Abstract
On the basis of achieving shortcut nitrification in a lab-scale SBR, the effects of constant pH and unsteady pH at different free ammonia concentrations on shortcut nitrification for landfill leachate treatment was investigated. The results indicate that under the condition of DO of 0.5 ± 0.2 mg/L and temperature of 30 ± 2 °C, the absolute value of nitrite accumulation increased significantly with the increase in free ammonia (FA) concentration from 5.30 to 48.67 mg/L; however, the nitrite accumulation rate remained almost constant at a constant pH of 8.0 ± 0.1. Ammonia oxidation and the nitrite accumulation become slow with the pH decreased from 8.0 ± 0.1 to 7.5 ± 0.2, and the activities of ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB) were severely inhibited when the pH further decreased to 6.5. More importantly, this study confirmed that the pH decrease from 8.0 to 6.5 within a short time exhibited significant negative effect on the ammonia oxidation rather than the FA concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Landfill leachate treatment has received significant attention in recent years as it contains high concentration of organics, ammonia, and other toxic pollutants. The conventional biological process for leachate treatment is nitrification and denitrification, which is widely studied (Renou et al. 2008; Sri Shalini and Joseph 2012). However, some problems have been encountered in this traditional nitrogen removal process, such as high oxygen and energy consumption, high land requirement, and high sludge production (Guo et al. 2009; Khin and Annachhatre 2004; Yang et al. 2007). The shortcut biological nitrogen removal (SBNR) utilizes the concept of direct nitrite reduction to nitrogen gas (Turk and Mavinic 1989; Hellinga et al. 1998). The SBNR process can reduce 25 % of oxygen supply needed for nitrification and approximately 40 % of electron donors needed for denitrification, compared to the conventional biological nitrogen removal (BNR) processes (Hellinga et al. 1998; Yoo et al. 1999; Turk and Mavinic 1986). These characteristics of SBNR make it appropriate for ammonia-rich wastewaters treatment such as landfill leachate. The SBNR process is strongly influenced by environmental factors, such as pH, free ammonia (FA), dissolved oxygen (DO), temperature, mixed liquor suspended solids (MLSS), and so on (Morgenroth et al. 2000; Vadivelu et al. 2007; Wu et al. 2007). It is generally accepted that the optimal pH range for ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB) are 7.0 ~ 8.5 and 6.0 ~ 7.5 (Groeneweg et al. 1994), respectively. And both AOB and NOB are inhibited by FA, but the NOB is more sensitive to FA than AOB, giving the inhibition range of 0.1 ~ 1.0 and 10 ~ 150 mg/L (Anthonisen et al. 1976), respectively. According to Anthonisen et al., the pH value is the key factor that determines the FA concentration in equilibrium with NH4 +-N concentration. Therefore, pH value and FA concentration are the important parameters to achieve stable shortcut nitrification.
The purpose of this study was to investigate the comparing effects of constant pH value and unsteady pH value at different FA concentrations on ammonia oxidation and nitrite accumulation by using batch experiments.
Materials and methods
Cultivation and domestication
The seeding sludge was obtained from an activated sludge tank of Lijiao municipal wastewater treatment plant in Guangzhou. The concentration of synthetic landfill leachate (after dilution) fall within the range of real landfill leachate in China (Peng et al. 2006; Yang and Zhou 2005; Li et al. 2009), with COD of 400 ~ 800 mg/L, NH4 +-N of 50 ~ 450 mg/L, and other inorganic medium (in mg/L): PO4 3+ 8.5 ~ 77.6; Ca2+ 1.0 ~ 5.0; Fe2+ 0.8 ~ 2.0; K+ 3.2 ~ 3.6; Mg2+ 5.0 ~ 9.6; Zn2+ 0.4 ~ 1.5; Cr3+ 0.12 ~ 0.2; and Cu2+ 0.1 ~ 0.2. The experiment was carried out in the lab-scale sequencing batch reactor (SBR) shown in Fig. 1. The working volume was 10 L, with an internal diameter of 18 cm and a liquid height of 40 cm. The SBR was operated with a 12-h cycle, consisting of five phases: transient filling; 8 h aeration, 0.5 h settling, 0.5 h drawing, and 3 h idle. The air was provided by an air pump through an aeration diffuser placed at the bottom of the reactor with the DO concentration of 0.5 ± 0.2 mg/L, and the temperature was kept at 30 ± 2 °C by using a cartridge heater. The pH was regulated constantly at 8.0 ± 0.1 by titrating NaHCO3 solution. Sample and analyze every cycle.
Analytical methods
NH4 +-N, NO2 −-N, NO3 −-N, and MLSS were measured according to Standard Methods (N. E. P. A. 2002). NH4 +-N concentration was determined colorimetrically. Nitrate nitrogen (NO3 −-N) and nitrite nitrogen (NO2 −-N) concentrations were measured with an ion chromatograph (ICS-1000, Dironex, USA). Samples of NH4 +-N, NO2 −-N, and NO3 −-N were obtained via a 0.45-μm filter paper. DO and pH were measured online by WTW pH/OXi 340i meters with a dissolved oxygen electrode and a pH electrode. Free ammonia (FA) concentration was estimated using the following equation (Anthonisen et al. 1976):
where FA is the free ammonia concentration (mg NH3/L), [NH4 +-N] is the ammonia concentration (mg/L), and T is the temperature (°C).
Batch experiments
The activated sludge was washed and settled in fresh water before use. All batch experiments were conducted in a 2-L reactor containing synthetic landfill leachate, which had the same substrate concentration as that of the cultivation experiment except NH4 +-N concentrations. For all the batch tests, the concentration of MLSS was designed as 3,800 ± 500 mg/L, and the temperature and DO concentration were kept at 30 ± 2 °C and 0.5 ± 0.2 mg/L, respectively, as same as that of the cultivation experiment. The effects of constant pH and unsteady pH on shortcut nitrification were studied with the initial FA concentrations of 5.30, 12.19, 33.19, and 48.67 mg/L. Each initial FA concentration gradient test was triplicate and divided into two groups: the pH at group I was regulated constantly at 8.0 ± 0.1 by titrating NaHCO3 solution during aeration, while no steps were taken to regulate the pH at group II. Samples were analyzed every 30 mins. The NH4 +-N concentrations designed for the batch experiments at different initial FA concentrations are listed in Table 1.
Results
Start-up of the shortcut nitrification
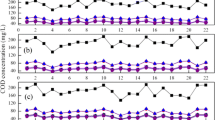
Figure 2 presents NH4 +-N removal performances during start-up period. The influent NH4 +-N concentrations increased stepwise from 50.1 to 405.7 mg/L during the start-up period, and the effluent NH4 +-N concentrations were below 16.42 mg/L during the first 20 cycles, then increased gradually with the NH4 +-N concentration increased to 405.7 mg/L, giving the maximum value of 45.51 mg/L. Excellent treatment efficiencies were obtained during the whole period, as we can see in Fig. 2. In general, an increasing trend was observed in NH4 +-N removal rate from 73.69 % at the beginning of the period to 90.94 % at the end of the period with the increasing influent NH4 +-N concentration.
The NO2 −-N and NO3 −-N accumulation performances during start-up period are shown in Fig. 3. It was clear that plenty of effluent NO2 −-N concentration was detected during the start-up period, and the NO2 −-N concentration increased rapidly from 7.61 to 116.99 mg/L, with the increasing influent NH4 +-N concentration from 50.1 to 405.7 mg/L. While at the same time, the effluent NO3 −-N concentration decreased stepwise from 16.30 to 2.89 mg/L. In the case of NO2 −-N accumulation rate, it increased significantly from 31.83 to 86.44 % during the first 20 cycles, and then maintained between 90.27 and 98.01 % during the last 14 cycles. It indicated that AOB showed high activities and that of NOB has been inhibited severely.
Nitrification at different FA concentration under constant pH and unsteady pH
Figure 4 shows the results of NH4 +-N removal performances of constant pH and unsteady pH at different FA concentration. The initial FA concentrations were set as 5.30, 12.19, 33.19, and 48.67 mg/L, respectively. As shown in Fig. 5, the NH4 +-N concentration decreased in a linear manner with relative high regression coefficients (R 2 > 0.93) in all FA concentration gradient test in group I. While in group II, the NH4 +-N concentration decreased become slow when it appear 3 points on the NH4 +-N curves, point A1, A2, and A3 in subpanels b, c, and d of Fig. 4, which corresponded to FA concentration of 12.19, 33.19, and 48.67 mg/L, respectively. An exception was at FA concentration of 5.30 mg/L, where the NH4 +-N curves almost has the same decreasing trend.
The pH value in group I was kept constantly at 8.0 ± 0.1 by titrating NaHCO3 solution during aeration, while no steps were taken to regulate the pH value in group II. The pH value decreased continuously during the aerobic phase in all case. In Fig. 4a, the pH value decreased slowly to a minimum value of 7.21. However, in subpanels b, c, and d of Fig. 4, the pH value decreased very fast from 8.0 to less than 6.5. It should be noted that points A1, A2, and A3 on the NH4 +-N curves correspond to pH values of 7.31, 7.25, and 7.37, respectively. Meanwhile, the FA concentrations decreased rapidly to 0 with the fast decreasing pH in all case in group II, as well as the cases of initial FA concentration of 5.30 and 12.19 mg/L in group I. However, the FA concentration maintained relative high level all along at initial FA concentration of 33.19 and 48.67 mg/L in group I.
Nitrite accumulation at different FA concentration under constant pH and unsteady pH
The NO2 −-N accumulations at different FA concentration under constant pH and unsteady pH are shown in Fig. 6. Considerable NO2 −-N accumulation was observed regardless of FA concentration in group I, and the absolute value of NO2 −-N accumulation increased with the increase in FA concentration from 5.30 to 48.67 mg/L, giving the maximum value of 48.08, 86.83, 85.21, and 121.54 mg/L, respectively.
Similar with Fig. 4, the NO2 −-N accumulated began to become slow when it appears 3 points on the NO2 −-N curves in group II, points B1, B2, and B3 in subpanels b, c, and d of Fig. 6, corresponding to FA concentration of 12.19, 33.19, and 48.67 mg/L, respectively. As for the initial FA concentration of 5.30 mg/L, the NO2 −-N curves had a similar increasing trend in group I and II.
Figure 7 presents the effluent NO2 −-N and NO3 −-N at different FA concentrations in group I and group II. The absolute value of nitrite accumulation increased significantly with the increase in FA concentration from 5.30 to 48.67 mg/L in group I, while the value varies irregularly in group II. Although the absolute values of effluent NO2 −-N and NO3 −-N in group II were lower than that in group I, the NO2 −-N accumulation rate has little difference between group I and group II, in addition, it varies insignificantly with the increase in FA concentration, averaging 94.03, 94.78, 94.31, and 95,51 % in group I and 95.37, 94.29, 91.76, and 91.54 % in group II, respectively, at FA concentration of 5.30, 12.19, 33.19, and 48.67 mg/L.
Discussion
The primary environmental parameters to obtain a shortcut biological nitrogen removal process are the pH value, free ammonia, temperature, and DO concentration. Many studies have been conducted to investigate the start-up of shortcut nitrification, under a variety of environmental and operational conditions, such as low DO level (Blackburne et al. 2008), high temperature (Hellinga et al. 1998), and free ammonia (Park et al. 2009). In this study, during start-up period, the temperature and the DO concentration in the reactor were kept at 30 ± 2 °C and 0.5 ± 0.2 mg/L, respectively. The pH value was regulated constantly at 8.0 ± 0.1. As a result, excellent treatment efficiencies, both NH4 +-N removal rate and NO2 −-N accumulation rate, were obtained during the whole period. The NO2 −-N accumulation rate increased significantly from 31.83 to 86.44 % within 20 cycles, and then stably maintained between 90.27 and 98.01 % at the end of the start-up period. It is generally accepted that the optimal pH range for AOB and NOB are 7.0 ~ 8.5 and 6.0 ~ 7.5 (Groeneweg et al. 1994), respectively. In addition, NOB have been shown to have lower affinity for oxygen than AOB, and the half-saturation constant (K o) for dissolved oxygen is 0.16 and 0.54 mg O2/L for AOB and NOB, respectively (Laanbroek et al. 1994; Hunik et al. 1994). Therefore, the SBR system can achieve stable shortcut nitrification soon in the operation conditions of 30 ± 2 °C, pH 8.0 ± 0.1, and DO of 0.5 ± 0.2 mg/L, and the result confirm that high temperature, high pH, and low DO strategy was feasible for the start-up of shortcut nitrification.
FA concentration has a large influence on ammonia oxidation as it is the actual substrate for AOB instead of NH4 +-N, and the equilibrium between FA and NH4 +-N is strongly depends on the pH value (Suzuki et al. 1974; Anthonisen et al. 1976). Oxidation of ammonia to nitrite is an acidifying process, in this study, a great amount of ammonia was oxidized to nitrite, resulting in the greatly decrease in pH and the subsequently decrease in FA concentration. NH4 +-N decrease began to become slow with the pH value decreased to 7.30 ± 0.1, as the further decrease in pH to 6.5 the NH4 +-N concentration almost no longer decreased, indicating the activities of AOB have been inhibited severely. This was in good agreement with the observations made by Hellinga et al. (1999) and van Dongen et al. (2001), who stated that at pH lower than 6.5, nitrification does not take place. However, when the pH value was regulated constantly at 8.0 ± 0.1, the FA concentration maintained relative high level all the time, even so NH4 +-N concentration decreased in a linear manner with relative high regression coefficients (R 2 > 0.93), meaning that the relationship of NH4 +-N consumption and time was linearly fitted and ammonia oxidation rate remained almost constant, these indicated that the oxidation of ammonia follows the zero-order reaction kinetics. Therefore, we can conclude that the FA concentration in the range of 5.30 ~ 48.67 mg/L had a limited effect on NH4 +-N oxidation, that the great decrease in pH within a short time had a significant effect on NH4 +-N oxidation. Similar with this study, Kim et al. (2008) studied the comparison study of the effects of temperature and free ammonia concentration on nitrification and nitrite accumulation, demonstrated that at a given temperature, the specific ammonium oxidation rate remained almost constant in spite of the variation in FA concentration. Despite a wide divergence of the reported effects of pH on nitrification, there seems to be a consensus that the optimal pH for ammonium oxidizers lies between 7 and 8 (Van Hulle et al. 2010). The experiments presented herein confirm that the greatly decrease in pH within a short time had a significant effect on NH4 +-N oxidation, thus, to maintain a stable pH range has great significance for the biological nitrogen removal process, especially in the case of ammonia-rich wastewater such as municipal landfill leachate.
Comparing Fig. 4 with Fig. 6, it is clear that there was a high correlation between NH4 +-N oxidation curve and NO2 −-N accumulation curve, that is to say, the NH4 +-N almost oxidized to NO2 −-N completely. We define nitrite accumulation rate as the NO2 −-N to NO x −-N ratio in the effluent. Our result demonstrated that the absolute value of NO2 −-N accumulation increased with the increase in FA concentration from 5.30 to 48.67 mg/L at a constant pH of 8.0 ± 0.1, and even though the pH decreased rapidly to 6.5, the NO2 −-N accumulation rate varies insignificantly. It indicated that both different FA concentrations and greatly decrease in pH have a negligible effect on the NO2 −-N accumulation rate. This result is consistent with that reported by Kim et al. (2008), who showed that there was no significant difference in the nitrite accumulation rate among different FA concentrations at a certain temperature. Moreover, Bae et al. (2001) reported that when the FA concentration was relatively low, the accumulated nitrite was rapidly converted to nitrate. But according to our result, even though the FA concentration decreased into 0, the NO2 −-N accumulation rate remained a high value; this was due to the inhibition of activities of both AOB and NOB by a low pH value of 6.5.
In summary, shortcut nitrification can be achieved rapidly under the operation conditions of 30 ± 2 °C, pH 8.0 ± 0.1, and DO of 0.5 ± 0.2 mg/L in 50 cycles. When pH value was below 6.5, activities of both AOB and NOB were inhibited severely. The absolute value of nitrite accumulation increased significantly with the increase in FA concentration from 5.30 to 48.67 mg/L at a constant pH of 8.0 ± 0.1. The greatly decrease in pH within a short time had a significant effect on NH4 +-N oxidation, while had a limited effect on the NO2 −-N accumulation rate. In addition, FA concentration was confirmed as a relative unimportant role on the NH4 +-N oxidation and the NO2 −-N accumulation rate almost remained constant with the increase in FA concentration from 5.30 to 48.67 mg/L. Therefore, pH value is an important parameter on SBNR processes, to maintain a stable pH range has great significance for landfill leachate treatment by SBNR.
Reference
Anthonisen AC, Loehr RC, Prakasam TBS, Srinath EG (1976) Inhibition of nitrification by ammonia and nitrous acid. Water Pollut Control Fed 48:835–852
Bae W, Baek S, Chung J, Lee Y (2001) Optimal operational factors for nitrite accumulation in batch reactors. Biodegradation 12(5):359–366
Blackburne R, Yuan Z, Keller J (2008) Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor. Biodegradation 19(2):303–312
Chinese N. E. P. A. (2002) Water and wastewater monitoring methods. Chinese Environmental Science Publishing House, Beijing, 354. (in Chinese)
Groeneweg J, Sellner B, Tappe W (1994) Ammonia oxidation in Nitrosomonas at NH3 concentrations near K m: effects of pH and temperature. Water Res 28(12):2561–2566
Guo J, Peng Y, Wang S, Zheng Y, Huang H, Wang Z (2009) Long-term effect of dissolved oxygen on partial nitrification performance and microbial community structure. Bioresour Technol 100(11):2796–2802
Hellinga C, Schellen AAJC, Mulder JW, Van Loosdrecht MCM, Heijnen JJ (1998) The SHARON process: an innovative method for nitrogen removal from ammonium-rich waste water. Water Sci Technol 37(9):135–142
Hellinga C, Van Loosdrecht MCM, Heijnen JJ (1999) Model based design of a novel process for nitrogen removal from concentrated flows. Math Comput Model Dyn 5(4):351–371
Hunik JH, Tramper J, Wijffels RH (1994) A strategy to scale up nitrification processes with immobilized cells of Nitrosomonas europaea and Nitrobacter agilis. Bioprocess Eng 11(2):73–82
Khin T, Annachhatre AP (2004) Novel microbial nitrogen removal processes. Biotechnol Adv 22(7):519–532
Kim JH, Guo X, Park HS (2008) Comparison study of the effects of temperature and free ammonia concentration on nitrification and nitrite accumulation. Process Biochem 43(2):154–160
Laanbroek HJ, Bodelier PL, Gerards S (1994) Oxygen consumption kinetics of Nitrosomonas europaea and Nitrobacter hamburgensis grown in mixed continuous cultures at different oxygen concentrations. Arch Microbiol 161(2):156–162
Li HS, Zhou SQ, Sun YB, Feng P, Li JD (2009) Advanced treatment of landfill leachate by a new combination process in a full-scale plant. J Hazard Mater 172(1):408–415
Morgenroth E, Obermayer A, Arnold E, Brl A, Wagner M, Wilderer P (2000) Effect of long-term idle periods on the performance of sequencing batch reactors. Water Sci Technol 41(1):105–113
Park S, Bae W, Rittmann BE (2009) Operational boundaries for nitrite accumulation in nitrification based on minimum/maximum substrate concentrations that include effects of oxygen limitation, pH, and free ammonia and free nitrous acid inhibition. Environ Sci Technol 44(1):335–342
Peng Y, Zhang S, Zheng S, Wang S (2006) Heavy metal ions in the biological treatment of municipal landfill leachate. Chin J Env Eng 7(1):1–5 (in Chinese)
Renou S, Givaudan JG, Poulain S, Dirassouyan F, Moulin P (2008) Landfill leachate treatment: review and opportunity. J Hazard Mater 150(3):468–493
Sri Shalini S, Joseph K (2012) Nitrogen management in landfill leachate: application of SHARON, ANAMMOX and combined SHARON–ANAMMOX Process. Waste Manag 32(12):2385–2400
Suzuki I, Dular U, Kwok SC (1974) Ammonia or ammonium ion as substrate for oxidation by Nitrosomonas europaea cells and extracts. J Bacteriol 120(1):556–558
Turk O, Mavinic DS (1986) Preliminary assessment of a shortcut in nitrogen removal from wastewater. Can J Civ Eng 13(6):600–605
Turk O, Mavinic DS (1989) Maintaining nitrite build-up in a system acclimated to free ammonia. Water Res 23(11):1383–1388
Vadivelu V, Keller J, Yuan Z (2007) Free ammonia and free nitrous acid inhibition on the anabolic and catabolic processes of Nitrosomonas and Nitrobacter. Water Sci Technol 56(7):89–97
van Dongen UGJM, Jetten MS, Van Loosdrecht MCM (2001) The SHARON®-Anammox® process for treatment of ammonium rich wastewater. Water Sci Technol 44(1):153–160
Van Hulle SW, Vandeweyer HJ, Meesschaert BD, Vanrolleghem PA, Dejans P, Dumoulin A (2010) Engineering aspects and practical application of autotrophic nitrogen removal from nitrogen rich streams. Chem Eng J 162(1):1–20
Wu C, Chen Z, Liu X, Peng Y (2007) Nitrification–denitrification via nitrite in SBR using real-time control strategy when treating domestic wastewater. Biochem Eng J 36(2):87–92
Yang Z, Zhou S (2005) Investigation and analysis of hazardous constituents in landfill leachate from Datianshan landfill in Guangzhou. J Chem Ind Eng 56(11):2183–2188 (in Chinese)
Yang Q, Wang S, Yang A, Guo J, Bo F (2007) Advanced nitrogen removal using pilot-scale SBR with intelligent control system built on three layer network. Front Environ Sci Eng 1(1):33–38
Yoo H, Ahn KH, Lee HJ, Lee KH, Kwak YJ, Song KG (1999) Nitrogen removal from synthetic wastewater by simultaneous nitrification and denitrification (SND) via nitrite in an intermittently-aerated reactor. Water Res 33(1):145–154
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC) (Grant No. 51178125, 51478127) and the S&T Planed Project of Guangdong Province (Grant No. 2012B030800005)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, C., Zhang, S., Zhang, L. et al. Effects of constant pH and unsteady pH at different free ammonia concentrations on shortcut nitrification for landfill leachate treatment. Appl Microbiol Biotechnol 99, 3707–3713 (2015). https://doi.org/10.1007/s00253-014-6340-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6340-0