Abstract
Mannitol is a natural polyol with multiple industrial applications. In this work, mannitol production by Lactobacillus reuteri CRL 1101 was studied at free- and controlled-pH (6.0–4.8) fermentations using a simplified culture medium containing yeast and beef extracts and sugarcane molasses. The activity of mannitol 2-dehydrogenase (MDH), the enzyme responsible for mannitol synthesis, was determined. The effect of the initial biomass concentration was further studied. Mannitol production (41.5 ± 1.1 g/l), volumetric productivity (Q Mtl 1.73 ± 0.05 g/l h), and yield (Y Mtl 105 ± 11 %) were maximum at pH 5.0 after 24 h while the highest MDH activity (1.66 ± 0.09 U/mg protein) was obtained at pH 6.0. No correlation between mannitol production and MDH activity was observed when varying the culture pH. The increase (up to 2000-fold) in the initial biomass concentration did not improve mannitol formation after 24 h although a 2-fold higher amount was produced at 8 h using 1 or 2 g cell dry weight/l comparing to the control (0.001 g cell dry weight/l). Finally, mannitol isolation under optimum fermentation conditions was achieved. The mannitol production obtained in this study is the highest reported so far by a wild-type L. reuteri strain and, more interestingly, using a simplified culture medium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mannitol is a natural sugar alcohol or polyol produced by certain bacteria (Kets et al. 1996), several fungi (Stoop and Mooibroek 1998; Voegele et al. 2005), algae (Iwamoto and Shiraiwa 2005; Rousvoal et al. 2011), and plants (Stoop et al. 1996). This polyol is industrially used for chemical, medical, and pharmaceutical applications (Monedero et al. 2010; Saha and Racine 2011; Vrancken et al. 2010). Mannitol, which is about half as sweet as sucrose, is used as low-calorie sweetener in the food industry. As it is a non-metabolizable compound, it can be applied in food products for diabetic patients (Livesey 2003). As food ingredient, it is also used as a texturing agent or in “breath-freshening” products due to its positive enthalpy of dissolution [120.9 kJ/kg; (Lawson 1997)]. Also, mannitol is used in the manufacture of different pharmaceutical products because of its capability to mask undesirable tastes of many compounds. In addition, it has been claimed to display health-promoting (antioxidant and anticariogenic) effects (Shen et al. 1997).
Current industrial mannitol synthesis is made by a catalytic hydrogenation of a 50/50 glucose/fructose mixture at high temperatures (100–130 °C) and pressures (14–55 bar) using Raney-nickel catalyst (Wisnlak and Simon 1979). The strong temperature and pressure conditions used as well as the requirement of pure substrates are the main disadvantages of this process, contributing to increase the global cost of the product. Moreover, the low purity and yield associated with this process due to the poor selectivity of the catalyst used is another negative feature. Purification steps are necessary as, typically, a 30/70 mannitol/sorbitol final mixture is obtained (Soetaert et al. 1999; Wisnlak and Simon 1979) making this chemical process expensive and time-consuming.
Lactic acid bacteria (LAB) have been historically used for their capacity to acidify different food matrices, thus preserving them from spoilage. In addition, LAB impart unique signature flavors besides other health-promoting benefits which may be desirable for consumers. Besides lactic acid, some LAB can produce other metabolites such as ethanol, acetic and formic acids, aroma compounds (acetoin and diacetyl), exopolysaccharides, vitamins, and polyols (Hugenholtz 2008). Among this bacterial group, heterofermentative lactobacilli are efficient mannitol producers from fructose due to the action of the mannitol 2-dehydrogenase (MDH) enzyme (Hahn et al. 2003; Korakli and Vogel 2003; Ortiz et al. 2013; Ortiz et al. 2012; Saha 2004; Sasaki et al. 2005). The conversion of fructose into mannitol increases the ATP yield by leading acetyl-phosphate towards acetate rather than ethanol (Axelsson 1998); thus, mannitol-producing strains display higher growth rates than non-producing ones (Wisselink et al. 2002) (Fig. 1). A complete conversion of fructose into mannitol without co-formation of side-products like sorbitol, mild production conditions, and no requirement of highly purified substrates are some advantages of the fermentative process comparing to its chemical synthesis (Soetaert et al. 1995; von Weymarn et al. 2002).
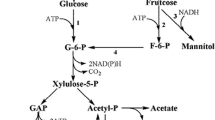
Proposed pathway for hexose metabolism of heterofermentative LAB. (1) Glucose permease; (2) fructose permease; (3) glucokinase; (4) glucose 6-phosphate dehydrogenase; (5) 6-phosphogluconate dehydrogenase; (6) epimerase; (7) phosphoketolase; (8) glucose phosphate isomerase; (9) fructokinase; (10) mannitol 2-dehydrogenase; (11) glyceraldehyde 3-phosphate dehydrogenase and phosphoglycerate kinase; (12) phosphoglyceromutase and enolase; (13) pyruvate kinase; (14) lactate dehydrogenase; (15) phosphate acetyltransferase; (16) acetaldehyde dehydrogenase and alcohol dehydrogenase; (17) acetate kinase; question mark indicates unknown mannitol transport system. Modified from Wisselink et al. (2002)
To achieve an efficient and competitive mannitol biotechnological productive process, low-cost culture media and optimized culture conditions are needed. Molasses, one of the main by-products of the sugarcane industry, is typically composed of carbohydrates, as main components, and a wide range of substances such as metallic ions, nitrates, sulfates, amino acids, vitamins, gums, dextrans, and ashes. Among carbohydrates, molasses contains mainly sucrose that could not be crystallized during the sucrose refining process, and glucose and fructose as free monosaccharides originated from sucrose hydrolysis (Castillo and Forero 2007). The range of applications of molasses in different productive processes is constantly expanding as in the production of ethanol (Mukhtar et al. 2010; Sheoran et al. 1998), bacterial cellulose (Bae and Shoda 2004), ergosterol (He et al. 2007), and lactic acid (Dumbrepatil et al. 2008; Wee et al. 2004) constituting an important carbon source for the production of valuable compounds.
In a previous study (Ortiz et al. 2012), we showed that Lactobacillus reuteri CRL 1101 produced mannitol in a rich culture medium containing sugarcane molasses as carbon source. Mannitol production was evaluated in free-pH cultures during 48 h using different molasses concentrations (3–10 %, w/v), temperatures (30 and 37 °C), and under static or agitating conditions. The Lactobacillus strain converted glucose into lactic acid, acetic acid, and ethanol while fructose was used as an alternative electron acceptor and was reduced into mannitol. Maximum mannitol concentrations of 178–184 mM (33–34 g/l) were attained using 7.5 % or 10 % (w/v) of total sugars at 37 °C after 24 h of incubation. Under agitated cultures, high mannitol values (145 ± 40 mM = 26 ± 7 g/l) were achieved at 8 h of fermentation as compared to the static ones (6 ± 3 mM = 1.1 ± 0.5 g/l). The highest mannitol concentration value attained was 211 ± 16 mM (38 ± 3 g/l) after 24-h fermentation period.
In this work, we aimed to achieve an efficient mannitol production by the wild-type strain L. reuteri CRL 1101 using a simplified, and hence more economical, culture medium. Constant-pH fermentations in a range between 6.0 and 4.8 were performed; the effect of the initial biomass concentration on mannitol formation was also studied.
Materials and methods
Bacterial strain and growth conditions
L. reuteri CRL 1101 (Culture Collection of Centro de Referencia para Lactobacilos (CERELA), San Miguel de Tucumán, Argentina) was previously selected for its ability to produce mannitol. Stock cultures were maintained at −20 °C in 10.0 % reconstituted skim milk supplemented with 0.5 % yeast extract, 1.0 % glucose, and 10.0 % glycerol (w/v).
Prior to experimental use, cultures were propagated (2 %, v/v) twice in MRS broth (g/l; peptone, 10.0; yeast extract, 5.0; beef extract, 10.0; glucose, 20.0; dipotassium phosphate, 2.0; sodium acetate, 5.0; triammonium citrate, 2.0; magnesium sulfate heptahydrate, 0.2; manganese sulfate monohydrate, 0.05; and Tween-80, 1.0 ml/l) and incubated for 10–15 h at 37 °C. Cultures were washed twice with cold 50 mM buffer sodium phosphate pH 7.0 and then used as inoculum. For each experiment, media were inoculated with cells to reach an optical density at 600 nm (OD600)= 0.07 ± 0.03.
Bacterial growth and mannitol production in a simplified culture medium
In preliminary experiments (data not shown), we evaluated the effect (by omission or addition) of each MRS component on cell growth (OD600) of L. reuteri CRL 1101 at free-pH fermentations at 37 °C for 24 h. Based on the obtained results, mannitol production was evaluated using a simplified culture medium formulated with those MRS components necessary to support growth, but replacing glucose by sugarcane molasses. The culture medium contained (%, w/v) total molasses sugars, 7.5; yeast extract, 0.5; and peptone, soy peptone, or beef extract, 1.0. L. reuteri CRL 1101 was cultured using these media (namely, A to D, Table 1) under free-pH conditions at 37 °C for 24 h. As L. reuteri CRL 1101 was not able to grow in these simplified media without yeast extract (data not shown), this component was always included. Molasses-supplemented MRS broth (MRS-molasses), where glucose was replaced by sugarcane molasses, was used as control.
Molasses used in this work was obtained from a local sugar factory. A stock solution of sugarcane molasses was prepared as described previously (Ortiz et al. 2012) and was added to the medium to reach a final total sugar concentration of 7.5 % (w/v). This sugar concentration was selected considering that higher concentration (10.0 %, w/v) did not lead to a significant increase in mannitol synthesis (Ortiz et al. 2012). As the composition of sugarcane molasses varies depending on its origin, sugar content was determined prior to experimental use. Molasses used in this study contained 49.5 % (w/w) total sugars: 38.7 % sucrose, 4.9 % fructose, and 5.9 % glucose.
Cell growth was determined by cell count (colony-forming units per ml, CFU/ml) as the presence of molasses interfered with OD measurements. Cell count was done plating diluted samples prepared in physiological solution (0.85 % NaCl, w/v) in MRS agar (12 g agar/l). Cell growth, maximum specific growth rate (μmax, h−1), and pH were evaluated in 100-ml-containing flasks at free-pH fermentations. Fructose consumption, mannitol formation, and yield were determined at 24 h. All assays were carried out in triplicates; values represent the means ± SD. The culture medium where mannitol production was the highest (Table 1 line D) was selected for optimization studies.
Mannitol production under controlled-pH fermentations
To evaluate mannitol production by L. reuteri CRL 1101, controlled-pH fermentations using the selected simplified medium were performed in a 2-l fermentor (New Brunswick Scientific Co. Inc., Edison, USA) under gentle agitation (100 rpm) at 37 °C for 24 h. The culture pH was kept constant by the automatic addition of sterile 5 M NaOH. The effect of different constant pH values (6.0, 5.8, 5.6, 5.4, 5.2, 5.0, and 4.8) on cell growth (cell count), sugar consumption, organic acid, and mannitol production were determined at 0, 4, 8, and 24 h of incubation. Mannitol volumetric productivities and yields were calculated at 24 h. All assays were carried out in triplicates; values represent the means ± SD. Free-pH fermentation values were used as control.
Analytical methods
Determination of residual carbohydrates (glucose, fructose, and sucrose), mannitol, organic acids (lactic and acetic acids), and ethanol concentrations was carried out by high-performance liquid chromatography (HPLC) as described previously (Ortiz et al. 2012). Briefly, all measurements were done using a Smartline pump 100, a WellChrom K-2301 refractive index (RI) detector, a Smartline autosampler 3800 Plus (Knauer GmbH & Co., Berlin, Germany), and a ZC-90 oven (Zeltec, Buenos Aires, Argentina). An Aminex HPX-87P column (Bio-Rad Laboratories Inc., Hercules, CA, USA) at 85 °C with MilliQ water as mobile phase (flow rate 0.6 ml/min) was used for determining carbohydrates and mannitol, and a Rezex ROA-Organic Acid H+ (8 %) (Phenomenex Laboratories Inc., Torrance, CA, USA) column at 41 °C with 5 mM H2SO4 as mobile phase (flow rate: 0.6 ml/min) was used to determine organic acids (lactic and acetic acids) and ethanol.
Volumetric productivities of mannitol (Q Mtl, g/l h) were calculated as the increase in mannitol concentrations in a defined period of time. Specific productivities (Q X , g mannitol/g cell dry biomass) were calculated as the amount of mannitol produced per gram of biomass in a specific period of time. Yields (Y Mtl, mol/mol*100) represent the conversion efficiencies of fructose into mannitol calculated as the ratio between mannitol increase and total fructose (free fructose plus fructose from sucrose hydrolysis) consumption in a defined time interval × 100 (von Weymarn 2002).
Measurement of mannitol 2-dehydrogenase activity
MDH is the enzyme responsible for the one-step conversion of fructose into mannitol. For determining the intracellular MDH activity in L. reuteri CRL 1101, cells were harvested (4000×g, 10 min, 4 °C) at 8 h of incubation and washed three times with cold 50-mM sodium phosphate buffer (pH 5.5). Pellets were mixed with glass beads (acid-washed, 150–212 μm, Sigma-Aldrich Chemical Co., USA) in a 1:2:1 cells/buffer/beads ratio, and cells were disrupted using a Mini Bead Beater-8 (Biospec Products) for 10 min at maximum speed (with 2-min interruption on ice after each 2-min cycle). Cell debris, unbroken cells, and glass beads were removed by centrifugation (14,500×g, 5 min, 4 °C), and the supernatants were immediately used for enzyme assays.
MDH activity was determined according to the method described previously (Ortiz et al. 2012); only slight modifications were done. Briefly, MDH was measured spectrophotometrically on a Cary 50 MPR microplate reader (Varian Inc., USA); the disappearance of NADPH (Sigma-Aldrich Chemical Co.) was monitored by measuring the absorbance at 340 nm (ε340, 6220/M cm) for 5 min. The protein concentration of cell-free extracts was measured using the Bio-Rad dye reagent concentrate (Bio-Rad Laboratories, USA) following the manufacturer’s instructions and using bovine serum albumin as standard. Cell-free extract samples were diluted to obtain a protein concentration of 0.5 mg prot/ml.
The enzymatic assay was done in a 200-μl volume; the reaction mixture contained 50 μl 200 mM sodium phosphate buffer (pH 5.5), 50 μl 2 mM NADPH, 50 μl of MilliQ water, and 10 μl of diluted cell-free extract. The reaction mixture was maintained at 37 °C for 2 min, and the reaction was started by adding 40 μl of 1 M fructose (Sigma-Aldrich Chemical Co.). One unit (U) of MDH activity was defined as the amount of enzyme required to catalyze the disappearance (fructose reducing direction) of 1 μmol of NADPH per minute under the experimental conditions used. Specific MDH activity was expressed in units per milligram of cell protein. All enzyme assays were done in duplicate of two independent experiments.
Effect of initial biomass concentration on mannitol production
Previous experiments were performed using an initial biomass concentration of about 0.001 g cell dry weight/l, corresponding to an initial cell count of 1 × 106 CFU/ml. To study the effect of the initial biomass concentration on mannitol production, fermentations were carried out using the simplified culture medium previously selected under optimized conditions. Ten-, 100-, 1000-, and 2000-fold (corresponding to 0.01, 0.1, 1.0, and 2.0 g cell dry weight/l, respectively) increase in the biomass concentration, as compared to that used in previous assays (0.001 g/l), were evaluated. Cells were grown for 8 h (log growth phase) and harvested by centrifugation (10,000×g, 10 min) from a given volume of fermented medium necessary to obtain the desired biomass amount as determined previously. Cells were resuspended in simplified culture medium and transferred into the bioreactor. Cell dry weight determinations were carried out using the thermogravimetric infrared humidity analyzer MA100C-000230 V1 (Sartorius AG, Goettingen, Germany). Biomass concentration assays were performed in duplicate in independent experiments. Culture grown under optimized conditions with an initial biomass of 0.001 g/l was used as control.
Mannitol isolation
Twenty-four-hour fermentations were carried out culturing L. reuteri CRL 1101 under optimized culture conditions using the selected simplified medium. Cells were removed by centrifugation (10,000×g, 20 min, 4 °C) and supernatants were five-fold concentrated for mannitol precipitation. To prevent foaming during the concentration step, 0.5 ml of a 50 % (v/v; 1/800 final dilution) food-grade antifoam solution (RE806, Biochemical S.A., Buenos Aires, Argentina) were added to 200 ml of culture supernatant. Medium concentration was done by evaporation at 50 °C, 50 rpm for 50 min using a rotary evaporator Hei-VAP Advantage (Heidolph, Schwabach, Germany). The concentrated supernatant was cooled down to 4 °C to induce crystallization and held at that temperature for 48 h. Given that mannitol precipitates in a concentration greater than 180 g/l at 4 °C, a pure mannitol solution of 190 g/l was used as crystallization control. To recover the formed crystals, a funnel with a filter paper (Double Rings 102 Medium Qualitative Flow) was used. Filtration was carried out immediately after the cooling step to prevent crystal dissolution.
Statistical analysis
Comparisons among different conditions for each studied parameter were performed using one-way ANOVA (analysis of variance) followed by Tukey’s honest significant difference (HSD) test. A value of p < 0.05 was considered to be statistically significant.
Results
Growth and mannitol production by L. reuteri CRL 1101 in a simplified culture medium at free-pH
Different nitrogen (soy peptone, peptone, or beef extract) sources were added to a simplified medium containing carbon (sugarcane molasses) and vitamin (yeast extract) sources (Media A-D, Table 1) to evaluate cell growth and mannitol production by L. reuteri CRL 1101. These media components were earlier selected based on preliminary studies (data not shown). Cell growth, carbohydrate consumption, mannitol production, and yields by the Lactobacillus strain incubated under free-pH fermentations at 37 °C for 24 h are shown in Table 1. L. reuteri CRL 1101 displayed complex amino acid requirements for cell growth since it was not able to ferment glucose in presence of soy peptone as sole protein source (Medium C) but it grew well when animal protein was present in the culture medium (media B and D, Table 1). As mannitol production by L. reuteri CRL 1101 is growth-associated, the presence of animal protein is needed for polyol formation. The highest cell viability (log CFU/ml 8.80 ± 0.07), Δ cell viability (log CFU/ml: 2.26 ± 0.34), and μ max (0.85 ± 0.02 h−1) values were found in medium D, these values being similar to those obtained in MRS-molasses (log CFU/ml 9.06 ± 0.03; Δ log CFU/ml 2.35 ± 0.08; μ max 0.87 ± 0.09 h−1). Also, the highest mannitol production value (148 ± 7 mM = 26.96 ± 1.28 g/l) was obtained in medium D after 24 h. Although this amount was statistically lower than that achieved in the control medium (184 ± 10 mM = 33.52 ± 1.82 g/l), the simplified medium D was selected for further studies.
Fermentation kinetics of growth, mannitol production, and end-product formation by L. reuteri CRL 1101 in medium D at free-pH are shown in Fig. 2. The strain grew well and reached the stationary phase at 8 h of incubation (log CFU/ml 8.65 ± 0.22); a final cell count of log CFU/ml 8.91 ± 0.04 was obtained at 24 h. Lactic acid production was growth-associated, a concentration of 110 ± 6 mM was produced at 24 h (Fig. 2a); this value being 1.5-fold greater than the acetic acid concentration (73 ± 2 mM), while a scarce ethanol production (13 ± 5 mM) was observed. Carbohydrates present in the culture medium were not completely depleted after the 24-h fermentation period; moreover, the concentration of free glucose remained constant until the end of the fermentation (t 0 48 ± 3 mM, t 24 43 ± 4 mM) (Fig. 2b). In contrast, free fructose concentration decreased approximately 80 % from 60 ± 7 mM (t 0) to 12 ± 3 mM (t 24) while sucrose consumption was around 67 % (from 144 ± 16 mM at t 0 to 48 ± 8 mM at t 24). Mannitol formation started at 4 h of incubation with the concomitant fructose consumption, mannitol concentration being maximum (148 ± 7 mM = 26.96 ± 1.28 g/l) at 24 h. At this time point, 100 % of consumed fructose (151 ± 13 mM = 27.20 ± 2.34 g/l) was converted into mannitol (Y Mtl 98 ± 3 %), indicating that fructose was exclusively used as an alternative electron acceptor.
Mannitol production under controlled-pH fermentations
To improve mannitol production, controlled-pH fermentations in a pH range between 6.0 and 4.8 at 37 °C for 24 h were carried out. Free-pH fermentation condition was used as control. Representative fermentation kinetics of pH 6.0 (Fig. 3a, b), 5.0 (Fig. 3c, d), and 4.8 (Fig. 3e, f) are shown as similar fermentation patterns were obtained at different pH cultures.
Under controlled-pH conditions, similar growth kinetics for L. reuteri CRL 1101 were observed (Fig. 3a, c, e). Stationary growth phase was attained at 8 h of incubation while cell count values between log CFU/ml 8.98 ± 0.05 (pH 6.0) and 9.52 ± 0.20 (pH 5.2) were reached at 24 h (Table 2); these values were similar to that at free pH (log CFU/ml, t 24 8.91 ± 0.04). Conversely to free-pH fermentations, all carbohydrates present in the culture medium were almost depleted after 24 h. In all cases, consumption of sucrose and free-fructose as well as the concomitant mannitol production started after 4 h of incubation. Interestingly, mannitol production was markedly improved (166–228 mM) under constant-pH fermentations as compared to free-pH cultures (148 ± 7 mM) (Table 2), the highest mannitol production (228 ± 6 mM = 41.5 ± 1.1 g/l), yield (105 ± 11 %), and volumetric productivity (1.73 ± 0.05 g/l h) values being achieved at pH 5.0 after 24 h (Fig. 3d, Table 2). Decreasing the pH values from 6.0 to 5.0 led to increased mannitol formation and yields. At pH 6.0 (Fig. 3b), 5.8 and 4.8 (Fig. 3f) fructose was partially converted into mannitol; the strain only used 77 % (pH 6.0 and 5.8) and 79 % (pH 4.8) of total consumed fructose (216 ± 8, 231 ± 12, and 224 ± 7 mM, respectively) for mannitol synthesis (166 ± 8, 178 ± 5, and 177 ± 6 mM, respectively) reaching volumetric productivities of 1.26 ± 0.02, 1.35 ± 0.01, and 1.34 ± 0.03 g/l h, respectively (Table 2). The partial conversion of fructose into mannitol indicates that fructose was used both as electron acceptor for mannitol production and as substrate fermentation. At pH values between 5.6 and 5.0―and similarly to what occurred in free-pH fermentations―the consumed fructose (207 ± 8 and 220 ± 19 mM, respectively) was mainly used as an external electron acceptor and converted into mannitol (194 ± 9 and 228 ± 6 mM, respectively), reaching yield values of 91 ± 6 % (pH 5.4) and 105 ± 11 % (pH 5.0) (Table 2).
Regarding organic acids and ethanol production, lactic acid was produced in greater concentration than acetic acid, and both organic acids were formed in larger amounts than ethanol at all pH values assayed (Table 2). At pH 5.4, maximum organic acids (lactic acid 236 ± 14 and acetic acid 127 ± 12 mM) and ethanol (95 ± 18 mM) concentrations were attained after 24 h of incubation. No ethanol production was detected until an 8-h incubation period.
Mannitol 2-dehydrogenase activity
To evaluate the correlation between mannitol production and MDH activity at all assayed pH values, enzyme activity measurements were done in cell-free extracts from 8-h cultures (Fig. 4). The highest MDH activity value 1.66 ± 0.09 U/mg cell protein was found in cells grown at pH 6.0 being this value not statistically different from those attained at the other studied pH, except for that at pH 4.8 (1.00 ± 0.24 U/mg prot), which was the lowest one observed.
Mannitol 2-dehydrogenase activity in cell-free extracts from 8 h-cultures of L. reuteri CRL 1101 grown in a molasses-based simplified culture medium under constant-pH. Data represent the mean ± standard deviation (SD). a, b Means with different letters are significantly different (p < 0.05); comparisons among different MDH activity values were performed using one-way ANOVA followed by Tukey’s HSD test
Effect of initial biomass concentration on mannitol production
Previous experiments were performed using an initial biomass concentration of about 0.001 g cell dry weight/l corresponding approximately to an initial cell count of 1 × 106 CFU/ml. The effect of increasing the initial biomass concentration on mannitol production by L. reuteri CRL 1101 using the simplified medium at optimized culture conditions (pH 5.0, 100 rpm, 37 °C and 24 h) was aimed here. Ten-, 100-, 1000-, and 2000-fold increases in the biomass concentration (corresponding to 0.01, 0.1, 1.0, and 2.0 g/l, respectively) were evaluated. Results are shown in Table 3 and Fig. 5. The increase in the initial biomass concentration caused a decrease in cell growth and final cell viability after 24 h comparing to the cultures with 0.001 g cell dry weight/l of initial biomass (control) (log CFU/ml: 9.34 ± 0.11, Fig. 3c; Δlog CFU/ml 2.91 ± 0.33, Table 3). This fact was specially noted in the culture inoculated with 2 g cell dry weight/l (log CFU/ml 8.82 ± 0.34, Fig. 5g; Δlog CFU/ml 0.33 ± 0.12, Table 3) after 24 h. In all cases, cells exhibited an intense metabolic activity showing a high glucose (238–324 mM) and fructose (220–336 mM) uptake up to 24 h of incubation (Table 3) due to the growth stage (logarithmic phase) of the inoculum. Sugar consumption was more pronounced when a larger number of cells were used. All carbohydrates were exhausted at the end of fermentation in cultures inoculated with 1 or 2 g cell dry weight/l (Fig. 5f, h, respectively). In these two cultures and at 8 h of incubation, mannitol production (132 ± 8 and 132 +- 21 mM, respectively) and the volumetric productivity values (Q Mtl 3.01 ± 0.14 and 2.98 ± 0.27 g/l h, respectively) were higher than the control (61 ± 17 mM and 1.73 g/l h, respectively, Fig. 3d) although yields were lower (data not shown by Table S1). The highest mannitol production (228 ± 6 mM, 42 g/l) was obtained in the culture inoculated with 0.001 g cell dry weight/l (control) after 24 h. Also, increasing the initial biomass (i.e., up to 1 or 2 g cell weight/l) caused a decrease in mannitol yield (59 and 65 %, respectively) and volumetric productivities (1.50 and 1.54 g/l h, respectively) compared to the control (105 %; 1.73 g/l h). At 24 h, the specific productivity values (Table S1) were similar in all cultures and higher than those obtained at 8 h. The results obtained suggest that increasing the initial biomass did not improve mannitol production after 24 h. At 8 h, an opposite effect of the initial biomass was observed; however, the largest mannitol production value was half of that obtained at 24 h.
Mannitol isolation
Fermentations were carried out in the simplified culture medium at pH 5.0, 100 rpm, with an initial biomass concentration of 0.001 g/l, 37 °C for a 24-h incubation. Under these conditions, L. reuteri CRL 1101 produces approximately 42 g mannitol/l (Table 3). Since mannitol crystallizes at concentrations higher than 180 g/l at 4 °C, it was necessary to concentrate five times the cell-free fermented culture. Mannitol formation was observed as small needle-shaped crystals in the filtered concentrated supernatant after keeping at 4 °C for 48 h (Fig. 6a) and by optical microscopy (objective ×40, ocular ×10) (Fig. 6b). The obtained crystals were dissolved in water and further analyzed by HPLC. Mannitol was detected as the main component of the crystals although a small contamination with sucrose, glucose, and fructose, derived from the culture medium, was observed (data not shown).
Discussion
One of the main challenges for the optimization of the productive process of an interesting compound is the replacement or reduction of expensive components of the culture medium allowing optimal product formation at lower cost. In this work, a simplified culture medium was used at free- and controlled-pH conditions to evaluate mannitol production by L. reuteri CRL 1101. The correlation between MDH activity and mannitol production when varying the culture pH was studied. Finally, the effect of the initial biomass concentration on mannitol synthesis and mannitol isolation was assayed.
The substitution of peptone or beef extract by soy peptone as a low-cost nitrogen source in a simplified culture medium for growth and mannitol production by L. reuteri CRL 1101 was studied; although the strain could grow well in the soy peptone containing medium, mannitol production was practically null. Amino acids in animal protein are present in different amounts comparing to vegetable protein. Ala, Lys, Met, and Tyr can be found in higher concentrations (between two- and three-fold) in beef extract than in soy peptides (Ikeda et al. 2011; Storcksdieck et al. 2007). These amino acids play fundamental roles for cell growth, while Ala and Lys are part of the peptidoglycan of the bacterial cell wall, all protein synthesis begins with Met (or formyl-methionine in bacteria), and Tyr constitutes the main catalytic residue of the enzyme topoisomerase (Champoux 2001) that catalyzes the DNA winding and unwinding during cell replication and protein synthesis.
In preliminary studies, it was shown that manganese sulfate was a necessary salt for growth of L. reuteri CRL 1101; however, its omission did not affect cell viability or mannitol formation by the strain when glucose was replaced by sugarcane molasses as carbon source (data not shown); this fact suggests that molasses may contain Mn2+ ions or another component displaying the same positive effect. Therefore, this salt was not included in the formulation of molasses-based culture media. Saha (2006) used molasses and fructose syrup as inexpensive carbon sources to replace pure and expensive glucose and fructose for mannitol production by L. intermedius NRRL B-3693. Moreover, alternative organic and inorganic inexpensive nitrogen sources were assayed to replace peptone and yeast extract. The strain L. intermedius NRRL B-3693 produced 105 g mannitol/l using both molasses and fructose-rich syrup (in a 1:1 molasses:syrup ratio, 150 g/l total sugars, and a 4:1 fructose:glucose ratio) and soy peptone (5 g/l instead of peptone) after 22 h of incubation. Fontes et al. (2009) used cashew apple juice as alternative substrate to evaluate mannitol production by LAB. Cashew apple juice is an industrial waste rich in fructose and glucose, fibers, vitamins, and salts (Campos et al. 2002). This substrate―alone or mixed with sucrose―was evaluated for mannitol production by Leuconostoc mesenteroides B-512F in a two-step process, which consisted in growing the strain first at pH 6.5 for 8 h and then at free-pH. The addition of sucrose enhanced mannitol yield (85 %) but decreased the volumetric productivity (1.6 g/l h) as compared with the values obtained using cashew apple juice only (70 % and 2.2 g/l h, respectively). Maximal mannitol production (18 g/l), yield (67 %), and volumetric productivity (1.8 g/l h) were obtained combining cashew apple juice and sucrose after 10 h of incubation. In another study, Carvalheiro et al. (2011) used carob syrup to evaluate growth and mannitol production by eight mannitol-producing LAB strains. Carbohydrates typically present in carob syrup are sucrose (>50 % of total sugars), fructose, and glucose. To increase free fructose and glucose content, sucrose hydrolysis was performed; the studied LAB strains produced mannitol with relatively high efficiency (0.7 g mannitol/g fructose). The best mannitol production (43.7 g/l), volumetric productivity (2.36 g/l h), and conversion efficiency (yield, 100 %) were exhibited by the L. fructosum NRRL B-2041 strain after 30 h of incubation. These results are similar to those obtained in our study (mannitol production 41.6 ± 1.1 g/l; Y Mtl 105 ± 11 %) except for the volumetric productivity, which was lower (1.73 ± 0.05 g/l h). Interestingly, our results were reached using a simplified molasses-based culture medium in a shorter fermentation period (24 h) instead of a supplemented-MRS (rich) medium after 30 h. Moreover, the molasses (sucrose) hydrolysis step was not necessary since L. reuteri CRL 1101 was capable to hydrolyze it as previously reported (Bae and Shoda 2004; Dumbrepatil et al. 2008).
Recently, Papagianni and Legisa (2014) optimized mannitol production by a L. reuteri strain through a metabolic engineering strategy. A truncated version of the tpfkA gene (encoding 6-P-1-fructokinase from Aspergillus niger NRRL 2270) into the mannitol producer strain L. reuteri ATCC 55730 was introduced. The transformant strain had an enhanced carbon flux through the Embden–Meyerhof pathway and was able to deal with an elevated glucose concentration (75 g/l). The NADH cofactor demand was satisfied, allowing fructose to be used more efficiently as an electron acceptor, being exclusively transformed into mannitol. An enhanced mannitol production (56 g/l compared to 10 g/l by the parental strain) was achieved from fructose (75 g/l) by the transformant strain after 102 h of fermentation.
Organic acid production, mainly lactic acid, by LAB during growth decreases environmental pH inhibiting cell growth and reducing end-product formation. Consequently, a controlled-pH strategy is useful to improve microbial viability and mannitol synthesis, which it has been established to be cell growth-associated for L. reuteri CRL 1101. In this work, controlled-pH fermentations using the formulated simplified medium were applied to enhance mannitol production by L. reuteri CRL 1101. The highest mannitol production (228 ± 6 mM = 41.5 ± 1.1 g/l), yield (105 ± 11 %), and volumetric productivity (1.73 ± 0.05 g/l h) were reached at pH 5.0. This fact was observed for this strain and for L. fermentum CRL 573 in a modified MRS medium containing a glucose/fructose mixture (1:6.5) as carbon source (Rodriguez et al. 2012); however, mannitol production was much lower as a value of 122 ± 6 mM = 22.2 ± 1.1 g/l was attained after 24 h of incubation. Coincidentally, Saha and Racine (2010) reported an optimal pH value of 5.0 for mannitol production by L. intermedius NRRL B-3693.
LAB are acid-tolerant fermentative bacteria capable to grow at pH values ranging from neutral to pH of 3.5 (Kashket 1987; McDonald et al. 1990; Russell 1991). A particular feature of these bacteria is the ability to decrease its internal pH (pHin) as the external pH (pHex) decreases during growth (Cook and Russell 1994; Nannen and Hutkins 1991) maintaining a constant ΔpH between inside and outside the cell rather than a constant pHin. Siegumfeldt et al. (2000) found that different LAB species incubated at pHex 5.0 showed pHin values of around 5.5, resulting in a ΔpH of 0.5–0.8 pH units. As the optimal pH reported for MDH enzyme activity of L. reuteri ATCC 53608 is 5.4 (Sasaki et al. 2005) and 5.5 for L. intermedius NRRL B-3693 (Saha 2004), MDH enzymes may operate at their optimum pH (pHin around 5.5–5.8) when these LAB are incubated at pH 5.0, explaining thus the high mannitol production obtained at this pH value. Conversely, Yue et al. (2013) found that the highest specific MDH activity (0.42 ± 0.09 U/mg protein) and specific mannitol formation rate (2.54 h−1) were obtained at pH 4.5 using the mutant strain L. brevis 3-A5; the authors suggested that a constant pH 4.5 promoted mannitol synthesis probably due to the high MDH activity attained at this pH value and concluded that at both free- and controlled-pH fermentations mannitol production was mainly associated to cell growth.
With respect to pH, four pH values that do not necessarily coincide should be distinguished: (i) the pH of maximum microbial growth, (ii) the pH of maximum enzyme production, (iii) the pH of maximum enzyme stability, and (iv) the pH of maximum enzyme activity (García Garibay et al. 2004). Regulation of a particular pH value during fermentation is often required to ensure maximum metabolite production and to avoid changes that may affect the enzymatic activity. In some cases, the pH of optimum microbial growth and enzyme production, especially for intracellular enzymes, do not match with the pH value of maximum activity. For example, dextransucrase from L. mesenteroides is secreted with high productivity at pH 6.5, suitable for bacterial growth, being the pH of maximum enzyme activity and stability at 5.2 (García Garibay et al. 2004). In our study, the MDH activity was similar at all assayed pH values (except for pH 4.8), probably indicating that the enzyme production was similar under all assayed conditions; however, depending on the pHin, the enzyme activity was higher or lower explaining differences in mannitol production among the different assessed pH values.
Acid is an important environmental stress, which occurs during industrial fermentation and processing using LAB. In response to acid stress, LAB employ different strategies to prevent acid damage, including the maintenance of pHin homeostasis. The induction of the proton-translocating ATPase is the most important mechanism used by LAB to regulate the pHi (De Angelis and Gobbetti 2004; Wu et al. 2013). The activity of this enzyme was found to be optimal at pH values between 5.0 and 5.5 (Bender and Marquis 1987); this could be a reason why L. reuteri CRL 1101 can successfully deal against acid stress at pH 5.0. Another strategy to manage acid stress is through the regulation of the aspartate and arginine metabolism; some LAB shift the metabolic pathway by increasing the flux from aspartate to arginine (arginine deiminase pathway, ADI) and decreasing the flux from aspartate to asparagine. The ADI system has been identified as another acid-stress response mechanism to protect cells in a several bacteria (Matsui and Cvitkovitch 2010; Mols and Abee 2011; Vrancken et al. 2009). The increase in the acid resistance of lactobacilli may be due to the restoration of the optimal pHin through arginine catabolism and the concomitant production of NH4 + (De Angelis and Gobbetti 2004). Some enzymes of the ADI system were upregulated in fermentations at pH 5.0 of L. reuteri CRL 1101, as detected in proteomic studies (unpublished data).
The increase in the initial biomass concentration did not enhance mannitol production and the specific productivity after 24 h under the studied conditions. In contrast, the volumetric productivity (Q Mtl) values were higher at 8 h than at 24 h in cultures inoculated with high biomass concentration (1 and 2 g of cell dry weight/l). However, preparation and centrifugation of large volumes of culture medium (1.5 l at laboratory scale) are needed to obtain the cell pellet required for fermentations with high biomass concentration, limiting microbial application at industrial scale. Thus, we conclude that increasing the initial inoculum neither improves mannitol production nor accelerates its synthesis by L. reuteri CRL 1101 under the assayed conditions.
In this work, a simplified culture medium containing sugarcane molasses as carbon source, yeast extract, and beef extract for mannitol production by L. reuteri CRL 1101 was used to efficiently produce mannitol at a constant pH of 5.0. Partial isolation of mannitol crystals was also achieved. To our knowledge, this is the highest mannitol production obtained by a wild-type L. reuteri strain. The application of an inexpensive and more competitive culture medium for the biotechnological production of mannitol by L. reuteri CRL 1101 represents an important step for its previous synthesis at pilot scale.
References
Axelsson L (1998) Lactic acid bacteria: classification and physiology. In: Salminen S, von Wright A (eds) Lactic acid bacteria: microbiology and functional aspects, 2nd edn. Dekker, M., New York, pp. 1–72
Bae S, Shoda M (2004) Bacterial cellulose production by fed-batch fermentation in molasses medium. Biotechnol Prog 20:1366–1371. doi:10.1021/bp0498490
Bender GR, Marquis RE (1987) Membrane ATPases and acid tolerance of Actinomyces viscosus and Lactobacillus casei. Appl Environ Microbiol 53:2124–2128
Campos DCP, Santos AS, Wolkoff DB, Matta VM, Cabral LMC, Couri S (2002) Cashew apple juice stabilization by microfiltration. Desalination 148:61–65. doi:10.1016/S0011-9164(02)00654-9
Carvalheiro F, Moniz P, Duarte LC, Esteves MP, Girio FM (2011) Mannitol production by lactic acid bacteria grown in supplemented carob syrup. J Ind Microbiol Biotechnol 38:221–227. doi:10.1007/s10295-010-0823-5
Castillo EEF, Forero SCS (2007) Evaluation of sugarcane molasses as substrate for production of Saccharomyces cerevisiae. Bachelor Thesis, Pontificia Universidad Javeriana
Cook GM, Russell JB (1994) The effect of extracellular pH and lactic acid on pH homeostasis in Lactococcus lactis and Streptococcus bovis. Curr Microbiol 28:165–168. doi:10.1007/bf01571059
Champoux JJ (2001) DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem 70:369–413. doi:10.1146/annurev.biochem.70.1.369
De Angelis M, Gobbetti M (2004) Environmental stress responses in Lactobacillus: a review. Proteomics 4:106–122. doi:10.1002/pmic.200300497
Dumbrepatil A, Adsul M, Chaudhari S, Khire J, Gokhale D (2008) Utilization of molasses sugar for lactic acid production by Lactobacillus delbrueckii subsp. delbrueckii mutant Uc-3 in batch fermentation. Appl Environ Microbiol 74:333–335. doi:10.1128/AEM.01595-07
Fontes CP, Honorato TL, Rabelo MC, Rodrigues S (2009) Kinetic study of mannitol production using cashew apple juice as substrate. Bioprocess Biosyst Eng 32:493–499. doi:10.1007/s00449-008-0269-6
García Garibay M, Quintero Ramirez R, López-Mujía Canales A (2004) Biotecnología Alimentaria, 5th edn. Editorial Limusa S.A, México
Hahn G, Kaup B, Bringer-Meyer S, Sahm H (2003) A zinc-containing mannitol-2-dehydrogenase from Leuconostoc pseudomesenteroides ATCC 12291: purification of the enzyme and cloning of the gene. Arch Microbiol 179:101–107. doi:10.1007/s00203-002-0507-2
He X, Guo X, Liu N, Zhang B (2007) Ergosterol production from molasses by genetically modified Saccharomyces cerevisiae. Appl Microbiol Biotechnol 75:55–60. doi:10.1007/s00253-006-0807-6
Hugenholtz J (2008) The lactic acid bacterium as a cell factory for food ingredient production. Int Dairy J 18:466–475. doi:10.1016/j.idairyj.2007.11.015
Ikeda K, Kitagawa S, Tada T, Iefuji H, Inoue Y, Izawa S (2011) Modification of yeast characteristics by soy peptides: cultivation with soy peptides represses the formation of lipid bodies. Appl Microbiol Biotechnol 89:1971–1977. doi:10.1007/s00253-010-3001-9
Iwamoto K, Shiraiwa Y (2005) Salt-regulated mannitol metabolism in algae. Mar Biotechnol (NY) 7:407–415. doi:10.1007/s10126-005-0029-4
Kashket ER (1987) Bioenergetics of lactic acid bacteria: cytoplasmic pH and osmotolerance. FEMS Microbiol Lett 46:233–244. doi:10.1111/j.1574-6968.1987.tb02463.x
Kets EP, Galinski EA, de Wit M, de Bont JA, Heipieper HJ (1996) Mannitol, a novel bacterial compatible solute in Pseudomonas putida S12. J Bacteriol 178:6665–6670
Korakli M, Vogel RF (2003) Purification and characterisation of mannitol dehydrogenase from Lactobacillus sanfranciscensis. FEMS Microbiol Lett 220:281–286
Lawson ME (1997) Sugar alcohols. In: Kroschwitz JI, Howe-Grant M (eds) Kirk-Othmer encyclopedia of chemical technology, 4th edn. John Wiley & Sons Inc., New York, pp. 93–119
Livesey G (2003) Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutr Res Rev 16:163–191. doi:10.1079/NRR200371
Matsui R, Cvitkovitch D (2010) Acid tolerance mechanisms utilized by Streptococcus mutans. Future Microbiol 5:403–417. doi:10.2217/fmb.09.129
McDonald LC, Fleming HP, Hassan HM (1990) Acid tolerance of Leuconostoc mesenteroides and Lactobacillus plantarum. Appl Environ Microbiol 56:2120–2124
Mols M, Abee T (2011) Bacillus cereus responses to acid stress. Environ Microbiol 13:2835–2843. doi:10.1111/j.1462-2920.2011.02490.x
Monedero V, Perez-Martinez G, Yebra MJ (2010) Perspectives of engineering lactic acid bacteria for biotechnological polyol production. Appl Microbiol Biotechnol 86:1003–1015. doi:10.1007/s00253-010-2494-6
Mukhtar K, Asgher M, Afghan S, Hussain K, Zia-Ul-Hussnain S (2010) Comparative study on two commercial strains of Saccharomyces cerevisiae for optimum ethanol production on industrial scale. J Biomed Biotechnol 2010:419586. doi:10.1155/2010/419586
Nannen NL, Hutkins RW (1991) Intracellular pH effects in lactic acid bacteria. J Dairy Sci 74:741–746. doi:10.3168/jds.S0022-0302(91)78219-2
Ortiz ME, Bleckwedel J, Raya RR, Mozzi F (2013) Biotechnological and in situ food production of polyols by lactic acid bacteria. Appl Microbiol Biotechnol 97:4713–4726. doi:10.1007/s00253-013-4884-z
Ortiz ME, Fornaguera MJ, Raya RR, Mozzi F (2012) Lactobacillus reuteri CRL 1101 highly produces mannitol from sugarcane molasses as carbon source. Appl Microbiol Biotechnol 95:991–999. doi:10.1007/s00253-012-3945-z
Papagianni M, Legisa M (2014) Increased mannitol production in Lactobacillus reuteri ATCC 55730 production strain with a modified 6-phosphofructo-1-kinase. J Biotechnol 181:20–26. doi:10.1016/j.jbiotec.2014.04.007
Rodriguez C, Rimaux T, Fornaguera MJ, Vrancken G, de Valdez GF, De Vuyst L, Mozzi F (2012) Mannitol production by heterofermentative Lactobacillus reuteri CRL 1101 and Lactobacillus fermentum CRL 573 in free and controlled pH batch fermentations. Appl Microbiol Biotechnol 93:2519–2527. doi:10.1007/s00253-011-3617-4
Rousvoal S, Groisillier A, Dittami SM, Michel G, Boyen C, Tonon T (2011) Mannitol-1-phosphate dehydrogenase activity in Ectocarpus siliculosus, a key role for mannitol synthesis in brown algae. Planta 233:261–273. doi:10.1007/s00425-010-1295-6
Russell JB (1991) Resistance of Streptococcus bovis to acetic acid at low pH: relationship between intracellular pH and anion accumulation. Appl Environ Microbiol 57:255–259
Saha BC (2004) Purification and characterization of a novel mannitol dehydrogenase from Lactobacillus intermedius. Biotechnol Prog 20:537–542. doi:10.1021/bp034277p
Saha BC (2006) A low-cost medium for mannitol production by Lactobacillus intermedius NRRL B-3693. Appl Microbiol Biotechnol 72:676–680. doi:10.1007/s00253-006-0364-z
Saha BC, Racine FM (2010) Effects of pH and corn steep liquor variability on mannitol production by Lactobacillus intermedius NRRL B-3693. Appl Microbiol Biotechnol 87:553–560. doi:10.1007/s00253-010-2552-0
Saha BC, Racine FM (2011) Biotechnological production of mannitol and its applications. Appl Microbiol Biotechnol 89:879–891. doi:10.1007/s00253-010-2979-3
Sasaki Y, Laivenieks M, Zeikus JG (2005) Lactobacillus reuteri ATCC 53608 mdh gene cloning and recombinant mannitol dehydrogenase characterization. Appl Microbiol Biotechnol 68:36–41. doi:10.1007/s00253-004-1841-x
Shen B, Jensen RG, Bohnert HJ (1997) Mannitol protects against oxidation by hydroxyl radicals. Plant Physiol 115:527–532
Sheoran A, Yadav BS, Nigam P, Singh D (1998) Continuous ethanol production from sugarcane molasses using a column reactor of immobilized Saccharomyces cerevisiae HAU-1. J Basic Microbiol 38:123–128. doi:10.1002/(SICI)1521-4028(199805)38:2<123::AID-JOBM123>3.0.CO;2-9
Siegumfeldt H, Bjorn Rechinger K, Jakobsen M (2000) Dynamic changes of intracellular pH in individual lactic acid bacterium cells in response to a rapid drop in extracellular pH. Appl Environ Microbiol 66:2330–2335. doi:10.1128/AEM.66.6.2330-2335.2000
Soetaert W, Buchholz K, Vandamme EJ (1995) Production of D-mannitol and D-lactic acid by fermentation with Leuconostoc mesenteroides. Agro Food Ind Hi Tec 6:41–44
Soetaert W, Vanhooren PT, Vandamme EJ (1999) The production of mannitol by fermentation. In: Bucke C (ed) Carbohydrate biotechnology protocols, vol 10. Methods in biotechnology™. Humana Press, New York, pp. 261–275. doi:10.1007/978-1-59259-261-6_21
Stoop JM, Mooibroek H (1998) Cloning and characterization of NADP-mannitol dehydrogenase cDNA from the button mushroom Agaricus bisporus, and its expression in response to NaCl stress. Appl Environ Microbiol 64:4689–4696
Stoop JMH, Williamson JD, Mason Pharr D (1996) Mannitol metabolism in plants: a method for coping with stress. Trends Plant Sci 1:139–144. doi:10.1016/S1360-1385(96)80048-3
Storcksdieck S, Bonsmann G, Hurrell RF (2007) Iron-binding properties, amino acid composition, and structure of muscle tissue peptides from in vitro digestion of different meat sources. J Food Sci 72:S019–S029. doi:10.1111/j.1750-3841.2006.00229.x
Voegele RT, Hahn M, Lohaus G, Link T, Heiser I, Mendgen K (2005) Possible roles for mannitol and mannitol dehydrogenase in the biotrophic plant pathogen Uromyces fabae. Plant Physiol 137:190–198. doi:10.1104/pp.104.051839
von Weymarn N (2002) Process development for mannitol production by lactic acid bacteria. PhD Thesis, Helsinki University of Technology
von Weymarn N, Hujanen M, Leisola M (2002) Production of D-mannitol by heterofermentative lactic acid bacteria. Process Biochem 37:1207–1213. doi:10.1016/S0032-9592(01)00339-9
Vrancken G, Rimaux T, De Vuyst L, Mozzi F (2010) Low-calorie sugars produced by lactic acid bacteria. In: Mozzi F, Raya RR, Vignolo GM (eds) Biotechnology of lactic acid bacteria: novel applications. Wiley-Blackwell, New York, pp. 193–209
Vrancken G, Rimaux T, Weckx S, De Vuyst L, Leroy F (2009) Environmental pH determines citrulline and ornithine release through the arginine deiminase pathway in Lactobacillus fermentum IMDO 130101. Int J Food Microbiol 135:216–222. doi:10.1016/j.ijfoodmicro.2009.07.035
Wee Y-J, Kim J-N, Yun J-S, Ryu H-W (2004) Utilization of sugar molasses for economical L(+)-lactic acid production by batch fermentation of Enterococcus faecalis. Enzyme Microb Technol 35:568–573. doi:10.1016/j.enzmictec.2004.08.008
Wisnlak J, Simon R (1979) Hydrogenation of glucose, fructose, and their mixtures. Ind Eng Chem Prod Res Dev 18:50–57. doi:10.1021/i360069a011
Wisselink HW, Weusthuis RA, Eggink G, Hugenholtz J, Grobben GJ (2002) Mannitol production by lactic acid bacteria: a review. Int Dairy J 12:151–161. doi:10.1016/S0958-6946(01)00153-4
Wu C, Zhang J, Du G, Chen J (2013) Aspartate protects Lactobacillus casei against acid stress. Appl Microbiol Biotechnol 97:4083–4093. doi:10.1007/s00253-012-4647-2
Yue M, Cao H, Zhang J, Li S, Meng Y, Chen W, Huang L, Du Y (2013) Improvement of mannitol production by Lactobacillus brevis mutant 3-A5 based on dual-stage pH control and fed-batch fermentations. World J Microbiol Biotechnol 29:1923–1930. doi:10.1007/s11274-013-1357-6
Acknowledgments
We acknowledge the financial support of CONICET (PIP 2010-0062), FONCyT (Préstamo BID PICT 2008-933), and CIUNT from Argentina. M. E. Ortiz is a recipient of a doctoral fellowship from CONICET, Argentina.
Conflict of interest
The authors declare that they have no competing interests.
Ethical statement
The authors state that principles of ethical and professional conduct have been followed in this research and in the preparation of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Table S1
(PDF 208 kb)
Rights and permissions
About this article
Cite this article
Ortiz, M.E., Raya, R.R. & Mozzi, F. Efficient mannitol production by wild-type Lactobacillus reuteri CRL 1101 is attained at constant pH using a simplified culture medium. Appl Microbiol Biotechnol 99, 8717–8729 (2015). https://doi.org/10.1007/s00253-015-6730-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6730-y