Abstract
Anaerobic digestion of lignocellulosic waste is considered to be an efficient way to answer present-day energy crisis and environmental challenges. However, the recalcitrance of lignocellulosic material forms a major obstacle for obtaining maximum biogas production. The use of biological pretreatment and bioaugmentation for enhancing the performance of anaerobic digestion is quite recent and still needs to be investigated. This paper reviews the status and perspectives of recent studies on biotechnology concept and investigates its possible use for enhancing biogas production from lignocellulosic waste with main emphases on biological pretreatment and bioaugmentation techniques.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Producing biogas from waste organic material by anaerobic digestion (AD) increasingly attracts the worldwide interest in recent years. This technology not only could reply the increasing of energy demands but also handle the problem of environmental pollution (Pöschl et al. 2010; Jiang et al. 2011). Additionally, the residual produced during AD is a kind of high quality organic fertilizer, which is rich in nitrogen, phosphorus, and other microelements (Surendra et al. 2014). Applying the digestate to land is the most attractive option in terms of increasing agricultural production and control of soil degeneration (Feng et al. 2009).
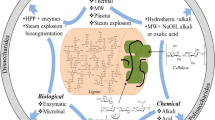
The feedstock used for biogas fermentation is very abundant, in which, lignocellulosic material is the most common and easily accessible, such as crop stalks, livestock manure, domestic waste, and some kinds of industrial waste. However, due to the recalcitrance of lignocellulosic material, the biogas yield usually is not high (Wei et al. 2014). Therefore, in order to increase biogas production from lignocellulosic material, some measurements like pretreatment and/or bioaugmentation should be adopted. At present, the pretreatment methods used for biogas production can be divided into three categories: physical, chemical, and biological pretreatments (Zhao et al. 2014b). Physical and chemical pretreatments like microwave, steam explosion, acid, alkali, or combined processes could destroy lignocellulosic structure in a short time, thus increasing the biological degradability. However, these methods make the process more expensive and possibly generate environmental toxic compounds or inhibitors (Zheng et al. 2014). Additionally, some chemicals like acid or alkali are needed for recovery or neutralization after pretreatment, which would make the process more complicated. Biological pretreatment using enzyme or microorganism to pretreat lignocellulosic material for producing biogas is a promising technology due to its environmental friendliness and cost-effectiveness, although the process is time-consuming compared with physical and chemical pretreatment (Zheng et al. 2014). The biological pretreatment in terms of enhancing biogas production will be discussed in more detail in the “Biological pretreatment” section.
Biogas fermentation is a very complicated biochemical process, which is commonly divided into four sequential stages, i.e., hydrolysis, acidogenesis, acetogenesis, and methanogenesis (Garcia et al. 2000). In the hydrolysis stage, complex organic substrates are degraded into simple organic compounds by hydrolytic microorganisms, and in the second stage, metabolites are converted into various volatile fatty acids (VFAs) and other small molecular compounds by fermentative bacteria; then homoacetogenic or syntrophic bacteria produce acetate at the third stage; lastly, the small molecular metabolites produced by bacteria, like H2, CO2, acetate, formate, and simple methylated compounds are converted to methane by methanogenic archaea (Fig. 1). It has been confirmed that each stage is carried out by different microorganisms, the successive methanogenesis depends on the balance of the four steps, and any rate-limiting step would limit the overall rate of biogas production (Vanwonterghem et al. 2014). Therefore, using bioaugmentation technology to increase the activity of microorganisms involved in the rate-limiting stage is an attractive option to increase the biogas yield. In this paper, I reviewed the progress in biotechnology with more focus on increasing biogas production from lignocellulosic materials of recent years.
Lignocellulosic biomass and the decomposers
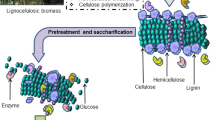
Lignocellulosic biomass is mainly composed of cellulose (35–45 %), hemicellulose (25–40 %), and lignin (5–25 %) (Zhang et al. 2015). The first two are carbohydrates while the last one is an aromatic tridimensional polymer (Rodrjguez et al. 1997). These components are tightly associated with each other to form a rigid and recalcitrant structure. Structures of cellulose, hemicelluloses, and lignin are described in several reviews (see references (Nanda et al. 2013; Payne et al. 2015; Kameshwar and Qin 2016; Kuhad et al. 2016)). The composition of lignocellulose varies from species to species, and even the same lignocellulose biomass may differ in composition between batches harvested in different seasons (Van Dyk and Pletschke 2012). As reported by Vasco-Correa and Li (2015), time of harvest had a great impact on the biogas production from Miscanthus sinensis, and the performance of delignification even if the pretreatment was carried out by the same fungus.
Cellulose is a polymer consisting of glucose units that are connected to each other by β-1-4 glycosidic bonds (Li et al. 2014). The complete hydrolysis of cellulose is synergistic carried out by at least three groups of cellulolytic activities, including endoglucanase (EC 3.2.1.4), exoglucanase (E.C.3.2.1.176) (EC 3.2.1.91), and β-glucosidase (EC3.2.1.21) (Juturu and Wu 2014). The first two kinds of enzymes act together to hydrolyze cellulose liberating cellobiose or cellooligosaccharides as major products, and the β-glucosidase further hydrolyzes the soluble oligosaccharides (mainly cellobiose) to glucose (Kuhad et al. 2016). Normally, cellulase-producing microbes produce two kinds of cellulases; one is extracellular enzyme complex, which is typically secreted by aerobes; the other is multienzyme cellulase complexes, known as cellulosomes, and most are expressed on the surface of anaerobes (Tsavkelova and Netrusov 2012). Presently, the degradation mechanism of these two kinds of cellulases still needs to be further studied, but it is clear that the aerobes and anaerobes operate in different systems.
In natural environment, there are a wide variety of microbes that could produce cellulase, including fungi, bacteria, actinomycetes, and yeast (Kuhad et al. 2016), but only relatively few could produce high titers of cellulase required at industrial scale and most are not capable of producing all the three cellulases for complete degradation of crystalline cellulose (Sukumaran et al. 2005). In present, thermophilic cellulolytic fungi have been studied in detail due to their higher cellulase productivity and the ability to produce thermostable cellulases, which could be used in a variety industry including animal feed, food, textiles, and detergents and in the paper industry (Bhat and Bhat 1997). In recent years, psychrophilic cellulolytic microorganisms are becoming more attractive because of their potential industrial applications. However, most isolated cold active cellulase-producing microorganisms are not true psychrophiles but facultative psychrophiles and can grow at 30–35 °C (Kasana and Gulati 2011).
Hemicellulose, which includes xylan, glucuronoxylan, arabinoxylan, glucomannan, and xyloglucan, is more varied in structure and composition than cellulose (Van Dyk and Pletschke 2012). It contains many different sugar monomers, such as glucose, xylose, mannose, galactose, rhamnose, and arabinose as well as sugar acids like methylglucuronic and galaturonic acids (Perez et al. 2002). In contrast to cellulose, hemicellulose is relatively easy for hydrolysis, and the produced monomeric sugars and acetic acid can be subjected to bioconversion for biogas and other useful byproducts (Nanda et al. 2013). Due to the complicated composition, biodegradation of hemicellulose requires a large number of enzymes, including endo-xylanase, acetyl xylan esterase, β-xylosidase, endo-mannanase, β-mannosidase, α-L-arabinofuranosidase, α-glucuronidase, ferulic acid esterase, α-galactosidase, and p-coumaric acid esterase. These enzymes can be divided into two categories: one is depolymerising enzymes, which cleave the backbone, and the other is to remove substituents, which may pose steric hindrances to the depolymerising enzymes (Van Dyk and Pletschke 2012). The major hemicellulose-degrading enzymes are endo-xylanase and endo-mannanase (Singh et al. 2010). Many species of fungi and bacteria including actinomycetes could produce these kinds of enzymes (Beg et al. 2001).
Lignin is a complex polyphenyl aromatic compound linked with ester bonds and tightly binds with cellulose and hemicellulose to form plant primary and secondary cell wall (Nanda et al. 2013). The monolignol monomers of lignin are p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol, which are assembled through the dehydrogenative in varying proportions (Chen et al. 2012). Under anaerobic condition, lignin is resistant to degradation and thus forms a major obstacle for the effective utilization of cellulose and hemicellulose (van Kuijk et al. 2015). Therefore, destroying the structure of lignin is one of the key ways to enhance the degradation efficiency of cellulose and hemicellulose and thus facilitate biogas production. A wide range of microorganisms such as bacteria, actinomycetes, cyanobacteria, and fungi are found to be efficient in degrading lignin (Kuhad et al. 2013). Among them, white rot fungi are most effective in delignification due to their unique ligninolytic systems (Kuhad et al. 2013). Up to now, five major groups of extracellular oxidative enzymes, which play a key role in lignin decomposition, have been discovered; they are lignin peroxidases (EC1.11.1.14), manganese peroxidases (EC 1.11.1.13), versatile peroxidases (EC1.11.1.16), laccases (EC 1.10.3.2), and a new class of enzyme of dye-decolorizing peroxidases (EC 1.11.1.19) (van Dyk and Pletschke 2012). Besides, several accessory enzymes including glyoxal oxidases and alcohol oxidases have been found to play a role in the lignin degradation (Furukawa et al. 2014).
Since lignin is a source of aromatics, the degradation of lignin will release phenolic compounds and furan derivatives like furfural and 5-hydroxymethyl-2-furaldehyde (HMF), which have been proven to have an inhibitory effect on biogas production (Schroyen et al. 2015). Hernandez and Edyvean (2008) confirmed that biogas production from the digestion of glucose, yeast extract, and nutrient broth could be reduced with 20 % by 25 mg phenolic compounds per gram volatile suspended solids. However, the amount of inhibitors released from the destruction of lignin varies between different feedstocks due to different lignin content. Schroyen et al. (2014, 2015) compared the effects of ligninolytic enzymes pretreatment on the production of phenolic compounds and the biomethane potential of various lignocellulosic substrates. Their results showed that the individual phenolic compounds did not reach the reported inhibition levels even though the initial concentration of total phenolic compounds was higher in the substrates containing more lignin. Despite this, the anaerobic digestion of the substrates containing more lignin resulted in a significantly lowered biomethane production. Although some kinds of fungi could digest phenolic compounds released from the decomposition of lignin and reduce the potential inhibition to the subsequent biogas production (Wan and Li 2012), the higher lignin concentrations are still disadvantageous to AD due to the inhibiting compounds, acting as toxins, together with the remaining lignin seal around the cellulose structure (Kudahettige et al. 2016). Therefore, when using lignin-containing material for biogas production, the lignin content as well as the pretreatment method is very important.
Biological pretreatment
Currently, the biological pretreatment used for enhancing biogas production from lignocellulosic material can be divided into three categories: pure culture, mixed culture, and enzymatic pretreatment. Table 1 shows these three biological pretreatments along with their advantages and disadvantages.
Enzymatic pretreatment, compared to microbial pretreatment, is attracting more interest in treating lignocellulosic material for biogas production due to several merits of enzymes: (1) the working condition of enzyme is simpler than that of living cells since enzymes do not need nutrient for growth; (2) enzymes can work in the presence of bacteriophage, various toxins, and inhibitors of some kinds of microbial metabolism; and (3) it is easy for enzymes to access the substrate due to their smaller size, higher solubility, and mobility than microbes (Romero-Güiza et al. 2016). Presently, crude or commercial enzymes, which mainly produced by fungi, have been frequently used to pretreat lignocellulosic biomass for enhancing biogas production (Table 2). In which, cellulase, xylanase, pectinase, and laccase are the most commonly used (Rouches et al. 2016a). Due to the complication of feedstock, enzymes are always used in combination or with other pretreatment method, like alkali pretreatment. As an example, Michalska et al. (2015) pretreated two species of energy crops of Miscanthus giganteus and Sida hermaphrodita with sodium hydroxide solution followed by enzymatic pretreatment of cellulase and cellobiase. The AD results showed that the two-step pretreatment process was more efficient with regard to biogas production by about 30 % higher than the sole enzymatic hydrolysis. However, how to combine the enzymes and ascertain the ratio are dependent on the components of feedstock, since the content of cellulose, hemicellulose, and lignin is different in different lignocellulosic materials. The biogas yield would be different using different feedstocks even if pretreated with the same enzymes. For example, since the lignin content of sugar beet pulp is lower than that of spent hops, after pretreatment by the same enzymes, the former produced much higher reducing sugars and as a result, much higher biogas production (Zieminski et al. 2012). In addition, the biogas yield from the same feedstock might be different if it was pretreated with different enzymes. Passos et al. (2016) studied the biogas production from microalgal biomass pretreated with commercial enzymes of cellulase, glucohydrolase, and an enzyme mix composed of cellulase, glucohydrolase, and xylanase; their results showed the methane yield was significantly higher for the enzyme mix (15 %) and cellulase (8 %) as compared to that for the control.
Actually, the performance of enzymatic pretreatment is determined by many factors including species of enzymes, substrates, incubation time, system configuration, and the environmental conditions, like pH and temperature (Romano et al. 2009). The pretreatment conditions could be configured according to the characterization of the enzyme, while other factors may require a great deal of research to address for obtaining higher biogas production. For instance, a lot of researchers in the literature have studied the effects of enzyme/substrate ratio on saccharification and the biogas production, and their results showed that appropriate ratio was needed due to the fact that the cost of enzyme is high and higher enzymes concentration do not always cause further increase in soluble carbohydrate concentrations (Antonopoulou and Lyberatos 2012). Another situation that should be considered during the enzymatic pretreatment is that reducing sugars released from the hydrolization might be consumed by indigenous feedstock microorganisms. Therefore, sterilization might be important. However, this process is energy-consuming and would increase the overall cost of enzymatic pretreatment.
In comparison to enzymatic pretreatment, directly adding enzyme to the digesters is an attractive design because it eliminates the need of additional reactors or equipments, although this method is not considered to be a pretreatment. However, the situation in the literature is not optimistic. Romano et al. (2009) added an enzyme mixture of cellulase, hemicellulase, and β-glucosidase directly to a single-stage digester using wheat grass as substrate for AD at 50 °C; results showed that the enzymes had no significant effect on methane generation. Likewise, a negligible improvement was found by Sutaryo et al. (2014) after adding a mixture of enzymes to batch and continuous anaerobic reactors treating dairy cattle manure. Donoso-Bravo et al. (2016) even obtained negative results when using a commercial enzyme mixture containing hemicellulase, cellulase, xylanase, and pectinase, and other activities to treat olive mill waste; their results showed that less methane production was attained with more addition of the enzymatic mix. Other studies using sludge as the feedstock for AD reported different results on the effectiveness of enzyme addition, with some positive results (Donoso-Bravo and Fdz-Polanco 2013; Yu et al. 2013) and some negative results (Diak et al. 2012). This may be explained by the low specificity of those commercial enzyme mixtures as well as by the presence of other degradable compounds (Donoso-Bravo et al. 2016).
Presently, the effect of enzymatic pretreatment on enhancing biogas production in most cases is still lower than that of physical or chemical pretreatments, but it is still promising due to its low energy requirement and environmental friendliness. Another drawback of enzymatic pretreatment that should be noted is the high enzyme cost, additional research for improving enzyme production on strain mutation, genetic engineering, protoplast fusion, and process optimization might be helpful. Interestingly, a number of lignocellulase genes with thermostability, alkalostability, overcoming feed-back inhibition, and other economically important traits have been cloned and expressed (Kuhad et al. 2016). Lima et al. (2016) used four Aspergillus nidulans recombinant strains to simultaneously produce a multi-enzymatic cocktail of arabinofuranosidase, endo-1,4-xylanase, endo-1,5-arabinanase, and xyloglucan-specific endo-β-1,4-glucanase; the recombinant enzymatic pretreatment was residue-free and seemed to be more efficient than the applied alkaline method. With the developing of biotechnology, the use of enzymatic pretreatment for enhancing biogas production from lignocellulosic materials could be unquestionable economically wise in the future.
The ultimate advantage of microbial pretreatment over free enzymes is due to the fact that microorganism can regenerate and produce different enzymes depending on the given substrate (Parawira 2012). Additionally, microbial pretreatment avoids the fussy steps to isolate and purify enzymes. However, the regeneration and growth of microorganism requires extended time periods (usually several days to weeks) and would consume the substrates, thus negatively affect the performance of subsequent AD. Numerous researchers have observed the dry mass loss of feedstock after pretreated with microorganism, and dry mass loss increases with the prolonging of pretreatment time. For instance, Liu et al. (2014a) pretreated corn stover silage using Phanerochaete chrysosporium in solid-state fermentation to enhance methane production. The highest methane yield was achieved on the 25th day of pretreatment, which was 23.0 % higher than that of the untreated corn stover silage, while the maximum dry mass loss of 14.2 % was reached at the 30th day of pretreatment. Thus, an effective pretreatment time requires a tradeoff between the dry mass loss and the degradation of lignocellulosic material.
The performance of microbial pretreatment varies according to the stains, cultivation conditions, and the type of lignocellulosic materials (Table 3). Inappropriate pretreatment could not increase or even decrease the biogas production compared with the untreated group. For instance, López et al. (2013) pretreated four kinds of lignocellulosic wastes (wood fiber, grass, corn stover, and wheat straw) with fungus Phanerochaete flavidoalba to improve the AD. The pretreatment had a positive effect on improving anaerobic biodegradability of corn stover, grass, and wheat straw, but failed to improve the subsequent biogas production; the biogas production was enhanced only in wood fiber group. Nuchdang et al. (2015) observed a negative effect on biogas production when using fungi Coprinopsis cinerea and Polyporus tricholoma to pretreat paragrass, although the fungi could shift the maximum methane production rate to an earlier date compared to the control; the methane yield of the pretreated paragrass was approximately 15 % lower than that of the untreated paragrass.
Due to the highly efficient lignocellulolytic enzyme system, the pure culture pretreatment mainly focus on fungal pretreatment. Rot fungi, including brown-, white-, and soft-rot, are the most usually used for pretreatment of lignocellulosic material (Zheng et al. 2014). In addition, some kinds of mushrooms also can be used for pretreating and improving biogas production (Miiller and Trfisch 1986; Bisaria er al. 1990; Mackuľak et al. 2012). The other specific species, which can produce high titer lignocellulase, also can be used for pretreatment. Munoz et al. (2014) showed a microalgal pretreatment method using cellulolytic bacteria that naturally degrades microalgae in their native habitat. They pretreated Nannochloropsis gaditana with Raoultella rnithinolytica strains MC3 and MA5 for improving biogas production, and the results showed that the pretreatment could increase the yield of methane by 140.32 and 158.68 %, respectively, over that from nonpretreated microalgae. Besides, ensilage, which is mainly carried out by Lactobacillus species, is also considered to be a potential pretreatment method to stimulate biogas production. During the ensiling process, silage bacteria act on the cellulose and carbohydrates to produce VFAs such as acetic, propionic, lactic, and butyric acids. Many researchers have proved that silage is a useful feedstock for AD. For instance, Liu et al. (2014b) investigated the effects of Phanerochaete chrysosporium pretreatment on the biodegradability and subsequent anaerobic production of biogas from corn stover and corn stover silage. Their results showed that the peak levels of daily biogas production and the CH4 yield from corn stover silage were approximately twice that of corn stover.
Since the type of lignocellulolytic enzymes produced by single stain is limited, one of the strategies to increase the biogas production of pure culture pretreatment is to combine it with other pretreatment methods. For example, Alexandropoulou et al. (2016) compared the biogas production from willow sawdust pretreated with different methods, i.e., fungal pretreatment via the white rot fungi Leiotrametes menziesii and Abortiporus biennis, alkaline, and the combined alkaline and fungous. The maximum methane production was observed for the combined alkaline and A. biennis pretreatment and was 12.5 and 50.1 % higher than the corresponding alkaline and fungal pretreatment alone and 115 % higher than the raw willow sawdust.
In order to decrease the dry mass loss and prevent indigenous feedstock microorganism from consuming the reducing sugars released from the hydrolization in the pretreatment process, pure culture pretreatment is usually conducted under sterilized conditions. However, the research carried out by Zhao et al. (2014a) showed that methane yields from fungal pretreatment of unsterilized yard trimmings using yard trimmings pre-colonized with Ceriporiopsis subvermispora as an inoculum were comparable to those obtained by using the traditional method requiring feedstock sterilization. The technique can save about 501–789 kJ/kg of dry yard trimmings processed, which is about half of the total biogas energy produced by the AD. Another strategy of decreasing the dry mass loss is to use anaerobic microorganism to pretreat the feedstock; as under anaerobic condition, the loss of carbon dioxide is usually lower than that of at aerobic condition. The prevalent approach presently is to add the anaerobic lignocellulolytic microorganism directly into the biogas reactor, which is known as bioaugmentation, and will be discussed in the “Bioaugmentation” section.
Mixed cultures, i.e., microbial consortium, increasingly attracts more attention in pretreating lignocellulosic material for AD due to several advantages: (1) microbial consortium contains complex enzymes needed in the degradation of lignocellulosic material and thus could effectively improve the degradation rate, while most pure culture only contains one or some of the enzymes, and thus makes the degradation inefficiently; (2) each microbe in the microbial consortium works synergistically and comes into being a functional microecosystem with a wide range of tolerance to various physical and chemical conditions; and (3) it is not necessary to sterilize feedstock for mixed culture pretreatment, as the activity of indigenous feedstock microorganism is inhibited by the strong function of microbial consortium (Yu et al. 2016). Presently, there are two strategies to obtain the microbial consortium; one is screened and constructed from natural environments, and the other is directly obtained from some specific environments, like rumen and anaerobic digesters. Several efficient microbial consortiums in terms of improving biogas production have been constructed by different research groups. MC1, a structurally stable, thermophilic consortium, was constructed by a succession of enrichment cultures from compost, and it was found to be capable of effectively increasing methane production from various lignocellulosic materials such as cotton stalk, rotted silage maize straw, municipal solid waste, filter paper, office paper, newspaper, and cardboard (Yuan et al. 2012, 2014, 2016; Hua et al. 2016). In another research group’s work, Poszytek et al. (2016) isolated over 100 strains of cellulose-degrading bacteria from sewage sludge, agricultural biogas sludge, cattle slurry, and manure, and chose 16 strains with high cellulolytic activity (consisting of Bacillus, Providencia, and Ochrobactrum genera) to construct a microbial consortium, called MCHCA, which is capable of efficient hydrolysis of maize silage, and increases biogas production by even 38 %. In addition, a mesophilic lignocellulolytic microbial consortium BYND-5, mainly composed of Firmicutes, Bacteroidetes, Deferribacteres, Proteobacteria, Lentisphaerae, Fibrobacteraceae, and uncultured bacterium, was established by successive subcultivation by Yan et al. (2012). BYND-5 can degrade more than 49 % of rice straw within 7 days at 30 °C under static conditions and increase total biogas yield by 9.3 % more than control.
The use of natural microbial consortium like rumen liquid and liquid fraction of some special digestates should be cost-effective, as which avoids the steps of screening and isolation. Zhang et al. (2016) reported that rice straw was pretreated for 24 h at 39 °C with rumen fluid under anaerobic conditions, resulting in 66.5 % more biogas production, 82.6 % more methane yield, and 40.0 % shorter technical digestion time compared with those under the control. Baba et al. (2013) evaluated the effect of rumen fluid-pretreatments on the methane production of waste paper at 37 °C. Their results showed that 6-h pretreatment was considered the optimal, resulting in 2.6 times higher of the best daily methane yield, 73.4 % of the theoretical methane yield compared with the untreated paper. Hu et al. (2015) used the liquid fraction of digestate from anaerobic digester with corn stover as substrate to promote anaerobic biogasification of corn stover at ambient temperature (20 ± 1 °C). Their results showed that 3-day pretreatment was considered to be optimal, resulting in 70.4 % more biogas production, 66.3 % more biomethane yield, and 41.7 % shorter technical digestion time compared with those in the untreated stover. Wei et al. (2015) compared the effects of liquid fraction of digestate, ammonia solution, and NaOH pretreatments on the process of mesophilic anaerobic co-digestion of cattle manure and corn stover. The results showed that the biological pretreatment not only achieved the same effects as chemical pretreatment at the performance of AD but also reduced the technical digestion time and improved the buffer capacity of AD system.
Interestingly, the microbes in the mixed cultures are not always lignocellulolytic. For example, Kato et al. (2004) found the cellulose degradation performance of Clostridium straminisolvens CSK1 was remarkably lower than that of the original microflora. However, when C. straminisolvens CSK1 was mixed with aerobic non-cellulolytic bacteria isolated from the original microflora; the cellulose degradation performance was increased significantly. The non-cellulolytic bacteria might essentially contribute to the cellulose degradation by supplying suitable environment conditions, and/or by consuming metabolites, which otherwise deteriorate the cellulolytic activity (Kato et al. 2004). Usually, the structures of microbial consortium obtained both by the artificial screening and from some specific natural environment conditions are very stable, as the former is often screened for decades of generations, like MC1 which composition did not change after more than 20 subcultures (Kato et al. 2005), as well as the later, which had been screened under the natural environmental conditions for a very long time (Hu et al. 2015). However, when they were used to pretreat the unsterilized feedstock, the microbial composition still could be changed little or more depending on the ratio of the inoculums to the indigenous feedstock microorganism. For example, when Yu et al. (2016) used MC1 to accelerate the acidification of corn stalks and cow dung to improve the biogas production under unsterilized and sterilized conditions, the microbial composition did not change obviously in the sterilized system, while the abundance of members of MC1, such as Bacillus and Clostridium, increased clearly on day 3 under unsterilized system, and with the prolonging of pre-cultivation time, MC1 nearly disappeared from the unsterilized system. Nevertheless, MC1 clearly improved the organic acid production on day 3 and which was enough to improve the biogas production (Yu et al. 2016).
Bioaugmentation
Bioaugmentation is the practice of adding selected strain/s or mixed cultures to biological systems to improve the catabolism of specific compounds, e.g., refractory organics. This method is especially used in soil and water bioremediation when indigenous microorganisms are rare or not able physiologically to perform the degradation process (Semrany et al. 2012; Herrero and Stuckey 2015). Recently, it was introduced into AD processes as an alternative method to eliminate some refractory compounds such as lignocellulosic materials and to increase the yield of biofuel products like ethanol, hydrogen, and methane.
Using bioaugmentation technique to improve biogas production from lignocellulosic materials has several potential advantages over the biological pretreatment methods: (1) saving the time used for biological pretreatment; (2) simplifying the process and thus reducing the costs, as the microorganism used for bioaugmentation is added directly into the anaerobic reactors, which eliminates the need of additional reactors or equipments; (3) avoiding the problem of dry matter loss that frequently appears in the aerobic pretreatment process, and this would increase the potential methane production since the organic carbons would have more chances to be converted into methane than carbon dioxide compared to that of under aerobic pretreatment conditions; and (4) delimiting the toxicity result from the accumulation of organic acids or ammonia (Westerholm et al. 2012; Fotidis et al. 2013; Fotidis et al. 2014; Town and Dumonceaux 2016). Basically, there are two options for performing bioaugmentation in anaerobic digester; one is the addition of a pure strain, and the other is the addition of a consortia. As mentioned above (“Biological pretreatment” section), the use of microbial consortia might be a better choice than pure culture.
Since the four sequential steps of biogas production are carried out by different microorganisms, hydrolytic bacteria/fungus, hydrogen-producing bacteria, acetate-type fermentation bacteria, and methanogenic achaea, all could be used theoretically for bioaugmentation in enhancing biogas production, and many studies in the literature confirmed that this was indeed the case. However, when lignocellulosic material is used as the sole feedstock, the step of hydrolysis is considered to be rate-limiting; therefore, bioaugmenting the lignocellulolytic microbes is considered to be able to accelerate acidification and significantly increase the biogas production. Table 4 shows the performance of recent studies on the bioaugmentation techniques used for enhancing biogas production from various lignocellulosic materials.
It should be noted that the successfulness of bioaugmentation requires that the introduced strain/s could survive for a long time in the anaerobic digester. However, the selected strain/s often fail to grow or to be active due to predation or competition with the indigenous microorganism, presence of bacteriophages and protozoa, or to a lack of acclimation to the environmental conditions (Herrero and Stuckey 2015). Many studies in the literature found that the positive effects of bioaugmentation on the performance of bioreactor were only maintained for a short time after inoculation. For example, when anaerobic fungi isolated from feces or rumen fluid of cows and deer were tested for their ability to integrate into the anaerobic bacterial ecosystem used for biogas production from energy crops, Procházka et al. (2012) found that the fungi improved the biogas production by 4–22 % depending on the substrates and the fungi species used. However, all the anaerobic fungi did not show long-term survival in the fermenters. Similarly, in Cater’s work, Pseudobutyrivibrio xylanivorans Mz5T, Fibrobacter succinogenes S85, Clostridium cellulovorans, and Ruminococcus flavefaciens 007C were used to bioaugment lignocellulosic substrate hydrolysis for enhancing the biogas production. The bioaugmentation was proved to be successful in methane production enhancement but most of the introduced strains were undetectable in the microbial community at the end of the experiment (day 30) (Cater et al. 2015). In order to keep the bioaugmented microorganism stable in the fermentation process, regular resupplementing of the introduced microorganism would be a promising approach to elevate the biogas production. In several recent researches, the effects of different bioaugmentation patterns on the biogas production were compared. For example, Martin-Ryals et al. (2015) and Yang et al. (2016) reported that the routine and repeated batch bioaugmentation were more effective in improving methane production than the one-time bioaugmentation pattern. Additionally, immobilization technique of bioaugmented microbial cells is also an efficient option to increase the survival time of introduced strain/s, but this method is mostly used in wastewater treatment (Weiß et al. 2010). As for the solid-state biogas fermentation, immobilizing bioaugmented microorganism is still difficult.
Another situation that should be pointed out is that the microbial community could be changed after the bioaugmentation candidates are introduced into the bioreactor, and these changes are dependent on the species of bioaugmented microorganism, the ratio of bioaugmented microorganism to biogas inoculum, and the bioaugmentation patterns. Neumann and Scherer (2011) used compost to augment the continuous anaerobic digestion of fodder beet silage; the results showed the addition of compost induced a methanogenic community change towards hydrogenotrophic methanogens. Acs et al. (2015) demonstrated the mechanism of bioaugmentation by a single mesophilic hydrogen-producing bacteria (Enterobacter cloacae) added to the natural biogas-producing microbial community. After the addition of E. cloacae, the community underwent pronounced changes and a group of unknown Clostridiales and a close relative of C. pasteurianum increased in abundance spectacularly. Yang et al. (2016) studied the effects of bioaugmentation patterns and the ratio of enriched microbial consortia to seed sludge on methane production from effluents of hydrogen-producing stage of potato slurry, as well as on the indigenous bacterial community; they found that bioaugmentation pattern strongly altered bacterial community structure, and increasing the ratio of bioaugmented consortia to seed sludge led to a stepwise increase in the relative abundances of some kinds of bacteria and archaea, respectively. The changes of the microbial community might be due to the competition for substrate and/or specific ecological niches between bioaugmented microorganism and indigenous populations, the inhibition from antibiotics or some kinds of metabolic inhibitors (Veen et al. 1997).
Conclusions and future perspectives
The use of biological pretreatment and bioaugmentation technique to maximize biogas production from lignocellulosic waste is attracting more attention due to their capacity of being environment friendly and cost-effective. To date, using enzyme to pretreat lignocellulosic waste for increasing biomethane production in the full-scale biogas plant is still limited due to the high cost. How to greatly diminish the cost of enzyme is urgently needed to be further studied, since the enzymatic pretreatment is obviously advantageous compared to the microbial pretreatment except the cost. Besides, the addition of lignocellulolytic enzymes directly to biogas reactor is very convenient, but which is little favorable to enhancing biogas production. The reason and the mechanism are not clear yet. Hence, it will be interesting if the biogas production could be greatly increased by adding the lignocellulolytic enzymes directly to anaerobic reactor.
Recombinant strains, which rely on plasmids for foreign gene expression, can simultaneously produce various enzymes like cellulase, hemicellulase, and ligninase or achieve new capability such as wide pH tolerance, toxin resistance, etc. as required. Using recombinant strains to treat lignocellulosic waste or bioaugment, the hydrolysis for enhancing biogas production is interesting although it is still scarce in the available literature. However, this technique is gaining attentions in other fields like biohydrogen and bioethanol production. With the developing of biotechnology, recombinant strains would be frequently used in the AD system since they have the potential to increase the biogas production.
References
Acs N, Bagi Z, Rakhely G, Minarovics J, Nagy K, Kovacs KL (2015) Bioaugmentation of biogas production by a hydrogen-producing bacterium. Bioresour Technol 186:286–293
Akila G, Chandra TS (2010) Stimulation of biomethanation by Clostridium sp. PXYL1 in coculture with a Methanosarcina strain PMET1 at psychrophilic temperatures. J Appl Microbio 108:204–213
Alexandropoulou M, Antonopoulou G, Fragkou E, Ntaikou I, Lyberatos G (2016) Fungal pretreatment of willow sawdust and its combination with alkaline treatment for enhancing biogas production. J Environ Manage, in press, corrected proof
Antonopoulou G, Lyberatos G (2012) Effect of pretreatment of sweet sorghum biomass on methane generation. Waste Biomass Valori 4:583–591
Aydin S (2016) Enhancement of microbial diversity and methane yield by bacterial bioaugmentation through the anaerobic digestion of Haematococcus pluvialis. Appl Microbiol Biotechnol 100:5631–5637
Baba Y, Tada C, Fukuda Y, Nakai Y (2013) Improvement of methane production from waste paper by pretreatment with rumen fluid. Bioresour Technol 128:94–99
Beg QK, Kapoor M, Mahajan L, Hoondal GS (2001) Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol 56:326–338
Bhat MK, Bhat S (1997) Cellulose degrading enzymes and their potential industrial applications. Biotechnol Adv 15:583–620
Bisaria R, Vasudevan P, Bisaria VS (1990) Utilization of spent agro-residues from mushroom cultivation for biogas production. Appl Microbiol Biotechnol 33:607–609
Cater M, Fanedl L, Malovrh S, Logar RM (2015) Biogas production from brewery spent grain enhanced by bioaugmentation with hydrolytic anaerobic bacteria. Bioresour Technol 186:261–269
Chen YR, Sarkanen S, Wang YY (2012) Lignin-degrading enzyme activities. Methods Mol Biol 908:251–268
Diak J, Ormeci B, Kennedy KJ (2012) Effect of enzymes on anaerobic digestion of primary sludge and septic tank performance. Bioprocess Biosyst Eng 35:1577–1589
Donoso-Bravo A, Fdz-Polanco M (2013) Anaerobic co-digestion of sewage sludge and grease trap: assessment of enzyme addition. Process Biochem 48:936–940
Donoso-Bravo A, Ortega-Martinez E, Ruiz-Filippi G (2016) Impact of milling, enzyme addition, and steam explosion on the solid waste biomethanation of an olive oil production plant. Bioprocess Biosyst Eng 39:331–340
Ehimen EA, Holm-Nielsen JB, Poulsen M, Boelsmand JE (2013) Influence of different pre-treatment routes on the anaerobic digestion of a filamentous algae. Renew Energ 50:476–480
El-Mashad HM (2015) Biomethane and ethanol production potential of Spirulina platensis algae and enzymatically saccharified switchgrass. Biochem Eng J 93:119–127
Feng T, Cheng S, Min Q, Li W (2009) Productive use of bioenergy for rural household in ecological fragile area, Panam County, Tibet in China: the case of the residential biogas model. Renew Sust Energ Rev 13:2070–2078
Fotidis IA, Karakashev D, Angelidaki I (2013) Bioaugmentation with an acetate-oxidising consortium as a tool to tackle ammonia inhibition of anaerobic digestion. Bioresour Technol 146:57–62
Fotidis IA, Wang H, Fiedel NR, Luo G, Karakashev DB, Angelidaki I (2014) Bioaugmentation as a solution to increase methane production from an ammonia-rich substrate. Environ Sci Technol 48:7669–7676
Furukawa T, Bello FO, Horsfall L (2014) Microbial enzyme systems for lignin degradation and their transcriptional regulation. Front Biol 9:448–471
Garcia JL, Patel BKC, Ollivier B (2000) Taxonomic, phylogenetic, and ecological diversity of methanogenic archaea. Anaerobe 6:205–226
Hernandez JE, Edyvean RG (2008) Inhibition of biogas production and biodegradability by substituted phenolic compounds in anaerobic sludge. J Hazard Mater 160:20–28
Herrero M, Stuckey DC (2015) Bioaugmentation and its application in wastewater treatment: a review. Chemosphere 140:119–128
Hu Y, Pang Y, Yuan H, Zou D, Liu Y, Zhu B, Chufo WA, Jaffar M, Li X (2015) Promoting anaerobic biogasification of corn stover through biological pretreatment by liquid fraction of digestate (LFD). Bioresour Technol 175:167–173
Hua B, Dai J, Liu B, Zhang H, Yuan X, Wang X, Cui Z (2016) Pretreatment of non-sterile, rotted silage maize straw by the microbial community MC1 increases biogas production. Bioresour Technol 216:699–705
Jiang X, Sommer SG, Christensen KV (2011) A review of the biogas industry in China. Energ Policy 39:6073–6081
Juturu V, Wu JC (2014) Microbial cellulases: engineering, production and applications. Renew Sust Energ Rev 33:188–203
Kameshwar AK, Qin W (2016) Recent developments in using advanced sequencing technologies for the genomic studies of lignin and cellulose degrading microorganisms. Int J Biol Sci 12:156–171
Karray R, Hamza M, Sayadi S (2015) Evaluation of ultrasonic, acid, thermo-alkaline and enzymatic pre-treatments on anaerobic digestion of Ulva rigida for biogas production. Bioresour Technol 187:205–213
Karray R, Hamza M, Sayadi S (2016) Production and characterization of enzymatic cocktail produced by Aspergillus niger using green macroalgae as nitrogen source and its application in the pre-treatment for biogas production from Ulva rigida. Bioresour Technol 216:622–628
Kasana RC, Gulati A (2011) Cellulases from psychrophilic microorganisms: a review. J Basic Microbiol 51:572–579
Kato S, Haruta S, Cui ZJ, Ishii M, Igarashi Y (2004) Effective cellulose degradation by a mixed-culture system composed of a cellulolytic Clostridium and aerobic non-cellulolytic bacteria. FEMS Microbiol Ecol 51:133–142
Kato S, Haruta S, Cui ZJ, Ishii M, Igarashi Y (2005) Stable coexistence of five bacterial strains as a cellulose-degrading community. Appl Environ Microbiol 71:7099–7106
Kinet R, Destain J, Hiligsmann S, Thonart P, Delhalle L, Taminiau B, Daube G, Delvigne F (2015) Thermophilic and cellulolytic consortium isolated from composting plants improves anaerobic digestion of cellulosic biomass: toward a microbial resource management approach. Bioresour Technol 189:138–144
Kovacs KL, Acs N, Kovacs E, Wirth R, Rakhely G, Strang O, Herbel Z, Bagi Z (2013) Improvement of biogas production by bioaugmentation. Biomed Res Int 2013:482653
Kudahettige NRL, Holmgren M, Madavi B, Nilsson RT, Sellstedt A (2016) Adaptability of Trametes versicolor to the lignocellulosic inhibitors furfural, HMF, phenol and levulinic acid during ethanol fermentation. Biomass Bioenerg 90:95–100
Kuhad RC, Deswal D, Sharma S, Bhattacharya A, Jain KK, Kaur A, Pletschke BI, Singh A, Karp M (2016) Revisiting cellulase production and redefining current strategies based on major challenges. Renew Sust Energ Rev 55:249–272
Kuhad RC, Kuhar S, Sharma KK, Shrivastava B (2013) Microorganisms and enzymes involved in lignin degradation vis-à-vis production of nutritionally rich animal feed: an overview. Biotechnol Environ Manage Resource Recov 3–44
Lü F, Ji J, Shao L, He P (2013) Bacterial bioaugmentation for improving methane and hydrogen production from microalgae. Biotechnol Biofuels 6:11
López MJ, Suárez-Estrella F, Vargas-García MC, López-González JA, Verstichel S, Debeer L, Wierinck I, Moreno J (2013) Biodelignification of agricultural and forest wastes: effect on anaerobic digestion. Biomass Bioenerg 58:343–349
Lalak J, Kasprzycka A, Martyniak D, Tys J (2016) Effect of biological pretreatment of Agropyron elongatum ‘BAMAR’ on biogas production by anaerobic digestion. Bioresour Technol 200:194–200
Li Q, Ng WT, Wu JC (2014) Isolation, characterization and application of a cellulose-degrading strain Neurospora crassa S1 from oil palm empty fruit bunch. Microb Cell Factories 13:157–165
Lima MS, Damasio AR, Crnkovic PM, Pinto MR, da Silva AM, da Silva JC, Segato F, de Lucas RC, Jorge JA, Polizeli Mde L (2016) Co-cultivation of Aspergillus nidulans recombinant strains produces an enzymatic cocktail as alternative to alkaline sugarcane bagasse pretreatment. Front Microbiol 7:583
Liu S, Li X, Wu S, He J, Pang C, Deng Y, Dong R (2014a) Fungal pretreatment by Phanerochaete chrysosporium for enhancement of biogas production from corn stover silage. Appl Biochem Biotechnol 174:1907–1918
Liu S, Wu S, Pang C, Li W, Dong R (2014b) Microbial pretreatment of corn stovers by solid-state cultivation of Phanerochaete chrysosporium for biogas production. Appl Biochem Biotechnol 172:1365–1376
Mackuľak T, Prousek J, Švorc Ľ, Drtil M (2012) Increase of biogas production from pretreated hay and leaves using wood-rotting fungi. Chem Pap 66:649–653
Mahdy A, Mendez L, Ballesteros M, González-Fernández C (2014) Enhanced methane production of Chlorella vulgaris and Chlamydomonas reinhardtii by hydrolytic enzymes addition. Energ Convers Manag 85:551–557
Martin-Ryals A, Schideman L, Li P, Wilkinson H, Wagner R (2015) Improving anaerobic digestion of a cellulosic waste via routine bioaugmentation with cellulolytic microorganisms. Bioresour Technol 189:62–70
Michalska K, Bizukojć M, Ledakowicz S (2015) Pretreatment of energy crops with sodium hydroxide and cellulolytic enzymes to increase biogas production. Biomass Bioenerg 80:213–221
Miiller HW, Trfisch W (1986) Screening of white-rot fungi for biological pretreatment of wheat straw for biogas production. Appl Microbiol Biotechnol 24:180–185
Munoz C, Hidalgo C, Zapata M, Jeison D, Riquelme C, Rivas M (2014) Use of cellulolytic marine bacteria for enzymatic pretreatment in microalgal biogas production. Appl Environ Microbiol 80:4199–4206
Mutschlechner M, Illmer P, Wagner AO (2015) Biological pre-treatment: enhancing biogas production using the highly cellulolytic fungus Trichoderma viride. Waste Manag 43:98–107
Nanda S, Mohammad J, Reddy SN, Kozinski JA, Dalai AK (2013) Pathways of lignocellulosic biomass conversion to renewable fuels. Biomass Convers Biorefin 4:157–191
Neumann L, Scherer P (2011) Impact of bioaugmentation by compost on the performance and ecology of an anaerobic digester fed with energy crops. Bioresour Technol 102:2931–2935
Nkemka VN, Gilroyed B, Yanke J, Gruninger R, Vedres D, McAllister T, Hao X (2015) Bioaugmentation with an anaerobic fungus in a two-stage process for biohydrogen and biogas production using corn silage and cattail. Bioresour Technol 185:79–88
Nuchdang S, Vatanyoopaisarn S, Phalakornkule C (2015) Effectiveness of fungal treatment by Coprinopsis cinerea and Polyporus tricholoma on degradation and methane yields of lignocellulosic grass. Int Biodeter Biodegr 104:38–45
Ometto F, Quiroga G, Psenicka P, Whitton R, Jefferson B, Villa R (2014) Impacts of microalgae pre-treatments for improved anaerobic digestion: thermal treatment, thermal hydrolysis, ultrasound and enzymatic hydrolysis. Water Res 65:350–361
Pöschl M, Ward S, Owende P (2010) Evaluation of energy efficiency of various biogas production and utilization pathways. Appl Energ 87:3305–3321
Parawira W (2012) Enzyme research and applications in biotechnological intensification of biogas production. Crit Rev Biotechnol 32:172–186
Passos F, Hom-Diaz A, Blanquez P, Vicent T, Ferrer I (2016) Improving biogas production from microalgae by enzymatic pretreatment. Bioresour Technol 199:347–351
Payne CM, Knott BC, Mayes HB, Hansson H, Himmel ME, Sandgren M, Stahlberg J, Beckham GT (2015) Fungal cellulases. Chem Rev 115:1308–1448
Peng X, Borner RA, Nges IA, Liu J (2014) Impact of bioaugmentation on biochemical methane potential for wheat straw with addition of Clostridium cellulolyticum. Bioresour Technol 152:567–571
Perez J, Munoz-Dorado J, de la Rubia T, Martinez J (2002) Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int Microbiol 5:53–63
Poszytek K, Ciezkowska M, Sklodowska A, Drewniak L (2016) Microbial consortium with high cellulolytic activity (MCHCA) for enhanced biogas production. Front Microbiol 7:324
Procházka J, Mrázek J, Štrosová L, Fliegerová K, Zábranská J, Dohányos M (2012) Enhanced biogas yield from energy crops with rumen anaerobic fungi. Eng Life Sci 12:343–351
Rodrjguez J, Ferraz A, Nogueira RFP, Ferrer I, Esposito E, Duran N (1997) Lignin biodegradation by the ascomycete Chrysondia sitophila. Appl Biochem Biotec 62:233–242
Rollini M, Sambusiti C, Musatti A, Ficara E, Retino I, Malpei F (2014) Comparative performance of enzymatic and combined alkaline-enzymatic pretreatments on methane production from ensiled sorghum forage. Bioprocess Biosyst Eng 37:2587–2595
Romano RT, Zhang R, Teter S, McGarvey JA (2009) The effect of enzyme addition on anaerobic digestion of Jose Tall wheat grass. Bioresour Technol 100:4564–4571
Romero-Güiza MS, Vila J, Mata-Alvarez J, Chimenos JM, Astals S (2016) The role of additives on anaerobic digestion: a review. Renew Sust Energ Rev 58:1486–1499
Rouches E, Herpoël-Gimbert I, Steyer JP, Carrere H (2016a) Improvement of anaerobic degradation by white-rot fungi pretreatment of lignocellulosic biomass: a review. Renew Sust Energ Rev 59:179–198
Rouches E, Zhou S, Steyer JP, Carrere H (2016b) White-rot fungi pretreatment of lignocellulosic biomass for anaerobic digestion: impact of glucose supplementation. Process Biochem, in press, corrected proof
Schroyen M, Vervaeren H, Van Hulle SW, Raes K (2014) Impact of enzymatic pretreatment on corn stover degradation and biogas production. Bioresour Technol 173:59–66
Schroyen M, Vervaeren H, Vandepitte H, Van Hulle SW, Raes K (2015) Effect of enzymatic pretreatment of various lignocellulosic substrates on production of phenolic compounds and biomethane potential. Bioresour Technol 192:696–702
Semrany S, Favier L, Djelal H, Taha S, Amrane A (2012) Bioaugmentation: possible solution in the treatment of bio-refractory organic compounds (Bio-ROCs). Biochem Eng J 69:75–86
Singh P, Sulaiman O, Hashim R, Rupani PF, Peng LC (2010) Biopulping of lignocellulosic material using different fungal species: a review. Rev Environ Sci Bio 9:141–151
Sukumaran RK, Singhania RR, Pandey A (2005) Microbial cellulases—production, applications and challenges. J Sci Ind Res 64:832–844
Surendra KC, Takara D, Hashimoto AG, Khanal SK (2014) Biogas as a sustainable energy source for developing countries: opportunities and challenges. Renew Sust Energ Rev 31:846–859
Sutaryo S, Ward AJ, Moller HB (2014) The effect of mixed-enzyme addition in anaerobic digestion on methane yield of dairy cattle manure. Environ Technol 35:2476–2482
Thomsen ST, Londono JE, Ambye-Jensen M, Heiske S, Kadar Z, Meyer AS (2016) Combination of ensiling and fungal delignification as effective wheat straw pretreatment. Biotechnol Biofuels 9:16
Town JR, Dumonceaux TJ (2016) Laboratory-scale bioaugmentation relieves acetate accumulation and stimulates methane production in stalled anaerobic digesters. Appl Microbiol Biotechnol 100:1009–1017
Tsavkelova EA, Netrusov AI (2012) Biogas production from cellulose-containing substrates: a review. Appl Biochem Microbiol 48:421–433
van Dyk JS, Pletschke BI (2012) A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—factors affecting enzymes, conversion and synergy. Biotechnol Adv 30:1458–1480
van Kuijk SJ, Sonnenberg AS, Baars JJ, Hendriks WH, Cone JW (2015) Fungal treated lignocellulosic biomass as ruminant feed ingredient: a review. Biotechnol Adv 33:191–202
Vanwonterghem I, Jensen PD, Ho DP, Batstone DJ, Tyson GW (2014) Linking microbial community structure, interactions and function in anaerobic digesters using new molecular techniques. Curr Opin Biotechnol 27:55–64
Vasco-Correa J, Li Y (2015) Solid-state anaerobic digestion of fungal pretreated Miscanthus sinensis harvested in two different seasons. Bioresour Technol 185:211–217
Veen JA, Overbeek LS, Elsas JD (1997) Fate and activity of microorganisms introduced into soil. Microbiol Mol Biol Rev 61:121–135
Wan C, Li Y (2012) Fungal pretreatment of lignocellulosic biomass. Biotechnol Adv 30:1447–1457
Weiß S, Tauber M, Somitsch W, Meincke R, Müller H, Berg G, Guebitz GM (2010) Enhancement of biogas production by addition of hemicellulolytic bacteria immobilised on activated zeolite. Water Res 44:1970–1980
Wei S, Zhang H, Cai X, Xu J, Fang J, Liu H (2014) Psychrophilic anaerobic co-digestion of highland barley straw with two animal manures at high altitude for enhancing biogas production. Energ Convers Manag 88:40–48
Wei Y, Li X, Yu L, Zou D, Yuan H (2015) Mesophilic anaerobic co-digestion of cattle manure and corn stover with biological and chemical pretreatment. Bioresour Technol 198:431–436
Weiss S, Somitsch W, Klymiuk I, Trajanoski S, Guebitz GM (2016) Comparison of biogas sludge and raw crop material as source of hydrolytic cultures for anaerobic digestion. Bioresour Technol 207:244–251
Wen B, Yuan X, Li QX, Liu J, Ren J, Wang X, Cui Z (2014) Comparison and evaluation of concurrent saccharification and anaerobic digestion of Napier grass after pretreatment by three microbial consortia. Bioresour Technol 175:102–111
Westerholm M, Leven L, Schnurer A (2012) Bioaugmentation of syntrophic acetate-oxidizing culture in biogas reactors exposed to increasing levels of ammonia. Appl Environ Microbiol 78:7619–7625
Yan L, Gao Y, Wang Y, Liu Q, Sun Z, Fu B, Wen X, Cui Z, Wang W (2012) Diversity of a mesophilic lignocellulolytic microbial consortium which is useful for enhancement of biogas production. Bioresour Technol 111:49–54
Yang Z, Guo R, Xu X, Wang L, Dai M (2016) Enhanced methane production via repeated batch bioaugmentation pattern of enriched microbial consortia. Bioresour Technol 216:471–477
Yu J, Zhao Y, Liu B, Zhao Y, Wu J, Yuan X, Zhu W, Cui Z (2016) Accelerated acidification by inoculation with a microbial consortia in a complex open environment. Bioresour Technol 216:294–301
Yu S, Zhang G, Li J, Zhao Z, Kang X (2013) Effect of endogenous hydrolytic enzymes pretreatment on the anaerobic digestion of sludge. Bioresour Technol 146:758–761
Yuan X, Cao Y, Li J, Wen B, Zhu W, Wang X, Cui Z (2012) Effect of pretreatment by a microbial consortium on methane production of waste paper and cardboard. Bioresour Technol 118:281–288
Yuan X, Ma L, Wen B, Zhou D, Kuang M, Yang W, Cui Z (2016) Enhancing anaerobic digestion of cotton stalk by pretreatment with a microbial consortium (MC1). Bioresour Technol 207:293–301
Yuan X, Wen B, Ma X, Zhu W, Wang X, Chen S, Cui Z (2014) Enhancing the anaerobic digestion of lignocellulose of municipal solid waste using a microbial pretreatment method. Bioresour Technol 154:1–9
Zhang H, Zhang P, Ye J, Wu Y, Fang W, Gou X, Zeng G (2016) Improvement of methane production from rice straw with rumen fluid pretreatment: a feasibility study. Int Biodeter Biodeg 113:9–16
Zhang J, Guo RB, Qiu YL, Qiao JT, Yuan XZ, Shi XS, Wang CS (2015) Bioaugmentation with an acetate-type fermentation bacterium Acetobacteroides hydrogenigenes improves methane production from corn straw. Bioresour Technol 179:306–313
Zhang Q, He J, Tian M, Mao Z, Tang L, Zhang J, Zhang H (2011) Enhancement of methane production from cassava residues by biological pretreatment using a constructed microbial consortium. Bioresour Technol 102:8899–8906
Zhao J, Ge X, Vasco-Correa J, Li Y (2014a) Fungal pretreatment of unsterilized yard trimmings for enhanced methane production by solid-state anaerobic digestion. Bioresour Technol 158C:248–252
Zhao J, Zheng Y, Li Y (2014b) Fungal pretreatment of yard trimmings for enhancement of methane yield from solid-state anaerobic digestion. Bioresour Technol 156:176–181
Zheng Y, Zhao J, Xu F, Li Y (2014) Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog Energ Combust Sci 42:35–53
Zhong W, Zhang Z, Luo Y, Sun S, Qiao W, Xiao M (2011) Effect of biological pretreatments in enhancing corn straw biogas production. Bioresour Technol 102:11177–11182
Zieminski K, Kowalska-Wentel M (2015) Effect of enzymatic pretreatment on anaerobic co-digestion of sugar beet pulp silage and vinasse. Bioresour Technol 180:274–280
Zieminski K, Romanowska I, Kowalska M (2012) Enzymatic pretreatment of lignocellulosic wastes to improve biogas production. Waste Manag 32:1131–1137
Acknowledgments
This research was financially supported by the Innovation Support for Young Teachers in Colleges and Universities in Tibet Autonomous Region (Grant No. QC2015-39).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
The author confirms that the article does not contain any studies with human participants or animals.
Conflict of interest
The author declares that she has no conflict of interest.
Rights and permissions
About this article
Cite this article
Wei, S. The application of biotechnology on the enhancing of biogas production from lignocellulosic waste. Appl Microbiol Biotechnol 100, 9821–9836 (2016). https://doi.org/10.1007/s00253-016-7926-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7926-5