljoen AM (2010) Geraniol—a review of a commercially important fragrance material. S Afr J Bot 76:643–651
De Castro KA, Byun EY, Rhee H (2009) Diketene and acetylated Meldrum’s acid: agents for a facile teprenone synthesis via Carroll rearrangement. Bull Kor Chem Soc 30:2155–2157
Eaton-Rye JJ (2011) Construction of gene interruptions and gene deletions in the cyanobacterium Synechocystis sp. strain PCC 6803. Methods Mol Biol 684:295–312
Falara V, Alba JM, Kant MR, Schuurink RC, Pichersky E (2014) Geranyllinalool synthases in Solanaceae and other angiosperms constitute an ancient branch of diterpene synthases involved in the synthesis of defensive compounds. Plant Physiol 166:428–441
Formighieri C, Melis A (2014) Regulation of β-phellandrene synthase gene expression, recombinant protein accumulation, and monoterpene hydrocarbons production in Synechocystis transformants. Planta 240:309–324
Formighieri C, Melis A (2015) A phycocyanin•phellandrene synthase fusion enhances recombinant protein expression and β-phellandrene (monoterpene) hydrocarbons production in Synechocystis (cyanobacteria). Metab Eng 32:116–124
Formighieri C, Melis A (2016) Sustainable heterologous production of terpene hydrocarbons in cyanobacteria. Photosynth Res. doi:10.1007/s11120-016-0233-2
Gershenzon J, Dudareva N (2007) The function of terpene natural products in the natural world. Nat Chem Biol 3:408–414
Heiling S, Schuman MC, Schoettner M, Mukerjee P, Berger B, Schneider B, Jassbi AR, Baldwin IT (2010) Jasmonate and ppHsystemin regulate key malonylation steps in the biosynthesis of 17-hydroxygeranyllinalool diterpene glycosides, an abundant and effective direct defense against herbivores in Nicotiana attenuata. Plant Cell 22:273–292
Jassbi AR, Gase K, Hettenhausen C, Schmidt A, Baldwin IT (2008) Silencing geranylgeranyl diphosphate synthase in Nicotiana attenuata dramatically impairs resistance to tobacco hornworm. Plant Physiol 146:974–986
Kirst H, Formighieri C, Melis A (2014) Maximizing photosynthetic efficiency and culture productivity in cyanobacteria upon minimizing the phycobilisome light-harvesting antenna size. Biochim Biophys Acta - Bioenergetics 1837(10):1653–1664
Lapczynski A, Bhatia SP, Letizia CS, Api AM (2008) Fragrance material review on geranyl linalool. Food Chem Toxicol 46:S176–S178
Lemaire M, Nagnan P, Clement J, Lange C, Peru L, Basselier J (1990) Geranyllinalool (diterpene alcohol): an insecticidal component of pine wood and termites (Isoptera: Rhinotermitidae) in four European ecosystems. J Chem Ecology 16:2067–2079
Liberton M, Page LE, O’Dell WB, O’Neill H, Mamontov E, Urban VS, Pakrasi HB (2013) Organization and flexibility of cyanobacterial thylakoid membranes examined by neutron scattering. J Biol Chem 288:3632–3640
Lindberg P, Park S, Melis A (2010) Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab Eng 12:70–79
Masuda T, Polle JEW, Melis A (2002) Biosynthesis and distribution of chlorophyll among the photosystems during recovery of the green alga Dunaliella salina from irradiance stress. Plant Physiol 128:603–614
Meeks JK, Castenholz RW (1971) Growth and photosynthesis in an extreme thermophile, Synechococcus lividus (Cyanophyta). Arch Microbiol 78:25–41
Nagy G, Posselt D, Kovács L, Holm JK, Szabó M, Ughy B, Rosta L, Peters J, Timmins P, Garab G (2011) Reversible membrane reorganizations during photosynthesis in vivo: revealed by small-angle neutron scattering. Biochem J 436:225–230
Snook ME, Johnson AW, Severon RF, Teng Q, White RA Jr, Sisson VA, Jackson DM (1997) Hydroxygeranyllinalool glycosides from tobacco exhibit antibiosis activity in the tobacco budworm [Heliothis virescens (F.)]. Agric Food Chem 45:2299–2308
Tholl D, Sohrabi R, Huh JH, Lee S (2011) The biochemistry of homoterpenes: common constituents of floral and herbivore-induced plant volatile bouquets. Phytochemistry 72:1635–1646
Wang S, Liu H, Yang Y, Fan W, Wilson IW, Wang Q, Qiu D (2015) Studies on the production of (E,E)-geranyllinalool in E. coli. J Microbiol Biotech 4:68–71
Acknowledgements
We thank the CNR Biological Imaging Facility at UC Berkeley for assistance with the microscope imaging analysis, and Dr. Zhongrui Zhou at the QB3, Chemistry Mass Spectrometry Facility, UC Berkeley, for the mass spectrometry analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Cinzia Formighieri and Anastasios Melis have read and approved the manuscript.
Electronic supplementary material
ESM 1
(PDF 112 kb)
Rights and permissions
About this article
Cite this article
Formighieri, C., Melis, A. Heterologous synthesis of geranyllinalool, a diterpenol plant product, in the cyanobacterium Synechocystis . Appl Microbiol Biotechnol 101, 2791–2800 (2017). https://doi.org/10.1007/s00253-016-8081-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-8081-8