Abstract
Ammonia inhibition of methane fermentation is one of the leading causes of failure of anaerobic digestion reactors. In a batch anaerobic digestion reactor with 429 mM NH3-N/L of ammonia, the addition of 25 mM phosphate resulted in an increase in methane production rate. Similar results were obtained with the addition of disodium phosphate in continuous anaerobic digestion using an upflow anaerobic sludge blanket (UASB) reactor. While methane content and production rate decreased in the presence of more than 143 mM NH3-N/L of ammonium chloride in UASB, the addition of 5 mM disodium phosphate suppressed ammonia inhibition at 214 mM NH3-N/L of ammonium chloride. The addition prevented acetate/propionate accumulation, which might be one of the effects of the phosphate on the ammonia inhibition. The effects on the microbial community in the UASB reactor was also assessed, which was composed of Bacteria involved in hydrolysis, acidogenesis, acetogenesis, and dehydrogenation, as well as Archaea carrying out methanogenesis. The change in the microbial community was observed by ammonia inhibition and the addition of phosphate. The change indicates that the suppression of ammonia inhibition by disodium phosphate addition could stimulate the activity of methanogens, reduce shift in bacterial community, and enhance hydrogen-producing bacteria. The addition of phosphate will be an important treatment for future studies of methane fermentation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anaerobic digestion is widely used for wastewater treatment with the production of renewable biogas, mainly methane. It is applicable to a wide range of organic materials at low temperatures (35–60 °C). Methane fermentation is carried out by a complex and phylogenetically diverse community of microorganisms (Archaea and Bacteria) with different metabolic functions (Tabatabaei et al. 2010). The metabolic pathway leading to methane fermentation can be divided into several steps. First, proteins, carbohydrates, and lipids of complex organic matter are degraded and converted to amino acids, oligosaccharides, and long chain fatty acids (hydrolysis). These intermediate compounds are then converted to organic acids (acidogenesis), and subsequently to acetate, CO2, and H2 (acetogenesis and dehydrogenation). These initial steps are bacterial processes. In the final step (methanogenesis), acetate, CO2, H2, and other C1 compounds such as formate, are converted to methane by Archaea (Chen et al. 2008).
Despite its significance, the methane fermentation system has not been extensively utilized because of process instability and operational difficulties. One of the reasons for its instability is a high sensitivity to ammonia; i.e., methane formation is usually inhibited by high concentrations of ammonia accumulated during the anaerobic digestion. The inhibition causes an adverse shift in the microbial population or inhibition of bacterial growth, and is usually indicated by a decrease of the steady-state rate of methane gas production and accumulation of organic acids (Chen et al. 2008). Concentrations of 2000–6000 mg/L (143–429 mM) of total ammonia nitrogen (TAN) have been reported to inhibit and reduce the methane production by 39-90 % (Krylova et al. 1997; Sung and Liu 2003). This inhibition has been attributed to a decrease in cell density of Archaea at TAN values greater than 3000 mg NH3-N/L (Sawayama et al. 2004). Organic wastes are therefore usually diluted with water to avoid ammonia inhibition. Several other methods have been developed to prevent ammonia accumulation (Chen et al. 2008), such as ammonia stripping with pH and temperature control to remove ammonia from the waste samples prior to the anaerobic digestion (Rao et al. 2008; Yabu et al. 2011; Zhang and Jahng 2010). Ammonia stripping is based on changing the sample conditions to enable the transition of ammonium ion to ammonia gas, which is efficiently removed from the liquid phase by air (Arogo et al. 1999). However, this method requires high-energy costs for heating and blowing of air or biogas to volatilize ammonia, and is therefore inappropriate for waste treatments. Another promising and established method, the precipitation of magnesium ammonium phosphate hexahydrate (MgNH4PO4·6H2O, MAP) by addition of magnesium and phosphate, has been widely studied for removal of ammonium ion from anaerobic digestion (Pastor et al. 2010; Qiao et al. 2010; Quan et al. 2010; Türker and Celen 2007; Uludag-Demirer et al. 2008). In addition to high elimination rates, this treatment is cost effective because MAP precipitate can be used as a fertilizer by recovering phosphate and ammonia in the anaerobic digestion (Uysal et al. 2010). However, its application is mainly based on models from fluidized bulk reactors and stirred tanks (Ueno and Fujii 2001; Battistoni et al. 2001). The objective of this study is to investigate whether the addition of magnesium and phosphate is effective in preventing ammonia inhibition during anaerobic digestion in the batch reactor system and upflow anaerobic sludge blanket (UASB) reactor system. Changes in gas production rate, methane content, COD removal, accumulation of acetate and propionate, and microbial communities in the continuous reactors were also monitored in different concentrations of TAN and phosphate.
Materials and methods
Preparation of batch reactors of methane fermentation
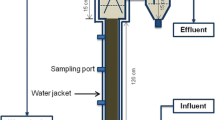
The batch reactors were prepared in 50-mL vials with 20 mL of nitrogenous seafood-processing wastewater provided by a seafood-processing company in Japan as substrate (chemical composition is shown in Table 1). As a source of microorganisms, 2.0 g lake sediment from Lake Sanaru (Hamamatsu, Shizuoka, Japan) was inoculated into the reactor. After purging with reduced nitrogen gas for 15 min, the vial bottles were sealed with butyl rubber caps and aluminum caps, and incubated at 35 °C. To assess the effects of cations and phosphates, 18 batch reactors were prepared for the six different conditions (in triplicate) by supplementing with: (i) MgCl2▪6H2O (25 mM final concentration), (ii) KCl (50 mM), (iii) K2HPO4 (25 mM), (iv) Na2HPO4 (25 mM), (v) MgCl2·6H2O (25 mM) + K2HPO4 (25 mM), and (vi) control (no additional compounds). The gas in the vial bottle headspace was subjected to gas chromatography to measure the methane and carbon dioxide contents (see “Analytical methods”). Concentration of total ammonia nitrogen (TAN) measured the dissolved ammonia nitrogen in the liquid of the batch reactor sample by ion chromatography (IC, see “Analytical methods”) after centrifugation (15,000×g for 10 min, 4 °C) and filtration through a 0.22-μm filter membrane of 2-mL sample.
Analytical methods
The methane and carbon dioxide contents in the gas were measured using a gas chromatograph system (GC-8A, SHIMADZU) equipped with a thermal conductivity detector and a Sunpak-A packed column (Shinwa Chemical Industries). Helium was used as carrier gas at a flow rate of 40 mL/min. The injection, detection, and column temperatures were set at 50 °C. The volume of produced biogas was measured by the water displacement method. TAN was analyzed by ion chromatography (IC, DIONEX ICS-1500, Thermo Scientific Dionex) equipped with a cation separation column (IonPac CS3, Thermo Scientific Dionex). Concentrations of acetate and propionate were analyzed using a high-performance liquid chromatography (HPLC) system equipped with a UV detector (UV-2075, JASCO) and an organic acid separation column (RSpak KC-811, Shodex). TOC was analyzed by TOC analyzer (TOC-V CPH, Shimadzu). Chemical oxygen demand (CODCr) was determined by standard methods (APHA 1989). Total nitrogen (TN) and phosphate concentration were measured following Japanese industrial standard methods (JIS K0101 1998).
Upflow anaerobic sludge blanket reactors
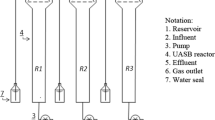
Two UASB reactors (A and B) were prepared with total volumes of 2.2 L. The reactors were initially filled with 1.0 L of granule sludge collected from a full-scale UASB wastewater treatment reactor at a Japanese domestic brewery. Cat food extract (pH 6.0) was fed into the reactors as the model wastewater because it was rich in ammonia (whose chemical composition is shown in Table 2). The reactors were operated with hydraulic retention time (HRT) of 6 h at 35 °C. COD of the model wastewater was started from 200 mg/L for 45 days followed by 400 mg/L for 100 days, and then increased to 1200 mg/mL (set to 0 day). Biogas produced from the reactors was collected in gasbags made of vinyl alcohol polymer film (Smart Bag PA, GL Science).
Analysis of effects of phosphate addition to the UASB reactors
Ammonia (ammonium chloride, NH4Cl) and phosphate (disodium phosphate, Na2HPO4) were added to the synthetic wastewater in a series of steps. We divided the steps into six phases (phase I to VI) according to the added concentrations of ammonium chloride and disodium phosphate. The concentrations of ammonium chloride in the influent to both reactors were increased from 0 (phase I) to 2000 mg (143 mM) NH3-N/L (phases II and III), 3000 mg (214 mM) NH3-N/L (phase IV), and 4000 mg (286 mM) NH3-N/L (phases V and VI). Disodium phosphate was added to the influent of reactor B with concentrations of 0 (phases I and II), 5 mM (phases III to V), and 10 mM (phase VI).
Analyses of microbial community structure
Granules in the reactors were sampled with a sterilized syringe at the end of each phase. DNA was extracted from the granules using PowerSoil® DNA Isolation Kit (MO BIO Laboratories, CA). 16S rRNA gene sequences of the granules were amplified with the extracted DNA using barcode-tagged-primer sets (515F–805R) (Table S1), and then the pool of tagged-16S rRNA gene sequences were examined by pyrosequencing using Roche 454 GS FLX+ (Roche Applied Science, Indianapolis, IN). The analyses of the resultant sequences were performed by quantitative insights into microbial ecology (QIIME, http://www.qiime.org/) (Caporaso et al. 2010). The operational taxonomic unit (OTU) was defined as a nucleotide sequence group that showed >97 % identity, and the representative sequence of the OTU was then subjected to a BLAST search. Each OTU was classified into families based on their identities in the BLAST search.
Nucleotide sequence accession numbers
The pyrosequencing run data of the 16S rRNA gene amplicons in the UASB reactors were deposited into the DDBJ Sequence Read Archive (DRA) (http://trace.ddbj.nig.ac.jp/dra/index_e.html; accession number DRA002312).
Results
Addition of magnesium and/or phosphate accelerated methane fermentation in the batch reactor
To assess the effects of magnesium and phosphate additions on ammonia inhibition in methane fermentation, batch reactors were prepared with sediments from Lake Sanaru (Hamamatsu, Japan) and nitrogenous seafood-processing wastewater. The TAN of the samples was 672 mg NH3-N/L (48 mM) before the fermentation (Table 1), and 5800–6300 mg NH3-N/L (414–450 mM) after 105 days of methane fermentation. Although ammonia concentration increased to the inhibitory concentration, the methane content of the samples reached and was maintained at 65–79 % after 54 days regardless of the addition of magnesium and/or phosphate (Fig. 1a). However, magnesium and/or phosphate addition accelerated the time in which samples reached the maximum methane content (65–70 %). The addition of phosphate was more effective, with samples reaching the maximum value 24 days earlier than without addition of phosphate (Fig. 1a). In samples with added magnesium and/or phosphate, the maximum carbon dioxide content (36–40 %) was obtained faster (at day 10) than the control (at day 24) (Fig. 1b). After reaching the peak, samples with phosphate (K2HPO4 and MgCl2 + K2HPO4) showed a rapid decrease in carbon dioxide content through day 39. The effect of adding potassium chloride was very similar to that of magnesium chloride and the addition of sodium phosphate was similar to that of potassium phosphate (Fig. S1). Disodium phosphate was routinely used as it is generally cheaper than dipotassium phosphate.
Disodium phosphate addition suppressed ammonia inhibition in the UASB reactor
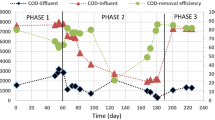
Two UASB reactors were prepared with model wastewater made from cat food (phase I to VI). Ammonium chloride was added to the model wastewater samples (influent) of both reactors (A and B) at phase II while disodium phosphate was only added to that of reactor B at phase III. Changes in gas production rate, methane content, accumulation of acetate and propionate, COD removal percentage, and pH were monitored in different concentrations of TAN and disodium phosphate (phosphate) (Fig. 2, phases I to VI). The changes in methane quantity (methane production rate, Fig. S2) were calculated from the gas production rate (Fig. 2a) and the methane content (Fig. 2b).
Performances of methane fermentation in UASB reactors, reactor A (blue open circles and lines, without phosphate) and reactor B (red open circles and lines, with phosphate addition): a gas production rate; b methane content; c the ratio of methane to carbon dioxide; d COD removal; e acetate concentration; f propionate concentration; g the ratio of acetate to propionate; h pH of influent (closed circles) and effluent (open circles). Phase I, no addition; II, 2000 mg (143 mM) NH3-N/L; III, 2000 mg NH3-N/L and 5 mM of phosphate; IV, 3000 mg (214 mM) NH3-N/L and 5 mM of phosphate; V, 4000 mg (286 mM) NH3-N/L and 5 mM of phosphate; VI, 4000 mg NH3-N/L and 10 mM of phosphate. Red arrows indicate the sampling points of DNA extraction to analyze the microbial community
Phases I to III
There were no differences observed in the performance of reactors A and B at phase I and II before and after ammonium chloride addition (Fig. 2). During these phases, fermentation seemed to be unstable because gas production rate, methane content, CH4/CO2 ratio, and COD removal were fluctuating (Fig. 2a–d). In phase II, the addition of 2000 mg NH3-N/L of TAN resulted in a decrease in gas production rate, methane content, CH4/CO2 ratio, and COD removal in both reactors, indicating its inhibitory effect on methane fermentation. This is further validated by the increase in concentrations of acetate and propionate in phase II effluent from 0.4 to 1.5 mM, and from 0.2 to 1.0 mM, respectively (Fig. 2e, f). Notably, influent pH of phase II decreased from 6.0 to 5.4 by the addition of ammonium chloride, which could be an additional factor that inhibited methane fermentation (Fig. 2h).
In phase III (addition of 5 mM disodium phosphate to the influent of reactor B), significant differences were detected between the two reactors. In reactor A, methane content (65–70 %) and CH4/CO2 ratio (3.3–3.9) were similar to late phase II levels, whereas those in reactor B increased up to 71–79 % (methane content) and 4.0–5.4 (CH4/CO2 ratio) (Fig. 2b, c). The COD removal percentage was better in reactor B (Fig. 2d). While acetate and propionate (especially acetate) accumulated in reactor A, their concentrations in reactor B decreased to levels similar to initial concentrations in phase I (Fig. 2e, f). Influent pH in reactor A was around 5.5, whereas that in reactor B increased from 5.4 to 6.5 (Fig. 2h), which may be due to the buffering action of disodium phosphate.
Phase IV
In phase IV, the concentration of ammonium chloride of the influent was adjusted to 3000 mg (214 mM) NH3-N/L of TAN in both reactors. In reactor A, gas production rate, methane content, CH4/CO2 ratio and COD removal ratio significantly decreased immediately after addition (Fig. 2a, b, c, d). An increase in acetate and propionate concentrations (especially acetate) was detected (Fig. 2e, f), resulting in an increase of the acetate/propionate ratio (>2.0, Fig. 2g), in the reactor effluent in the absence of disodium phosphate addition. All granules in reactor A were washed out by day 57, suggesting that the maintenance of granules is sensitive to high ammonia concentrations. In contrast, gas production rate, methane content, CH4/CO2 ratio and COD removal ratio increased in reactor B (Fig. 2a, b, c, d). Acetate and propionate concentrations, their ratio, and influent pH were maintained at levels similar to phase III (Fig. 2e, f, g, h).
Phases V and VI
Because all granules in reactor A were washed out, only reactor B was used for subsequent experiments where the concentration of ammonia was adjusted to 4000 mg (286 mM) NH3-N/L (phase V). Increased ammonia concentration induced a gradual decrease in gas production rate, methane content, CH4/CO2 ratio, and COD removal (Fig. 2a, b, c, d), and a corresponding increase in concentrations of acetate and propionate (around 2.0 mM and 1.5 mM, respectively) (Fig. 2e, f). In phase VI, disodium phosphate concentration was increased from 5 to 10 mM to suppress ammonia inhibition. Addition of 10 mM phosphate increased methane content from 72 to 76 % (Fig. 2b) and CH4/CO2 ratio from 4.0 to 4.5 (Fig. 2c). However, gas production rate and COD removal did not increase (Fig. 2a, d). Furthermore, concentrations of acetate and propionate did not change (Fig. 2e, f).
Changes in microbial community caused by the addition of ammonium chloride and phosphate
Microbial communities in both reactors were analyzed by sequencing PCR products of archaeal and bacterial 16S rRNA genes. The PCR products were obtained in the points of phase II to III, III to IV, IV, IV to V, and V to VI (Figs. 2a and 3). The number of reads of OTUs classified into families based on their identities in the BLAST search are shown in Tables S2-1 and S2-2. The ratio of archaea and bacteria to the total reads were shown in Fig. 3, which changed in different phases.
Microbial community composition in the granules. a Archaea, b Bacteria, c Bacteroidetes; d Firmicutes; e Synergistates, and f Proteobacteria. Upper chart, reactor A; lower chart, reactor B. Each phase corresponds with those of Fig. 2
Discussion
This study assessed the effects of phosphate addition on anaerobic digestion in the batch reactor system and UASB reactor system. In the batch reactor, during 105 days fermentation, the concentration of ammonia, which was 5800–6300 mg NH3-N/L (414–450 mM), got higher than the reported inhibitory concentration of 2000 mg (143 mM) NH3-N/L (Krylova et al. 1997; Zhou and Qiu 2006). Nevertheless, the methane content of samples was shown to be maintained at 65–79 % (Fig. 1a). Notably, the phosphate addition was effective in accelerating methane production rather than removing ammonia. This indicates that phosphate addition could stimulate carbon dioxide-utilizing microorganisms such as hydrogenotrophic methanogens. Ammonia precipitation was not observed and this may be attributed to 17-fold lower concentration of magnesium or phosphate added to the reactor (25 mM) compared to ammonia (414–450 mM). This fact indicates that the addition of the phosphate could suppress ammonia inhibition not by ammonia removal but by other mechanisms. Similar reports showed that phosphate addition accelerated methane production (Lei et al. 2010) and that addition of phosphorite ore stimulated methane production under high ammonia concentration (Krylova et al. 1997; Zhou and Qiu 2006). Because similar effects were obtained using potassium chloride instead of magnesium chloride (Fig. S1), the addition of cations (K+ and Mg2+) may have also been effective at increasing methane content by suppression of ammonia inhibition as previously reported (Kugelman and McCarty 1965). The cations may accelerate the microbial granulation by their adsorption and adhesion with each other through bridging between negatively charged groups on cell surfaces (Tiwari et al. 2006). These results led to the conclusion that phosphate and cation additions were effective in suppressing ammonia inhibition in the batch methane fermentation.
In comparisons of the two UASB reactors, addition of disodium phosphate to the reactor influent (phase III) clearly showed effects similar to those observed in batch fermentation, suggesting that the addition also suppresses ammonia inhibition in continuous methane fermentation. We did not measure the TAN in reactor B after addition of disodium phosphate. The granules were likely not exposed to 4000 mg/L TAN because the phosphate addition could precipitate some ammonia, although no particles were detected in the reactor (data not shown). Since the relative concentrations of added phosphate were lower (5–10 mM) than those of ammonia (143–214 mM), the possible reduction of TAN by the addition of phosphate was ignored in this study. In phase IV, at 3000 mg NH3-N/L of TAN, phosphate addition could effectively suppress the inhibitory effects of ammonia on methane fermentation. In phases V and VI, the acetate/propionate ratio decreased from 2.0 to 1.0 (Fig. 2g), probably because the consumption of propionate was reduced, which could be a key factor for the inhibition of methane production. The ammonia inhibition in the UASB was possibly caused by acidification of the influent samples (Fig. 2h, phase II). Such a low pH condition could result in prolonged inhibition of methanogenesis due to hydrogen ion, even after correction of pH to neutrality (Tiwari et al. 2006). However, the pH of effluents was still around neutral in both reactors (phase II, Fig. 2h), probably due to the buffering effect of granule as previously reviewed (Tiwari et al. 2006). The continuous low pH of influent for reactor A (phase II to IV, Fig. 2h) could cause the washout of all the granules at phase IV. The addition of disodium phosphate might result in an increasing of the influent pH, and this could explain the recovery of the phosphate-amended reactor. The accumulations of acids (Fig. 2e, f) were not affected by the addition of a higher concentration of disodium phosphate. These results indicate that high ammonia concentration could inhibit acetate-consuming microorganisms such as aceticlastic and acetate-oxidizing bacteria. On the other hand, disodium phosphate addition could stimulate carbon dioxide-utilizing microorganisms such as hydrogenotrophic methanogens. Notably, the addition of disodium phosphate was also effective for the COD removal (Fig. 2d), suggesting that the addition could activate consumption of the organic compounds in the reactor.
As for the microbial communities in the UASB reactors, most of the Archaea in the UASB reactors were methanogens (Fig. 3a), which are known to comprise five orders of Archaea, namely, Methanobacteriales, Methanococcales, Methanomicrobiales, Methanosarcinales, and Methanopyrales (Liu and Whitman 2008). Among them, Methanobacteriales, Methanomicrobiales, and Methanosarcinales were detected in granules of both UASB reactors in all phases. The former two orders are known as hydrogenotrophic methanogens that use H2 to convert CO2 to CH4, while the latter is an aceticlastic methanogen that produces methane from acetate. Methanosarcinales was the most abundant (Fig. 3a), suggesting that aceticlastic methanogens were dominant in both reactors. Furthermore, between the two families under Methanosarcinales, Methanosaetaceae and Methanosarcinaceae, the former was the dominant methanogen in both UASB reactors (Table S2-2), which is consistent with previous reports (Ban et al. 2013; Calli et al. 2006). Both of the aceticlastic and hydrogenotrophic methanogens decreased in the two reactors through phase II to IV (Fig. 3a) as the concentration of ammonium chloride increased from phase III to IV (Fig. 2), which may have caused the reduction of methane production (Fig. 2a). The decrease of aceticlastic methanogens in reactor A may have caused accumulation of acetate and propionate in phase IV (Fig. 2e, f). In general, methane gas production was affected by ammonia concentration, which may indicate that ammonia inhibition causes a decrease in methane production activity by methanogens in both reactors, and eventual cessation and granule washout in reactor A (Fig. 2). However, with phosphate addition to reactor B (phase III), suppression of ammonia inhibition stimulated the activity of aceticlastic and hydrogenotrophic methanogens, as indicated in the increased methane production and decreased accumulation of acetate and propionate.
As for the bacterial communities, the four major phyla, Bacteriodetes (13–45 %), Firmicutes (18–30 %), Proteobacteria (5.7–17 %), and Synergistetes (4.7–15 %) were detected in both reactors (Fig. 3b). The major order of Bacteroidetes was Bacteroidales, and one particular family of this order in reactor B, Porphyromonadaceae significantly increased (0.01 to 33 %) in the presence of high ammonium chloride concentration (Fig. 3c). Porphyromonadaceae has been reported as one of the major glucose fermenting bacterium in anaerobic degradation of waste samples (Li et al. 2009) that produce hydrogen, acetate, and propionate (Jabari et al. 2012; Oh et al. 2003). These bacteria may have played an important role in the anaerobic hydrolysis of complex and simple sugars to acids, acetate and propionate (Garcia et al. 2011). In fact, the amount of acetate and propionate increased in phases V to VI (Fig. 2e, f) when the ratio of Porphyromonadaceae increased to 33 % (V to VI, Fig. 3c). The major class of Firmicutes was Clostridia (Ruminococcaceae and Veillonellaceae) (Fig. 3d), which is known to use mono- and di-oligosaccharides to produce acetate, ethanol, hydrogen, and carbon dioxide (Abreu et al. 2011). No change was observed upon addition of ammonium chloride and phosphate (Fig. 3d), suggesting that their bacterial activity is not affected by either ammonium chloride or phosphate concentration. In Synergistetes, only class Synergistia (order Synergistales) was detected. The major families of Synergistales in the reactor were Dethiosulfovibrionaceae and Synergistaceae, with the latter increasing from 0.4 to 6.6 % in phase II to V in reactor B (Fig. 3e). Synergistetes are widely distributed throughout anaerobic environments, especially those associated with the digestive system of animals (Godon et al. 2005). Several bacteria belonging to this phylum are known as acetate-utilizing bacteria (Ito et al. 2011), and it is therefore possible that the observed increase in Synergistetes in reactor B (Fig. 3e, IV to V) resulted in a low accumulation of acetate in phase IV (Fig. 2e). Gammaproteobacteria, Deltaproteobacteria, and Betaproteobacteria were the major orders of Proteobacteria identified in the reactors. Among them, Gammaproteobacteria significantly fluctuated, particularly in reactor B (Fig. 3f). Enterobacteriaceae, known to be capable of producing hydrogen in anaerobic conditions (Zhang et al. 2011), was the most abundant family in this order (Table S2-2).
Although methanogens have been known to be more sensitive to ammonia than bacteria in anaerobic digestion (Gallert and Winter 1997), ammonium chloride had a greater effect on bacteria (at the bacterial order levels), causing a shift in their community (Fig. 3). As a result of ammonia inhibition, deterioration of methane fermentation by acetate and propionate accumulation may be explained by several potential mechanisms: (1) strong sensitivity of hydrogenotrophic methanogens (Methanobacteriales and Methanomicrobiales) to high ammonia concentration; (2) enhanced growth of acetate- and propionate-producing bacteria (Porphyromonadaceae) with high ammonium chloride concentration; and (3) inhibition of acetate- and propionate-oxidizing bacteria (Syntrophobacterales) by high ammonium chloride concentration.
This study clearly showed the suppression of ammonia inhibition in the batch reactor and UASB reactors by disodium phosphate addition. The addition could suppress the ammonia inhibition and consequently stimulate the activity of methanogens. The addition also could affect the bacterial community and enhance the growth of hydrogen-producing bacteria, resulting in an increase in methane production. Further experiments will be required with different cations and anions in different concentrations to understand and evaluate in greater detail the effects of cation and/or phosphate addition.
References
Abreu AA, Alves JI, Pereira MA, Sousa DZ, Alves MM (2011) Strategies to suppress hydrogen-consuming microorganisms affect macro and micro scale structure and microbiology of granular sludge. Biotechnol Bioeng 108(8):1766–1775. doi:10.1002/bit.23145
APHA (1989) Standard methods for the examination of water and wastewater, 17th edn. American Public Health Association, Washington D.C.
Arogo J, Zhang RH, Riskowski GL, Christianson LL, Day DL (1999) Mass transfer coefficient of ammonia in liquid swine manure and aqueous solutions. J Agric Eng Res 73(1):77–86. doi:10.1006/jaer.1998.0390
Ban Q, Li J, Zhang L, Jha AK, Zhang Y, Ai B (2013) Syntrophic propionate degradation response to temperature decrease and microbial community shift in an UASB reactor. J Microbiol Biotechnol 23(3):382–389. doi:10.4014/jmb.1210.10008
Battistoni P, De Angelis A, Pavan P, Prisciandaro M, Cecchi F (2001) Phosphorus removal from anaerobic supernatant by struvite crystallization. Water Res 35(9):2167–2178. doi:10.1016/S0043-1354(00)00498-X
Calli B, Mertoglu B, Roest K, Inanc B (2006) Comparison of long-term performances and final microbial compositions of anaerobic reactors treating landfill leachate. Bioresour Technol 97(4):641–647. doi:10.1016/j.biortech.2005.03.021
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336. doi:10.1038/nmeth.f.303
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Bioresour Technol 99(10):4044–4064. doi:10.1016/j.biortech.2007.01.057
Gallert C, Winter J (1997) Mesophilic and thermophilic anaerobic digestion of source-sorted organic wastes: effect of ammonia on glucose degradation and methane production. Appl Microbiol Biotechnol 48(3):405–410. doi:10.1007/s002530051071
Garcia SL, Jangid K, Whitman WB, Das KC (2011) Transition of microbial communities during the adaption to anaerobic digestion of carrot waste. Bioresour Technol 102(15):7249–7256. doi:10.1016/j.biortech.2011.04.098
Godon JJ, Morinière J, Moletta M, Gaillac M, Bru V, Delgènes JP (2005) Rarity associated with specific ecological niches in the bacterial world: the ‘Synergistes’ example. Environ Microbiol 7(2):213–224. doi:10.1111/j.1462-2920.2004.00693.x
Ito T, Yoshiguchi K, Ariesyady HD, Okabe S (2011) Identification of a novel acetate-utilizing bacterium belonging to Synergistes group 4 in anaerobic digester sludge. ISME J 5(12):1844–1856. doi:10.1038/ismej.2011.59
Jabari L, Gannoun H, Cayol JL, Hedi A, Sakamoto M, Falsen E, Ohkuma M, Hamdi M, Fauque G, Ollivier B, Fardeau ML (2012) Macellibacteroides fermentans gen. nov., sp. nov., a member of the family Porphyromonadaceae isolated from an upflow anaerobic filter treating abattoir wastewaters. Int J Syst Evol Microbiol 62(Pt 10):2522–2527. doi:10.1099/ijs.0.032508-0
JIS K0101 (1998) Testing methods for industrial water. Japanese Standards Association
Krylova N, Khabiboulline R, Naumova R, Nagel M (1997) The influence of ammonium and methods for removal during the anaerobic treatment of poultry manure. J Chem Technol Biotechnol 70(1):99–105. doi:10.1002/(SICI)1097-4660(199709)70:1<99::AID-JCTB684>3.0.CO;2-C
Kugelman IJ, McCarty PL (1965) Cation toxicity and stimulation in anaerobic waste treatment. J Water Pollut Control Fed 37:97–116
Lei Z, Chen J, Zhang Z, Sugiura N (2010) Methane production from rice straw with acclimated anaerobic sludge: effect of phosphate supplementation. Bioresour Technol 101(12):4343–4348. doi:10.1016/j.biortech.2010.01.083
Li T, Mazéas L, Sghir A, Leblon G, Bouchez T (2009) Insights into networks of functional microbes catalysing methanization of cellulose under mesophilic conditions. Environ Microbiol 11(4):889–904. doi:10.1111/j.1462-2920.2008.01810.x
Liu Y, Whitman WB (2008) Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann N Y Acad Sci 1125:171–189. doi:10.1196/annals.1419.019
Oh Y, Park M, Seol E, Lee S, Park S (2003) Isolation of hydrogen-producing bacteria from granular sludge of an upflow anaerobic sludge blanket reactor. Biotechnol Bioprocess Eng 8(1):54–57. doi:10.1007/BF02932899
Pastor L, Mangin D, Ferrer J, Seco A (2010) Struvite formation from the supernatants of an anaerobic digestion pilot plant. Bioresour Technol 101(1):118–125. doi:10.1016/j.biortech.2009.08.002
Qiao S, Matsumoto N, Shinohara T, Nishiyama T, Fujii T, Bhatti Z, Furukawa K (2010) High-rate partial nitrification performance of high ammonium containing wastewater under low temperatures. Bioresour Technol 101(1):111–117. doi:10.1016/j.biortech.2009.08.003
Quan X, Ye C, Xiong Y, Xiang J, Wang F (2010) Simultaneous removal of ammonia, P and COD from anaerobically digested piggery wastewater using an integrated process of chemical precipitation and air stripping. J Hazard Mater 178(1–3):326–332. doi:10.1016/j.jhazmat.2010.01.083
Rao A, Reddy T, Prakash S, Vanajakshi J, Joseph J, Jetty A, Reddy A, Sarma P (2008) Biomethanation of poultry litter leachate in UASB reactor coupled with ammonia stripper for enhancement of overall performance. Bioresour Technol 99(18):8679–8684. doi:10.1016/j.biortech.2008.04.016
Sawayama S, Tada C, Tsukahara K, Yagishita T (2004) Effect of ammonium addition on methanogenic community in a fluidized bed anaerobic digestion. J Biosci Bioeng 97(1):65–70. doi:10.1016/S1389-1723(04)70167-X
Sung S, Liu T (2003) Ammonia inhibition on thermophilic anaerobic digestion. Chemosphere 53(1):43–52. doi:10.1016/S0045-6535(03)00434-X
Tabatabaei M, Rahim R, Abdullah N, Wright A, Shirai Y, Sakai K, Sulaiman A, Hassan M (2010) Importance of the methanogenic archaea populations in anaerobic wastewater treatments. Process Biochem 45(8):1214–1225. doi:10.1016/j.procbio.2010.05.017
Tiwari MK, Guha S, Harendranath CS, Tripathi S (2006) Influence of extrinsic factors on granulation in UASB reactor. Appl Microbiol Biotechnol 71(2):145–154. doi:10.1007/s00253-006-0397-3
Türker M, Celen I (2007) Removal of ammonia as struvite from anaerobic digester effluents and recycling of magnesium and phosphate. Bioresour Technol 98(8):1529–1534. doi:10.1016/j.biortech.2006.06.026
Ueno Y, Fujii M (2001) Three years experience of operating and selling recovered struvite from full-scale plant. Environ Technol 22(11):1373–1381
Uludag-Demirer S, Demirer G, Frear C, Chen S (2008) Anaerobic digestion of dairy manure with enhanced ammonia removal. J Environ Manag 86(1):193–200. doi:10.1016/j.jenvman.2006.12.002
Uysal A, Yilmazel YD, Demirer GN (2010) The determination of fertilizer quality of the formed struvite from effluent of a sewage sludge anaerobic digester. J Hazard Mater 181(1–3):248–254. doi:10.1016/j.jhazmat.2010.05.004
Yabu H, Sakai C, Fujiwara T, Nishio N, Nakashimada Y (2011) Thermophilic two-stage dry anaerobic digestion of model garbage with ammonia stripping. J Biosci Bioeng 111(3):312–319. doi:10.1016/j.jbiosc.2010.10.011
Zhang L, Jahng D (2010) Enhanced anaerobic digestion of piggery wastewater by ammonia stripping: effects of alkali types. J Hazard Mater 182(1–3):536–543. doi:10.1016/j.jhazmat.2010.06.065
Zhang C, Lv FX, Xing XH (2011) Bioengineering of the Enterobacter aerogenes strain for biohydrogen production. Bioresour Technol 102(18):8344–8349. doi:10.1016/j.biortech.2011.06.018
Zhou H, Qiu G (2006) Inhibitory effect of ammonia nitrogen on specific methanogenic activity of anaerobic granular sludge. J Cent S Univ Technol 13(1):63–67. doi:10.1007/s11771-006-0108-3
Acknowledgments
We are very grateful to Prof. Dr. Stefano Ferri for his support and discussion. A part of this study was supported by a grant from Shizuoka Industrial Foundation and Japan Science and Technology Agency on Initiatives for Atomic Energy Basic and Generic Strategic Research (240408).
Compliance with ethical standards
ᅟ
Funding
This study was funded by grant from Shizuoka Industrial Foundation and Japan Science and Technology Agency on Initiatives for Atomic Energy Basic and Generic Strategic Research (240408).
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sho Suzuki and Masaki Shintani contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM. 1
(PDF 768 kb)
Rights and permissions
About this article
Cite this article
Suzuki, S., Shintani, M., Sanchez, Z.K. et al. Effects of phosphate addition on methane fermentation in the batch and upflow anaerobic sludge blanket (UASB) reactors. Appl Microbiol Biotechnol 99, 10457–10466 (2015). https://doi.org/10.1007/s00253-015-6942-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6942-1