Abstract
Exploration of cost-effective fermentation substrates for efficient lactate production is an important economic objective. Although some organic nitrogen sources are also cheaper, inorganic nitrogen salts for lactate fermentation have additional advantages in facilitating downstream procedures and significantly improving the commercial competitiveness of lactate production. In this study, we first established an application of diammonium phosphate to replace yeast extract with a reduced 90 % nitrogen cost for a thermotolerant Bacillus coagulans strain. In vivo enzymatic and transcriptional analyses demonstrated that diammonium phosphate stimulates the gene expression of L-lactate dehydrogenase, thus providing higher specific enzyme activity in vivo and increasing L-lactic acid production. This new information provides a foundation for establishing a cost-effective process for polymer-grade L-lactic acid production in an industrial setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactic acid is a valuable chemical that can be polymerized to biodegradable polylactic acid (PLA) (Lee et al. 2011). This polymer is attractive as it can be produced from renewable resources (Fukushima et al. 2004; John et al. 2007). The fermentative production of lactic acid has seen wide industrial application since biological processes produce optically pure isomers whereas chemical processes yield racemic mixtures (Okano et al. 2010; Li et al. 2013). Unlike Lactococcus lactis and Lactococcus rhamnosus, the most frequently used lactic acid producers, Bacillus species can withstand relatively low pH and various other harsh conditions (Peng et al. 2013). Additionally, Bacillus coagulans grows optimally at 50–55 °C, which is expected to minimize contamination in industrial-scale fermentations under non-sterile conditions and thereby reduce the operation costs and substrate loss through sterilization. Therefore, in recent years, there has been increasing interest in optically pure L-lactic acid production by B. coagulans (Wang et al. 2013; Wang et al. 2014). We previously isolated several B. coagulans strains that efficiently produce L-lactic acid with high productivity and optical purity (Qin et al. 2009; Wang et al. 2010; Peng et al. 2013). The strains were also found to utilize cellulosic hydrolyzate efficiently under open fermentation (without sterilization), indicating its great industrial potential for L-lactic acid production (Wang et al. 2010; Peng et al. 2014).
As lactic acid is one of the top 30 potential building-block chemicals from biomass, the search for cheap raw materials as lactate fermentation sources is an important economic objective (Abdel-Rahman et al. 2010). To date, various low-cost organic nitrogen materials such as soy protein hydrolysates (Hsieh et al. 1999), whey permeate/yeast extract (Adolf et al. 2002), defatted rice bran (Tanaka et al. 2006), and Baker’s yeast cells (Altaf et al. 2007) have been investigated as yeast extract (YE) substitutes during lactic acid production. Hujanen and Linko (1996) also studied the effect of less expensive organic nitrogen sources in combination with YE, peptone, and malt sprouts to reduce the YE concentration. Compared to these cheaper organic nitrogen sources, inorganic nitrogen substitutes for lactic acid fermentation have advantages in facilitating downstream decolorization, purification, and waste treatment processes (Zhou et al. 2003). Thus, inorganic nitrogen sources can significantly improve the commercial competitiveness of lactic acid production. However, investigations of inorganic nitrogen sources for production of high optical purity L-lactic acid are rare, especially never for the thermotolerant B. coagulans strains.
In this study, we first report the effective application of an inorganic nitrogen source, diammonium phosphate, for lactate fermentation by a B. coagulans strain. We further demonstrate the mechanism by which diammonium phosphate stimulates lactate production. This new finding provides useful information for modern L-lactic acid fermentation industries.
Materials and methods
Microorganism and cultural conditions
B. coagulans 2-6 was used as a homofermentative L-lactic acid producer in this study (Qin et al. 2009). The strain is deposited at the China General Microbiological Culture Collection Center (CGMCC No. 2184). The slant was cultivated at 50 °C for 48 h and stored at 4 °C. The medium for the seed culture had the following composition (g/L): glucose, 50; yeast extract, 10; and CaCO3, 30. The seed culture was prepared as follows: a loop of cells from a fully grown slant was inoculated into 20 mL of the above sterile medium in 50-mL conical flasks and incubated for 48 h at 50 °C without agitation. The seed culture was then inoculated into fermentation medium for L-lactate production. The inoculum volume was 10 % (v/v). Industrial grade YE was purchased from Angel Yeast Co., Ltd. (Hubei, China). All other chemicals were of analytical grade and commercially available.
Effect of inorganic salts on L-lactic acid production
The starting medium for studying the effect of inorganic salts on L-lactate production with poor nitrogen sources contained the following (g/L): glucose, 120; YE, 0.5; CaCO3, 72; (NH4)2SO4, 3; (NH4)2HPO4, 3; K2HPO4, 0.5; KH2PO4, 0.5; and ZnSO4, 0.2. To explore the role of the five inorganic salts for L-lactate production, the individual inorganic salt (K2HPO4 and KH2PO4 were combined) was added respectively in the medium with above concentrations of glucose, YE, and CaCO3. The performance of cells cultivated in the starting medium containing 10 g/L YE was used as a control. ZnSO4 and K2HPO4/KH2PO4 were also subtracted from the starting medium to demonstrate their negative effects on L-lactate production. All experiments were conducted in 100-mL flasks containing 50 mL medium at 50 °C, 120 rpm with an inoculum volume of 10 % (v/v) in triplicate.
Establishment of a low-cost medium for efficient L-lactate fermentation
To study the optimal (NH4)2HPO4 concentration, a medium with the following composition was used (g/L): glucose, 120; YE, 0.5; CaCO3, 72; and (NH4)2HPO4, 1–6. To further optimize the YE addition, the medium composition was set as follows (g/L): glucose, 120; (NH4)2HPO4, 3; CaCO3, 72; and YE, 0.5–1. The medium for optimization of betaine on L-lactate production contained 0–300 mg/L betaine, 3 g/L (NH4)2HPO4, 0.9 g/L YE, 120 g/L glucose, and 72 g/L CaCO3. The performance of cells cultivated in the medium containing 120 g/L glucose, 10 g/L YE, and 72 g/L CaCO3 was used as a control. Experiments were all carried out at 50 °C, 120 rpm in 100-mL Erlenmeyer flasks containing 50 mL medium. The inoculum volume was 10 % (v/v). Samples were taken at 50 h to determine the L-lactic acid production and glucose consumption.
Effect of various inorganic nitrogen sources on L-lactic acid production under the optimal cultivation condition
After optimization of the culture medium with (NH4)2HPO4 as the inorganic nitrogen source, we determined whether other inorganic nitrogen sources could also favor L-lactate production. The medium contained 120 g/L glucose, 72 g/L CaCO3, 0.9 g/L YE, 200 mg/L betaine, and various inorganic nitrogen salts with the same molar nitrogen concentration as 3 g/L (NH4)2HPO4. Samples were taken at 50 h to determine the L-lactic acid production and glucose consumption.
In vivo enzymatic activity assay
To assay enzymatic activity in vivo, whole-cell extracts were used as described previously (Wang et al. 2014). Fermentations were performed in the above fermentation medium supplemented with different inorganic nitrogen salts. Exponentially growing cells were harvested by centrifugation (5000×g, 10 min, 4 °C) and washed with 0.85 % (w/v) physiological saline. Cell pellets were subsequently suspended in 100 mM potassium phosphate buffer (pH 7.0) and disrupted by sonication in an ice bath. After centrifugation at 12,000×g for 10 min, the supernatants were used as the crude cell extracts. The reaction mixture contained 100 mM sodium phosphate (pH 6.5), 0.2 mM NADH, 20 mM pyruvate, and 0.1 mg/mL crude cell extract. The reaction was initiated by adding enzyme. The NADH oxidation activity without pyruvate was used as a blank to subtract the non-specific activities associated with the non-lactate dehydrogenase (LDH) reaction. One unit was defined as the amount of enzyme converting 1 μM NADH per minute. Specific activity is expressed as units per milligram protein. The D-LDH activity was detected by HPLC for quantitative analysis of produced D-lactic acid.
Quantitative real-time PCR
Quantitative real-time (RT) PCR was employed to determine the transcription levels of the genes encoding key enzymes under different fermentation conditions. B. coagulans 2-6 was cultivated in optimized medium containing (NH4)2HPO4 or NH4Cl as the sole inorganic nitrogen source. Cells were harvested at the logarithmic phase by centrifugation (5000×g for 10 min, 4 °C) for RNA isolation using an E.Z.N.A.™ Bacterial RNA Kit (Omega). The total RNA concentration was determined by the absorbance at 260 nm (NanoVue spectrophotometer; GE). cDNA copies were synthesized with a FastQuant RT kit (with gDNase) (Tiangen, China) and were amplified with SYBR Premix Ex Taq (TaKaRa, China) by primers as indicated previously (Wang et al. 2014). The 2−△△Ct relative quantification method was used to determine the messenger RNA (mRNA) levels, and 16S rRNA was used as the internal reference. From these results, a ratio of the concentration of gene-specific mRNA in the sample was calculated. Reported results are the average of at least three experiments with variation less than 15 %.
Comparative active staining of LDHs in cells cultivated in medium with (NH4)2HPO4 or NH4Cl
In vivo enzymatic activities were also confirmed by active staining of LDHs, after native PAGE, as described previously (Wang et al. 2014). Briefly, B. coagulans 2-6 was cultured in the optimized medium containing (NH4)2HPO4 or NH4Cl as the sole inorganic nitrogen source. Crude enzymes were separated using 4–20 % gradient native PAGE. The gels were soaked with 100 mM Tris-HCl buffer (pH 8.0) containing 0.1 mM phenazinemethosulfate, 0.1 mM nitrotetrazolium blue chloride, 2 mM NAD, and 100 mM D-lactate or L-lactate (Sigma-Aldrich).
Analytical methods
The concentration of residual glucose and l-lactate was measured by a SBA-40C biosensor analyzer (Institute of Biology, Shandong Academy of Sciences, China). The optical purity of L-lactic acid was determined by HPLC equipped with a chiral column (4.6 mm ID × 50 mm; MCI GEL CRS10W, Japan) at 254 nm. The mobile phase was 2 mM CuSO4 at a flow rate of 0.5 mL/min (25 °C). The optical purity of L-lactic acid was defined as follows: L-lactic acid/(l-lactic acid + d-lactic acid) × 100 %.
Results
Effect of various inorganic nitrogen sources on L-lactic acid production
To decrease cost, mineral nutrient salts are desirable as the nitrogen source for lactate fermentation. To establish an efficient fermentation medium, the roles of mineral salts in the routine fermentation medium were first individually investigated. A small amount of YE (0.05 %, w/v) was initially added to provide the essential vitamins for cell growth since without YE in the medium, the cell growth and lactate production are rather low, which makes the process less advisable (Yoo et al. 1997). Surprisingly, the combination of all mineral salts did not give the highest titer; the addition of (NH4)2HPO4 alone served as the best nitrogen source for lactate production (Fig. 1). Subtracting potassium phosphates yielded a better titer, and ZnSO4 had a negative effect on lactate fermentation, probably because it formed phosphate salt precipitates.
The effects of inorganic salts in the routine fermentation medium on L-lactate production by Bacillus coagulans 2-6. The experiments were carried out in 100-mL flasks containing 50 mL fresh medium. A small amount of yeast extract (0.05 %, w/v) was added to provide trace nutritional elements for cell growth. The titer for medium with 1 % yeast extract and no inorganic salts was set as 100 %. All the experiments were carried out at 50 °C, 120 rpm. Error bars indicate the standard deviations of three parallel replicates. Triangles indicate subtraction. All inorganic salts were added to the starting medium as described in “Materials and methods”
Optimization of the fermentation medium for L-lactic acid production
Since (NH4)2HPO4 experimentally favored L-lactate production, we optimized its concentration to establish a cost-effective fermentation medium (Fig. 2a). Increasing the (NH4)2HPO4 concentration from 1 to 6 g/L first increased the resultant lactic acid concentration and then decreased it slightly. We found that 3 g/L (NH4)2HPO4 was optimal with 0.05 % (w/v) YE. To further increase the titer, we varied the YE concentration from 0.05 to 0.1 % (w/v). As expected, the lactate titer increased with increasing YE. From an economic point of view, we limited the concentration of YE to 0.09 % since there was very little difference between the titers with 0.1 and 0.09 % YE (Fig. 2b). Furthermore, betaine has been shown to promote microbial fermentation as an effective exogenous osmoprotectant, and it has been applied in fermentative production of lactic acid in Lactobacilli (Zou et al. 2013). To explore the effects of betaine on L-lactate production in B. coagulans, we added different concentrations of betaine (0, 50, 100, 150, 200, and 300 mg/L) to medium supplemented with 3 g/L (NH4)2HPO4 and 0.09 % YE. Increasing the betaine concentration from 50 to 200 mg/L gradually increased lactate production, with no appreciable change upon increasing to 300 mg/L; therefore, we selected 200 mg/L betaine as the optimal concentration (Fig. 2c).
Optimization of (NH4)2HPO4, betaine, and yeast extract concentrations for L-lactate fermentation. a (NH4)2HPO4 concentration, b yeast extract concentration, and c betaine concentration were varied. All the experiments were carried out at 50 °C, 120 rpm. Addition of 1 % yeast extract without (NH4)2HPO4 and betaine was used as the control. Error bars indicate the standard deviations of three parallel replicates
After optimization, the combination of 0.09 % YE, 3 g/L (NH4)2HPO4, and 200 mg/L betaine produced L-lactate equivalent to the control (1 % YE) with a high yield of 96.76 ± 0.40 % (g/g). The optical purity of L-lactate in the broth reached 99.8 %, which meets the requirement for lactic acid polymerization. The price of industrial grade YE is ~10,000 USD per ton in China, whereas the price of (NH4)2HPO4 is 20-fold lower (~500 USD per ton). Although betaine is needed, a 0.02 % addition at ~3000 USD per ton still significantly reduces the substrate cost compared to 1 % YE. Therefore, we successfully used the much cheaper inorganic salt (NH4)2HPO4 to substitute for >90 % of the YE.
Diammonium phosphate enhances lactate productivity by increasing the specific lactate dehydrogenase activity in vivo
Since (NH4)2HPO4 was an effective inorganic nitrogen source for lactate production by B. coagulans, we investigated its mechanism of action. Since nitrogen sources are vital to lactate fermentation, we first investigated whether other inorganic nitrogen sources also favored lactate production. With the optimal concentrations of YE and betaine, 3 g/L (NH4)2HPO4 had the best lactate production performance and support for cell growth among the five inorganic nitrogen sources, each provided at the same molar concentration of nitrogen as (NH4)2HPO4 (Fig. 3).
The effects of various nitrogen sources on L-lactic acid production. a L-Lactic acid production with different nitrogen sources. b Cell growth curves of Bacillus coagulans 2-6 cultivated using different nitrogen sources. All the experiments were carried out at 50 °C, 120 rpm in medium supplemented with 0.09 % YE, 200 mg/L betaine, and the respective inorganic nitrogen source with a constant molar concentration of nitrogen calculated based on 3 g/L (NH4)2HPO4. Error bars indicate the standard deviations of three parallel replicates
These results confirmed that (NH4)2HPO4 alone enhanced lactic acid production. As LDH plays a key role in lactic acid production, its specific enzyme activity levels in cells grown in different fermentation media were studied to explore the mechanism (Table 1). Under the same cultivation conditions, LDH activity varied with the added nitrogen source. The specific LDH activity was highest by ~3-fold for cells cultivated in medium containing (NH4)2HPO4.
Diammonium phosphate stimulates the transcription levels of genes encoding the key enzyme for lactate formation
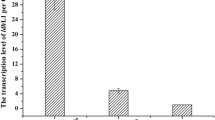
To further study the mechanism of (NH4)2HPO4 on L-lactic acid production, quantitative real-time (RT) PCR was employed to determine the transcription levels of the genes encoding key enzymes for lactate formation with (NH4)2HPO4 and NH4Cl. In our previous research, we found that B. coagulans 2-6 harbored three key enzymes responsible for lactic acid metabolism: L-LDH, D-LDH, and a glycolate oxidase enzyme (GO) (Wang et al. 2014). The transcription level of ldhL (encoding L-LDH) in (NH4)2HPO4-cultivated cells increased ~12-fold over that of cells cultivated with NH4Cl, whereas there was no obvious change in transcription of ldhD (encoding D-LDH) (Fig. 4). The specific enzyme activity of LDH for cells cultivated by diammonium phosphate was 2.09 ± 0.148 U/mg, while the data for NH4Cl was only 0.69 ± 0.061 U/mg (Table 1). The difference of specific enzyme activity was consistent with the difference of mRNA analysis. Although the transcription level of go (encoding GO) increased ~6-fold, this result does not counter the positive effects of (NH4)2HPO4 on L-lactate production since GO catalyzes the oxidation of lactic acid to pyruvate and, most importantly, its in vivo enzymatic activity was undetectable in our previous study (Wang et al. 2014).
The relative transcription levels of ldhD, ldhL, and go in Bacillus coagulans cells cultivated with (NH4)2HPO4 or NH4Cl. The fold change represents the transcriptional levels in (NH4)2HPO4-cultivated cells versus those of NH4Cl-cultivated cells. Error bars indicate the standard deviations of three parallel replicates. go, the gene responsible for glycolate oxidase
The increased gene expression of L-LDH in vivo was also confirmed by active staining. The band for L-LDH from (NH4)2HPO4-cultivated cells on the active staining gel (lane 2) was much thicker than that from NH4Cl-cultivated cells (lane 1) (Fig. 5). No bands associated with D-LDH activities were detected either in (NH4)2HPO4- or NH4Cl-cultivated B. coagulans 2-6 cells. Therefore, the transcription and active staining results further explain the beneficial effect of (NH4)2HPO4 on L-lactic acid production: diammonium phosphate stimulates expression of L-LDH, increasing the amount of enzyme to give a higher specific activity in vivo and increasing L-lactic acid production.
Active staining of lactate dehydrogenases from Bacillus coagulans 2-6 grown in (NH4)2HPO4 or NH4Cl medium. Extracts of cells grown in NH4Cl medium stained with l-lactate (lane 1) and d-lactate (lane 3) or cells grown in (NH4)2HPO4 medium stained with l-lactate (lane 2) and d-lactate (lane 4), respectively. Approximately 5.5 μg protein was applied to each well
Discussion
YE is a major factor affecting the economy of lactic acid production (Altaf et al. 2007). Therefore, development of an efficient and cost-effective process for lactic acid fermentation from cheap nitrogen sources is highly desired (Abdel-Rahman et al. 2010). In this study, we successfully established an application of (NH4)2HPO4 to replace commercial YE with a reduced 90 % nitrogen substrate cost. Additionally, the significantly reduced YE in the fermentation broth also facilitated the downstream of decolorization and purification processes and thus reduced the operation costs simultaneously (Zhou et al. 2003).
Singhvi et al. (2013) reported that the combination of (NH4)2HPO4 and peptone could increase the D-lactic acid production by L. lactis but did not enhance the titer and productivity of L-lactic acid production by Lactobacillus delbrueckii Uc-3. They suggested that the incremental effect of (NH4)2HPO4 was only selective for D-lactic acid production. In this study, we found that additional (NH4)2HPO4 alone could stimulate L-lactate production by the thermotolerant B. coagulans strain under low YE conditions, which revealed different responses to (NH4)2HPO4 between species. Thus, we further investigated the mechanism of (NH4)2HPO4 stimulation of L-lactate production by B. coagulans.
As LDH plays a key role in lactic acid production, its level in the cells grown in different fermentation media was studied. In our previous report, we found that the D-LDH activity in B. coagulans 2-6 was too low to be detected both by in vitro enzymatic activity measurement and native PAGE, although its expression could be detected by western blotting, of which the sensitivity is much higher (Wang et al. 2014). This could also explain the result from RT-PCR analysis in this study, in which the transcription of D-LDH-encoding gene was indeed detected (Fig. 4). However, the contribution of transcription of D-LDH-encoding gene under diammonium phosphate could be ignored in this study since only slight increase (about 0.3-fold) was found and, importantly, no detectable D-LDH activity was observed in cells cultivated with all of the inorganic nitrogen salts. Thus, the measured LDH activity should be specific to L-LDH activity. The increased L-LDH activity of cells grown in medium with (NH4)2HPO4 was correlated with enhanced lactic acid production. Therefore, the increase in specific L-LDH enzyme activity levels in cells grown with (NH4)2HPO4 was attributed to enhanced production of lactate. Although betaine has been reported to be an effective osmoprotectant for L-LDH activity (Zou et al. 2013; Xu and Xu 2014), the variation of specific L-LDH activities in this study should be mainly due to the different inorganic nitrogen sources in the medium since a constant amount of betaine (200 mg/L) was used in all experiments.
Diammonium phosphate was widely reported as a nutritional supplement in many fermentative production processes (Acourene and Ammouche 2012; Berbert-Molina et al. 2001; Deed et al. 2011; Isar et al. 2006; Zhu and Xu 2010). However, its mechanism of action in replacing YE has rarely been investigated. In this study, we first demonstrated the mechanism for diammonium phosphate stimulation of L-lactate production by a thermotolerant B. coagulans strain at in vivo enzymatic and transcriptional levels. Although the molecular mechanism of such induction effect on L-LDH remains unknown, this new finding provides important information for industrial L-lactic acid production and other fermentative process. Additionally, the genome sequence of B. coagulans 2-6 shares a high similarity (>99 %) with its phylogenetically close type strain B. coagulans DSM1, although the genome size of DSM1 is larger than that of strain 2-6 (Su et al. 2011; Johnson et al. 2015). However, unexpected result was found that diammonium phosphate could not stimulate the L-lactate production in B. coagulans DSM1 under the same conditions with B. coagulans 2-6 (data not shown). It sounds like that the diammonium phosphate-stimulating phenomenon is strain-specific. As no specific protein factors or enzymes have been previously reported to be responsible for stimulating L-LDH transcription, comparative transcriptional analysis by RNseq under the respective diammonium phosphate and NH4Cl cultivation conditions in B. coagulans 2-6, as well as comparative genome analysis of the two strains, are under investigation in our laboratory.
In summary, the mechanism of (NH4)2HPO4-stimulated enhancement of L-lactate production in thermotolerant B. coagulans strain was first demonstrated at in vivo enzymatic and transcriptional levels. (NH4)2HPO4 stimulates the transcription of L-LDH, thus increasing specific enzyme activity in the cells and resulting in enhanced L-lactate production. This new information provides a foundation for establishing a cost-effective process for polymer-grade L-lactic acid production.
References
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2010) Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: overview and limits. J Biotechnol 156:286–301
Acourene S, Ammouche A (2012) Optimization of ethanol, citric acid, and α-amylase production from date wastes by strains of Saccharomyces cerevisiae, Aspergillus niger, and Candida guilliermondii. J Ind Microbiol Biotechnol 39:759–766
Adolf WS, Jules T, Christophe L (2002) Lactobacillus helveticus growth and lactic acid production during pH-controlled batch cultures in whey permeate/yeast extract medium. Part I. Multiple factor kinetic analysis. Enzym Microb Technol 30:176–186
Altaf M, Naveena BJ, Reddy G (2007) Use of inexpensive nitrogen sources and starch for L-(+)-lactic acid production in anaerobic submerged fermentation. Bioresour Technol 98:498–503
Berbert-Molina MA, Sato S, Silveira MM (2001) Ammonium phosphate as a sole nutritional supplement for the fermentative production of 2,3-butanediol from sugar cane juice. Z Naturforsch C 56:787–791
Deed NK, van Vuuren HJ, Gardner RC (2011) Effects of nitrogen catabolite repression and di-ammonium phosphate addition during wine fermentation by a commercial strain of S. cerevisiae. Appl Microbiol Biotechnol 89:1537–1549
Fukushima K, Sogo K, Miura S, Kimura Y (2004) Production of D-lactic acid by bacterial fermentation of rice starch. Macromol Biosci 4:1021–1027
Hsieh CM, Yang FC, Iannotti EL (1999) The effect of soy protein hydrolyzates on fermentation by Lactobacillus amylovorus. Proc Biochem 34:173–179
Hujanen M, Linko YY (1996) Effect of temperature and various nitrogen sources on L(+) lactic acid production by Lactobacillus caesi. Appl Microbiol Biotechnol 45:307–313
Isar J, Agarwal L, Saran S, Gupta P, Saxena RK (2006) Effect of process parameters on succinic acid production in Escherichia coli W3110 and enzymes involved in the reductive tricarboxylic acid cycle. Can J Microbiol 52:893–902
John RP, Nampoothiri KM, Pandey A (2007) Fermentative production of lactic acid from biomass: an overview on process developments and future perspectives. Appl Microbiol Biotechnol 74:524–534
Johnson SL, Daligault HE, Davenport KW, Jaissle J, Frey KG, Ladner JT, Broomall SM, Bishop-Lilly KA, Bruce DC, Gibbons HS, Coyne SR, Lo C-C, Meincke L, Munk AC, Koroleva GI, Rosenzweig CN, Palacios GF, Redden CL, Minogue TD, Chain PS (2015) Complete genome sequences for 35 biothreat assay-relevant Bacillus species. Genome Announc. doi:10.1128/genomeA.00151-15
Lee JW, Kin HU, Choi S, Yi J, Lee SY (2011) Microbial production of building block chemicals and polymers. Curr Opin Biotech 22:1–10
Li Y, Wang LM, Ju JS, Yu B, Ma YH (2013) Efficient production of polymer-grade D-lactate by Sporolactobacillus laevolacticus DSM442 with agricultural waste cottonseed as the sole nitrogen source. Bioresour Technol 142:186–191
Okano K, Tanaka T, Ogino C, Fukuda H, Kondo A (2010) Biotechnological production of enantiomeric pure lactic acid from renewable resources: recent achievements, perspectives, and limits. Appl Microbiol Biotechnol 85:413–423
Peng LL, Wang LM, Che CC, Yang G, Yu B, Ma YH (2013) Bacillus sp. strain P38: an efficient producer of L-lactate from cellulosic hydrolysate, with high tolerance for 2-furfural. Bioresour Technol 149:169–176
Peng LL, Xie NZ, Guo L, Wang LM, Yu B, Ma YH (2014) Efficient open fermentative production of polymer-grade L-lactate from sugarcane bagasse hydrolysate by thermotolerant Bacillus sp. strain P38. PLoS one 9:e107143
Qin JY, Zhao B, Wang XW, Wang LM, Yu B, Ma YH, Ma CQ, Tang HZ, Sun JB, Xu P (2009) Production of L-lactic acid by a newly isolated thermophilic strain Bacillus sp. 2-6. PLoS ONE 4:e4359
Singhvi M, Jadhav A, Gokhale D (2013) Supplementation of medium with diammonium hydrogen phosphate enhanced the D-lactate dehydrogenase levels leading to increased D-lactic acid productivity. Bioresour Technol 146:736–739
Su F, Yu B, Sun JB, Ou HY, Zhao B, Wang LM, Qin JY, Tang HZ, Tao F, Jarek M, Scharfe M, Ma CQ, Ma YH, Xu P (2011) Genome sequence of thermophilic strain Bacillus coagulans 2-6, an efficient producer of high optical purity L-lactic acid. J Bacteriol 193:4563–4564
Tanaka T, Hoshina M, Tanabe S, Sakai K, Ohtsubo S, Taniguchi M (2006) Production of D-lactic acid from defatted rice bran by simultaneous saccharification and fermentation. Bioresour Technol 97:211–217
Wang LM, Zhao B, Liu B, Yu B, Ma CQ, Su F, Hua DL, Li QG, Ma YH, Xu P (2010) Efficient production of l-lactic acid from corncob molasses, a waste by-product in xylitol production, by a newly isolated xylose utilizing Bacillus sp. strain. Bioresour Technol 101:7908–7915
Wang LM, Xue ZW, Zhao B, Yu B, Xu P, Ma YH (2013) Jerusalem artichoke powder: A useful material in producing high-optical-purity L-lactate using an efficient sugar-utilizing thermophilic Bacillus coagulans strain. Bioresour Technol 130:174–180
Wang LM, Cai YM, Zhu LF, Guo HL, Yu B (2014) Major role of NAD-dependent lactate dehydrogenases in high-optical-pure L-lactic acid production by thermophilic Bacillus coagulans. Appl Environ Microbiol 80:7134–7141
Xu K, Xu P (2014) Betaine and beet molasses enhance L-lactic acid production by Bacillus coagulans. PLoS one 9:e100731
Yoo IK, Chang HN, Lee EG, Chang KC, Moon SH (1997) Effect of B vitamins on the lactic acid fermentation by Lactobacillus casei. J Ferment Bioeng 84:172–175
Zhou S, Causey TB, Hasona A, Shanmugan KT, Ingram LO (2003) Production of optically pure D-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl Environ Microbiol 69:399–407
Zhu BF, Xu Y (2010) A feeding strategy for tetramethylpyrazine production by Bacillus subtilis based on the stimulating effect of ammonium phosphate. Bioprocess Biosyst Eng 33:953–959
Zou H, Wu Z, Xian M, Liu H, Cheng T, Cao YJ (2013) Not only osmoprotectant: betaine increased lactate dehydrogenase activity and L-lactate production in Lactobacilli. Bioresour Technol 148:591–595
Acknowledgments
The work was supported by grants from the National Natural Science Foundation of China (31270108), the Chinese National Programs for High Technology Research & Development (2011AA02A202), and the Key Deployment Project of Chinese Academy of Sciences (KSZD-EW-Z-016-3). BY is supported by the Youth Innovation Promotion Association, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Sun, L., Li, Y., Wang, L. et al. Diammonium phosphate stimulates transcription of L-lactate dehydrogenase leading to increased L-lactate production in the thermotolerant Bacillus coagulans strain. Appl Microbiol Biotechnol 100, 6653–6660 (2016). https://doi.org/10.1007/s00253-016-7379-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7379-x