Abstract
Glucosylglycerol (GG) has a range of potential applications in health, pharmacy, and cosmetics due to its physiological, protein-stabilizing, and antioxidative properties. In addition to chemical synthesis and enzymatic catalysis, GG can be produced as a protective osmolyte in salt-stressed bacteria, such as the cyanobacterium Synechocystis sp. PCC 6803. Here, we presented an efficient GG production and secretion by genetically modified and encapsulated Synechocystis cells grown in a semicontinuous manner. We improved the production and secretion of GG in Synechocystis by first disrupting both the ggtC and ggtD genes, which encode the subunits of a GG uptake transporter, as well as the ggpR gene, which encodes a repressor for GG synthesis. Then, we confirmed that the rapid GG release from salt-stressed cells of Synechocystis depended on the ion gradient across the cell membrane. Finally, we proved the feasibility of an agar gel encapsulation method in supporting cell growth and the GG production of Synechocystis under semicontinuous culturing conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to their photosynthetic ability and clear genetic background, cyanobacteria have been genetically modified to produce varieties of biofuel molecules (Lu 2010; Machado and Atsumi 2012; Quintana et al. 2011). However, most of cyanobacteria-derived biofuels are still under development and are not economically competitive with fossil fuels in the near future (Quintana et al. 2011). Under these circumstances, it is more viable to produce other higher-value bioactive compounds, such as glycosylglycerol (Ducat et al. 2011).
As its stabilizing effect on protein structure and the ability to activate cell-protective enzymes, such as superoxide dismutase, GG can be used as a protein drug stabilizer (Sawangwan et al. 2010) and cosmetic additive (Klein and Stumm 2010). GG has also been found to be a bioactive compound in some Japanese foods, including sake (Takenaka et al. 2000). A few Japanese companies have patented applications of GG in human health care (Aizawa Kyo et al. 2013; Krutmann et al. 2009; Okumura Hidenobu 2012). GG can be de novo synthesized and accumulated as an osmo-protectant by heterotrophic bacteria, such as Pseudomonas (Pocard et al. 1994) and Stenotrophomonas (Roder et al. 2005) strains, or cyanobacteria with moderate salt tolerance, such as Synechocystis sp. PCC 6803 (hereafter, Synechocystis; Fig. 1) (Klähn and Hagemann 2011) and Synechococcus sp. PCC 7002 (Xu et al. 2013). However, GG titers in these salt-stressed bacteria, such as 28.7 mg/l for Stenotrophomonas (Roder et al. 2005) and ∼100 mg/l for wild type Synechocystis in our lab, are far from the requirements necessary for industrial applications. Generally, GG is produced by chemical synthesis (Takenaka et al. 2000) or in vitro enzyme catalytic synthesis (Goedl et al. 2008). However, the hurdle of low efficiency and high cost makes the scalable production of GG difficult. To explore the feasibility of the cyanobacteria production of GG, this present study applied targeted genetic engineering and a novel cultivation strategy.
Diagram of GG biosynthetic pathways of Synechocystis, genetic engineering strategy, and response mechanism of Synechocystis to salt shock (modified from (Hagemann 2011)). When salt-shocked (indicated by the white filled circles), GgpS and GgpP (glucosylglycerol-phosphate phosphatase) were induced and activated by NaCl. By catalyzing with the GgpS-GgpP protein complex, the substrates ADP-glucose (ADP-G) and glycerol-3-phosphate (G-3-P) were sequentially converted to GG (indicated by red lines). Extracellular GG, which might be actively or passively released from the cells, will be actively captured by the GG transporter Ggt encoded by ggtA, ggtB, ggtC, and ggtD (indicated by red lines). In this study, the ggpR, ggtC, and ggtD genes were deleted to increase the ggpS transcripts and the amounts of extracellular GG (indicated by the red crosses). 3PG, 3-phosphoglycerate; 6PG, 6-P-gluconate; ADP-G, ADP-glucose; DHAP, dihydroxyacetone phosphate; F6P, fructose 6-phosphate; G1P, glucose 1-phosphate; G-3-P, glycerol-3-phosphate; G6P, glucose 6-phosphate; GA3P, glyceraldehyde 3-phosphate; PSI, photosystem I; PSII, photosystem II; Ru5P, ribulose 5-phosphate; RuBP, ribulose 1, 5-bis-phosphate; Suc, sucrose; Suc-6-P, sucrose-6-phosphate; UDP-G, UDP-glucose. GgpR, a repressor of ggpS transcription; GlgA, glycogen synthase; GlgC, ADP glucose pyrophosphorylase; GlpD/GpsA, glycerol-3-phosphate dehydrogenase; Gnd, 6-phosphogluconate dehydrogenase; Pgi, glucose-6-phosphate isomerase; Pgm, phosphoglucose mutase; Rbc, ribulose-1,5-bis-phosphate carboxylase/oxygenase; Spp, sucrose-phosphate phosphatase; Sps, sucrose phosphate synthase; Ugp, UDP glucose pyrophosphorylase; Zwf, glucose 6-phosphate dehydrogenase
Material and methods
Chemicals and reagents
Standard glucosylglycerol was purchased from Bitop AG (Germany). Oligonucleotide primers were synthesized by Shanghai Sunny Biotechnology (China). Taq DNA polymerases, Pfu DNA polymerase, T4 DNA ligase, and all restriction endonucleases were from Fermentas (Burlington, Canada) or Takara (Japan). The kits used for molecular cloning were from Omega (Norcross, USA).
Construction of plasmids
Escherichia coli DH5α was used for plasmids construction and were grown in Lysogeny broth (LB) medium. Antibiotics (50 μg/ml ampicillin, kanamycin, or spectinomycin) were supplemented into the LB medium when needed. Recombinant plasmids and strains used in this study are listed in Table S1. All oligonucleotide primers used in this study are listed in Table S2. The ggtCD targeting plasmid pWD12 was constructed as described in our previous work (Du et al. 2013). The upstream and downstream DNA fragments of the ggpR gene were amplified by PCR with primers ggpR-Fwd and ggpR-Rev. The DNA fragments were cloned into the pMD18-T simple vector (Takara, Japan), resulting in the plasmid pWD37. The kanamycin resistance gene cassette was cut from the plasmid pRL446 (Elhai and Wolk 1988) with BamHI, blunted by T4 DNA polymerase (Fermentase), and ligated with the HpaI-digested pWD37, resulting in the ggpR targeting plasmid pWD41.
Construction of mutant strains of Synechocystis
The glucose-tolerance strain of Synechocystis PCC 6803, grown in BG11 medium (Rippka et al. 1979), was used as the starting strain for genetic engineering. Antibiotics (25 μg/ml kanamycin or 20 μg/ml spectinomycin) were supplemented into the BG11 medium as needed. First, the pWD12 plasmid was used to transform Synechocystis according to a previous report (Tan et al. 2011), resulting in the spectinomycin-resistant ggtCD mutant strain ΔggtCD. The ΔggtCD strain was then transformed by the plasmid pWD41, resulting in the ΔggtCDΔggpR double mutant with spectinomycin and kanamycin resistance. The genotypes of these two mutant strains were characterized by PCR to ensure they were completely segregated.
Liquid culturing and salt shock of Synechocystis strains
Synechocystis strains were first inoculated into 30 ml BG11 media in a 100-ml flask and cultured at 30 °C under constant light (30 μE/m2/s) for 1 week on a shaker (140 rotation per minute; rpm). Then, the culture was reinoculated into 1,000 ml BG11 media in a 2,000-ml flask at a ratio of 1:100 and grown under an air-aeration condition (30 °C, 30 μE/m2/s constant light, aeration with air) until the culture reached the stationary phase. Then, 200 ml of the resultant culture was transferred into a column photobioreactor (580 mm × 30 mm glass column with a rubber plug) and grown under a CO2-enriched condition (30 °C, 100 μE/m2/s constant light, aeration with 5 % CO2) until the culture reached the stationary phase. For salt shocks, an appropriate volume of the saturated NaCl solution prepared with the BG11 medium was added into the column photobioreactor (600 mM NaCl for all strains of Synechocystis). The salt-shocked culture was continuously grown under the same CO2-enriched condition for 6 days and sampled every 2 days to monitor the cell growth and GG production.
Extraction and determination of the intra- and extracellular GG
Based on the methods of our previous study (with minor modifications) (Du et al. 2013), 1 ml of liquid salt-shocked culture was centrifuged at 12,000 rpm for 5 min. The resultant supernatant was filtered by 0.22-μm filters for the extracellular GG determination. For the extraction of intracellular GG, harvested cells were resuspended with the same volume of 80 % ethanol, incubated at 65 °C for 4 h, and centrifuged at 12,000 rpm for 5 min. The resulting supernatant was evaporated under nitrogen at 55 °C, dissolved in 1 ml of deionized water, and filtered by a 0.22-μm filter for the further intracellular GG determination. The GG samples were analyzed by an ICS-3000 ion-exchange chromatograph system (DIONEX) equipped with an electrochemical cell (DIONEX) and a CarboPac® MA1 analytical column. The column was equilibrated and eluted with 800 mM NaOH at a flow rate of 0.4 ml/min. The varying concentrations of GG standards were also analyzed in the same manner for obtaining a standard curve of GG to quantitatively determine GG contents in the samples.
Hypoosmotic shock of salt-stressed Synechocystis to release intracellular GG
For liquid cultures, salt-shocked cells of Synechocystis were harvested by centrifugation at 6,000 rpm for 10 min, resuspended by the same volume of distilled water or fresh BG11 medium with or without 600 mM NaCl, and incubated at 30 °C under constant light (30 μE/m2/s) in a shaker (140 rpm) for 0.25 or 2 h. Then, the suspensions were centrifuged again at 6,000 rpm for 10 min, and the GG concentration in the supernatant was determined by the above method. GG release efficiencies were calculated by dividing intracellular GG concentrations of salt-stressed cells by released GG concentrations.
Semicontinuous liquid culture of Synechocystis strain
According to the above procedure, ΔggtCDΔggpR cells were grown under CO2-enriched conditions in column photobioreactors until reaching a stationary growth phase. Then, cultures were salt-stressed by 600 mM NaCl. After the 8-day salt shock, the extracellular GG (“secreted GG”) was recovered by centrifugation at 6,000 rpm for 10 min, and the cells were hypoosmotically shocked for 2 h with fresh BG11 medium without NaCl, as previously described. After the 2-h hypoosmotic shock, the suspensions were centrifuged at 6,000 rpm for 10 min, and the GG in the supernatant (“released GG”) was recovered. To start a new growth cycle, the cells were salt-shocked again by suspending them with the same volume of the BG11 medium containing 600 mM NaCl. In this manner, cultures were semicontinuously grown cycle-by-cycle until the onset of cell death. The cultures were sampled every 2 days for monitoring the cell growth. Aliquots (1 ml) from the 8 days salt-shocked culture (for a determination of the secreted GG) and the 2-h hypoosmotically shocked culture (for a determination of the “released GG”) at the end of each growth cycle were sampled. The extracellular GG were collected and analyzed as described above.
Agar gel encapsulation of Synechocystis cells
The gel encapsulation of ΔggtCDΔggpR cells was performed as previously described (Erdmann et al. 1992) with minor modifications. Fifty milliliters of ΔggtCDΔggpR stationary culture (OD730 = 4–5) was mixed well with 125 ml agar solution (3 % w/v, 50 °C) prepared with BG11 medium, and sucked into silicone tubings (3-mm inner diameter) by a vacuum pump. After gelling, gels harboring the Synechocystis cells were extruded from the tubings and cut into cylinders with lengths no longer than 10 mm.
Semicontinuous culturing of agar gel-encapsulated cells and free cells of Synechocystis
The 50 ml ΔggtCDΔggpR stationary culture (OD730 = 4–5) was encapsulated by agar gel and transferred into the column photobioreactor. As control, a 50-ml volume of the same ΔggtCDΔggpR stationary culture (OD730 = 4–5) was directly transferred into the column photobioreactor. BG11 medium was added into the bioreactors containing free or gel-encapsulated cells of Synechocystis for a total volume of 250 ml. Then, cultures were grown under the CO2-enriched condition for 2 days and salt-stressed by 600 mM NaCl for another 8 days. The liquid culture was hypoosmotically shocked and semicontinuously grown by the method described above. The gel culture was hypoosmotically shocked by filtering with a sterilized gauze, resuspending in the same volume of fresh BG11, and incubating at 30 °C under constant light (100 μE/m2/s) with a 5 % CO2 aeration for 4 h. The hypoosmotically shocked gel cultures were filtered and started each new growth cycle with a salt shock at 600 mM NaCl. Aliquots of 1 ml of both the liquid and agar-encapsulated cultures were sampled at the end of the salt treatment and the hypoosmotic shock in each growth cycle and centrifuged at 12,000 rpm for 5 min. The resultant supernatants were filtered by 0.22-μm filters, and the GG concentrations in the media were determined by the previously described methods.
Laser scanning confocal microscope analysis
For monitoring cell distribution and growth in encapsulated cells, pieces of gels with Synechocystis cells were characterized by a laser scanning confocal microscope (FluoView™ FV1000, Olympus, Japan). The resulting images were reconstructed into a three-dimensional image and analyzed by the Imaris software (Bitplane, Switzerland).
Results
GG secretion by disrupting the ggtCD gene
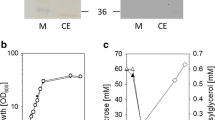
The ggtA, ggtB, ggtC, and ggtD genes in the Synechocystis genome encode the subunits of an ABC transporter for the reuptake of osmo-protective compounds from the medium, including GG, sucrose, and trehalose (Fig. 1) (Mikkat and Hagemann 2000). To prevent GG reuptake, we deleted both ggtC and ggtD genes in Synechocystis by replacing them with the spectinomycin resistance gene, resulting in a ΔggtCD mutant strain (Fig. S1a). The cell growths and intercellular and extracellular GG concentrations of both the wild type and the ΔggtCD strain were measured and compared. As shown in Fig. 2a, there were no significant differences in growth between the two strains under either normal or salt-stressed culturing conditions. After salt shock, the intracellular GG concentrations in both strains increased sharply from 0 to approximately 100 mg/l within 2 days and were maintained for six additional days (Fig. 2b). There was no GG detected in the medium of the salt-stressed wild type strain of Synechocystis (Fig. 2c). After salt shock for 2 days, approximately 17 mg/l of GG appeared in the medium of the ggtCD mutant, and the concentration of the extracellular GG increased continuously to 149 mg/l in the following 6 days (Fig. 2c). In total over 8 days, under salt-stressed conditions, the ggtCD mutant produced more than 250 mg/l of GG, which was 2.5-folds that of the wild type (Fig. 2d).
GG production and release from different Synechocystis strains. Growth curves of the wild type (WT, circles), ΔggtCD (squares), and ΔggtCDΔggpR (triangles) in column photobioreactors (a). The time point of salt shock is indicated as the arrow. The intracellular (b), extracellular (c), and total (d) GG production by wild type (WT, circles), ΔggtCD (squares), and ΔggtCDΔggpR (triangles) under salt-stressed conditions were determined. Error bars indicate s.d. (n = 3). The release of intracellular GG from salt-stressed cells of the ΔggtCDΔggpR strain by a hypoosmotic shock (e). The salt-stressed cells of the ΔggtCDΔggpR were resuspended in H2O or BG11 without or with 600 mM NaCl, respectively. After incubation for 0.25 or 2 h, the GG released from the cells was measured (presented in each column). The efficiencies of the GG release were calculated and provided at the top of the columns. Time course for the cell growth of the ΔggtCDΔggpR strain under the semicontinuous liquid culture (f). The stationary cultures of the ΔggtCDΔggpR were repeatedly treated with 6 days salt stress and 2 h hypoosmotic shock until the onset of cell death. Time points for the hypoosmotic shock are indicated by arrows. GG production of the ΔggtCDΔggpR by semicontinuous cultures (g). The “secreted GG” represents the GG recovered from the 6-day salt stress, while the “released GG” represents the GG recovered from the 2-h hypoosmotic treatment in each growth cycle. Error bars indicate s.d. (n = 3)
Improvement of GG titer by deleting the ggpR gene
The ggpR gene was previously identified as a repressor regulating the transcription of ggpS, which encodes glucosyl-glycerol-phosphate synthase and is a key gene in the GG biosynthesis pathway of Synechocystis (Marin et al. 1998). The deletion of ggpR will enable ggpS expression under low-salt conditions and increase GG accumulation under salt-stress conditions (Klahn et al. 2010). To further improve GG production, we disrupted the ggpR gene in the ΔggtCD mutant strain by inserting a kanamycin-resistant gene in its open reading frame, resulting in the ΔggtCDΔggpR double mutant (Fig. S1b). Both the intracellular and the extracellular GG yields of the ΔggtCDΔggpR strain slightly increased after the deletion of ggpR, and the total GG yield was increased to approximately 291 mg/l, which was 16 % higher than that of the ΔggtCD (Fig. 2b, c). In addition, the ΔggtCDΔggpR mutant grew better than both the wild type and the ΔggtCD mutant under salt-stressed conditions (Fig. 2a), suggesting that higher intracellular GG concentrations benefit Synechocystis cell growth.
GG release by the hypoosmotic shock
Some metabolites with low molecular weight, such as GG, were found to be released from the salt-stressed cyanobacterial cells in few minutes after the cells were hypoosmotically shocked by rapidly reducing extracellular NaCl concentration (Fig. S2a) (Erdmann et al. 1992; Reed et al. 1986; Xu et al. 2013). To release the intracellular GG of the salt-stressed Synechocystis, we applied the hypoosmotic treatment and evaluated the effects of BG11 nutrient salts, NaCl, and the hypoosmotic treatment duration on the efficiency of GG release. Cells of the ΔggtCDΔggpR mutant stressed with 600 mM NaCl for 8 days were collected and resuspended in H2O or fresh BG11 medium with or without 600 mM NaCl. Approximately 73 and 67 % of the intracellular GG were released from the cells after the hypoosmotic treatment with H2O after 0.25 and 2 h, respectively; approximately 63 and 59 % were released after treatment with BG11 medium for 0.25 and 2 h, respectively (Fig. 2e and Fig. S2a). This suggested that 0.25 h is a sufficient amount of time for the GG to be liberated from the salt-stressed cells by resuspending with either water or fresh BG11. However, no GG were released when cells were resuspended in BG11 with 600 mM NaCl (Fig. 2e and Fig. S2b), indicating that the GG release out of cells depended on the ion gradient across the cell membrane. A similar GG releasing efficiency with the ΔggtCDΔggpR mutant was achieved in a hypoosmotic shock of wild type Synechocystis (Fig. S3). These results coincide with the previous observations in other cyanobacterial strains (Erdmann et al. 1992; Reed et al. 1986).
GG production by the ΔggtCDΔggpR strain under semicontinuous culturing condition
To make the process for GG production sustainable, we semicontinuously grew the ΔggtCDΔggpR strain according to the “Material and methods”. Each growth cycle included a 6-day salt shock and a subsequent 2-h hypoosmotic shock. Thus, a fraction of the GG was actively secreted into the supernatant during the 6 days salt shock, and another fraction of the GG was passively released from the cells during the 2-h hypoosmotic shock in each growth cycle. Figure 2f shows the growth curve and the different fraction of GG recovered in each growth cycle. From the 2nd growth cycle, the final OD730 of the culture increased compared with the 1st growth cycle (Fig. 2f), indicating that the refreshing medium released some growth inhibitions caused by limited nutrients or accumulated extracellular metabolites in the stationary phase. However, on the 3rd day of the 5th growth cycle, the culture turned a yellow color, showing that the ΔggtCDΔggpR cells could not survive more than four growth cycles (i.e., 24 days). This might be because periodic oscillations in osmotic stress caused irreversible damages to the cell structure. In each growth cycle, a similar GG titer (ranging from 210.39 to 295.85 mg/l) was achieved, which suggested that the ΔggtCDΔggpR cells could stably produce and secrete GG in four growth cycles. In total, there was 981.8 ± 26.12 mg/l GG recovered from the ΔggtCDΔggpR cells after culture for 24 days (Fig. 2g). This showed that a semicontinuous cultivation strategy with periodic salt-stresses and hypoosmotic shocks could enhance the GG production of Synechocystis.
GG production by the agar gel-encapsulated cells of the ΔggtCDΔggpR strain
Although the above semicontinuous culturing significantly extended the potential GG production by Synechocystis, it would still not be economically feasible for future applications because it relies on frequent, costly cell harvesting by centrifugation. Sol-gel encapsulation has been used to immobilize cells of some cyanobacteria strains for the production of hydrogen (Dickson et al. 2009), sucrose, GG, and ammonia (Erdmann et al. 1992; Reed et al. 1986). In this method, cyanobacteria cells are embedded in a gel, and the secreted products are separated from the encapsulated cells by simple filtration instead of centrifugation.
To establish a cost- and labor-effective culturing system for GG production, we explored the feasibility of an agar-based gel encapsulation method for supporting cell growth and the production and secretion of GG from Synechocystis under semicontinuous culturing conditions. As stated in the “Material and methods” section, liquid and gel-encapsulated cultures were seeded with the same cell densities and were subjected to periodic salt-stress and hypoosmotic shock in each growth cycle (8 days per growth cycle). The liquid cultures survived 24 days culture (i.e., three growth cycles), whereas the gel-encapsulated cultures survived 32 days (i.e., 4 cycles), showing that the agar gel encapsulation cultures could better support cell growth under a semicontinuous condition than traditional liquid cultures. As revealed by laser scanning confocal microscope analysis, cells in the gel samples were found to be dispersed at 0 day (Fig. 3a and Fig. S4a) and grew to be grape-like clusters in subsequent days (Fig. 3b and Fig. S4b). The grape-like cell clusters may have been formed by a single cell that was movement-restricted in the gel with a definite elasticity. These results also showed that the agar gel-encapsulated cells of the ΔggtCDΔggpR mutant could survive and grow.
Agar gel encapsulation of Synechocystis cells for GG production and secretion. a, b 3-D images reconstructed from single images of gels sampled from the a 0-day and b 3-day cultures. c GG production by free and agar gel-encapsulated (GE) cells of the ΔggtCDΔggpR strain under semicontinuous culturing conditions. Error bars indicate s.d. (n = 3)
The comparison between GG titers of cell cultures from different growth cycles is shown in Fig. 3c. Within three growth cycles, there was no significant difference in the GG titer between the two culturing methods. However, the total GG titer from the gel-encapsulated culture was 1.64 g/l and 1.96-folds of that from the liquid culture. This was because gel cultures accomplished an additional growth cycle compared with liquid cultures. The results show that the agar-encapsulated cells of the ΔggtCDΔggpR mutants could produce and secrete GG as well as liquid culture cells.
Discussions
In conclusion, the photosynthetic and extracellular production of GG was significantly improved with a combination of biotechnologies, including the genetic engineering of the transporter protein and the transcription regulator, the semicontinuous cultivation with periodic salt-stresses and hypoosmotic shocks, and the gel encapsulation of cyanobacteria cells. Our work provides a potential technical route for GG production. And the agar gel encapsulation procedure was believed to be a relatively economic treatment method for culturing Synechocystis strains, especially for the extracellular production of high-value compounds. In future studies, additional genetic modifications can be carried out to further enhance GG production. For instance, because glycogen biosynthesis is competitive with GG biosynthesis (Fig. 1), the glycogen synthase gene can be deleted to provide additional availability of the ADP-glucose precursor for GG synthesis, just as the similar strategy applied on the production of soluble sugars in Synechococcus PCC 7002 (Xu et al. 2013). Because the native ggpS is strictly regulated at both transcriptional (Klahn et al. 2010; Marin et al. 2002) and posttranslational (Stirnberg et al. 2007) levels in Synechocystis, screening mutants of the native ggpS or the ggpS from other cyanobacteria strains may be useful for GG production than the expression of the native one. Therefore, given these options, there remains the potential to improve the GG titer further by genetic, enzymatic, and metabolic engineering. An additional optimization of the gel encapsulation process could include testing gels of different strengths and sizes. Furthermore, changing the ratio of the gel solution volume to culture volume to improve the liquid-solid mass transfer could extend the growth duration. The research presented is applicable for vast biotechnological applications of GG in health, pharmacy, and cosmetics fields.
References
Aizawa Kyo S T, Kotani Yasuhiro, Iwata Koushi, Doi Kazuhisa (2013) Keratoconjunctival protecting agent, or keratoconjunctival disorder inhibiting agent. WO/2013/077433. WO/2013/077433
Dickson DJ, Page CJ, Ely RL (2009) Photobiological hydrogen production from Synechocystis sp. PCC 6803 encapsulated in silica sol–gel. Int J Hydrogen Energy 34:204–215
Du W, Liang F, Duan Y, Tan X, Lu X (2013) Exploring the photosynthetic production capacity of sucrose by cyanobacteria. Metab Eng 19:17–25
Ducat DC, Way JC, Silver PA (2011) Engineering cyanobacteria to generate high-value products. Trends Biotechnol 29:95–103
Elhai J, Wolk CP (1988) A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene 68:119–138
Erdmann N, Zuther E, Abarzua S (1992) Comparative studies on the photoproduction of nonhydrogenous resources by cyanobacteria. Curr Microbiol 25:83–87
Goedl C, Sawangwan T, Mueller M, Schwarz A, Nidetzky B (2008) A high-yielding biocatalytic process for the production of 2-O-(α-D-glucopyranosyl)-sn-glycerol, a natural osmolyte and useful moisturizing ingredient. Angew Chem 47:10086–10089
Hagemann M (2011) Molecular biology of cyanobacterial salt acclimation. FEMS Microbiol Rev 35:87–123
Klähn S, Hagemann M (2011) Compatible solute biosynthesis in cyanobacteria. Environ Microbiol 13:551–562
Klahn S, Hohne A, Simon E, Hagemann M (2010) The gene ssl3076 encodes a protein mediating the salt-induced expression of ggpS for the biosynthesis of the compatible solute glucosylglycerol in Synechocystis sp. strain PCC 6803. J Bacteriol 192:4403–4412
Klein J, Stumm, Gerhard (2010) Use of glucosylglycerol. WO/2010/020424
Krutmann J, Lentzen, Georg, Schwarz, Thomas (2009) Osmolytes for the treatment of allergic or viral respiratory diseases. WO/2009/027069
Lu X (2010) A perspective: photosynthetic production of fatty acid-based biofuels in genetically engineered cyanobacteria. Biotechnol Adv 28:742–746
Machado IM, Atsumi S (2012) Cyanobacterial biofuel production. J Biotechnol 162:50–56
Marin K, Huckauf J, Fulda S, Hagemann M (2002) Salt-dependent expression of glucosylglycerol-phosphate synthase, involved in osmolyte synthesis in the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol 184:2870–2877
Marin K, Zuther E, Kerstan T, Kunert A, Hagemann M (1998) The ggpS gene from Synechocystis sp. strain PCC 6803 encoding glucosyl-glycerol-phosphate synthase is involved in osmolyte synthesis. J Bacteriol 180:4843–4849
Mikkat S, Hagemann M (2000) Molecular analysis of the ggtBCD gene cluster of Synechocystis sp. strain PCC6803 encoding subunits of an ABC transporter for osmoprotective compounds. Arch Microbiol 174:273–282
Okumura Hidenobu U S (2012) Blood glucose level suppressant and food inhibiting sharp increase in blood glucose level. JP2003-129931
Pocard JA, Smith LT, Smith GM, Le Rudulier D (1994) A prominent role for glucosylglycerol in the adaptation of Pseudomonas mendocina SKB70 to osmotic stress. J Bacteriol 176:6877–6884
Quintana N, Van der Kooy F, Van de Rhee MD, Voshol GP, Verpoorte R (2011) Renewable energy from cyanobacteria: energy production optimization by metabolic pathway engineering. Appl Microbiol Biotechnol 91:471–490
Reed RH, Warr SRC, Kerby NW, Stewart WDP (1986) Osmotic shock-induced release of low-molecular-weight metabolites from free-living and immobilized cyanobacteria. Enzyme Microb Technol 8:101–104
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Roder A, Hoffmann E, Hagemann M, Berg G (2005) Synthesis of the compatible solutes glucosylglycerol and trehalose by salt-stressed cells of Stenotrophomonas strains. FEMS Microbiol Lett 243:219–226
Sawangwan T, Goedl C, Nidetzky B (2010) Glucosylglycerol and glucosylglycerate as enzyme stabilizers. Biotechnol J 5:187–191
Stirnberg M, Fulda S, Huckauf J, Hagemann M, Krämer R, Marin K (2007) A membrane-bound FtsH protease is involved in osmoregulation in Synechocystis sp. PCC 6803: the compatible solute synthesizing enzyme GgpS is one of the targets for proteolysis. Mol Microbiol 63:86–102
Takenaka F, Uchiyama H, Imamura T (2000) Identification of alpha-D-glucosylglycerol in sake. Biosci, Biotechnol, Biochem 64:378–385
Tan X, Yao L, Gao Q, Wang W, Qi F, Lu X (2011) Photosynthesis driven conversion of carbon dioxide to fatty alcohols and hydrocarbons in cyanobacteria. Metab Eng 13:169–176
Xu Y, Tiago Guerra L, Li Z, Ludwig M, Charles Dismukes G, Bryant DA (2013) Altered carbohydrate metabolism in glycogen synthase mutants of Synechococcus sp. strain PCC 7002: cell factories for soluble sugars. Metab Eng 16:56–67
Acknowledgments
This work was supported by the Excellent Youth Award of the Shandong Natural Science Foundation (JQ201306 to X. Lu), the Shandong Taishan Scholarship (X. Lu), the National Science Foundation of China (31301018), the Shandong Province Science and Technology Development Project (2014GSF121033), and the Youth Innovation Promotion Association (X. Tan).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 339 kb)
Rights and permissions
About this article
Cite this article
Tan, X., Du, W. & Lu, X. Photosynthetic and extracellular production of glucosylglycerol by genetically engineered and gel-encapsulated cyanobacteria. Appl Microbiol Biotechnol 99, 2147–2154 (2015). https://doi.org/10.1007/s00253-014-6273-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6273-7