Abstract
A drawback of biotechnological processes, where microorganisms convert biomass constituents, such as starch, cellulose, hemicelluloses, lipids, and proteins, into wanted products, is the economic feasibility. Particularly the cost of nitrogen sources in biotechnological processes can make up a large fraction of total process expenses. To further develop the bioeconomy, it is of considerable interest to substitute cost-intensive by inexpensive nitrogen sources. The aim of this mini-review was to provide a comprehensive insight of utilization methods of protein-rich residues, such as fish waste, green biomass, hairs, and food waste. The methods described include (i) production of enzymes, (ii) recovery of bioactive compounds, and/or (iii) usage as nitrogen source for microorganisms in biotechnological processes. In this aspect, the utilization of protein-rich residues, which are conventionally considered as waste, allows the development of value-adding processes for the production of bioactive compounds, biomolecules, chemicals, and materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The finiteness of fossil resources and negative ecological impact of fossil oil-based consumables and fuels caused increasing interest in sustainable, “green,” compounds by industries and consumers. Due to the increasing preference to substitute petroleum-based compounds, various biotechnological processes for the production of bio-based compounds from residues of low value have been developed and intensively studied (Koutinas et al. 2014).
Many microorganisms are able to take up and utilize amines as organic nitrogen sources (Burkovski and Krämer 2002; Pleissner and Eriksen 2012; Pleissner et al. 2011; Richter et al. 2015). For instance, amino acids, belonging to the group of amines, can be linked together by a peptide bond to small peptides, polypeptides, and proteins. On the other hand, proteins and peptides can be hydrolyzed into amino acids and used as nitrogen sources for microorganisms in biotechnological processes. For instance, lactic acid bacteria are limited in their capability to synthesize amino acids, and thus strongly dependent on an external supply to build up peptides and proteins and eventually for growth (Ummadi and Curic-Bawden 2010).
A complex source conventionally used in fermentations is yeast extract (Tejayadi and Cheryan 1995). Other complex nitrogen sources with the potential to be used in fermentations are hydrolysates from protein-rich plants, vegetables, food residues in particular meat, animal tissues and body liquids, fish waste, feathers, cheese whey permeate, and grass press juice (Beaulieu et al. 2009; Klompong et al. 2012; Lasekan et al. 2013; Lazzi et al. 2013; Lü et al. 2007; Mark Hsieh et al. 1999; Papendiek and Venus 2014; Pleissner et al. 2013; Safari et al. 2012; Serrano-Carreon et al. 1993; Tejayadi and Cheryan 1995; Vasileva-Tonkova et al. 2007; Venus 2009; Zhao et al. 2014). The hydrolysis of protein-rich materials can be carried out using proteases, acids, and bases and at high temperature and pressure (Animox 2012; Brandelli 2008; Gao et al. 2006; Solaiman et al. 2011). Furthermore, a sub-critical water hydrolysis has been applied (Esteban et al. 2010). The hydrolysate contains a mixture of peptides, peptone, and amino acids. The complexity of hydrolytic products may stimulate and improve the metabolic activity and growth of microorganisms (Pasupuleti et al. 2010). Protein-rich materials may further be used for the production of protein hydrolysates to be applied as feed, source of antibacterial and antioxidant agents, and plant growth regulator (Bataille and Bataille 1983; Benhabiles et al. 2013; Chalamaiah et al. 2012; Dalev 1994; Fakhfakh et al. 2013; Gunasekaran et al. 2014; Lasekan et al. 2013; Najafian and Babji 2012). Hence, protein-rich materials pose a great potential for the production of value-added products.
The aim of this mini-review is to provide a comprehensive insight in the utilization of protein-rich materials in biotechnological processes. The focus is particularly on residues to be considered of no or low value and appropriate to serve as alternatives to cost-intensive nitrogen sources and substrates for many microorganisms. Examples of fermentations are presented, which illustrate the potential of protein hydrolysates to be used in processes following the approach of white biotechnology for the production of enzymes, antioxidants and antibacterial agents, and value-added chemicals and materials.
Utilization of protein-rich materials
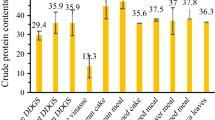
The utilization of protein-rich materials can occur by the following approaches: (i) microbial hydrolysis of the material and recovery of enzymes secreted by microbes used, (ii) hydrolysis of the material and recovery of bioactive compounds from the hydrolysate, and/or (iii) hydrolysis of the material and use of hydrolysate as nitrogen source for microorganisms in subsequently carried out biotechnological processes (Fig. 1). The protein contents can vary between different materials derived from vegetables or animals (Table 1). Hair, feather, horn, and wool contain a protein content of nearly 100 % (w/w), and thus are desirable nitrogen sources. Contrarily, food, meat and fish, wastes contain between 10 and 20 % (w/w) protein.
Some of the materials listed in Table 1 are waste streams and considered of no or low value. A utilization of these waste streams in biotechnological processes provides the opportunity to treat it in a way to exploit its full potential.
Production of enzymes
Many microorganisms, such as fungi (Aspergillus fumigatus and Aspergillus flavus) and bacteria (Bacillus sp. and Streptomyces sp.), are able to degrade keratinous substrates (e.g., hairs and wool) by the secretion of extracellular keratinases (Brandelli 2008). The secretion of extracellular keratinases seems to be triggered by either the presence of keratinous substrates or by a limitation in nitrogen and consequently the pressure for microorganisms to make non-conventional substrates available as nitrogen sources (Adıgüzel et al. 2009; Wang and Shih 1999). Streptomyces thermoviolaceus has been found to secrete a thermostable and alkaline keratinase when cultured in the presence of feather, hair, nail, or collagen (Chitte et al. 1999). Bacillus cereus strains IZ-06b and IZ-06r secrete sequentially collagenolytic, elastolytic, and keratinolytic proteases in peptide-limited cultures (Adıgüzel et al. 2009). First collagenolytic and elastolytic proteases are secreted in order to make organic nitrogen sources available. If this does not result in the release of utilizable nitrogen sources, keratinases to degrade keratin are secreted (Adıgüzel et al. 2009). Chrysobacterium sp. kr6 was found to produce keratinases when cultured in the presence of chicken feather (Fontoura et al. 2014). The use of keratinous substrates in biotechnological processes does not only result in the production of collagenolytic, elastolytic, and keratinolytic proteases with various industrially applications, such as in dehairing and deskinning processes in the leather industry, but also in the production of a hydrolysate to be used as source of bioactive compounds and/or nitrogen sources in biotechnological processes (Fontoura et al. 2014; Gupta and Ramnani 2006).
Production of bioactive compounds
Protein hydrolysates can lead to the production of antioxidative and antibacterial peptides. Antioxidative peptides are inactive within the sequence of the precursor protein and released and activated by hydrolysis (Chalamaiah et al. 2012). During the process of hydrolysis, peptide bonds are cleaved and active peptides are released, which are capable of sequestering oxygen radicals, chelating prooxidant metal ions and inhibiting lipid peroxidation in food systems (You et al. 2010). Antibacterial peptides usually have less than 50 amino acids and are also obtained by hydrolysis of proteinous material. Antibacterial peptides are involved in host defense mechanisms by interacting with bacteria (Najafian and Babji 2012).
When shrimp shell waste was demineralized and deproteinized in Pseudomonas aeruginosa A2 fermentation, 90 % of protein was hydrolyzed. The hydrolysate exhibited a 1,1-diphenyl-2-picrylhydrazyl radical-scavenging activity (Ghorbel-Bellaaj et al. 2012a). Similar results of demineralization and deproteinization were obtained when shrimp shell waste was treated in Bacillus pumilus A1, Bacillus mojavencis A21, Bacillus licheniformis RP1, B. cereus SV1, Bacillus amyloliquefaciens An6 or Bacillus subtilis A26 fermentation (Ghorbel-Bellaaj et al. 2012b). When fish meat, obtained from sardinelle, zebra blenny, goby, or ray, was hydrolyzed using B. subtilis A26, hydrolysates with antioxidant activities were produced. Furthermore, these hydrolysates showed antibacterial activities against Gram-positive bacteria (Jemil et al. 2014). Ruthu et al. (2014) used carb fish head and 10 % (w/w) dextrose for the fermentative production of a protein hydrolysate with antibacterial effects. The strains used in fermentations were Enterococcus faecium NCIM5335, Pediococcus acidilactici FD3, and P. acidilactici NCIM5368. Cai et al. (2015) hydrolyzed grass carb skin using Alcalase for 115 min at 52 °C and pH 8.5 and isolated antioxidant peptides. Furthermore, the production of angiotensin-I converting enzyme inhibitory peptide by Lactobacillus helveticus LB10 was shown (Pan and Guo 2010). This enzyme inhibitor is used in medical treatments of hypertension. At an initial pH of 7.5, 39 °C, and 4 % (v/w) of skin milk inoculum, the predicted angiotensin-I converting enzyme inhibitory activity was 72.3 %.
Use of protein hydrolysate for the production of biomolecules, chemicals and materials
Protein hydrolysates obtained using various hydrolytic methods from various protein-rich materials have been used in biotechnological processes as alternatives to cost-intensive nitrogen sources (Table 2). A large number of the processes described in literature deal with the production of lactic acid. This is probably due to the capability of the lactic acid bacteria to utilize various complex nitrogen sources. Fitzpatrick and O’Keeffe (2001) used whey protein hydrolysate, obtained by hydrolyzing the retentate from ultrafiltration of whey using a serine protease from B. licheniformis, together with permeate in L. helveticus lactic acid fermentation. They emphasized on evaluating the minimum amount of whey protein hydrolysate required to obtain high lactose conversion and lactic acid yields. The goal was to keep raw material cost low and to avoid costly purification steps of lactic acid in order to remove impurities introduced by the hydrolysate. It was found that a 3–4 % (w/w) whey protein hydrolysate, accounting for 0.06–0.09 % (w/w) nitrogen, is appropriate to convert 50 g L−1 lactose into lactic acid within 30–40 h. It was, however, admitted that the amount of whey protein hydrolysate required depends on the protein concentration of the retentate. The retentate used in their study had a protein content of 10 % (w/w). Gao et al. (2006) utilized fish wastes in lactic acid fermentations. They first grinded the waste material and used autoclavation at 121 °C for 20 min as pretreatment. Afterwards, supernatant and residue were separated. The pH of residues was then adjusted to 1 by adding 6 M HCl and the suspension autoclaved again at 121 °C for 20 min (Gao et al. 2006). The supernatants of pretreatment and acid hydrolysis were mixed and used as low-cost nutrient source in Lactobacillus rhamnosus fermentation. The authors reached a lactic acid concentration of 79 g L−1 within 29 h when they added 5.1 g L−1 nitrogen to 100 g L−1 glucose. This fermentation was remarkably faster than control fermentations carried out in the presence of 20 g L−1 yeast extract; however, it was also indicated that the addition of fish waste hydrolysate results in higher separation costs due to impurities introduced (Gao et al. 2006). An interesting study on reducing nutrient cost in lactic acid fermentations was published by Ma et al. (2014). They used hydrolyzed excess sludge from a sewage disposal plant, which was rich in protein, lipid, and carbohydrate, and carried out non-sterile Bacillus coagulans lactic acid fermentations. This innovative approach links wastewater treatment to white biotechnology. In their study, a lactic acid concentration of 79 g L−1 and a productivity of 2.8 g L−1 h−1 were obtained in the presence of 1 % (w/w) excess sludge hydrolysate and 100 g L−1 glucose (Ma et al. 2014). Yao et al. used untreated crude protein from dairy manure in order to supply an inexpensive nitrogen source in Rhizopus orzyae lactic fermentations (Yao et al. 2009). They used 1.3 g L−1 crude protein and 240 g L−1 glucose and achieved a lactic acid yield of 57.2 %. Another example dealing with the use of non-conventional nitrogen sources in lactic acid fermentations was provided by Vodnar et al. (2010) who applied lucerne press juice. Using lucerne press juice, no hydrolysis is needed in order to make nitrogen compounds available for microorganisms. In the study by Vodnar et al. (2010), Lactobacillus paracasei efficiently utilized 2 g L−1 nitrogen from lucerne press juice and produced around 50 g L−1 l-lactic acid from 55 g L−1 glucose within 20 h. The optical purity of l-lactic acid was 97 %. Winter et al. (2015) used animal by-products as cheap source of peptones to be used as nitrogen source in B. coagulans lactic acid fermentation. Hydrolysis was carried out at 180–220 °C for 25–40 min and 50–75 bar (Animox 2012). The application of the obtained hydrolysate did not negatively affect lactic acid fermentation, and after around 30 h, more than 90 g L−1 was obtained from 120 g L−1 glucose (Table 2).
The organic fraction of municipal solid waste can be rich in proteins due to wasted food. Food waste usually consists of meat, noodles, vegetables, and fruits, which can be hydrolyzed to create carbon and nitrogen-rich fermentation feedstocks (Pleissner and Lin 2013) (Table 2). An economic way to hydrolyze starch and protein in food waste, in order to treat and utilize food waste, and for providing an inexpensive fermentation medium, is fungal hydrolysis (Kwan et al. 2015). Fungal hydrolysis refers to a process where fungal biomass (Aspergillus awamori and Aspergillus oryzae) added to a blend of food waste secretes extracellular glycolytic and proteolytic enzymes. Up to 90 % of initial food waste can be hydrolyzed using this approach (Pleissner et al. 2014a). The advantage is that no cost-intensive enzymes are required. About 10 % (w/w) of food waste can be proteins and under consideration that 1.3 billion tons appear globally per year, food waste is a remarkable protein source to be used in fermentations (Pleissner et al. 2014a). Sun et al. (2014) used food waste hydrolysate, consisting of 0.28 g L−1 free amino nitrogen, as feedstock for the fermentative production of succinic acid and obtained promising results. When Actinobacillus succinogenes was used in fermentations, 24.1 g L−1 succinic acid and 13.7 g L−1 of by-products were produced from 31.9 g L−1 glucose. However, when a recombinant strain of Escherichia coli was used, 29.9 g L−1 of succinic acid was obtained from 31.9 g L−1 glucose (Table 2).
Food waste hydrolysate is not only an appropriate nutrient source for bacteria but also for microalgae and yeasts (Table 2) (Lau et al. 2014; Pleissner et al. 2015; Ryu et al. 2013). For instance, Pleissner et al. (2015) presented a process for fatty acid feedstock preparation as an integrated process in mixed restaurant food and bakery wastes treatment. In their study, fed-batch cultivation of the heterotrophic microalgal strain Chlorella pyrenoidosa was carried out using a food waste hydrolysate containing 106.9 g L−1 glucose and 1.9 g L−1 free amino nitrogen as feed. More than 50 g L−1 biomass was produced within 5 days containing 9 g L−1 lipids and 2.7 g L−1 alpha-linolenic acid. Lau et al. (2014) applied food waste hydrolysate as nutrient medium in Chlorella vulgaris batch fermentation. The hydrolysate contained around 18 g L−1 glucose, 0.5 g L−1 fructose, 0.3 g L−1 free amino nitrogen, and 0.2 g L−1 phosphate. From the biomass produced, they obtained 1.1 g L−1 lipids and 0.2 g L−1 alpha-linolenic acid. It should, however, be admitted here that the growth rate of C. pyrenoidosa and C. vulgaris was strongly dependent on the food waste hydrolysate concentration applied. C. pyrenoidosa did grow much slower at glucose concentration above 30 g L−1 (Pleissner et al. 2015). C. vulgaris exhibited a reduced growth rate when the cultivation was carried in food waste hydrolysate exceeding the abovementioned concentrations (Lau et al. 2014). Hence, preliminary experiments are required in order to identify the appropriate food waste hydrolysate concentration to be used in Chlorella sp. fermentations. As indicated, the biomasses of both algal strains are sources of the polyunsaturated fatty acid alpha-linolenic acid, which is an essential fatty acid for mammals, and thus a desired food and feed supplement. However, it should be admitted here that food and feed supplements produced from waste streams may pose risks for animal and human health. For instance, the EU prohibited the use of animal protein materials as farmed animal feed due to the bovine spongiform encephalopathy problem (García et al. 2005). This restriction may also apply for the use of food and feed supplements produced from food waste via biotechnological processes. Furthermore, the presence of heavy metals and dioxins in organic waste makes an analysis of fermentation products necessary (García et al. 2005).
Ryu et al. (2013). presented another approach to produce fatty acids from inexpensive feedstocks. They used untreated spent yeast as nitrogen source in fermentations of the oleaginous yeast Cryptococcus curvatus. The hydrolysis of spent yeast was carried out by the process of autolysis. In the study of Ryu et al. (2013), a biomass concentration of 50.4 g L−1 and a total fatty acid concentration of 19 g L−1 were obtained when 30 g L−1 spent yeast was supplied with glycerol as carbon source. Another product obtainable from the fermentative treatment of food waste hydrolysate, and in particular bakery waste hydrolysate, is polyhydroxybutyrate, a highly wanted bio-plastic (Table 2). Pleissner et al. (2014b) reported the fermentative production of 2.6 g L−1 polyhydroxybutyrate using Halomonas boliviensis cultured in bakery waste hydrolysate containing 62.5 g L−1 glucose, 20 g L−1 fructose, and 0.2 g L−1 free amino nitrogen.
The quality of nitrogen source is particularly important when the production of a desired product requires special amino acids. Solaiman et al. (2011) used hydrolysates of meat and bone meal for the fermentative production of cyanophycin, a poly(arginyl-aspartate) biopolymer. A recombinant E. coli strain with the ability to intracellularly store cyanophycin was employed. Interesting enough, using meat and bone meal hydrolysates, Solaiman et al. (2011) obtained 33–35 % of a crude cyanophycin product compared to the product when reference casamino acids were used. The result is particularly of interest as it shows that waste streams can be utilized for the supply of special amino acids (Table 2).
Conclusions and future perspectives
Protein-rich materials are appropriate substrates for the production of enzymes, hydrolysates with antioxidant and antimicrobial activities, and hydrolysates rich in organic nitrogen to be used as nitrogen sources in biotechnological processes. The presented examples of biotechnological processes for the production of various value-added products illustrate the potential of protein-rich materials to serve as complex nitrogen sources for many microorganisms. In particular, the use of keratinous materials, such as hair, wool, and horn, is not only an effective utilization approach but also a treatment to minimize the amount of those recalcitrant materials, which are conventionally considered as waste. It should, however, be noted that the application of complex nitrogen sources in biotechnological processes may complicate downstream processing. Complex nitrogen sources may contain impurities, which need to be removed in order to obtain pure products. Hence, additional purification steps may be necessary. Nevertheless, the low cost of residues may counterbalance the increased process costs. It can be concluded that the number of industrial processes based on protein-rich materials will increase in the future due to economic benefits.
References
Adıgüzel AC, Bitlisli BO, Yaşa İ, Eriksen NT (2009) Sequential secretion of collagenolytic, elastolytic, and keratinolytic proteases in peptide-limited cultures of two Bacillus cereus strains isolated from wool. J Appl Microbiol 107(1):226–234. doi:10.1111/j.1365-2672.2009.04200.x
Animox (2012) Method for producing protein hydrolysates. EP 1835816 B1
Bataille MP, Bataille PF (1983) Extraction of proteins from shrimp processing waste. J Chem Technol Biotechnol 33(4):203–208. doi:10.1002/jctb.280330402
Beaulieu L, Desbiens M, Thibodeau J, Thibault S (2009) Pelagic fish hydrolysates as peptones for bacterial culture media. Can J Microbiol 55(11):1240–1249. doi:10.1139/W09-084
Benhabiles MS, Abdi N, Drouiche N, Lounici H, Pauss A, Goosen MFA, Mameri N (2013) Protein recovery by ultrafiltration during isolation of chitin from shrimp shells Parapenaeus longirostris. Food Hydrocoll 32(1):28–34. doi:10.1016/j.foodhyd.2012.11.035
Brandelli A (2008) Bacterial keratinases: useful enzymes for bioprocessing agroindustrial wastes and beyond. Food Bioprocess Technol 1(2):105–116. doi:10.1007/s11947-007-0025-y
Burkovski A, Krämer R (2002) Bacterial amino acid transport proteins: occurrence, functions, and significance for biotechnological applications. Appl Microbiol Biotechnol 58(3):265–274. doi:10.1007/s00253-001-0869-4
Cai L, Wu X, Zhang Y, Li X, Ma S, Li J (2015) Purification and characterization of three antioxidant peptides from protein hydrolysate of grass carp (Ctenopharyngodon idella) skin. J Funct Foods 16:234–242. doi:10.1016/j.jff.2015.04.042
Chalamaiah M, B D k, R H, T J (2012) Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chem 135(4):3020–3038. doi:10.1016/j.foodchem.2012.06.100
Chitte RR, Nalawade VK, Dey S (1999) Keratinolytic activity from the broth of a feather-degrading thermophilic Streptomyces thermoviolaceus strain SD8. Lett Appl Microbiol 28(2):131–136. doi:10.1046/j.1365-2672.1999.00484.x
Dalev PG (1994) Utilisation of waste feathers from poultry slaughter for production of a protein concentrate. Bioresour Technol 48(3):265–267. doi:10.1016/0960-8524(94)90156-2
El SN (1995) Evaluating protein quality of meats using collagen content. Food Chem 53(2):209–210. doi:10.1016/0308-8146(95)90790-E
Esteban MB, García AJ, Ramos P, Márquez MC (2010) Sub-critical water hydrolysis of hog hair for amino acid production. Bioresour Technol 101(7):2472–2476. doi:10.1016/j.biortech.2009.11.054
Fakhfakh N, Ktari N, Siala R, Nasri M (2013) Wool-waste valorization: production of protein hydrolysate with high antioxidative potential by fermentation with a new keratinolytic bacterium, Bacillus pumilus A1. J Appl Microbiol 115(2):424–433. doi:10.1111/jam.12246
Fitzpatrick JJ, U O’K (2001) Influence of whey protein hydrolysate addition to whey permeate batch fermentations for producing lactic acid. Process Biochem 37(2):183–186. doi:10.1016/S0032-9592(01)00203-5
Fontoura R, Daroit DJ, Correa APF, Meira SMM, Mosquera M, Brandelli A (2014) Production of feather hydrolysates with antioxidant, angiotensin-I converting enzyme- and dipeptidyl peptidase-IV-inhibitory activities. New Biotechnol 31(5):506–513. doi:10.1016/j.nbt.2014.07.002
Gao M-T, Hirata M, Toorisaka E, Hano T (2006) Acid-hydrolysis of fish wastes for lactic acid fermentation. Bioresour Technol 97(18):2414–2420. doi:10.1016/j.biortech.2005.10.002
Garcia AJ, Esteban MB, Márquez MC, Ramos P (2005) Biodegradable municipal solid waste: characterization and potential use as animal feedstuffs. Waste Manage 25(8):780–787. doi:10.1016/j.wasman.2005.01.006
Gehle MH, Speers GM, Miller DL, Balloun SL (1967) Nutritive value of hydrolyzed hog hair as a protein source for chicks and poults. Poult Sci 46(1):156–164. doi:10.3382/ps.0460156
Ghorbel-Bellaaj O, Jridi M, Khaled HB, Jellouli K, Nasri M (2012a) Bioconversion of shrimp shell waste for the production of antioxidant and chitosan used as fruit juice clarifier. Int J Food Sci Technol 47(9):1835–1841. doi:10.1111/j.1365-2621.2012.03039.x
Ghorbel-Bellaaj O, Younes I, Maâlej H, Hajji S, Nasri M (2012b) Chitin extraction from shrimp shell waste using Bacillus bacteria. Int J Biol Macromol 51(5):1196–1201. doi:10.1016/j.ijbiomac.2012.08.034
Gunasekaran J, Kannuchamy N, Kannaiyan S, Chakraborti R, Gudipatti V (2014) Protein hydrolysates from shrimp (Metapenaeus dobsoni) head waste: optimization of extraction conditions by response surface methodology. J Aquat Food Prod Technol 24(5):429–442. doi:10.1080/10498850.2013.787134
Gupta R, Ramnani P (2006) Microbial keratinases and their prospective applications: an overview. Appl Microbiol Biotechnol 70(1):21–33. doi:10.1007/s00253-005-0239-8
Jemil I, Jridi M, Nasri R, Ktari N, R BS-BS, M M, M H, M N (2014) Functional, antioxidant and antibacterial properties of protein hydrolysates prepared from fish meat fermented by Bacillus subtilis A26. Process Biochem 49(6):963–972. doi:10.1016/j.procbio.2014.03.004
Klompong V, Benjakul S, Kantachote D, Shahidi F (2012) Use of protein hydrolysate from yellow stripe trevally (Selaroides leptolepis) as microbial media. Food Bioprocess Technol 5(4):1317–1327. doi:10.1007/s11947-010-0402-9
Koutinas AA, Vlysidis A, Pleissner D, Kopsahelis N, Lopez Garcia I, Kookos IK, Papanikolaou S, Kwan TH, Lin CSK (2014) Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem Soc Rev 43(8):2587–2627. doi:10.1039/C3CS60293A
Kwan TH, Pleissner D, Lau KY, Venus J, Pommeret A, Lin CSK (2015) Techno-economic analysis of a food waste valorization process via microalgae cultivation and co-production of plasticizer, lactic acid and animal feed from algal biomass and food waste. Bioresour Technol 198:292–299. doi:10.1016/j.biortech.2015.09.003
Lasekan A, Abu Bakar F, Hashim D (2013) Potential of chicken by-products as sources of useful biological resources. Waste Manage 33(3):552–565. doi:10.1016/j.wasman.2012.08.001
Lau KY, Pleissner D, Lin CSK (2014) Recycling of food waste as nutrients in Chlorella vulgaris cultivation. Bioresour Technol 170:144–151. doi:10.1016/j.biortech.2014.07.096
Lazzi C, Meli F, Lambertini F, Bottesini C, Nikolaev I, Gatti M, Sforza S, Koroleva O, Popov V, Neviani E, Dossena A (2013) Growth promotion of Bifidobacterium and Lactobacillus species by proteinaceous hydrolysates derived from poultry processing leftovers. Int J Food Sci Technol 48(2):341–349. doi:10.1111/j.1365-2621.2012.03192.x
Lü F, He P-J, Shao L-M, Lee D-J (2007) Effects of ammonia on hydrolysis of proteins and lipids from fish residues. Appl Microbiol Biotechnol 75(5):1201–1208. doi:10.1007/s00253-007-0935-7
Ma K, Maeda T, You H, Shirai Y (2014) Open fermentative production of L-lactic acid with high optical purity by thermophilic Bacillus coagulans using excess sludge as nutrient. Bioresour Technol 151:28–35. doi:10.1016/j.biortech.2013.10.022
Mark Hsieh C, Yang F-C, Iannotti EL (1999) The effect of soy protein hydrolyzates on fermentation by Lactobacillus amylovorus. Process Biochem 34(2):173–179. doi:10.1016/S0032-9592(98)00081-8
Mohamed AA, Rayas-Duarte P (1995) Composition of Lupinus albus. Cereal Chem 72(6):643–647
Najafian L, Babji AS (2012) A review of fish-derived antioxidant and antimicrobial peptides: their production, assessment, and applications. Peptides 33(1):178–185. doi:10.1016/j.peptides.2011.11.013
Pan D, Guo Y (2010) Optimization of sour milk fermentation for the production of ACE-inhibitory peptides and purification of a novel peptide from whey protein hydrolysate. Int Dairy J 20(7):472–479. doi:10.1016/j.idairyj.2010.01.007
Papendiek F, Venus J (2014) Cultivation and fractionation of leguminous biomass for lactic acid production. Chem Biochem Eng Q 28(3):33–40
Pasupuleti VK, Holmes C, Demain AL (2010) Applications of protein hydrolysates in biotechnology. In: Pasupuleti VK, Demain AL (eds) Protein hydrolysates in biotechnology. Springer, Dordrecht, Heidelberg, London, New York, pp. 1–9
Pleissner D, Eriksen NT (2012) Effects of phosphorous, nitrogen, and carbon limitation on biomass composition in batch and continuous flow cultures of the heterotrophic dinoflagellate Crypthecodinium cohnii. Biotechnol Bioeng 109(8):2005–2016. doi:10.1002/bit.24470
Pleissner D, Kwan TH, Lin CSK (2014a) Fungal hydrolysis in submerged fermentation for food waste treatment and fermentation feedstock preparation. Bioresour Technol 158:48–54. doi:10.1016/j.biortech.2014.01.139
Pleissner D, Lam WC, Han W, Lau KY, Cheung LC, Lee MW, Lei HM, Lo KY, Ng WY, Sun Z, Melikoglu M, Lin CSK (2014b) Fermentative polyhydroxybutyrate production from a novel feedstock derived from bakery waste. BioMed Res Int 2014:8. doi:10.1155/2014/819474
Pleissner D, Lam WC, Sun Z, Lin CSK (2013) Food waste as nutrient source in heterotrophic microalgae cultivation. Bioresour Technol 137:139–146. doi:10.1016/j.biortech.2013.03.088
Pleissner D, Lau KY, Schneider R, Venus J, Lin CSK (2015) Fatty acid feedstock preparation and lactic acid production as integrated processes in mixed restaurant food and bakery wastes treatment. Food Res Int 73:52–61. doi:10.1016/j.foodres.2014.11.048
Pleissner D, Lin CSK (2013) Valorisation of food waste in biotechnological processes. Sus Chem Proc 1:6. doi:10.1186/2043-7129-1-21
Pleissner D, Wimmer R, Eriksen NT (2011) Quantification of amino acids in fermentation media by isocratic HPLC analysis of their α-hydroxy acid derivatives. Anal Chem 83(1):175–181. doi:10.1021/ac1021908
Richter PR, Liu Y, An Y, Li X, Nasir A, Strauch SM, Becker I, Krüger J, Schuster M, Ntefidou M, Daiker V, Haag FWM, Aiach A, Lebert M (2015) Amino acids as possible of Euglena gracilis Z in life support systems. Life Sci Space Res 4:1–5. doi:10.1016/j.lssr.2014.11.001
Ruthu MPS, Rai AK, Bhaskar N (2014) Fermentative recovery of lipids and proteins from freshwater fish head waste with reference to antimicrobial and antioxidant properties of protein hydrolysate. J Food Sci Technol 51(9):1884–1892. doi:10.1007/s13197-012-0730-z
Ryu B-G, Kim J, Kim K, Choi Y-E, Han J-I, Yang J-W (2013) High-cell-density cultivation of oleaginous yeast Cryptococcus curvatus for biodiesel production using organic waste from the brewery industry. Bioresour Technol 135:357–364. doi:10.1016/j.biortech.2012.09.054
Safari R, Motamedzadegan A, Ovissipour M, Regenstein J, Gildberg A, Rasco B (2012) Use of hydrolysates from yellowfin tuna (Thunnus albacares) heads as a complex nitrogen source for lactic acid bacteria. Food Bioprocess Technol 5(1):73–79. doi:10.1007/s11947-009-0225-8
Serrano-Carreon L, Cortes-Fernandez J, Gutierrez-Rojas M, Roussos S, Viniegra-Gonzalez G, Lonsane BK (1993) Utilization of slaugther-house byproducts as nitrogen source for fiamentous fungi in submerged fermentation, with simultaneous deodorization. Micol Neotrop Apl 6:15–26
Solaiman DKY, Garcia RA, Ashby RD, Piazza GJ, Steinbüchel A (2011) Rendered-protein hydrolysates for microbial synthesis of cyanophycin biopolymer. New Biotechnol 28(6):552–558. doi:10.1016/j.nbt.2011.03.025
Solli L, Bergersen O, Sørheim R, Briseid T (2014) Effects of a gradually increased load of fish waste silage in co-digestion with cow manure on methane production. Waste Manage 34(8):1553–1559. doi:10.1016/j.wasman.2014.04.011
Sun Z, Li M, Qi Q, Gao C, Lin C (2014) Mixed food waste as renewable feedstock in succinic acid fermentation. Appl Biochem Biotechnol 174(5):1822–1833. doi:10.1007/s12010-014-1169-7
Tejayadi S, Cheryan M (1995) Lactic acid from cheese whey permeate. Productivity and economics of a continuous membrane bioreactor. Appl Microbiol Biotechnol 43(2):242–248. doi:10.1007/BF00172819
Ummadi M, Curic-Bawden M (2010) Use of protein hydrolysate in industrial starter culture fermentations. In: Pasupuleti VK, Demain AL (eds) Protein hydrolysates in biotechnology. Springer, Dordrecht, Heidelberg, London, New York, pp. 91–114
Vasileva-Tonkova E, Nustorova M, Gushterova A (2007) New protein hydrolysates from collagen wastes used as peptone for bacterial growth. Curr Microbiol 54(1):54–57. doi:10.1007/s00284-006-0308-y
Venus J (2009) Continuous mode lactic acid fermentation based on renewables. Res J Biotechnol 4(2):8
Vodnar DC, Venus J, Schneider R, Socaciu C (2010) Lactic acid production by Lactobacillus paracasei 168 in discontinuous fermentation using lucerne green juice as nutrient substitute. Chem Eng Technol 33(3):468–474
Wang JJ, Shih JCH (1999) Fermentation production of keratinase from Bacillus licheniformis PWD-1 and a recombinant B. subtilis FDB-29. J Ind Microbiol Biotechnol 22(6):608–616. doi:10.1038/sj.jim.2900667
Winter B, Höhling A, Venus J (2015) Tierische proteinhydrolysate als N-Quelle für die Milchsäurefermentation. BIOspektrum 2:228–229. doi:10.1007/s12268-015-0562-2
Wu YV, Sexson KR, Lagoda AA (1984) Protein-rich residue from wheat alcohol distillation: fractionation and characteriation. Cereal Chem 61(5):423–427
Yao W, Zhu J, Sun B, Miller C (2009) Development and optimization of a culture medium for L-lactic acid production by Rhizopus oryzae using crude protein from dairy manure as a nitrogen source. J Environ Sci Health A 44(12):1306–1313. doi:10.1080/10934520903140157
You L, Zhao M, Regenstein JM, Ren J (2010) Purification and identification of antioxidative peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Res Int 43(4):1167–1173. doi:10.1016/j.foodres.2010.02.009
Young G, Mebrahtu T (1998) Protein, fiber, and lipid content of vegetable soybean. J Am Diet Assoc 98(9, Supplement):A44. doi:10.1016/S0002-8223(98)00461-1
Zhao H, Wan C, Zhao M, Lei H, Mo F (2014) Effects of soy protein hydrolysates on the growth and fermentation performances of brewer’s yeast. Int J Food Sci Technol 49(9):2015–2022. doi:10.1111/ijfs.12503
Zoccola M, Aluigi A, Tonin C (2009) Characterisation of keratin biomass from butchery and wool industry wastes. J Mol Struct 938(1–3):35–40. doi:10.1016/j.molstruc.2009.08.036
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Pleissner, D., Venus, J. Utilization of protein-rich residues in biotechnological processes. Appl Microbiol Biotechnol 100, 2133–2140 (2016). https://doi.org/10.1007/s00253-015-7278-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7278-6