Abstract
l-Asparaginases (EC 3.5.1.1) are enzymes that catalyze the hydrolysis of l-asparagine to l-aspartic acid and found in a variety of organisms from microorganisms to mammals. However, they are mainly expressed and produced by microorganisms. Microbial l-asparaginases have received sustained attention due to their irreplaceable role in the therapy of acute lymphoblastic leukemia and for their inhibition of acrylamide formation during food processing. In this article, we review the application of microbial l-asparaginases in medical treatments and acrylamide mitigation. In addition, we describe in detail recent advances in the existing sources, purification, production, properties, molecular modification, and immobilization of l-asparaginase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
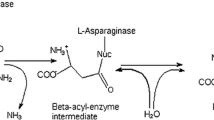
l-Asparaginases (l-asparagine amidohydrolase; EC 3.5.1.1), which catalyze the hydrolysis of the amide group of the side-chain of l-asparagine into aspartic acid and ammonia (Fig. 1), are widely distributed among living creatures, including animals, plants, and microorganisms (Verma et al. 2007b). For more than four decades, l-asparaginases have been a hallmark of multidrug chemotherapeutic regimens broadly used for the treatment of lymphoid systems malignancies, childhood acute lymphoblastic leukemia, Hodgkin’s lymphoma, lymphosarcoma, and melanosarcoma (Stecher et al. 1999; Duval et al. 2002). Moreover, l-asparaginases have been used as a diagnostic biosensor for l-asparagine due to the large amount of ammonia produced by the enzymatic reaction and its direct correlation to the level of l-asparagine in a patient’s blood (Verma et al. 2007a). Apart from its clinical usage, l-asparaginases have also been characterized successfully as inhibitors of the formation of acrylamide in heated food (Kornbrust et al. 2009); acrylamide is classified as a probable human carcinogen by the International Agency for Research on Cancer (IARC 1994), suggesting that l-asparaginases have great potential for use in the food industry.
Although l-asparaginase has been reviewed by Savitri and Azmi (2003) and Verma et al. (2007b), here we focus on recent advancement in aspects such as the sources, purification, and characterization of l-asparaginase and its application in the pharmaceutical and food industries. The l-asparaginase production by fermentation as well as its modification and immobilization are also reviewed in this paper.
Application of microbial l-asparaginase
Pharmaceutical application
l-Asparaginases are a cornerstone of treatment protocols for childhood acute lymphoblastic leukemia (ALL) and are used for remission induction and intensification treatment in all pediatric regimens and most adult treatment protocols. There are three main l-asparaginase drug preparations used currently; these include the native l-asparaginase from Escherichia coli (E. coli asparaginase), a PEGylated (PEG: polyethylene glycol) form of l-asparaginase (PEG-asparaginase), and l-asparaginase derived from Erwinia chrysanthemi (Erwinia asparaginase) (Pieters et al. 2011). E. coli asparaginase or PEG-asparaginase is used as a first-line treatment of childhood ALL in current treatment protocols. Typically, patients showing sensitivity to one formulation of l-asparaginase are switched to another to ensure they receive the most efficacious treatment. Erwinia asparaginase has been adopted in European and US protocols for second- or third-line treatments. In addition to the abovementioned three drugs, a novel recombinant E. coli asparaginase preparation is currently being subjected to clinical evaluation. The original data show that this recombinant enzyme has an efficacy and toxicity profile similar to the native E. coli asparaginase. An l-asparaginase encapsulated into homologous red blood cells has recently been proposed as a new approach to maintain enzyme activity, while reducing the formation of anti-asparaginase antibodies (Agrawal et al. 2013). In addition, a PEGylated form of recombinant Erwinia asparaginase is under preclinical study (Allas et al. 2009).
Unlike normal cells that can synthesize l-asparagine by l-asparagine synthetase, tumor cells require abundant exogenous l-asparagine to ensure rapid growth (Verma et al. 2007b). Therefore, the presence of l-asparaginase can deprive lymphatic tumor cells of this essential amino acid, thereby causing the cells to starve to death (Fig. 2). The toxic effects of most l-asparaginases are associated with their l-glutaminase activities, causing a prolonged low level l-glutamine intake by normal cells of the liver and pancreas, thereby leading to liver enzyme elevations and pancreatitis (Patil et al. 2011). According to Asselin et al. (1989), E. coli asparaginase causes more coagulation abnormalities and yields a better anti-leukemia effect than Erwinia asparaginase due to its more effective and sustained ability to deplete l-asparagine.
Application as a food processing aid
l-Asparaginase came under scrutiny by food experts when acrylamide was initially detected in some fried and baked foods, which are rich in carbohydrates (Tareke et al. 2002). Compared with other methods, l-asparaginase has the advantage of mitigating acrylamide levels without altering the final sensorial experience typical of these products (Hendriksen et al. 2009). In previous studies, l-asparaginase pretreatment of potato and cereal foods achieved a final acrylamide content reduction of 30–97 % (Ciesarová et al. 2006; Pedreschi et al. 2008; Hendriksen et al. 2009; Pedreschi et al. 2011; Kumar et al. 2014a, b). Pedreschi et al. (2011) reported that traditional blanching in combination with l-asparaginase treatment could effectively reduce acrylamide levels by 90 % in potato chips.
As described above, l-asparaginase catalyzes the conversion of l-asparagine into l-aspartic acid, which is one of the two important precursors (asparagine and reducing sugar) for acrylamide synthesis (Fig. 3) (Hedegaard et al. 2008). Several articles show that the activity of l-asparaginase is related to enzyme dose, reaction conditions, and the ingredients used in processing. There are also direct correlations between lowered asparagine levels and the reduction in acrylamide according to the complexity of the processing system (Anese et al. 2011a, b; Hendriksen et al. 2009). Anese et al. (2011b) demonstrated that the existence of fat might reduce both the enzyme activity and the contact between the substrate and l-asparaginase.
l-asparaginases produced by Aspergillus oryzae (Hendriksen et al. 2009) and Aspergillus niger (Kornbrust et al. 2009) have been universally accepted in recent years because of their advantageous properties, such as an optimum pH of 6.0–7.0 and high activity at 60 °C, and because they have been carefully evaluated by the Joint FAO/WHO Expert Committee on Food Additives. Commercially available l-asparaginase produced from genetically modified organisms (A. oryzae and A. niger) is now permitted for use in the United States, Australia, New Zealand, Switzerland, China, México, Russia, and in several EU countries (Anese et al. 2011a). Canada has recommended amending regulations to allow the application of l-asparaginase as a food additive, and some EU countries have also authorized bakeries to use the enzyme in food processing (Anese et al. 2011a).
Purification and characterization of microbial l-asparaginase
Occurrence in microorganisms
l-Asparaginases are extensively distributed in all three domains of life, from microorganisms (bacteria, molds, yeasts, actinomycetes, and algaes) to higher organisms (plants, vertebrates, and animal tissues). l-Asparaginase plays a critical role in amino acid metabolism by hydrolyzing l-asparagine to l-aspartic acid, which, can be transformed to oxaloacetate, which can enter the Kreb’s cycle (Savitri and Azmi 2003). l-Asparaginase is also important in the biosynthesis of the aspartic family of amino acids (Martin 1989). l-Asparaginase is produced constitutively, and its role may be as an overflow enzyme, decomposing excess l-asparagine into l-aspartic acid, which is the precursor of lysine, threonine, and methionine (Savitri and Azmi 2003). In addition, the product of the l-asparaginase catalytic reaction in plants, l-asparagine, is used for nitrogen storage and transport, and is vital for the utilization of this essential resource (Bruneau et al. 2006).
Microbes can produce several asparaginase types that differ in their cellular location and properties, namely periplasmic asparaginase, extracellular asparaginase, intracellular asparaginase, and glutaminase-asparaginase, which plays a role in basic metabolism (Cedar and Schwartz 1967; Hüser et al. 1999). Borek and Jaskólki (2001) compared almost all known protein sequences encoding l-asparaginase. Based on phylogenetic conservation and other biochemical and crystallographic data, they concluded that the l-asparaginases could be divided into three different and unrelated families that they termed bacterial-type, plant-type, and Rhizobium-type. There are two designated bacterial-types: type I and type II. Type I l-asparaginases display high K m values toward l-asparagine and also show catalytic activity toward l-glutamine, while type II l-asparaginases exhibit high affinity to l-asparagine and low-to-negative activity toward l-glutamine (Cedar and Schwartz 1967, 1968; Jerlström et al. 1989).
Novel microbial sources
Microorganisms have been considered as the most important source of l-asparaginase since it was first reported in E. coli (Mashburn and Wriston Jr 1964). Although l-asparaginase has also been isolated from plant and animals sources, microorganisms, such as bacteria, fungi, yeasts, actinomycetes, and algaes, have proven to be abundant sources of this enzyme. Although the sources of l-asparaginase have been reviewed before 2007 (Savitri and Azmi 2003; Verma et al. 2007b), there have been many other novel microorganisms reported in recent years.
Sahu et al. (2007) screened six actinomycetes strains (Streptomyces aureofasciculus, Streptomyces chattanoogenesis, Streptomyces hawaiiensis, Streptomyces orientalis, Streptomyces canus, and Streptomyces olivoviridis) from estuarine fish. Two Helicobacter pylori species were reported to produce l-asparaginase (Shibayama et al. 2011; Gladilina et al. 2008). Basha et al. (2009), using solid-state and submerged fermentation, screened three strains (S3, S4, and K8) of marine actinomycetes. l-Asparaginase has been reported from two species of Serratia, Serratia marcescens SK-07 (Agarwal et al. 2011) and S. marcescens NCIM 2919 (Ghosh et al. 2013). Eisele et al. (2011) reported the first characterized l-asparaginase from a basidiomycete, Flammulina velutipes. Fungal isolates from rhizosphere soils were screened for l-asparaginase production by using modified Czapek Dox agar containing l-asparagine and phenol red as an indicator. The 16S rDNA sequence analysis indicated that one of these strains was most closely related to Fusarium equiseti (Hosamani and Kaliwal 2011).
-
Kumar et al. (2011a) identified another glutaminase-free l-asparaginase from Pectobacterium carotovorum MTCC 1428. A moderate halophilic bacterium, Bacillus sp. BCCS 034, was screened for both intra- and extracellular l-asparaginase production (Ebrahiminezhad et al. 2011). Penicillium digitatum was screened for the production of extracellular l-asparaginase (Shrivastava et al. 2012). A protease-deficient Bacillus aryabhattai ITBHU02 for l-asparaginase production was isolated from soil contaminated with hospital waste (Singh and Srivastava 2012). Sudhir et al. (2012) screened a new strain, Streptomyces ABR2, which showed the highest l-asparaginase activity among all screened organisms. Chlorella vulgaris was identified as a novel microalgal source for l-asparaginase production (Ebrahiminezhad et al. 2014). Recently, three extremely thermostable l-asparaginases have been characterized from hyperthermophilic archaeon strains, including Thermococcus kodakaraensis KOD1 (Chohan and Rashid 2013), Thermococcus gammatolerans EJ3 (Zuo et al. 2014), and Pyrococcus furiosus (Bansal et al. 2010). However, these l-asparaginases from T. kodakaraensis KOD1 and P. furiosus displayed no L-glutaminase activity, while the T. gammatolerans l-asparaginase had relatively high hydrolysis activity toward l-glutamine (Zuo et al. 2014).
Purification and characterization
l-Asparaginase is an injectable drug used for the treatment of tumors. The sensitivity of the application of this enzyme requires a high degree of purity. The majority of microbial l-asparaginases are intracellular. Most purification procedures are comprised of conventional methods, such as ammonium sulfate fractionation combined with gel filtration or ion exchange column chromatography, while recombinant l-asparaginase harboring a 6× hisidine-tag was purified to homogeneity using one-step nickel-affinity chromatography. Several novel methods have also been applied to purify this enzyme. In situ extraction of intracellular l-asparaginase using a thermo-separating aqueous two-phase system was compared with the conventional aqueous two-phase extraction process, and the purification of l-asparaginase by this novel method produced greater yield and higher specific activity (Zhu et al. 2007). The recovery of the enzyme was further enhanced when Zhu and Liu (2008) combined high-pressure homogenization with aqueous two-phase extraction for the purification of intracellular E. coli l-asparaginase. Ferrara et al. (2010) studied the extraction of periplasmic l-asparaginase from recombinant Pichia pastoris harboring the Saccharomyces cerevisiae ASP3 gene. They obtained high extraction yields and enzyme recovery using freeze–thaw cycles, ethanol treatment and alkaline extraction in the presence and absence of cysteine.
l-Asparaginases from different organisms vary in their biochemical properties. Generally, the optimum temperature for l-asparaginase activity is between 30 and 40 °C; several Yersinia and Streptomyces species have higher optimum temperatures (40–60 °C), while the hyperthermophilic l-asparaginases from archaea Thermococcus kodakarensis KOD1, T. gammatolerans EJ3, and P. furiosus have optimum temperatures at 85, 85, and 80 °C, respectively (Table 1). The enzyme displayed activity across a wide pH range, with optimum activity in the range of 6.0–9.5. Most of the enzymes have an optimum at alkaline pH, while only a few strains have maximum enzymatic activity below pH 7.0 (Table 1). Streptomyces gulbargensis l-asparaginase was more stable at alkaline pH, and it retained 55 % activity at 80 °C for 1 h (Amena et al. 2010).
Different ions have different influences on the activity of l-asparaginase. Fe3+ strongly inhibited Bacillus subtilis B11-06 l-asparaginase activity. Rhizobium etli l-asparaginase activity was reduced in the presence of Mn2+, Zn2+, Ca2+, and Mg2+ (Moreno-Enriquez et al. 2012). Complete inhibition of l-asparaginase activity from P. carotovorum MTCC 1428 was observed in the presence of Cu2+, Cd2+, and Hg2+ (Kumar et al. 2011a). The addition of the metal chelating agent EDTA had no effect on enzyme activity, revealing that the enzyme was not a metalloprotein (Jia et al. 2013; Kumar et al. 2011a; Chohan and Rashid 2013; Singh et al. 2013; Kumar and Manonmani 2013).
Production of l-asparaginase by fermentation
There have been many reports examining the production of l-asparaginase under diverse conditions by different microorganisms. l-Asparaginase was produced normally during submerged fermentation and solid-state fermentation. Many methods were employed to optimize the production of l-asparaginase, such as the Plackett–Burman experimental design, genetic algorithm, and artificial neural network-based design models, central composite rotatable design, and response surface methodology.
Prakasham et al. (2007) described the interaction of fermentation process parameters (carbon and nitrogen sources, incubation temperature, medium pH, aeration and agitation, and inoculum levels) for the production of l-asparaginase by Staphylococcus sp. –6A. The production of Streptomyces albidoflavus l-asparaginase under submerged fermentation was optimized, and the maximum level of enzyme production was found at pH 7.5 and 35 °C in culture medium supplemented with maltose and yeast extract as carbon and nitrogen sources, respectively (Narayana et al. 2008). Application of the Plackett–Burman experimental design and response surface methodology for the optimization of l-asparaginase production from P. carotovorum MTCC 1428 in submerged fermentation was described for the first time by Sanjeeviroyar et al. (2010). A new isolate, S. gulbargensis, was studied for the production of extracellular l-asparaginase under submerged fermentation conditions using groundnut cake extract (Amena et al. 2010). A three-level central composite design of the response surface methodology and the artificial neural network-linked genetic algorithm were applied to optimize the operating conditions for the enhanced production of l-asparaginase by submerged fermentation of Aspergillus terreus MTCC 1782 (Baskar and Renganathan 2012). The production of Streptomyces noursei MTCC 10469 l-asparaginase by submerged fermentation was conducted using a tryptone, glucose, and yeast extract broth (Dharmaraj 2011).
Bacillus circulans MTCC 8574 l-asparaginase production was optimized under solid-state fermentation using different agricultural materials, such as red gram husk, Bengal gram husk, coconut, and groundnut cake, with red gram husk yielding the maximum enzyme production (Hymavathi et al. 2009). The optimization of l-asparaginase production by A. niger using solid-state fermentation on sesame cake was performed using genetic algorithms and artificial neural network-based design models (Babu et al. 2010). Different physical and chemical parameters were optimized under solid-state fermentation conditions for F. equiseti l-asparaginase production (Hosamani and Kaliwal 2011). Kumar et al. (2013b) reported the production and optimization of l-asparaginase from Cladosporium sp. using agricultural residues and solid-state fermentation. The classical one-factor-at-a-time and response surface methodology have been applied to optimize l-asparaginase production by S. marcescens (NCIM 2919) under solid-state fermentation conditions using coconut oil cake (Ghosh et al. 2013). The production of extracellular l-asparaginase from marine actinomycetes was conducted in both solid-state and submerged fermentation conditions (Basha et al. 2009).
Many frequently used methods were applied to optimize the culture medium and fermentation production of l-asparaginase. The production of l-asparaginase from P. carotovorum MTCC 1428 has been studied several times since it was first reported. The Plackett–Burman experimental design has been applied to maximize the production of a novel glutaminase-free l-asparaginase from P. carotovorum MTCC 1428 (Kumar et al. 2009). Kumar et al. (2010) also studied the effect of various carbon and nitrogen sources on the production of l-asparaginase by P. carotovorum MTCC 1428. Among the tested carbon sources, l-asparagine or the combination of l-asparagine and glucose were the best carbon sources. Kumar et al. (2011b) optimized the physical process conditions (initial pH, temperature, rotation rate of the shaking incubator, and inoculum size) for the production of l-asparaginase from P. carotovorum MTCC 1428.
Central composite rotatable design was used to optimize the chemical and physical parameters to enhance the production of l-asparaginase from a novel isolated S. marcescens SK-07 in a batch bioreactor, and the maximum l-asparaginase production was obtained at an initial pH of 6.5 and a dissolved oxygen level of 40 % (Agarwal et al. 2011). The production of l-asparaginase from E. coli ATCC 11303 was reported using response surface methodology (Kenari et al. 2011). Narta et al. (2011) evaluated the production of l-asparaginase by Bacillus brevis cultivated in the presence of three oxygen-vectors: liquid paraffin, silicone oil, and n-dodecane. The results showed that each of the three oxygen-vectors stimulated the enzyme yield. l-Asparaginase production by mangrove-derived Bacillus cereus MAB5 was optimized by response surface methodology (Thenmozhi et al. 2011). The production of l-asparaginase by Nocardia levis MK-VL_113 isolated from laterite soils of the Guntur region has been optimized (Kavitha and Vijayalakshmi 2012). Response surface methodology was found to be not as efficient as artificial neural network-linked genetic algorithms for maximizing production. Response surface methodology and genetic algorithm-based techniques were also implemented to improve the production of l-asparaginase by B. aryabhattai ITBHU02 (Singh and Srivastava 2012). The high-throughput production of l-asparaginase using a kinetic model-based approach has been developed (Singh and Srivastava 2014). Recombinant Erwinia carotovora l-asparaginase II in E. coli was produced using a robust fed-batch technique with pre-determined exponential feeding rates (Roth et al. 2013).
Molecular recombinant and modified l-asparaginase
Recombinant DNA technology is an important strategy to improve the protein yield. In recent years, many researchers used recombinant technology to increase l-asparaginase expression level successfully. Recombinant l-asparaginases were summarized in Table 2. And the comparison of amino acid sequences of l-asparaginases from various microorganisms was shown in Table 3. The heterologous expression of S. cerevisiae ASP3 gene in P. pastoris was first reported by Ferrara et al. (2006). The recombinant l-asparaginase was produced in shake flasks and in a 2-L instrumented bioreactor. Kotzia and Labrou (2007) reported the cloning, expression, and characterization of l-asparaginase from E. chrysanthemi 3937 in E. coli BL21(DE3) containing the plasmid pLysS. A novel l-asparaginase from the pathogenic strain H. pylori CCUG 17874 has been cloned and expressed in E. coli BL21(DE3); the recombinant enzyme displayed a strong preference for l-asparagine over l-glutamine (Cappelletti et al. 2008). Yano et al. (2008) first reported the overexpression of type I l-asparaginase of B. subtilis NBRC 3009 in E. coli Rosetta Gami B. The ansA gene from P. furiosus was cloned and expressed in E. coli, and the recombinant enzyme was purified to homogeneity (Bansal et al. 2010). The enzyme was found to be thermostable, dimeric in its native form, and glutaminase-free, with optimum activity at pH 9.0. Urea could not induce unfolding and enzyme inactivation; however, with guanidine hydrochloride (GdnCl), a two-state unfolding pattern was observed. Two E. carotovora l-asparaginase II constructions, with and without the signal peptide, were compared with each other, and the results show that the former has lower glutaminase activity (Wink et al. 2010).
Five l-asparaginase genes from Streptomyces strains, Streptomyces thermoluteus subsp. fuscus NBRC 14270, Streptomyces coelicolor, Streptomyces avermitilis, and Streptomyces griseus were expressed in Streptomyces lividans using the hyperexpression vector: pTONA5a (Hatanaka et al. 2011). Among those genes, only the genes from S. thermoluteus subsp. fuscus NBRC 14270 and S. griseus were successful for overexpression. Onishi et al. (2011) examined the expression of the ansZ gene encoding a putative l-asparaginase II from B. subtilis in E. coli. No expression was found in E. coli transformed with a plasmid containing the full-length ansZ gene. Three N-terminal truncated enzymes were constructed based on a comparison with the N-terminal sequences of other type II l-asparaginases. All of the truncated enzymes were successfully expressed. The ansB gene encoding l-asparaginase II from E. coli MTCC 739 was successfully expressed in E. coli DE3 cells (Vidya et al. 2011). Recombinant intracellular Rhodospirillum rubrum l-asparaginase with low l-glutaminase activity and antiproliferative effects has been reported (Pokrovskaya et al. 2012a). The l-asparaginase II gene from a moderately thermotolerant bacterial isolate belonging to Enterobacteriaceae was cloned in the pET20b vector with a pelB leader sequence (Vidya and Pandey 2012).
Pokrovskaya et al. (2012b) have cloned the ansB gene from Yersinia pseudotuberculosis Q66CJ2 and have constructed a stable inducible expression system that overexpresses l-asparaginase from Y. pseudotuberculosis in E. coli BL21 (DE3). A thermostable recombinant l-asparaginase from T. kodakaraensis KOD1 exhibited the highest ever reported enzyme activity (Chohan and Rashid 2013). The gene encoding l-asparaginase from a nonpathogenic strain of B. subtilis B11–06 was successfully expressed in B. subtilis 168 (Jia et al. 2013). A novel fungal l-asparaginase gene from Rhizomucor miehei was cloned and expressed in E. coli (Huang et al. 2014). Its deduced amino acid sequence shared only 57 % identity with those of other reported l-asparaginases.
The short half-life and high immunogenicity of native l-asparaginase were the greatest deficits identified for medical applications. The most commonly used method to circumvent these problems is to modify the enzyme to increase the half-life and lower immune reactivity. The E. coli D178P mutant was created by replacing Asp178 located within a hydrogen-bonded turn with proline. The results show that the mutant has higher thermostability without affecting the enzyme activity (Li et al. 2007). Three active-site mutants of P. furiosus l-asparaginase were developed to improve the substrate affinity and antineoplastic activity at physiological conditions (Bansal et al. 2012). The effect of surface charge on the stability of the enzyme l-asparaginase II was studied by site-directed mutagenesis of the cloned ansB gene from Escherichia sp. (Vidya et al. 2014). A library of enzyme variants was constructed by a staggered extension process using the genes coding for l-asparaginase from E. chrysanthemi and E. carotovora and subsequently screened using activity assays to determine the activity of all represented variants (Kotzia and Labrou 2011). A variant of the E. carotovora enzyme with undetectable glutaminase activity was found. Sequence analysis showed that this variant contained a single point mutation resulting in a single amino acid substitution (Leu71Ile).
Immobilization of l-asparaginase
Immobilization is a helpful method to modify l-asparaginase to increase half-life and lower immune reactivity (Table 4). Kotzia et al. (2007), using the site-directed mutagenesis technique followed by covalent coupling of methoxypoly (ethylene glycol) succinate N-hydroxysuccinimide ester (mPEG-SNHS) to the l-asparaginases from E. carotovora 1526, found that the modified enzyme retains most of its original activity (82 %), shows improved resistance to trypsin degradation, and displays higher thermal stability than the wild-type enzyme. The recombinant enzyme from E. chrysanthemi 3937 has been immobilized on epoxy-activated Sepharose CL-6B, and the immobilized enzyme retains 60 % of its original activity and exhibits high stability at 4 °C (Kotzia and Labrou 2007). This method offers the possibility of designing a bioreactor that can be operated over a long period of time with high efficiency, which can be used for leukemia therapy. Zhang et al. (2008) described a method of preparing silk fibroin nanoparticles and successfully used the method to immobilize l-asparaginase by glutaraldehyde cross-linking. The thermal stability of the immobilized enzyme increased markedly, and the optimal pH range became broader. The optimum temperature of the modified enzyme was also increased approximately 10 °C. Later, Zhang et al. (2011) reported another novel and highly efficient method to process fine crystalline silk fibroin nanoparticle-l-asparaginase bioconjugates in the presence of excess organic solvents. The bioconjugates could resist higher temperatures, were more resistant to trypsin digestion, and had better stability in serum. No leakage of the enzyme from the nanoparticles was found. Ghosh et al. (2012) described a novel polyaniline nanofiber immobilization matrix for the anti-leukemia enzyme l-asparaginase. Immobilized l-asparaginase showed greater stability toward decomposition or denaturation at a range of temperatures and pH conditions compared with the free enzyme. l-Asparaginase from Cladosporium sp. was modified with bovine serum albumin, ovalbumin by crosslinking using glutaraldehyde, N-bromosuccinimide, and mono-methoxy polyethylene glycol (Kumar et al. 2014a, b).
Future
Despite the wide application of l-asparaginase in medical treatment and acrylamide mitigation in the food industry, there are also remaining deficiencies and problems to be resolved. The side effects, short half-life, and low levels of enzyme production are major concerns for clinical usage. Firstly, further screening of microorganisms is needed to improve enzyme yield, activity, and l-asparagine specificity to reduce l-glutamine hydrolytic characteristics. Secondly, modification, directed evolution, and recombinant techniques should be applied to improve stability against proteolysis, to increase enzyme half-life, and to reduce immunogenicity. In addition, fermentation conditions need to be optimized to yield higher enzyme activity. For application in the food industry, enhancing the thermostability of l-asparaginase and its activity across a broad pH range is still the most important issue to overcome. There remains a lot to be explored about this useful enzyme.
References
Agarwal A, Kumar S, Veeranki VD (2011) Effect of chemical and physical parameters on the production of l-asparaginase from a newly isolated Serratia marcescens SK-07. Lett Appl Microbiol 52:307–313
Agrawal V, Hee Woo J, Borthakur G, Kantarjian H, Frankel AE (2013) Red blood cell-encapsulated l-asparaginase: potential therapy of patients with asparagine synthetase deficient acute myeloid leukemia. Protein Peptide Lett 20:392–402
Allas S, Sahakian P, Fichtner I, Abribat T (2009) Pharmacokinetics and pharmacodynamics in mice of a pegylated recombinant Erwinia chrysanthemi-derived l-asparaginase. Blood 114:2033
Amena S, Vishalakshi N, Prabhakar M, Dayanand A, Lingappa K (2010) Production, purification and characterization of l-asparaginase from Streptomyces gulbargensis. Braz J Microbiol 41:173–178
Anese M, Quarta B, Frias J (2011a) Modelling the effect of asparaginase in reducing acrylamide formation in biscuits. Food Chem 126:435–440
Anese M, Quarta B, Peloux L, Calligaris S (2011b) Effect of formulation on the capacity of l-asparaginase to minimize acrylamide formation in short dough biscuits. Food Res Int 44:2837–2842
Asselin BL, Ryan D, Frantz CN, Bernal SD, Leavitt P, Sallan SE, Cohen HJ (1989) In vitro and in vivo killing of acute lymphoblastic leukemia cells by l-asparaginase. Cancer Res 49:4363–4368
Babu UK, Ramagopal N, Reddy DSR (2010) Optimization of l-asparaginase production from isolated Aspergillus niger by using solid state fermentation on sesame cake via application of genetic algorithm, and artificial neural network-based design model. J Biotechnol 150:S538–S539
Bansal S, Gnaneswari D, Mishra P, Kundu B (2010) Structural stability and functional analysis of l-asparaginase from Pyrococcus furiosus. Biochemistry-Moscow 75:375–381
Bansal S, Srivastava A, Mukherjee G, Pandey R, Verma AK, Mishra P, Kundu B (2012) Hyperthermophilic asparaginase mutants with enhanced substrate affinity and antineoplastic activity: structural insights on their mechanism of action. FASEB J 26:1161–1171
Basha NS, Rekha R, Komala M, Ruby S (2009) Production of extracellular anti-leukaemic enzyme l-asparaginase from marine actinomycetes by solid-state and submerged fermentation: purification and characterisation. Trop J Pharm Res 8:353–360
Baskar G, Renganathan S (2012) Optimization of l-asparaginase production by Aspergillus terreus MTCC 1782 using response surface methodology and artificial neural network-linked genetic algorithm. Asia-Pac J Chem Eng 7:212–220
Borek D, Jaskólski M (2001) Sequence analysis of enzymes with asparaginase activity. Acta Biochim Pol 48:893–902
Bruneau L, Chapman R, Marsolais F (2006) Co-occurrence of both l-asparaginase subtypes in Arabidopsis: At3g16150 encodes a K+-dependent l-asparaginase. Planta 224:668–679
Cappelletti D, Chiarelli LR, Pasquetto MV, Stivala S, Valentini G, Scotti C (2008) Helicobacter pylori l-asparaginase: a promising chemotherapeutic agent. Biochem Biophys Res Commun 377:1222–1226
Cedar H, Schwartz JH (1967) Localization of the two l-asparaginases in anaerobically grown Escherichia coli. J Biol Chem 242:3753–3755
Cedar H, Schwartz JH (1968) Production of l-asparaginase II by Escherichia coli. J Bacteriol 96:2043–2048
Chohan SM, Rashid N (2013) TK1656, a thermostable l-asparaginase from Thermococcus kodakaraensis, exhibiting highest ever reported enzyme activity. J Biosci Bioeng 116:438–443
Ciesarová Z, Kiss E, Boegl P (2006) Impact of l-asparaginase on acrylamide content in potato products. J Food Nutr Res 45:141–146
Dharmaraj S (2011) Study of l-asparaginase production by Streptomyces noursei MTCC 10469, isolated from marine sponge Callyspongia diffusa. Iranian J Biotechnol 9:102–108
Duval M, Suciu S, Ferster A, Rialland X, Nelken B, Lutz P, Benoit Y, Robert A, Manel A-M, Vilmer E (2002) Comparison of Escherichia coli-asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized european organisation for research and treatment of cancer-children’s leukemia group phase 3 trial. Blood 99:2734–2739
Ebrahiminezhad A, Rasoul-Amini S, Ghasemi Y (2011) l-Asparaginase production by moderate halophilic bacteria isolated from maharloo salt lake. Indian J Microbiol 1:307–311
Ebrahiminezhad A, Rasoul-Amini S, Ghoshoon MB, Ghasemi Y (2014) Chlorella vulgaris, a novel microalgal source for l-asparaginase production. Biocatal Agr Biotechnol 3:214–217
Eisele N, Linke D, Bitzer K, Na’amnieh S, Nimtz M, Berger RG (2011) The first characterized asparaginase from a basidiomycete, Flammulina velutipes. Bioresour Technol 102:3316–3321
Ferrara MA, Severino NMB, Mansure JJ, Martins AS, Oliveira EMM, Siani AC, Pereira N Jr, Torres FAG, Bon EPS (2006) Asparaginase production by a recombinant Pichia pastoris strain harbouring Saccharomyces cerevisiae ASP3 gene. Enz Microb Tech 39:1457–1463
Ferrara MA, Bonomo Severino NM, Valente RH, Perales J, Bon EPS (2010) High-yield extraction of periplasmic asparaginase produced by recombinant Pichia pastoris harbouring the Saccharomyces cerevisiae ASP3 gene. Enz Microb Tech 47:71–76
Ghosh S, Chaganti SR, Prakasham R (2012) Polyaniline nanofiber as a novel immobilization matrix for the anti-leukemia enzyme l-asparaginase. J Mol Catal B-Enzym 74:132–137
Ghosh S, Murthy S, Govindasamy S, Chandrasekaran M (2013) Optimization of l-asparaginase production by Serratia marcescens (NCIM 2919) under solid state fermentation using coconut oil cake. Sustain Chem Process 1:1–8
Gladilina IA, Sokolov NN, Krasotkina IV (2008) Cloning, expression and purification of Helicobater pylori l-asparaginase. Biomed Khim 54:482–486
Hatanaka T, Usuki H, Arima J, Uesugi Y, Yamamoto Y, Kumagai Y, Yamasato A, Mukaihara T (2011) Extracellular production and characterization of two Streptomyces l-asparaginases. Appl Biochem Biotechnol 163:836–844
Hedegaard RV, Frandsen H, Skibsted LH (2008) Kinetics of formation of acrylamide and Schiff base intermediates from asparagine and glucose. Food Chem 108:917–925
Hendriksen HV, Kornbrust BA, Østergaard PR, Stringer MA (2009) Evaluating the potential for enzymatic acrylamide mitigation in a range of food products using an asparaginase from Aspergillus oryzae. J Agric Food Chem 57:4168–4176
Hosamani R, Kaliwal B (2011) Isolation, molecular identification and optimization of fermentation parameters for the production of l-asparaginase, an anticancer agent by Fusarium equiseti. Int J Microbiol Res 3:108–119
Huang L, Liu Y, Sun Y, Yan Q, Jiang Z (2014) Biochemical characterization of a novel l-asparaginase with low glutaminase activity from Rhizomucor miehei and its application in food safety and leukemia treatment. Appl Environ Microb 80:1561–1569
Hüser A, Klöppner U, Röhm KH (1999) Cloning, sequence analysis, and expression of ansB from Pseudomonas fluorescens, encoding periplasmic glutaminase/asparaginase. FEMS Microbiol Lett 178:327–335
Hymavathi M, Sathish T, Rao CS, Prakasham RS (2009) Enhancement of l-asparaginase production by isolated Bacillus circulans (MTCC 8574) using response surface methodology. Appl Biochem Biotechnol 159:191–198
International Agency for Research on Cancer (IARC) (1994) Monographs on the evaluation of carcinogenic risks to humans, some industrial chemicals. Acrylamide. 88:389–433. http://monographs.iarc.fr/ENG/Monographs/vol60/mono60-16.pdf
Jerlström PG, Bezjak DA, Jennings MP, Beacham IR (1989) Structure and expression in Escherichia coli K-12 of the l-asparaginase I-encoding ansA gene and its flanking regions. Gene 78:37–46
Jia M, Xu M, He B, Rao Z (2013) Cloning, expression and characterization of l-asparaginase from a newly isolated Bacillus subtilis B11-06. J Agric Food Chem 61:9428–9434
Kavitha A, Vijayalakshmi M (2012) A study on l-asparaginase of Nocardia levis MK-VL_113. Sci World J 2012:160434
Kenari SLD, Alemzadeh I, Maghsodi V (2011) Production of l-asparaginase from Escherichia coli ATCC 11303: optimization by response surface methodology. Food Bioprod Process 89:315–321
Kornbrust BA, Stringer MA, Lange NEK, Hendriksen HV, Whitehurst R, Oort Mv (2009) Asparaginase—an enzyme for acrylamide reduction in food products. In: Enzymes in Food Technology, 2nd edn. Wiley-Blackwell, UK, pp 59–87
Kotzia GA, Labrou NE (2007) l-Asparaginase from Erwinia chrysanthemi 3937: cloning, expression and characterization. J Biotechnol 127:657–669
Kotzia GA, Labrou NE (2011) Engineering substrate specificity of E. carotovora l-asparaginase for the development of biosensor. J Mol Catal B-Enzym 72:95–101
Kotzia GA, Lappa K, Labrou NE (2007) Tailoring structure-function properties of l-asparaginase: engineering resistance to trypsin cleavage. Biochem J 404:337–343
Kumar NSM, Manonmani HK (2013) Purification, characterization and kinetic properties of extracellular l-asparaginase produced by Cladosporium sp. World J Microbiol Biotechnol 29:577–587
Kumar S, Pakshirajan K, Dasu VV (2009) Development of medium for enhanced production of glutaminase-free l-asparaginase from Pectobacterium carotovorum MTCC 1428. Appl Biochem Biotechnol 84:477–486
Kumar S, Dasu VV, Pakshirajan K (2010) Localization and production of novel l-asparaginase from Pectobacterium carotovorum MTCC 1428. Process Biochem 45:223–229
Kumar S, Dasu VV, Pakshirajan K (2011a) Purification and characterization of glutaminase-free l-asparaginase from Pectobacterium carotovorum MTCC 1428. Bioresour Technol 102:2077–2082
Kumar S, Veeranki VD, Pakshirajan K (2011b) Assessment of physical process conditions for enhanced production of novel glutaminase-free l-asparaginase from Pectobacterium carotovorum MTCC 1428. Appl Biochem Biotechnol 163:327–337
Kumar NSM, Ramasamy R, Manonmani HK (2013) Production and optimization of l-asparaginase from Cladosporium sp. using agricultural residues in solid state fermentation. Ind Crop Prod 43:150–158
Kumar NM, Shimray CA, Indrani D, Manonmani H (2014a) Reduction of acrylamide formation in sweet bread with l-asparaginase treatment. Food Bioprocess Technol 7:741–748
Kumar NSM, Kishore V, Manonmani HK (2014b) Chemical modification of l-asparaginase from Cladosporium sp. for improved activity and thermal stability. Prep Biochem Biotechnol 44:433–450
Kumari PK, Sankar GG, Prabhakar T, Lakshmi SS (2013) Purification and characterization of l-asparaginase from Streptomyces griseoluteus WS3/1. Int J Pharm Sci Rev Res 23:198–202
Li LZ, Xie TH, Li HJ, Qing C, Zhang GM, Sun MS (2007) Enhancing the thermostability of Escherichia coli l-asparaginase II by substitution with pro in predicted hydrogen-bonded turn structures. Enz Microb Tech 41:523–527
Martin JF (1989) Molecular genetics of amino acid-producing Corynebacteria. Symp Soc Gen Microbiol 44:25–59
Mashburn LT, Wriston JC Jr (1964) Tumor inhibitory effect of l-asparaginase from Escherichia coli. Arch Biochem Biophys 105:450–453
Moreno-Enriquez A, Evangelista-Martinez Z, Gonzalez-Mondragon EG, Calderon-Flores A, Arreguin R, Perez-Ruedas E, Huerta-Saquero A (2012) Biochemical characterization of recombinant l-asparaginase (AnsA) from Rhizobium etli, a member of an increasing Rhizobial-type family of l-asparaginases. J Microbiol Biotechnol 22:292–300
Narayana KJP, Kumar KG, Vijayalakshmi M (2008) l-Asparaginase production by Streptomyces albidoflavus. Indian J Microbiol 48:331–336
Narta U, Roy S, Kanwar SS, Azmi W (2011) Improved production of l-asparaginase by Bacillus brevis cultivated in the presence of oxygen-vectors. Bioresour Technol 102:2083–2085
Onishi Y, Yano S, Thongsanit J, Takagi K, Yoshimune K, Wakayama M (2011) Expression in Escherichia coli of a gene encoding type II l-asparaginase from Bacillus subtilis, and characterization of its unique properties. Ann Microbiol 61:517–524
Patil S, Coutsouvelis J, Spencer A (2011) Asparaginase in the management of adult acute lymphoblastic leukaemia: is it used appropriately? Cancer Treat Rev 37:202–207
Pedreschi F, Kaack K, Granby K (2008) The effect of asparaginase on acrylamide formation in French fries. Food Chem 109:386–392
Pedreschi F, Mariotti S, Granby K, Risum J (2011) Acrylamide reduction in potato chips by using commercial asparaginase in combination with conventional blanching. LWT-Food Sci Technol 44:1473–1476
Pieters R, Hunger SP, Boos J, Rizzari C, Silverman L, Baruchel A, Goekbuget N, Schrappe M, Pui CH (2011) l-Asparaginase treatment in acute lymphoblastic leukemia. Cancer 117:238–249
Pokrovskaya M, Pokrovskiy V, Aleksandrova S, Anisimova NY, Andrianov R, Treschalina E, Ponomarev G, Sokolov N (2012a) Recombinant intracellular Rhodospirillum rubrum l-asparaginase with low l-glutaminase activity and antiproliferative effect. Biochemistry-Moscow 6:123–131
Pokrovskaya MV, Aleksandrova SS, Pokrovsky VS, Omeljanjuk NM, Borisova AA, Anisimova NY, Sokolov NN (2012b) Cloning, expression and characterization of the recombinant Yersinia pseudotuberculosis l-asparaginase. Protein Expres Purif 82:150–154
Prakasham RS, Rao CS, Rao RS, Lakshmi GS, Sarma PN (2007) l-Asparaginase production by isolated Staphylococcus sp-6A: design of experiment considering interaction effect for process parameter optimization. J Appl Microbiol 102:1382–1391
Roth G, Nunes J, Rosado L, Bizarro C, Volpato G, Nunes C, Renard G, Basso L, Santos D, Chies J (2013) Recombinant Erwinia carotovora l-asparaginase II production in Escherichia coli fed-batch cultures. Braz J Chem Eng 30:245–256
Sahu MK, Sivakumar K, Poorani E, Thangaradjou T, Kannan L (2007) Studies on l-asparaginase enzyme of actinomycetes isolated from estuarine fishes. J Environ Biol 28:465–474
Sanjeeviroyar A, Rajendran A, Muthuraj M, Basha KM, Thangavelu V (2010) Sequential optimization and kinetic modeling of l-asparaginase production by Pectobacterium carotovorum in submerged fermentation. Asia-Pac J Chem Eng 5:743–755
Savitri AN, Azmi W (2003) Microbial l-asparaginase: a potent antitumour enzyme. Indian J Biotechnol 2:184–194
Shibayama K, Takeuchi H, J-i W, Mori S, Arakawa Y (2011) Biochemical and pathophysiological characterization of Helicobacter pylori asparaginase. Microbiol Immunol 55:408–417
Shrivastava A, Khan AA, Shrivastav A, Jain SK, Singhal PK (2012) Kinetis studies of l-asparaginase from Penicillium digitatum. Prep Biochem Biotechnol 42:574–581
Singh Y, Srivastava S (2012) Statistical and evolutionary optimization for enhanced production of an anti-leukemic enzyme, l-asparaginase, in a protease-deficient Bacillus aryabhattai ITBHU02 isolated from the soil contaminated with hospital waste. Indian J Exp Biol 51:322–335
Singh Y, Srivastava S (2014) Performance improvement of Bacillus aryabhattai ITBHU02 for high-throughput production of a tumor-inhibitory l-asparaginase using a kinetic model based approach. J Chem Technol Biotechnol 89:117–127
Singh Y, Gundampati RK, Jagannadham MV, Srivastava S (2013) Extracellular l-asparaginase from a protease-deficient Bacillus aryabhattai ITBHU02: purification, biochemical characterization, and evaluation of antineoplastic activity in vitro. Appl Biochem Biotechnol 171:1759–1774
Stecher A, Morgantetti de Deus P, Polikarpov I, Abrahao-Neto J (1999) Stability of l-asparaginase: an enzyme used in leukemia treatment. Pharm Acta Helv 74:1–9
Sudhir AP, Dave BR, Trivedi KA, Subramanian RB (2012) Production and amplification of an l-asparaginase gene from actinomycete isolate Streptomyces ABR2. Ann Microbiol 62:1609–1614
Tareke E, Rydberg P, Karlsson P, Eriksson S, Törnqvist M (2002) Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem 50:4998–5006
Thenmozhi C, Sankar R, Karuppiah V, Sampathkumar P (2011) l-Asparaginase production by mangrove derived Bacillus cereus MAB5: optimization by response surface methodology. Asian Pac J Trop Med 4:486–491
Verma N, Kumar K, Kaur G, Anand S (2007a) E. coli K-12 asparaginase-based asparagine biosensor for leukemia. Artif Cell Blood Sub 35:449–456
Verma N, Kumar K, Kaur G, Anand S (2007b) l-Asparaginase: a promising chemotherapeutic agent. Crit Rev Biotechnol 27:45–62
Vidya J, Pandey A (2012) Recombinant expression and characterization of l-asparaginase II from a moderately thermotolerant bacterial isolate. Appl Biochem Biotechnol 167:973–980
Vidya J, Vasudevan UM, Soccol CR, Pandey A (2011) Cloning, functional expression and characterization of l-asparaginase II from E. coli MTCC 739. Food Technol Biotechnol 49:286–290
Vidya J, Ushasree MV, Pandey A (2014) Effect of surface charge alteration on stability of l-asparaginase II from Escherichia sp. Enz Microb Tech 56:15–19
Wink PL, Bogdawa HM, Renard G, Chies JM, Basso LA, Santos DS (2010) Comparison between two Erwinia carotovora l-asparaginase II constructions: cloning, heterologous expression, purification, and kinetic characterization. J Microbial Biochem Technol 2:13–19
Yano S, Minato R, Thongsanit J, Tachiki T, Wakayama M (2008) Overexpression of type I l-asparaginase of Bacillus subtilis in Escherichia coli, rapid purification and characterisation of recombinant type I l-asparaginase. Ann Microbiol 58:711–716
Zhang YQ, Xiang RL, Yan HB, Chen XX (2008) Preparation of silk fibroin nanoparticles and their application to immobilization of l-asparaginase. Chem J Chin Univ 29:628–633
Zhang YQ, Wang YJ, Wang HY, Zhu L, Zhou ZZ (2011) Highly efficient processing of silk fibroin nanoparticle-l-asparaginase bioconjugates and their characterization as a drug delivery system. Soft Matter 7:9728–9736
Zhu JH, Liu J (2008) Improving the extraction of intracellular l-asparaginase by high-pressure homogenization. Asia-Pac J Chem Eng 3:211–216
Zhu JH, Yan XL, Chen HJ, Wang ZH (2007) In situ extraction of intracellular l-asparaginase using thermoseparating aqueous two-phase systems. J Chromatogra A 1147:127–134
Zuo S, Xue D, Zhang T, Jiang B, Mu W (2014) Biochemical characterization of an extremely thermostable l-asparaginase from Thermococcus gammatolerans EJ3. J Mol Catal B Enzym 109:122–129
Acknowledgments
This work was supported by the 973 Project (No. 2012CB720802), the 863 Project (No. 2011AA100904), and the Support Project of Jiangsu Province (No. BK20130001) and Shaoxing City (No. 2013A23002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zuo, S., Zhang, T., Jiang, B. et al. Recent research progress on microbial l-asparaginases. Appl Microbiol Biotechnol 99, 1069–1079 (2015). https://doi.org/10.1007/s00253-014-6271-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6271-9