Abstract
We previously described an endo-acting rhamnogalacturonan (RG) lyase, termed PcRGL4A, of Penicillium chrysogenum 31B. Here, we describe a second RG lyase, called PcRGLX. We determined the cDNA sequence of the Pcrglx gene, which encodes PcRGLX. Based on analyses using a BLAST search and a conserved domain search, PcRGLX was found to be structurally distinct from known RG lyases and might belong to a new polysaccharide lyase family together with uncharacterized fungal proteins of Nectria haematococca, Aspergillus oryzae, and Fusarium oxysporum. The Pcrglx cDNA gene product (rPcRGLX) expressed in Escherichia coli demonstrated specific activity against RG but not against homogalacturonan. Divalent cations were not essential for the enzymatic activity of rPcRGLX. rPcRGLX mainly released unsaturated galacturonosyl rhamnose (ΔGR) from RG backbones used as the substrate from the initial stage of the reaction, indicating that the enzyme can be classified as an exo-acting RG lyase (EC 4.2.2.24). This is the first report of an RG lyase with this mode of action in Eukaryota. rPcRGLX acted synergistically with PcRGL4A to degrade soybean RG and released ΔGR. This ΔGR was partially decorated with galactose (Gal) residues, indicating that rPcRGLX preferred oligomeric RGs to polymeric RGs, that the enzyme did not require Gal decoration of RG backbones for degradation, and that the enzyme bypassed the Gal side chains of RG backbones. These characteristics of rPcRGLX might be useful in the determination of complex structures of pectins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
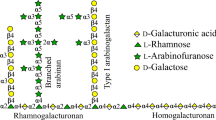
Pectins are acidic heteropolysaccharides and a major component of primary cell walls and intracellular middle lamellae of dicotyledons. They are complex macromolecules that basically consist of seven different polysaccharides, including homogalacturonan (HG), rhamnogalacturonan-I (RG-I), RG-II, xylogalacturonan, arabinan, arabinogalactan-I (AG-I), and AG-II. In general, HG and RG-I are the main backbones in pectin, although fine structures of pectins vary depending on plant origins. HG has a backbone that is composed of α-1,4-linked D-galacturonic acid (GalA) residues, which may be substituted by compounds such as methanol and acetic acid. RG-I has a backbone that is composed of repeating units of α-1,2-linked L-rhamnose (Rha) and α-1,4-linked GalA. The Rha residues of the RG-I backbone can be substituted at C4 with neutral sugar side chains such as arabinan and AG-I. The GalA residues of RG-I can be acetylated at positions C2 and/or C3 (Wong 2008; Voragen et al. 2009; Bonnin et al. 2014).
Pectolytic enzymes are very important in biotechnological applications such as fruit juice extraction and clarification, animal feed production, textile processing, and oil extraction (Jayani et al. 2005; Lara-Márquez et al. 2011). They are also powerful tools in analyzing fine structures of pectins due to their strict substrate specificities. So far, numerous HG-degrading enzymes have been extensively characterized in bacteria, fungi, and plants. However, the number of reports concerning RG-I degrading enzymes is limited. Systems for degradation of RG-I have only been well characterized in Aspergillus aculeatus and Bacillus subtilis. Schols and colleagues isolated the first RG-degrading enzyme, RG hydrolase (EC 3.2.1.171), from A. aculeatus (Schols et al. 1990). Since then, several RG-I-specific enzymes with different modes of action have been found in A. aculeatus, including RG acetylesterase (EC 3.1.1.86; Searle-van Leeuwen et al. 1992; Kauppinen et al. 1995), RG rhamnohydrolase (EC 3.2.1.174; Mutter et al. 1994), endo-RG lyase (EC 4.2.2.23; Mutter et al. 1996), and RG galacturonohydrolase (EC 3.2.1.173; Mutter et al. 1998). In contrast, B. subtilis was demonstrated to produce an exo-RG lyase (YesX, EC 4.2.2.24; Ochiai et al. 2007) and two unsaturated rhamnogalacturonyl hydrolase (YesR and YteR, EC 3.2.1.172; Itoh et al. 2006) in addition to an endo-RG lyase (YesW; Ochiai et al. 2007) and RG acetylesterase (YesT; Martínez-Martínez et al. 2008). YesR and YteR specifically hydrolyze unsaturated galacturonosyl rhamnose (ΔGR) released from RG oligosaccharides by the action of YesX. Based on the differences in enzymes produced by A. aculeatus and B. subtilis, their degradation pathways for RG-I are inferred to be different.
We previously isolated the Penicillium chrysogenum 31B strain that strongly degrades sugar beet fiber (SBF). When the culture filtrate of this strain was incubated with SBF, approximately 95 % of the total Rha in the fiber was solubilized. This strain has been shown to secrete an endo-RG lyase (PcRGL4A) belonging to polysaccharide lyase (PL) family 4. The enzyme cleaves the α-1,4-linkage between the Rha and GalA of RG-I backbones via a trans-elimination reaction in an endolytic fashion and releases oligomers with 4-deoxy-4,5-unsaturated GalA (ΔGalA) at the nonreducing end (Iwai et al. 2015). Furthermore, genome sequencing of P. chrysogenum Wisconsin 54–1255 indicates that this strain possesses two other putative RG lyases belonging to PL4 and two unsaturated rhamnogalacturonyl hydrolases belonging to glycoside hydrolase (GH) family 105. Based on these facts, P. chrysogenum seems to possess a degradation pathway of RG-I similar to that of B. subtilis. In order to elucidate the RG-I degrading system found in this strain, we purified, cloned, and overexpressed an exo-RG lyase (PcRGLX) in the present study. We also investigated the biochemical characteristics of its recombinant enzyme.
Materials and methods
Chemicals and reagents
DEAE-Toyopearl 650M was purchased from Tosoh Corp. (Tokyo, Japan). HiLoad 16/60 Superdex 75 and Mono Q HR 5/5 were obtained from GE Healthcare UK Ltd. (Little Chalfont, Buckinghamshire, UK). Amylose resin was from New England BioLabs Inc. (Ipswich, MA, USA). SBF was kindly provided by Nippon Beet Sugar Manufacturing Co., Ltd. (Tokyo, Japan). All other chemicals were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan) unless otherwise stated and were of certified reagent grade.
Strain, media, growth conditions, and plasmids
The P. chrysogenum 31B strain used in this study had been deposited in the International Patent Organism Depositary, National Institute of Advanced Industrial Science and Technology (acc. no. FERM P-19163; http://unit.aist.go.jp/ipod/cie/index.html).
To purify PcRGLX, a culture supernatant (5 l) of P. chrysogenum 31B was prepared in a medium containing 0.1 % glucose (Glc) and 2 % SBF as the carbon sources, as described previously (Shinozaki et al. 2014).
Escherichia coli DH5α, E. coli BL21 (DE3), and the plasmid pMAL-c2X (New England BioLabs, Ipswich, MA, USA) were used for cloning and expression. Transformants were grown in LB medium consisting of 1 % peptone, 0.5 % yeast extract, and 0.5 % NaCl (pH 7.0) supplemented with ampicillin (50 μg/ml).
Substrates
Alkali-soluble pectin (ASP) was extracted from SBF (15 g) as follows: SBF was mixed with 0.1 M NaOH (1 l), and the mixture was autoclaved at 121 °C for 20 min. The slurry was filtered through gauze, and the filtrate was centrifuged. The filtrate was neutralized with 1 M HCl, evaporated under reduced pressure, and mixed with three volumes of acetone. The precipitate formed was dried at 60 °C, dissolved in 20 mM K-phosphate buffer (pH 7.0), and dialyzed against the same buffer. The solution was loaded onto a DEAE-Toyopearl 650 M column (4 × 100 cm), which was equilibrated with dialysis buffer, and washed with the same buffer. The bound polysaccharides were eluted by a linear gradient of NaCl (0 to 1 M) in the same buffer. The bound fractions were pooled, concentrated, dialyzed against water, and finally lyophilized, resulting in ASP (911 mg).
Soybean RG was obtained from Megazyme International Ireland Ltd. (Wicklow, Ireland). RG oligosaccharides with ΔGalA at the nonreducing end, named usRG Oligo, were prepared by treating 10 ml of 0.5 % soybean RG with 100 mU of recombinant endo-RG lyase (rPcRGL4A) in 20 mM Tris-HCl buffer (pH 7.0) at 37 °C for 24 h followed by boiling the mixture for 5 min. usRG Oligo-Gal, which is usRG Oligo that the galactose (Gal) side chains are removed, was prepared by treating 3 ml of 0.5 % usRG Oligo with 20 mU of recombinant β-galactosidase (rPcBGAL35A) in 50 mM Na-acetate buffer (pH 5.0) at 37 °C for 24 h followed by boiling the mixture for 5 min. RG with a reduced quantity of neutral sugar side chains, named RG-AG, was prepared from soybean RG by treatment with mild acid and β-galactosidase as previously reported (Iwai et al. 2015). Sugar composition of RG-AG is as follows: GalA (70 %)/Rha (11 %)/Ara (0 %)/Gal (1 %)/Xyl (17 %)/Glc (1 %).
HG was obtained by hydrolysis of de-esterified water-soluble pectin from sugar beet according to the method of Thibault et al. (1993). HG was confirmed to be composed of only GalA and to contain no neutral sugar. HG oligosaccharides with ΔGalA at the nonreducing end, named usHG Oligo, were prepared by treating 2 ml of 0.25 % HG with 30 mU of recombinant endo-pectate lyase (rPelSWU) in 100 mM Tris-HCl buffer (pH 8.0) containing 0.1 mM CaCl2 at 60 °C for 24 h followed by boiling the mixture for 10 min.
Conditions of high-performance anion-exchange chromatography (HPAEC)
HPAEC for sugar analysis was performed using a Dionex DXc-500 system (Dionex Corp., Sunnyvale, CA, USA). Four different programs (Table 1) were selected for elution of sugars depending on the samples. The effluent was monitored with pulsed amperometric detection.
Sugar analysis
Uronic acids were quantified by the method of Blumenkrantz and Asboe-Hansen (1973). The neutral sugar composition of carbohydrates was determined by HPAEC after hydrolysis with 1 M sulfuric acid at 100 °C for 2 h. Monosaccharides were separated under the conditions of program 4 in Table 1.
Preparation of recombinant enzymes
Recombinant endo-RG lyase (rPcRGL4A) and endo-pectate lyase (rPelSWU) were prepared according to the methods of Iwai et al. (2015) and Sukhumsiirchart et al. (2009), respectively. Recombinant β-galactosidase (rPcBGAL35A; unpublished data) was isolated from a cell-free extract of E. coli Rosetta-gami B(DE3)pLysS (Novagen Inc., Madison, WI, USA) transformed with the pMAL-c2X vector containing the P. chrysogenum Pcbgal35A gene. The enzyme is a fusion protein consisting of a 42-kDa segment of maltose binding protein and PcBGAL35A.
Isolation of the oligosaccharides 1
The concentrated culture supernatant from P. chrysogenum was incubated with 35 ml of 2 % ASP in 20 mM K-phosphate buffer (pH 7.0) for 4 days at 37 °C and boiled for 10 min to inactivate the enzyme. The mixture was concentrated to 5 ml by evaporation and loaded onto a Bio-Gel P2 column (3.0 × 90 cm; Bio-Rad Laboratories Inc., Hercules, CA, USA) equilibrated with water. The sugars were eluted with water at a flow rate of 15 ml/h and 2-ml fractions were collected. The oligosaccharide 1 contents in the fractions were quantified by HPAEC under the conditions of program 1 in Table 1.
Determination of the reducing-end sugar of oligosaccharide 1
The reducing end of oligosaccharide 1 was reduced by treatment with 10 mM NaBH4 in 25 mM NaOH at 30 °C for 4 h. After neutralization of the mixture with 1 M HCl, the sample was hydrolyzed with 1 M sulfuric acid at 100 °C for 2 h. The resulting sugar alcohol was identified by HPAEC under the conditions of program 2 in Table 1. Standard sugars, including Rha, GalA, arabinose (Ara), Gal, and Glc, were also treated using the same protocol.
Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS)
MALDI-TOF MS was performed using an Autoflex II MALDI-TOF mass spectrometer (Bruker Daltonics, Bremen, Germany) as previously reported Iwai et al. (2015), except that the matrix solution was prepared by dissolving 10 mg of 2,5-dihydroxybenzoic acid in 1 ml of 20 % ethanol.
Enzyme assays
RG lyase activity was assayed using soybean RG as the substrate according to the method of Iwai et al. (2015), except that CaCl2 was not added to the reaction mixture.
ΔGR-releasing activity was assayed with soybean RG as the substrate. The reaction conditions are the same as described above for the RG lyase activity assay. The ΔGR contents in the reaction mixture were quantified by HPAEC under the conditions of program 3 in Table 1. One unit of enzyme activity was defined as the amount of enzyme that releases 1 μmol of ΔGR in 1 min under the above conditions.
Purification of PcRGLX
PcRGLX was purified from the culture filtrate of P. chrysogenum 31B as follows: The culture filtrate (5 l) was concentrated by ultrafiltration (10-kDa cutoff) and precipitated with ammonium sulfate at 80 % saturation. After centrifugation, the pellet was dissolved in 100 ml of 20 mM Na-acetate buffer (pH 5.0), dialyzed against the same buffer, and used as a crude enzyme solution. The enzyme solution was loaded onto a DEAE-Toyopearl 650 M column (2.5 × 20 cm) equilibrated with the dialysis buffer. The bound proteins were eluted using a linear gradient of NaCl (from 0 to 0.5 M) in the same buffer. The PcRGLX-containing fractions were pooled, concentrated by centrifugal filtration at 3,000×g with a 9-kDa cut-off filter (Pierce Concentrator 9K MWCO/20 ml; Thermo Fisher Scientific Inc., Rockford, IL USA) and loaded onto a size exclusion column of Superdex 75 equilibrated with 100 mM NaCl in 20 mM Na-acetate buffer (pH 5.0). Proteins were eluted with the same buffer at a flow rate of 1 ml/min. The active fractions were pooled, dialyzed against 20 mM Na-acetate buffer (pH 5.0), and loaded onto a Mono Q column equilibrated with the dialysis buffer. The bound proteins were eluted using a linear gradient of NaCl (from 0.2 to 0.5 M) at a flow rate of 1 ml/min.
Protein concentration was determined by measurement of the absorbance at 280 nm with bovine serum albumin (BSA) as the standard. Protein homogeneity and molecular mass were estimated by SDS-PAGE in a 10 % gel using the method of Laemmli (1970). The proteins were visualized with Coomassie Brilliant Blue R-250 staining.
The effect of temperature and pH on enzymatic activity
Standard RG lyase activity was assayed using soybean RG as the substrate. The optimal temperature and temperature stability were examined according to the method of Iwai et al. (2015), except that 20 mM Tris-HCl buffer (pH 7.0) and 20 mM Na-acetate buffer (pH 5.0) containing 50 μg/ml BSA were used, respectively. pH stability was examined by pre-incubating the enzyme at 4 °C for 16 h at various pHs using 100 mM glycine-HCl buffer (pH 3), Na-acetate buffer (pH 4 to 6), Tris-HCl buffer (pH 7, 8), glycine-NaOH buffer (pH 9, 10), and Na-phosphate-NaOH buffer (pH 11). The remaining activity was expressed as a percentage of the activity of the enzyme solution kept in 100 mM Tris-HCl buffer (pH 7) at 4 °C for 16 h. Optimum pH was determined by measuring activity at 37 °C for 30 min at pHs ranging from 4 to 10 using 100 mM of the above-described buffers.
Analysis of the internal amino acid sequence of PcRGLX using mass spectrometry
The internal amino acid sequence of PcRGLX was analyzed using liquid chromatography mass spectrometry equipped with an electrospray ionization source (LCMS IT-TOF; Shimadzu, Kyoto, Japan) and Mascot search software (http://www.matrixscience.com) linked with the NCBInr database (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Detailed conditions have been described previously (Iwai et al. 2015).
Cloning and expression of Pcrglx in E. coli
Pcrglx was cloned by the same method used for Pcrgl4A (Iwai et al. 2015) based on the sequence data of the Pc16g08050 gene of P. chrysogenum Wisconsin 54–1255. To construct the vector encoding mature PcRGLX, PCR was performed using the two primers rglx-Neco (5′-ATGGAATTCTTCAACTGCACCTCATCTTCC) and rglx-Chind (5′-CTAAAGCTTAAACGTCTATACCATCAAACC). The amplified fragment was digested with EcoRI and HindIII, ligated into the pMAL-c2X vector at the corresponding restriction enzyme sites, and sequenced. The recombinant plasmid was termed pMAL-rglx.
For the production of recombinant PcRGLX (rPcRGLX), the overnight culture of E. coli BL21 (DE3) transformant containing pMAL-rglx was inoculated into LB medium containing ampicillin (50 μg/ml) and then cultured at 37 °C for 2 h. Next, 0.1 mM isopropyl β-thiogalactopyranoside was added, and the culture was further incubated at 15 °C for 2 days. Preparation of the cell-free extract and purification of rPcRGLX was performed as described previously (Sakamoto et al. 2013) with minor modifications.
Nucleotide sequence
The nucleotide sequence of Pcrglx cDNA was submitted to the DDBJ/EMBL/GenBank database (acc. no. AB854357).
Sequence analysis
The closest matches to the PcRGLX deduced amino acid sequence were identified using the UniProtKB blast program (http://www.uniprot.org/blast/). Module sequences of the enzyme were analyzed using the NCBI Conserved Domain Search service (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
Kinetic parameters of rPcRGLX towards usRG Oligo and usRG Oligo-Gal
Kinetic parameters of rPcRGLX were assessed according to the method of Iwai et al. (2015), except that usRG Oligo and usRG Oligo-Gal were used as the substrates.
Substrate specificity
The reaction mixture containing 1 ml of 0.25 % usRG Oligo or usHG Oligo in 100 mM Tris-HCl buffer (pH 8.0) and rPcRGLX (10 mU) was incubated at 37 °C. A portion of the mixture was withdrawn at 10, 30, and 60 min followed by measuring the absorbance of the aliquot at 235 nm.
The effect of calcium ions (Ca2+) on the enzymatic activity of rPcRGLX
Both rPcRGLX and soybean RG were treated with ethylenediaminetetraacetic acid (EDTA) as described previously (Iwai et al. 2015). The usRG Oligo was prepared using soybean RG treated with EDTA by the same method described in the Substrate section. The reaction mixture, composed of 300 μl of 0.25 % EDTA-treated usRG Oligo in 20 mM Tris-HCl buffer (pH 7.0), rPcRGLX (7 mU), and CaCl2 (1 to 10 mM), was incubated at 37 °C for 20 min followed by measuring the absorbance of the mixture at 235 nm.
Results
Identification of the structure of oligosaccharide 1
When ASP was incubated with the concentrated culture supernatant of P. chrysogenum 31B extensively, an unidentified oligosaccharide (oligosaccharide 1) was detected in association with monomeric sugars via HPAEC (Fig. 1a). The reaction products were applied to a Bio-Gel P2 column to purify the oligosaccharide. HPAEC analysis of the products within fractions revealed that oligosaccharide 1 was eluted at around 240 ml and partially purified as shown in Fig. 1b.
HPAEC (a and b) and MALDI-TOF MS (c) analyses of the enzymatic products of ASP incubated with the concentrated culture supernatant of P. chrysogenum 31B. HPAEC was performed under the conditions of program 1 in Table 1. a Reaction products obtained from ASP with the concentrated culture supernatant of P. chrysogenum 31B. b oligosaccharide 1 purified with a Bio-Gel P2 column. c Purified oligosaccharide 1 was mixed with the matrix solution and dried on the sample plate, followed by analysis using MALDI-TOF MS. The experimental conditions are described in the text
For identification of the reducing-end sugar in oligosaccharide 1, the sugar was reduced with NaBH4. The resultant sugar alcohol was analyzed by HPAEC after acid hydrolysis. HPAEC chromatograms revealed that the reducing-end sugar was Rha by comparing the sample with standard sugar alcohols (data not shown). Products obtained from pectate via trans-eliminative reactions of pectate lyases contain ΔGalA residues at their non-reducing ends and have maximum absorption at 235 nm (Sakai et al. 1993). UV absorption of oligosaccharide 1 showed maximum absorption at 235 nm, indicating that it contains ΔGalA at the non-reducing end. MALDI-TOF MS revealed major ions with molecular masses of 345.6 and 361.6 (Fig. 1c). These were assumed to be the sodiated and potassiated molecules [M + Na]+ and [M + K]+, indicating that the molecular mass of the compound was 322. Based on the above results, oligosaccharide 1 was demonstrated to be ΔGR.
P. chrysogenum 31B has previously been shown to secrete an endo-RG lyase (PcRGL4A: Iwai et al. 2015), which mainly produces RG tetrasaccharides with ΔGalA at the non-reducing end. Considering all of these findings, an exo-acting RG lyase seems to exist in the culture supernatant of this strain.
Purification of an exo-RG lyase (PcRGLX)
PcRGLX was purified from 5 l of the culture filtrate of P. chrysogenum 31B by measuring ΔGR-releasing activity using soybean RG as the substrate. In the first chromatographic separation using a DEAE-Toyopearl column, the active enzyme was eluted at around 0.2 M NaCl as a single peak. PcRGLX was further purified by chromatography using Superdex 75 and Mono Q columns. This procedure represented a 3.4-fold purification of PcRGLX with a final yield of 1 % and specific activity of 55.6 mU/mg (Table 2). The purification fold was very low, probably because endo-RG lyases or side chain-degrading enzymes such as galactanase, which may have synergistic effects with PcRGLX on the degradation of RG-I, were eliminated during the purification steps. Based on the results of SDS-PAGE, the protein was highly purified (Fig. 2).
Physicochemical characteristics of PcRGLX
The molecular mass of PcRGLX was estimated to be 97 kDa by SDS-PAGE (Fig. 2). Endoglycosidase H treatment did not affect the molecular mass of PcRGLX (data not shown), suggesting that the enzyme did not contain N-linked carbohydrates. The activity of PcRGLX was highest at 40 °C and at pH 7–8. After incubation of the enzyme at pH 5.0 and 40 °C for 1 h, more than 80 % of the initial activity remained. However, the enzyme completely lost activity after treatment at 50 °C for 1 h. More than 80 % of the initial PcRGLX activity remained after 16 h of incubation at pH values ranging from 5 to 10.
The LCMS IT-TOF analysis detected seven PcRGLX peptides (F1: FDVPLKGEEYYDR, F2: LADTETWEPR, F3: AGQSWVNIPSGTR, F4: FAEALTRHTGEVDVYHIGDWK, F5: ISQPQYR, F6: TYGELDPQR, and F7: ISLLQAHSRLAAYAAYETK). A Mascot search identified a protein that matched peptides F1 to F7 with 100 % amino acid identity: a hypothetical protein from P. chrysogenum Wisconsin 54–1255 (Pc16g08050; NCBI acc. no. XP_002561127.1; Van den Berg et al. 2008). With a probability-based MOWSE score of 357, this protein was not classified into any PL family. Additional properties of PcRGLX were evaluated using a recombinant enzyme to exclude the possibility of contamination with other pectolytic enzymes. These results are described in the following sections.
Nucleotide and amino acid sequence alignments
The coding sequence of the Pcrglx cDNA is 2,781 bp and encodes a protein of 927 amino acids. SignalP (http://www.cbs.dtu.dk/services/SignalP) predicted that PcRGLX has an N-terminal signal peptide of 21 amino acids. After cleavage of the signal peptide, the calculated molecular mass would be 100,585 Da, which is in good agreement with that of the native protein (97 kDa). The internal amino acid sequences determined by LCMS IT-TOF were identified at positions 274–286 (F1), 326–335 (F2), 367–379 (F3), 609–629 (F4), 649–655 (F5), 681–689 (F6), and 837–855 (F7) in the deduced amino acid sequence. The deduced amino acid sequence of the Pcrglx gene showed high similarities with the sequences of other fungal uncharacterized proteins from P. chrysogenum Wisconsin 54–1255 (the Pc16g08050 protein; UniProtKB/TrEMBL acc. no. B6H7Q7; 100 % identity: Van den Berg et al. 2008), Nectria haematococca (C7ZK36; 66 % identity: Coleman et al. 2009), Aspergillus oryzae (I7ZQX6; 66 % identity), and Fusarium oxysporum f. sp. lycopersici (J9NAE6; 66 % identity: Ma et al. 2010). NCBI conserved domain search analysis did not find any significant matches to the deduced amino acid sequence of PcRGLX. Based on these results, PcRGLX may belong to a new family of RG lyases.
Overexpression of the Pcrglx gene in E. coli
The mature region of the Pcrglx gene was amplified by PCR, inserted into the pMAL-c2X vector, and transformed into E. coli. rPcRGLX was designed to be a fusion protein consisting of a maltose binding protein (42 kDa) attached to the N-terminus of PcRGLX. The fusion protein was purified from the cell-free extract by DEAE-Toyopearl, Amylose Resin, and Mono Q column chromatography. SDS-PAGE analysis of the purified rPcRGLX showed a protein band with molecular mass of 145 kDa (Fig. 2). Although the molecular mass of the fusion protein was much higher than that of the native enzyme, the recombinant enzyme successfully showed RG lyase activity. The recombinant enzyme demonstrated optimal activity at 40 °C and at pH 7.0, similar to the optimum temperature and pH of the native enzyme.
Mode of action of rPcRGLX
Soybean RG has a great number of neutral sugar side chains linked to the RG backbones. Therefore RG-AG, an RG that contains less side chains, was used as the substrate to facilitate the analysis of enzymatic reaction products with rPcRGLX. Using RG-AG as the substrate, the absorbance of the reaction mixture at 235 nm increased with time (Fig. 3a). HPAEC analysis of the reaction products revealed that rPcRGLX mainly released ΔGR from the initial stage of the reaction along with several trace products (Fig. 3b), indicating that the enzyme can be classified as an exo-acting RG lyase (EC 4.2.2.24).
Degradation of RG-AG with rPcRGLX. Enzyme activity was tested by incubating 4 mU of enzyme with 1 ml of 0.5 % substrate in 20 mM Tris-HCl buffer (pH 7.0) containing 1 mM CaCl2 at 37 °C for the indicated times periods. a Absorbance of the reaction mixture was measured at 235 nm. b Reaction products were analyzed using HPAEC under the conditions of program 1 in Table 1
We investigated whether rPcRGLX worked synergistically with the recombinant P. chrysogenum endo-RG lyase (rPcRGL4A) in the degradation of RG backbones. When soybean RG was used as the substrate, the addition of both enzymes to the reaction mixture dramatically increased the absorbance of the mixture at 235 nm compared to those of each enzyme individually (Fig. 4). These results demonstrated that rPcRGLX and rPcRGL4A synergistically degraded the substrate and that rPcRGLX preferred oligomeric RGs to polymeric RGs. Subsequently, reaction products obtained from soybean RG in combination with endo- and exo-RG lyases were analyzed using HPAEC. Surprisingly, several enzymatic products were detected in addition to ΔGR (Fig. 5a). The major products were then analyzed using MALDI-TOF MS. The peaks appearing in the spectrum were at m/z 345.3, 361.3, 507.4, 669.6, and 831.7 (Fig. 5b). Considering the structure of RG-I, these observed masses were indicated as ions: [ΔGalA-Rha + Na]+, [ΔGalA-Rha + K]+, [ΔGalA-Rha-Gal + Na]+, [ΔGalA-Rha-Gal2 + Na]+, [ΔGalA-Rha-Gal3 + Na]+. To confirm this speculation, the reaction products were further treated with recombinant β-galactosidase (rPcBGAL35A). The enzyme degraded Gal residues linked to ΔGR and released free ΔGR (Fig. 5a). These data indicate that rPcRGLX can bypass the Gal side chains of RG backbones and release ΔGR and ΔGR decorated with Gal residues. Moreover, it was demonstrated that rPcRGLX did not require Gal decoration of the RG backbones for degradation. However, when rPcRGLX was incubated with usRG Oligo or usRG Oligo-Gal, the enzyme exhibited higher activity against usRG Oligo-Gal (Fig. 6). This result indicates that the enzyme has a preference for RG lacking Gal side chains. The K m and V max values of rPcRGLX in reference to usRG Oligo and usRG Oligo-Gal were determined to be 1.71 mg/ml and 8.86 μmol/mg/min for the former and 0.96 mg/ml and 7.58 μmol/mg/min for the latter. These data suggest that the preference of the enzyme for usRG Oligo-Gal is due to the recognition phenomenon affecting the affinity.
Degradation of soybean RG in combination with rPcRGLX and rPcRGL4A. The reaction mixture consisted of 10 mU of the enzyme and 1.5 ml of 0.5 % soybean RG in 20 mM Tris-HCl buffer (pH 7.0) containing 1 mM CaCl2 and was incubated at 37 °C. The mixture (0.2 ml) was withdrawn at the times shown followed by measuring the absorbance at 235 nm. Closed circles, rPcRGLX (10 mU); closed squares, rPcRGL4A (10 mU); open circles, a mixture of rPcRGLX (10 mU) and rPcRGL4A (10 mU). Experiments were performed in duplicate
HPAEC (a) and MALDI-TOF MS (b) analyses of the enzymatic products of soybean RG incubated with rPcRGLX and rPcRGL4A. a The reaction mixture consisted of rPcRGLX (100 mU), rPcRGL4A (100 mU), and 2 ml of 0.5 % soybean RG in 25 mM ammonium acetate (pH 7.0) and was incubated at 37 °C overnight followed by analysis using HPAEC under the conditions of program 1 in Table 1. After the reaction with rPcRGLX and rPcRGL4A, a sample of the mixture (0.5 ml) was withdrawn, and the pH was adjusted to 5.0 with Na-acetate buffer. The mixture was added to rPcBGAL35A (25 mU) and incubated at 37 °C overnight followed by analysis using HPAEC. b Following the reaction with rPcRGLX and rPcRGL4A, the mixture was combined with the matrix solution and dried on the sample plate, followed by analysis using MALDI-TOF MS. The experimental conditions are described in the text
rPcRGLX degrading activity against usRG Oligo (closed circles) and usRG Oligo-Gal (open circles). rPcRGLX (1 mU) was incubated with 1.5 ml of 0.5 % usRG Oligo or usRG Oligo-Gal in 200 mM Tris-HCl buffer (pH 7.0) containing 1 mM CaCl2 at 37 °C for the indicated times. Absorbance of the reaction mixture was measured at 235 nm. Experiments were performed in duplicate
Substrate specificity of rPcRGLX was tested using usRG Oligo and usHG Oligo, which are oligosaccharides with ΔGalA at their non-reducing ends, obtained from soybean RG and sugar beet HG, respectively. When usRG Oligo was used as the substrate, the absorbance of the reaction mixture at 235 nm increased with time (data not shown). In contrast, the enzyme exhibited no activity when usHG Oligo was used, indicating that rPcRGLX specifically cleaves RG backbones.
To determine the effect of Ca2+ on the enzymatic activity of rPcRGLX, divalent ions were removed from the enzyme and the substrate used for enzyme assays via treatment with 1 mM EDTA. rPcRGLX exhibited enzymatic activity in the absence of metals, indicating that divalent cations are not essential for its activity (Table 3). However, Ca2+ did have a slight positive effect on its activity.
Discussion
Very recently, we reported the biochemical characteristics of a P. chrysogenum endo-RG lyase (PcRGL4A), which released RG tetrasaccharides with ΔGalA at the non-reducing ends as the major reaction products from soybean RG. In the present paper, we describe the isolation, characterization, gene cloning, and overexpression of the second RG lyase (PcRGLX) of this strain. To evaluate the mode of action towards RG-I, we used a recombinant enzyme to exclude the possibility of contamination with other pectolytic enzymes.
rPcRGLX degraded RG-I backbones via a β-elimination reaction in an exolytic manner to release ΔGR, leading to classification of the enzyme as an exo-RG lyase (EC 4.2.2.24). This is the first report of an RG lyase with this mode of action in Eukaryota. The available literature concerning RG lyases is quite limited. Characteristics of RG lyases reported so far are summarized in Table 4. To our knowledge, only one enzyme is known to release ΔGR from RG in an exolytic manner: YesX from B. subtilis (Ochiai et al. 2007). The deduced amino acid sequence of PcRGLX shows no homology to the sequence of the B. subtilis YesX. All of the RG lyases reported so far have been grouped into two PL families based on their amino acid sequences (PL4 and PL11; http://www.cazy.org/Polysaccharide-Lyases.html). Enzymes in PL4 have been found in bacteria and eukaryota, whereas enzymes in PL11 have been found predominantly in bacteria. However, the deduced amino acid sequence of PcRGLX has no similarity to the deduced amino acid sequences of enzymes belonging to these PL families. In contrast, the deduced amino acid sequence of the Pcrglx gene showed high similarities with the sequences of other uncharacterized proteins from N. haematococca (Coleman et al. 2009), A. oryzae, and F. oxysporum (Ma et al. 2010), which are not classified into any PL family. Furthermore, conserved domain search analysis did not find any significant matches to the deduced amino acid sequence of PcRGLX. This indicates that PcRGLX is distinct from known polysaccharide lyases in terms of protein structure and will belong to a new PL family together with these uncharacterized proteins.
Besides the amino acid sequence, several other properties of PcRGLX were completely different from those of the B. subtilis YesX (Ochiai et al. 2007). rPcRGLX was specifically active on RG-I but not on HG. In contrast, YesX shows slight activity towards polygalacturonan as well as RG-I. Moreover, rPcRGLX did not require divalent cations such as Ca2+ for its activity, whereas these are essential for the activity of RG lyases belonging to PL11, including YesX. The calculated molecular mass of PcRGLX (102,766 Da) was much larger than that of YesX (67,690 Da).
RG lyases from Pseudomonas cellulosa (McKie et al. 2001) and Clostridium cellulolyticum (Pagès et al. 2003) require Gal decoration of RG-I backbones for degradation. In contrast, rPcRGLX released ΔGR and ΔGR decorated with Gal residues from usRG Oligo, demonstrating that Gal decoration is not essential for this enzyme and that rPcRGLX bypasses Gal side chains linked to RG-I backbones. However, rPcRGLX was shown to have a preference for usRG Oligo lacking Gal side chains. Based on the kinetic parameters of the enzyme towards usRG Oligo with or without Gal residues, the preference seemed to be due to the recognition phenomenon affecting the affinity. P. chrysogenum rPcRGL4A also has similar enzymatic characteristics with regard to Gal side chains of RG-I; rPcRGL4A does not require Gal residues for RG-I degradation but prefers degalactosylated RG-I (Iwai et al. 2015). The ability of rPcRGLX to bypass Gal side chains liked to RG-I backbones in an exolytic manner might be useful in the determination of complex structures of RG-I. We are currently studying whether rPcRGLX has bypassing activity towards Ara side chains or acetyl groups linked to RG-I.
P. chrysogenum 31B was demonstrated to secrete at least two RG lyases (PcRGL4A and PcRGLX). These enzymes act in a synergistic manner towards soybean RG and release ΔGR and ΔGR decorated with Gal residues. These galactosylated RG oligosaccharides were shown to be hydrolyzed with β-galactosidase (PcBGAL35A) and convert to ΔGR. The enzymatic degradation pathway of RG-I by P. chrysogenum 31B supports the observation that ΔGR was accumulated in the reaction mixture when sugar beet ASP was incubated with the culture supernatant of this strain (Fig. 1a). We speculate that ΔGR is then incorporated into cells and degraded by an intracellular enzyme, unsaturated rhamnogalacturonyl hydrolase. Thus, P. chrysogenum seems to possess a degradation pathway for RG-I similar to that of B. subtilis (Ochiai et al. 2007).
ΔGR, called “lepidimoide”, has been isolated from the mucilage of germinated cress (Lepidium sativum L.) seeds and has been shown to promote growth of the hypocotyl of etiolated Amaranthus caudatus L. (Hasegawa et al. 1992). Based on its structure, lepidimoide was suggested to be derived from RG-I (Fry et al. 1993), and later it was found to be released as ΔGR from RG-I rich okra polysaccharide by the fungus Colletotrichum sp. AHU9748 (Saranpuetti et al. 2006). These findings suggest that PcRGLX and PcRGL4A may be beneficial for use in fields of plants by producing ΔGR.
References
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489
Bonnin E, Garnier C, Ralet MC (2014) Pectin-modifying enzymes and pectin-derived materials: applications and impacts. Appl Microbiol Biotechnol 98:519–532
Coleman JJ, Rounsley SD, Rodriguez-Carres M, Kuo A, Wasmann CC, Grimwood J, Schmutz J, Taga M, White GJ, Zhou S, Schwartz DC, Freitag M, Ma LJ, Danchin EGJ, Henrissat B, Coutinho PM, Nelson DR, Straney D, Napoli CA, Barker BM, Gribskov M, Rep M, Kroken S, Molnár I, Rensing C, Kennell JC, Zamora J, Farman ML, Selker EU, Salamov A, Shapiro H, Pangilinan J, Lindquist E, Lamers C, Grigoriev IV, Geiser DM, Covert SF, Temporini E, VanEtten HD (2009) The genome of Nectria haematococca: contribution of supernumerary chromosomes to gene expansion. PLoS Genet 5, e1000618
Fry SC, Aldington S, Hetherington PR, Aitken J (1993) Oligosaccharides as signals and substrates in the plant cell wall. Plant Physiol 103:1–5
Hasegawa K, Mizutani J, Kosemura S, Yamamura S (1992) Isolation and identification of lepidimoide, a new allelopathic substance from mucilage of germinated cress seeds. Plant Physiol 100:1059–1061
Itoh T, Ochiai A, Mikami B, Hashimoto W, Murata K (2006) A novel glycoside hydrolase family 105: the structure of family 105 unsaturated rhamnogalacturonyl hydrolase complexed with a disaccharide in comparison with family 88 enzyme complexed with the disaccharide. J Mol Biol 360:573–585
Iwai M, Yamada H, Ikemoto T, Matsumoto S, Fujiwara D, Takenaka S, Sakamoto T (2015) Biochemical characterization and overexpression of an endo-rhamnogalacturonan lyase from Penicillium chrysogenum. Mol Biotechnol. doi:10.1007/s12033-015-9847-4
Jayani RS, Saxena S, Gupta R (2005) Microbial pectinolytic enzymes: a review. Process Biochem 40:2931–2944
Kauppinen S, Christgau S, Kofod LV, Halkier T, Dörreich K, Dalbøge H (1995) Molecular cloning and characterization of a rhamnogalacturonan acetylesterase from Aspergillus aculeatus. Synergism between rhamnogalacturonan degrading enzymes. J Biol Chem 270:27172–27178
Kofod LV, Kauppinen S, Christgau S, Andersen LN, Heldt-Hansen HP, Dörreich K, Dalbøge H (1994) Cloning and characterization of two structurally and functionally divergent rhamnogalacturonases from Aspergillus aculeatus. J Biol Chem 269:29182–29189
Laatu M, Condemine G (2003) Rhamnogalacturonate lyase RhiE is secreted by the out system in Erwinia chrysanthemi. J Bacteriol 185:1642–1649
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lara-Márquez A, Zavala-Páramo MG, López-Romero E, Camacho HC (2011) Biotechnological potential of pectinolytic complexes of fungi. Biotechnol Lett 33:859–868
Ma LJ, van der Does HC, Borkovich KA, Coleman JJ, Daboussi MJ, Di Pietro A, Dufresne M, Freitag M, Grabherr M, Henrissat B, Houterman PM, Kang S, Shim WB, Woloshuk C, Xie X, Xu JR, Antoniw J, Baker SE, Bluhm BH, Breakspear A, Brown DW, Butchko RAE, Chapman S, Coulson R, Coutinho PM, Danchin EGJ, Diener A, Gale LR, Gardiner DM, Goff S, Hammond-Kosack KE, Hilburn K, Hua-Van A, Jonkers W, Kazan K, Kodira CD, Koehrsen M, Kumar L, Lee YH, Li L, Manners JM, Miranda-Saavedra D, Mukherjee M, Park G, Park J, Park SY, Proctor RH, Regev A, Ruiz-Roldan MC, Sain D, Sakthikumar S, Sykes S, Schwartz DC, Turgeon BG, Wapinski I, Yoder O, Young S, Zeng Q, Zhou S, Galagan J, Cuomo CA, Kistler HC, Rep M (2010) Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 464:367–373
Martínez-Martínez I, Navarro-Fernández J, Lozada-Ramírez JD, García-Carmona F, Sánchez-Ferrer A (2008) YesT: a new rhamnogalacturonan acetyl esterase from Bacillus subtilis. Proteins 71:379–388
McKie VA, Vincken JP, Voragen AGJ, van den Broek LAM, Stimson E, Gilbert HJ (2001) A new family of rhamnogalacturonan lyases contains an enzyme that binds to cellulose. Biochem J 355:167–177
Mutter M, Beldman G, Schols HA, Voragen AGJ (1994) Rhamnogalacturonan α-L-rhamnopyranohydrolase. A novel enzyme specific for the terminal nonreducing rhamnosyl unit in rhamnogalacturonan regions of pectin. Plant Physiol 106:241–250
Mutter M, Colquhoun IJ, Schols HA, Beldman G, Voragen AGJ (1996) Rhamnogalacturonase B from Aspergillus aculeatus is a rhamnogalacturonan α-L-rhamnopyranosyl-(1- > 4)-α-D-galactopyranosyluronide lyase. Plant Physiol 110:73–77
Mutter M, Beldman G, Pitson SM, Schols HA, Voragen AGJ (1998) Rhamnogalacturonan α-D-galactopyranosyluronohydrolase. An enzyme that specifically removes the terminal nonreducing galacturonosyl residue in rhamnogalacturonan regions of pectin. Plant Physiol 117:153–163
Ochiai A, Itoh T, Kawamata A, Hashimoto W, Murata K (2007) Plant cell wall degradation by saprophytic Bacillus subtilis strains: gene clusters responsible for rhamnogalacturonan depolymerization. Appl Environ Microbiol 73:3803–3813
Pagès S, Valette O, Abdou L, Bélaïch A, Bélaïch JP (2003) A rhamnogalacturonan lyase in the Clostridium cellulolyticum cellulosome. J Bacteriol 185:4727–4733
Sakai T, Sakamoto T, Hallaert J, Vandamme EJ (1993) Pectin, pectinase and protopectinase: production, properties, and applications. Adv Appl Microbiol 39:213–294
Sakamoto T, Nishimura Y, Makino Y, Sunagawa Y, Harada N (2013) Biochemical characterization of a GH53 endo-β-1,4-galactanase and a GH35 exo-β-1,4-galactanase from Penicillium chrysogenum. Appl Microbiol Biotechnol 97:2895–2906
Saranpuetti C, Tanaka M, Sone T, Asano K, Tomita F (2006) Determination of enzymes from Colletotrichum sp. AHU9748 essential for lepidimoide production from okra polysaccharide. J Biosci Bioeng 102:452–456
Schols HA, Geraeds CCJM, Searle-van Leeuwen MF, Kormelink FJM, Voragen AGJ (1990) Rhamnogalacturonase: a novel enzyme that degrades the hairy regions of pectins. Carbohydr Res 206:105–115
Searle-van Leeuwen MJF, van den Broek LAM, Schols HA, Beldman G, Voragen AGJ (1992) Rhamnogalacturonan acetylesterase: a novel enzyme from Aspergillus aculeatus, specific for the deacetylation of hairy (ramified) regions of pectins. Appl Microbiol Biotechnol 38:347–349
Shinozaki A, Kawakami T, Hosokawa S, Sakamoto T (2014) A novel GH43 α-L-arabinofuranosidase of Penicillium chrysogenum that preferentially degrades single-substituted arabinosyl side chains in arabinan. Enzyme Microb Technol 58–59:80–86
Silva IR, Larsen DM, Meyer AS, Mikkelsen JD (2011) Identification, expression, and characterization of a novel bacterial RGI lyase enzyme for the production of bio-functional fibers. Enzyme Microb Technol 49:160–166
Silva IR, Jers C, Otten H, Nyffenegger C, Larsen DM, Derkx PMF, Meyer AS, Mikkelsen JD, Larsen S (2014) Design of thermostable rhamnogalacturonan lyase mutants from Bacillus licheniformis by combination of targeted single point mutations. Appl Microbiol Biotechnol 98:4521–4531
Sukhumsiirchart W, Kawanishi S, Deesukon W, Chansiri K, Kawasaki H, Sakamoto T (2009) Purification, characterization, and overexpression of thermophilic pectate lyase of Bacillus sp. RN1 isolated from a hot spring in Thailand. Biosci Biotechnol Biochem 73:268–273
Thibault JF, Renard CMGC, Axelos MAV, Roger P, Crépeau MJ (1993) Studies of the length of homogalacturonic regions in pectins by acid-hydrolysis. Carbohydr Res 238:271–286
Van den Berg MA, Albang R, Albermann K, Badger JH, Daran JM, Driessen AJ, Garcia-Estrada C, Fedorova ND, Harris DM, Heijne WH, Joardar V, Kiel JA, Kovalchuk A, Martín JF, Nierman WC, Nijland JG, Pronk JT, Roubos JA, van der Klei IJ, van Peij NN, Veenhuis M, von Döhren H, Wagner C, Wortman J, Bovenberg RA (2008) Genome sequencing and analysis of the filamentous fungus Penicillium chrysogenum. Nat Biotechnol 26:1161–1168
Voragen AGJ, Coenen GJ, Verhoef RP, Schols H (2009) Pectin, a versatile polysaccharide present in plant cell walls. Struct Chem 20:263–275
Wong D (2008) Enzymatic deconstruction of backbone structures of the ramified regions in pectins. Protein J 27:30–42
Yoshino-Yasuda S, Karita S, Kato M, Kitamoto N (2012) Sequence analysis and heterologous expression of rhamnogalacturonan lyase A gene (AsrglA) from Shoyu Koji Mold, Aspergillus sojae KBN1340. Food Sci Technol Res 18:901–909
Acknowledgments
This work was supported by JSPS KAKENHI grant number 25450135.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iwai, M., Kawakami, T., Ikemoto, T. et al. Molecular characterization of a Penicillium chrysogenum exo-rhamnogalacturonan lyase that is structurally distinct from other polysaccharide lyase family proteins. Appl Microbiol Biotechnol 99, 8515–8525 (2015). https://doi.org/10.1007/s00253-015-6600-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6600-7