Abstract
Staphylococcus aureus is one of the most important pathogens in humans and animals. The formation of biofilm by S. aureus is considered an important mechanism of antimicrobial resistance. Therefore, finding effective drugs against the biofilm produced by S. aureus has been a high priority. Licochalcone A (LAA), a natural plant product, was reported to have antibacterial activities and showed good activity against all 21 tested strains of S. aureus biofilm and planktonic cells. To detect the possible molecular mechanism of LAA against S. aureus biofilm or planktonic cells, Affymetrix GeneChips were used to determine the global comparative transcription of S. aureus biofilm and planktonic cells triggered by treatment with sub-bactericidal and sub-inhibitory concentrations of LAA, respectively. LAA significantly altered (greater than a 2- or less than −2-fold change) the expression of 693 genes in planktonic cells and 817 genes in biofilm. The levels of genes encoding autolysis-associated proteins, cell wall proteins, pathogenic factors, protein synthesis genes, and enzymes involved in capsule synthesis were significantly altered in LAA-treated S. aureus. Furthermore, some differences observed in the microarray analysis were verified by real-time RT–PCR. To our knowledge, this is the first observation of phenotype and expression profiles of S. aureus biofilm and planktonic cells in response to LAA treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus is an important worldwide human pathogen that leads to a number of diseases including endocarditis, cellulitis, impetigo, etc. (Cameron et al. 2012). Greater than 60 % of S. aureus isolates are now resistant to methicillin, and some strains have developed resistance to more than 20 different antimicrobial agents. Resistance in clinical isolates is mostly related to the capacity for biofilm formation. The main component of the biofilm extracellular matrix in S. aureus is polysaccharide intercellular adhesin (PIA), which adheres the bacterial cells together and renders an increase in resistance to antimicrobial agents and host defenses (Rohde et al. 2001). Therefore, finding effective drugs against S. aureus biofilm has been a high priority.
Licochalcone A (LAA) (Fig. 1), a main phenolic component of the licorice species, Glycyrrhiza inflata, has been reported to have various biological activities, e.g., anti-inflammatory (Shibata 2000), anti-protozoal (Chen et al. 2001), anti-tumor (Shibata 2000), anti-oxidative (Wu et al. 2011), and antimicrobial (Liu et al. 2008) effects. LAA has antibacterial activities against both methicillin-sensitive and methicillin-resistant S. aureus (Liu et al. 2008). Our preliminary study has found that LAA has anti-biofilm activity against S. aureus strains (unpublished). However, the inhibitory characteristics and mechanism of LAA against S. aureus biofilms needs thorough investigation. Transcriptional profiles generated by GeneChip analysis of bacteria are a tool with which to investigate differential gene expression, exploring possible mechanisms of antimicrobial activity (Smith et al. 2010). Transcriptional profiles of S. aureus response to tigecycline, berberine, triclosan, and rhein have been previously performed (Wang et al. 2008; Jang et al. 2008). Moreover, virulence factors of S. aureus and Staphylococcus epidermidis were detected using an electrical protein array chip technology (Quiel et al. 2010). Although new techniques (such as tiling microarrays, protein biochips, and microfluidics) are gaining popularity, the reliability of results from cDNA GeneChip analyses has ensured that this widely used technique still has a place in gene expression studies.
In this study, we found than LAA had antimicrobial activity against S. aureus in planktonic or biofilm forms. Transcriptomic analysis showed the levels of genes encoding autolysis-associated proteins, cell wall proteins, pathogenic factors, protein synthesis, and enzymes involved in capsule synthesis were significantly altered in LAA-treated S. aureus biofilm and planktonic cells, and some of the microarray results were validated using real-time RT–PCR.
Materials and methods
Bacterial strains and materials
S. aureus ATCC 29213 was obtained from the China Medical Culture Collection Center. Twenty clinical samples of S. aureus (1078, 1018, 1524, 1628, 1932, 2001, 2005, 2027, 2233, 2441, 2484, 2750, 2796, 2871, 3076, 3218, 3198, 3212, 3701, and 3808) were isolated from the First Hospital of Jilin University, Changchun, China. LAA was purchased from Sigma-Aldrich (St Louis, MO, USA), and a stock solution was made in dimethyl sulfoxide (DMSO; Sigma-Aldrich). The final concentration of DMSO applied to culture systems was adjusted to 0.1 % (v/v), also in control groups.
Planktonic antimicrobial susceptibility testing
The minimum inhibitory concentrations (MICs) of LAA against the 21 S. aureus strains described above were determined according to the standard NCCLS procedures (National Committee for Clinical Laboratory Standards, also called CLSI 2005). The MIC was defined as the lowest concentration at which no visible growth was observed. The minimum bactericidal concentrations (MBCs) were identified as the lowest concentration to show no microbial growth on agar plates for 24 h at 37 °C. The assays were repeated in triplicate.
Establishment of microbial biofilms
Biofilms were established as previously described (Yu et al. 2008). To confirm slime production, microorganisms were cultured on Congo Red agar (CRA) (Yu et al. 2008), and the morphology of biofilms was observed under a microscope by staining with crystal violet, silver, and a LIVE/DEAD BacLight Bacterial Viability kit (Molecular Probes, Inc., Eugene, OR, USA).
Biofilm antimicrobial susceptibility testing
Biofilm antimicrobial susceptibility tests were performed as previously described (Yu et al. 2008). The minimum biofilm inhibition concentration (MBIC) was determined as the lowest concentration to show growth below or equal to that of the control. The minimum biofilm bactericidal concentration (MBBC) was identified as the lowest concentration demonstrating no bacterial growth.
Confocal laser scanning microscopy (CLSM)
Biofilm staining with a LIVE/DEAD BacLight Bacterial Viability kit (Invitrogen Molecular Probes, Inc., Eugene, OR, USA) was performed as previously described (Yu et al. 2008). CLSM images were collected using an Olympus FV1000 confocal laser scanning microscope (Olympus, Tokyo, Japan) with a ×40 objective lens. For detection of SYTO 9 (green channel), we used 488 nm excitation and 520 nm emission filter settings. For PI detection (red channel), we used 543 nm excitation and 572 nm emission filter settings. Image analyses and export were performed in Olympus Fluoview software version 1.7.3.0.
Growth curves
The growth curves of planktonic S. aureus ATCC 29213 were described in a previous study (Xing et al. 2012). The planktonic cell growth was spectrophotometrically monitored as the optical density (OD) at 600 nm, and the biofilm cell growth was spectrophotometrically monitored using an XTT assay at 540 nm, which was recorded at specific time intervals.
GeneChip analysis of planktonic and biofilm S. aureus with LAA treatment
The S. aureus strain ATCC 29213 was treated with LAA as previously described (Xing et al. 2012). In brief, S. aureus planktonic and biofilm cells were treated with LAA for 60 min at final concentrations of 1/2× MIC (2 μg mL−1) and 4× MIBC (64 μg mL−1), respectively. Construction of a genome-wide DNA microarray for S. aureus, RNA preparation, cDNA labeling, the GeneChip hybridization procedure, and microarray data processing were performed as described previously (Hutter et al. 2004). To select the differentially expressed genes, we used threshold values of ≥2 and ≤−2-fold change between three LAA-treated planktonic or biofilm samples and their controls. The false discovery rate (FDR) significance level was <5 %.
Quantitative real-time RT–PCR
RT–PCR was used to verify the microarray results. The RNA preparations from LAA-treated and control samples used in the microarray experiments were also used for RT–PCR follow-up studies. The cDNA was subjected to real-time PCR using the primer pairs listed in Table 1. RT–PCR was performed in triplicate using the 7000 Sequence Detection System (Applied Biosystems, USA) (Hutter et al. 2004).
Results
Phenotype analysis of S. aureus biofilms and planktonic cells with LAA treatment
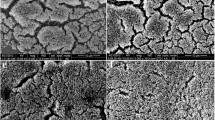
The most important step in biofilm research should be the construction of biofilm models. The ability of S. aureus to form biofilms was tested using the CRA method. As shown in Fig. 2a, biofilm producers generated black colonies. The morphology of the biofilms was further ascertained by microscopy using crystal violet, silver, and LIVE/DEAD bacterial viability kit staining (Fig. 2b–d).
The images of stained S. aureus 29213 biofilm grown on cover slide discs. a Ability to form biofilm was tested by the Congo red agar (CRA) method, which produced black colonies. b Two-day-old biofilm was washed gently three times with PBS and stained with 1 % crystal violet. c Two-day-old biofilm was washed gently three times with PBS with silver-staining. d Two-day-old biofilms were stained with the LIVE/DEAD bacterial viability kit. Live cells are stained with SYTO 9 and shown in green
In the assessment of the antimicrobial activities of LAA, the MIC and MBC values for the drug treatment against planktonic cells for all 21 strains ranged from 1 to 8 μg mL−1 and from 2 to 16 μg mL−1, respectively; in biofilms, the MBIC and MBBC values for LAA treatment ranged from 8 to 64 μg mL−1 and ≥1,024 μg mL−1, respectively (Table 2). This result showed that LAA effectively inhibited S. aureus planktonic cells and biofilm.
The effect of LAA against S. aureus ATCC 29213 biofilm grown on cover slides was observed by CLSM (Fig. 3a–b). With LAA treatment, Fig. 3 shows that there was a greatest decrease in Syto9 (green) staining (live cells) of biofilms, and there was a largest increase in PI (red) staining (dead cells) of biofilms, with changed drug concentrations (0–128 μg mL−1) applied for the same time period. The thickness of biofilm after treatment of LAA was quantified by CLSM by one-way ANOVA analysis (Fig. 4). Figure 4 shows that the biofilm had become thinner with treatment of 0 to 128 μg mL−1 LAA for same times, which suggested that LAA prevented the biofilm from reaching the same thickness as the intreated control groups (only containing 0.1 % v/v DMSO). As previously shown, this concentration of DMSO did not interfere with the testing system (You et al. 2013). The results highlighting the damage caused to the biofilm by LAA treatment was time dependent and concentration dependent.
Confocal laser scanning microscopy image of LIVE/DEAD®-stained S. aureus 29213 biofilm treated with LAA and grown on cover slide discs. a S. aureus 29213 were incubated at 37 °C for 24 h and treated with LAA. 1 24 h growth control; 2 1-day-old biofilm of S. aureus 29213 treated with 16 μg mL−1 LAA; 3 1-day-old biofilm of S. aureus 29213 treated with 64 μg mL−1 LAA; 4 1-day-old biofilm of S. aureus 29213 treated with 128 μg mL−1 LAA. b S. aureus 29213 were incubated at 37 °C for 48 h and treated with LAA. 1 48 h growth control; 2 2-day-old biofilm of S. aureus 29213 treated with 16 μg mL−1 LAA; 3 2-day-old biofilm of S. aureus 29213 treated with 64 μg mL−1 LAA; 4 2-day-old biofilm of S. aureus 29213 treated with 128 μg mL−1 LAA
The quantification of biofilm thickness (from S. aureus 29213 treated with LAA) by confocal laser scanning microscopy. Comparisons of mean values from three experiments were statistically evaluated by analysis of variance, followed by one-way ANOVA analysis. **p < 0.01 significant difference between 1-day-old biofilm and 2-day-old biofilm treated with LAA
In order to choose the suitable low LAA concentration against the S. aureus strain to be used in the transcription analysis, we determined growth curves of S. aureus ATCC 29213 with LAA. The growth curves of S. aureus ATCC 29213 with LAA treatment showed the OD of bacterial cultures increased steadily with 1, 2, and 2.5 μg mL−1 LAA treatment, but bacteria almost did not grow at 3 and 4 μg mL−1 of LAA (Fig. 5a). Compared with the control, the growth curve of biofilm showed that the optical density of cells decreased steadily with the addition of sub-MBBC concentrations of LAA (Fig. 5b). The results showed that there was a dose-dependent inhibitory activity of LAA against S. aureus and a low LAA concentration displayed a bacteriostatic action against S. aureus planktonic cells and biofilm.
Total alteration of gene transcription responses to LAA exposure
To study the effects of low LAA concentrations on the S. aureus ATCC 29213 strain, we used a microarray to examine the transcription of planktonic cells and biofilm at a sub-MIC concentration (1/2× MIC, 2 μg mL−1) and a sub-MBBC concentration (4× MIBC, 64 μg mL−1) of LAA at the 60 min time point. The GeneChip analysis of LAA against S. aureus planktonic cells and biofilm revealed that 693 and 817 genes were differentially regulated, respectively. Of these, the expression levels of 375 and 355 genes were markedly elevated in planktonic cells and biofilm, respectively, and 318 and 462 genes were significantly decreased. The distribution of LAA-responsive genes and their biological roles in planktonic cells and biofilm are shown in Figs. 5 and 6, respectively (not including hypothetical proteins and unknown genes). The microarray-related data were submitted to Gene Expression Omnibus under accession number GSE58938. A relatively complete list of all differentially expressed genes from bacteria treated by LAA can be found in the supplementary Tables S1 and S2 in the Supplementary Material.
Functional classification. a Differentially expressed genes from S. aureus 29213 planktonic cells treated with LAA (2 μg mL−1) are grouped by functional classification. The differentially regulated genes were divided into 27 functional categories. The number of genes up-regulated and down-regulated for each functional group is represented. b Differentially expressed genes from S. aureus 29213 biofilm treated with LAA (64 μg mL−1) are grouped by functional classification. The differentially regulated genes were divided into 26 functional categories. The number of genes up-regulated and down-regulated for each functional group is represented
Expression levels of autolysis-associated genes following treatment with LAA
To determine the effect of LAA against S. aureus planktonic cells and biofilm, autolysis genes were analyzed using the GeneChip. In planktonic cells, the main autolysin gene lytM was significantly down-regulated by 4.0-fold. The transcript levels of the negative regulator of autolysis lrgB was markedly increased by 5.1-fold, while the levels of the positive regulators, agrA, lytR, and RNAIII, were significantly decreased. The down-regulation of lytM and agrA and up-regulation of lrgB expressions were confirmed by RT–PCR (Table 3). Recent reports have demonstrated that extracellular DNA (eDNA), an essential matrix molecule in S. aureus biofilm, is released in cell autolysis (Otto 2012). The levels of the major autolysin genes atl, slel, and lytM were markedly down-regulated within LAA-treated biofilm. In S. aureus, cell death and lysis are controlled by the cid and lrg operons. The cidA gene encodes a murein hydrolase regulator that promotes cell lysis during biofilm development, whereas the lrg operon inhibits cell lysis (Sadykov and Bayles 2012). The transcript levels of the positive regulator cidA markedly decreased 2.6-fold, but the transcript levels of negative regulators of autolysis lrgA and lrgB notably increased 3.3- and 4.4-fold, respectively. In addition, the expression of agrA, a positive regulatory gene, was also down-regulated 1.5-fold. Surprisingly, transcription of the autolysin genes cidBC was significantly up-regulated. RT–PCR confirmed the decreased levels of atl, sle1, cidA, lytM, and agrA and observed increases in lrgA, lrgB, and cidB transcription levels (Table 4). This result suggests that LAA reduces S. aureus biofilm production by inhibiting autolysis in vitro, similar to our previous report in which magnolol reduced S. aureus biofilm production in vitro (Wang et al. 2011).
Influence of LAA on capsule synthesis and cell wall synthesis genes
To investigate whether LAA could undermine the protective barrier of S. aureus planktonic cells and biofilm, capsule synthesis and cell wall synthesis genes were analyzed. GeneChip analysis data showed that the expression levels of capsule synthesis genes betA, gbsA, and SA2175 were increased in planktonic cells (Table 3). Capsule synthesis has an important role not only in planktonic cells but also in the adherence and formation stages of biofilm formation. Compared with planktonic cells, levels of genes (capABCDEFGHIJKLMNOP) encoding the capsule polysaccharide synthesis enzymes were significantly up-regulated, but the levels of cap1A and cap1C were down-regulated in biofilm. The levels of asp23, which codes for alkaline shock protein 23, was increased. This result shows that biofilm production was affected by LAA treatment. RT–PCR confirmed the up-regulation of capC and capG levels (Table 4). The cell wall is important to resist damage from external factors. In planktonic cells treated with LAA, the transcript levels of cell wall synthesis genes SA0522 and SA0523 were significantly decreased, but the transcript levels of pbp4, sgtB, murZ, hmrA, gtaB, drp35, and SA0205 were markedly increased (Table 3). Resch et al. (2005) suggested that processes related to cell wall synthesis also play a key role in biofilm persistence. In biofilm cells challenged with LAA, the expression levels of cell wall synthesis genes lytM, lytH, tagA, tag, pbp3, pbp4, llm, dltA, dltC, murE, uppS, and fmhA were significantly down-regulated, but the transcript levels of drp35 and fmtB were significantly increased by 3.6- and 2.1-fold, respectively (Table 4). This suggests that bacterial activation of the stress response in an attempt to withstand the antimicrobial challenge was also inhibited by LAA.
Effect of LAA on protein synthesis genes
We further observed the effects of LAA on protein synthesis genes in S. aureus planktonic cells and biofilm. The expression levels of protein synthesis genes serS and ileS were significantly decreased, but transcript levels of leuS, encoding leucyl-tRNA synthetase, were up-regulated 3.4-fold (Table 3) in planktonic cells. As shown in Table 4, nine genes encoding ribosomal proteins in biofilm cells after LAA treatment were down-regulated by 2.0- to 4.3-fold. At the same time, expression of the initiation factor gene infC and the genes of the transcription factors EF and T were notably decreased; moreover, the expression levels of protein synthesis genes fmt, gatA, gatC, glyS, hisS, lepA, pheS, prfA, prfC, pth, and tyrS were significantly down-regulated. This result showed that LAA inhibited S. aureus planktonic cells or biofilm by inhibiting genes associated with protein synthesis.
Expression alteration of virulence-associated genes after LAA treatment
The presence of LAA also reduced the level of virulence-associated genes in S. aureus. GeneChip analysis data showed significant down-regulation of plc, spa, geh, set15, sdrCE, sbi, hlgC, clfB, lip, RNAIII, hysA, nuc1, splAFC, sak, SA0102, SA0587, SA1007, and eight additional hypothetical virulence genes in planktonic cells after LAA treatment. Furthermore, the levels of sspA and sspB were markedly decreased (2.8- and 3.3-fold, respectively). The S. aureus V8 protease, encoded by sspA, is a major serine protease (Rice et al. 2001). This serine protease affects autolytic activity via the expression of the autolysin gene atl and the proteolytic maturation of the cysteine protease SspB coded by sspB (Komatsuzawa et al. 2001). However, the levels of expression of fnbB, fmtA SA1577, SA1898, and SA2097 were significantly up-regulated in LAA-treated biofilm. RT–PCR confirmed the observed decreases in clfB and RNAIII levels and the observed increase in fnbB levels (Table 3). These data suggest that LAA could suppress planktonic cells by affecting virulence-associated genes.
In staphylococci, toxins are responsible for its lethal pathogenicity. Until now, 20 serologically different staphylococcal superantigens have been described, including TSST-1, the staphylococcal enterotoxins (enterotoxins A to E and G to J), and the staphylococcal enterotoxin-like toxins (Smith et al. 2010). As shown in Table 4, the levels of toxin genes set15, sen, yent2, yent1, sei, sem, seo, and truncated (hlb) were notably decreased in biofilm cells. In S. aureus, virulence gene expression is mainly regulated by at least seven two-component systems (TCSs) (ArlRS, SaeRS, AgrAC, SrrAB, LytRS, YycFG, and VraRS), the DNA-binding protein SarA, the SarA family (SarS, SarR, SarU, SarT, SarV, MgrA, and TcaR) (Novick 2003), and an alternative sigma factor (SigB). The accessory gene regulator agr suppresses the post-exponential phase expression of cell surface binding proteins and enhances the expression of secreted proteins. Unlike agr, the sar locus activates the synthesis of both extracellular and surface-bound proteins in S. aureus (Cheung and Zhang 2002) The two-component system lytSR is involved in the regulation of peptidoglycan hydrolases, and lrgA and lrgB are positively regulated by lytSR (Sadykov and Bayles 2012). Recent reports have revealed that lytSR and lrgAB are down-regulated by ArlRS (Liang et al. 2005). The levels of the response regulators agrA, lytR, lytS, mgrA, and arlR were reduced after LAA treatment in biofilm. In addition, the expression levels of the virulence genes coa, sdrCE, mapW (truncated), mapW (truncated), hlb (truncated), sbi, and isaA were markedly down-regulated, while icaA and icaD were both slightly down-regulated (1.8-fold) in biofilm after LAA exposure. This result showed that LAA could inhibit the expression of biofilm virulence-associated genes.
Virulence genes have been implicated in biofilm formation. The attachment of S. aureus cells to abiotic or biological surfaces is the key step in biofilm development, and this is mediated by the protein adhesins on the microbial surface (Smith et al. 2010). The well-characterized adhesins in S. aureus are the structurally related fibrinogen binding proteins ClfA and ClfB, fibronectin-binding proteins FnbA and FnbB, and the collagen adhesin Can (Wann et al. 2000). We observed that the virulence gene fnbB (encoding FnbB) is down-regulated 6.9-fold in biofilm cells treated with LAA. Previous reports have shown that expression of the fnb genes is enhanced by SarA (encoded by sarA) through promoter binding in vitro (Chien et al. 1999). The level of sarA decreased 5.9-fold in the presence of LAA, and this may have directly influenced the transcription of fnbB but not fnbA. The level of the virulence gene clfB, which encodes ClfB, a protein that mediates the adherence of S. aureus to immobilized and soluble fibrinogen (Entenza et al. 2000), was decreased 3.6-fold in biofilm. However, the levels of expression of ebhAB, fmtB (mrp), hlgB, and lip were significantly up-regulated. Real-time RT–PCR confirmed the decreased fnbB, isaA, and clfB levels (Table 4). This result showed that LAA might affect S. aureus biofilm by inhibiting the initial adhesion step.
Discussion
In a biofilm, a microbial community is attached to a surface and embedded in a self-produced matrix composed of extracellular polymeric substances. The bacterial cells grown in a biofilm have an increased resistance to grazing, desiccation, and antimicrobial agents compared to planktonic cells. In addition, the resistance of S. aureus biofilms were impacted of environmental conditions or sessile cells membrane fluidity with drug treatment (e.g., disinfectants) (Abdallah et al. 2014). In this study, we observed lawn-like bacteria that crumbled and overspread on cover slide discs visualized with crystal violet, silver, or SYTO 9-staining (Fig. 2b–d). These results indicated the successful formation of S. aureus biofilms in vitro. Previous reports have shown that LAA has antibacterial activities against S. aureus. We found that LAA not only strongly inhibited bacterial activity against 21 microorganisms tested as planktonic cells but also against biofilm (Table 2). We further corroborated the inhibitory activity of LAA against S. aureus biofilm by the CLSM (Fig. 3a–b).
Our previous reports have revealed that the compound concentration is of crucial importance for data quality in transcriptome analysis, and the best results were obtained with sub-inhibitory concentrations (concentrations that are just low enough not to affect the growth of the organism) (Yu et al. 2008). We chose the 60 min time point for drug treatment to avoid confounding secondary drug effects, and it has been claimed that compounds should be at a low concentration to lessen the effect on the growth of the organism and obtain optimal microarray results (Hutter et al. 2004).
When the S. aureus planktonic cells and biofilm were treated by LAA, the transcriptome analysis showed the expression of genes coding autolysis-associated protein, cell wall-associated protein, pathogenic factors, capsule synthesis, and protein synthesis were significantly regulated. Some of the same pathways were affected by LAA exposure in both S. aureus biofilm and planktonic cells, but most concrete genes were differentially regulated in biofilm compared with planktonic cells.
In staphylococcal biofilm, PIA is an adhesive molecule, and it is synthesized by the icaADBC-encoded proteins; the ica operon appears to be present in all S. aureus strains (Otto 2012). The ica genes, required for adhesion and biofilm formation, are up-regulated only at the beginning of biofilm formation, rather than during maturation and persistence (Resch et al. 2005). In this study, the microarray of LAA-treated S. aureus biofilm results showed that the levels of icaADBC decreased less than 2-fold. However, we used LAA to treat a mature 2-day biofilm; thus, the level of ica was only slightly changed by LAA treatment in this study.
In planktonic cells, the expression of fnbB was enhanced 2.1-fold, and clfB was decreased 2.0-fold after LAA treatment. Blickwede et al. (2005) reported similar findings that fnbB was up-regulated 2.0-fold in the S. aureus strain Newman exposed to a sub-lethal concentration clindamycin. At the same time, LAA treatment of biofilm appeared to increase the expression of the adhesin genes ebhA and ebhB. Their up-regulation was also reported in a study on tigecycline influence on the expression of virulence factors in biofilm cells in methicillin-resistant S. aureus (Smith et al. 2010). This result suggests that the binding capacity of S. aureus may be altered during drug-induced stress.
In addition, LAA also markedly inhibited the expression of autolysis-associated genes. The genes fmtA and fmtB were recently described as positive regulators of autolysis (Manna et al. 2004). Surprisingly, LAA treatment increased the level of fmtB by 2.1-fold in S. aureus planktonic cells, and the levels of fmtA were increased by 2.1-fold in S. aureus biofilm cells.
The majority of S. aureus isolates produce either a serotype 5 or 8 capsular polysaccharide that has been shown to enhance bacterial virulence. The expression of the cap5, cap8, and asp23 genes was up-regulated in LAA-challenged biofilms. In addition, LAA-treated S. aureus planktonic cells also showed up-regulated capsular gene (SA2175, SA2405, SA2406) expressions. Other studies have also shown that exposure of sensitive S. aureus to sub-inhibitory levels of vancomycin leads to the elevation of capsular gene expression. This up-regulation of genes may be part of the bacterial stress response, an attempt to withstand the antimicrobial challenge. However, LAA treatment significantly down-regulated the levels of the cap1A and cap1C gene expression. Thus, the reduction in the levels of capsule gene expression may be a consequence of the decrease in virulence factors in the presence of LAA, which renders the organism easily cleared by the host phagocytic immune response.
Unlike antibiotics, anti-virulence agents diminish bacterial virulence and may not lead to drug resistance. Many anti-virulence agents had a good effect against persistent S. aureus infection, e.g., indole derivatives (Lee et al. 2013). In this study, S. aureus RNAIII, encoding δ-hemolysin, plays a key role in the quorum-sensing-dependent central regulatory circuit and coordinately regulates several virulence-associated genes (Novick 2003). Previous studies have shown that RNAIII inhibits the expression of surface protein A (spa), which is one of the major virulence factors during exponential phase (Gao and Stewart 2004). In this study, the expression of RNAIII and spa in LAA-treated S. aureus planktonic cells decreased 2.5- and 8.5-fold, respectively. Our study has also shown that the expression of toxin genes was inhibited by LAA in S. aureus in planktonic cells and biofilm. At the same time, the differential expression of virulence factors in response to LAA may be coordinately regulated by these two-component signal transduction systems.
Unlike biofilm, planktonic cells challenged with LAA responded with significantly increased expression levels of the cell wall synthesis genes vraS, vraR, pbp4, sgtB, murZ, hmrA, gtaB, drp35, fmtA, SA1577, and SA0205. Previous studies of the S. aureus responses to the cell wall-active antibiotic vancomycin (Kuroda et al. 2003), oxacillin, bacitracin, and d-cycloserine (Utaida et al. 2003) identified a series of gene expression changes involved in cell wall synthesis, which seems to be predominantly regulated by the VraSR two-component regulatory system. McAleese et al. (2006) described that vancomycin treatment caused a core cell wall stress stimulation of 17 genes (including pbp2, pbp4, sgtB, murZ, hmrA, gtaB, and drp35). This result implies that although LAA harmed S. aureus biofilm, it also stressed S. aureus planktonic cells.
In addition to biofilm, the transcriptome analysis showed that LAA significantly inhibited the expression of initiation factors, elongation factors, ribosomal proteins, peptide chain release factor, tRNA synthetases (including glycyl-tRNA synthetase, histidyl-tRNA synthetase, and tyrosyl-tRNA synthetase), glutamyl-tRNA (Gln) amidotransferase, and GTP-binding protein of S. aureus. Previous reports indicated that the drug may elicit its antimicrobial effect on bacteria by binding to the 30S ribosomal subunit, preventing the incorporation of amino acid residues into the elongating peptide chain and inhibiting protein synthesis (Smith et al. 2010). In planktonic cells, LAA at a sub-lethal concentration inhibited the expression of tRNA synthetases (including glycyl-tRNA synthetase, histidyl-tRNA synthetase, and tyrosyl-tRNA synthetase). These results show that LAA also had effects on protein synthesis in S. aureus.
Bacterial biofilm infections can be very difficult to address, and the implanted (infected) device often has to be removed or replaced (Xing et al. 2012). Developing novel therapeutic agents or antibiotic alternatives may help solve the problem of biofilm infection. Plants and other natural materials may prove to be possible sources of new antibacterial and synergistic antibacterial compounds. In this study, we observed that LAA showed strong activity against S. aureus biofilm and planktonic cells, and it does influence the expression of some important genes in S. aureus biofilm and planktonic cells. These findings may have important implications for understanding the response mechanisms of S. aureus to LAA, and the results facilitate the further development of LAA as an antibacterial compound.
References
Abdallah M, Chataigne G, Ferreira-Theret P, Benoliel C, Drider D, Dhulster P, Chihib NE (2014) Effect of growth temperature, surface type and incubation time on the resistance of Staphylococcus aureus biofilms to disinfectants. Appl Microbiol Biotechnol 98:2597–2607
Blickwede M, Wolz C, Valentin-Weigand P, Schwarz S (2005) Influence of clindamycin on the stability of coa and fnbB transcripts and adherence properties of Staphylococcus aureus Newman. FEMS Microbiol Lett 252(1):73–78. doi:10.1016/j.femsle.2005.08.022
Cameron DR, Ward DV, Kostoulias X, Howden BP, Moellering RC, Eliopoulos GM, Peleg AY (2012) Serine/threonine phosphatase Stp1 contributes to reduced susceptibility to vancomycin and virulence in Staphylococcus aureus. J Infect Dis 205(11):1677–1687
Chen M, Zhai L, Christensen SB, Theander TG, Kharazmi A (2001) Inhibition of fumarate reductase in Leishmania major and L. donovani by chalcones. Antimicrob Agents Chemother 45(7):2023–2029
Cheung AL, Zhang G (2002) Global regulation of virulence determinants in Staphylococcus aureus by the SarA protein family. Front Biosci 7:d1825–d1842
Chien Y-T, Manna AC, Projan SJ, Cheung AL (1999) SarA, a global regulator of virulence determinants in Staphylococcus aureus, binds to a conserved motif essential for sar-dependent gene regulation. J Biol Chem 274(52):37169–37176
Clinical and Laboratory Standards Institute (CLSI) (2005) Performance standards for antimicrobial susceptibility testing. Fifteenth informational supplement. Document M100-S15. CLSI/NCCLS, Wayne, PA, USA
Entenza J, Foster T, Eidhin DN, Vaudaux P, Francioli P, Moreillon P (2000) Contribution of clumping factor B to pathogenesis of experimental endocarditis due to Staphylococcus aureus. Infect Immun 68(9):5443–5446
Gao J, Stewart GC (2004) Regulatory elements of the Staphylococcus aureus protein A (Spa) promoter. J Bacteriol 186(12):3738–3748
Hutter B, Schaab C, Albrecht S, Borgmann M, Brunner NA, Freiberg C, Ziegelbauer K, Rock CO, Ivanov I, Loferer H (2004) Prediction of mechanisms of action of antibacterial compounds by gene expression profiling. Antimicrob Agents Chemother 48(8):2838–2844
Jang HJ, Chang MW, Toghrol F, Bentley WE (2008) Microarray analysis of toxicogenomic effects of triclosan on Staphylococcus aureus. Appl Microbiol Biotechnol 78:695–707
Komatsuzawa H, Ohta K, Fujiwara T, Choi GH, Labischinski H, Sugai M (2001) Cloning and sequencing of the gene, fmtC, which affects oxacillin resistance in methicillin-resistant Staphylococcus aureus. FEMS Microbiol Lett 203(1):49–54
Kuroda M, Kuroda H, Oshima T, Takeuchi F, Mori H, Hiramatsu K (2003) Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol Microbiol 49(3):807–821
Lee JH, Cho HS, Kim Y, Kim JA, Banskota S, Cho MH, Lee J (2013) Indole and 7-benzyloxyindole attenuate the virulence of Staphylococcus aureus. Appl Microbiol Biotechnol 97:4543–4552
Liang X, Zheng L, Landwehr C, Lunsford D, Holmes D, Ji Y (2005) Global regulation of gene expression by ArlRS, a two-component signal transduction regulatory system of Staphylococcus aureus. J Bacteriol 187(15):5486–5492
Liu XL, Xu YJ, Go ML (2008) Functionalized chalcones with basic functionalities have antibacterial activity against drug sensitive Staphylococcus aureus. Eur J Med Chem 43(8):1681–1687
Manna AC, Ingavale SS, Maloney M, Van Wamel W, Cheung AL (2004) Identification of sarV (SA2062), a new transcriptional regulator, is repressed by SarA and MgrA (SA0641) and involved in the regulation of autolysis in Staphylococcus aureus. J Bacteriol 186(16):5267–5280
McAleese F, Wu SW, Sieradzki K, Dunman P, Murphy E, Projan S, Tomasz A (2006) Overexpression of genes of the cell wall stimulon in clinical isolates of Staphylococcus aureus exhibiting vancomycin-intermediate-S. aureus-type resistance to vancomycin. J Bacteriol 188:1120–1133
Novick RP (2003) Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48(6):1429–1449
Otto M (2012) Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med 64(1):175–188. doi:10.1146/annurev-med-042711-140023
Quiel A, Jürgen B, Piechotta G, Le Foll AP, Ziebandt AK, Kohler C, Köster D, Engelmann S, Erck C, Hintsche R, Wehland J, Hecker M, Schweder T (2010) Electrical protein array chips for the detection of staphylococcal virulence factors. Appl Microbiol Biotechnol 85:1619–1627
Resch A, Rosenstein R, Nerz C, Götz F (2005) Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl Environ Microbiol 71(5):2663–2676
Rice K, Peralta R, Bast D, de Azavedo J, McGavin MJ (2001) Description of staphylococcus serine protease (ssp) operon in Staphylococcus aureus and nonpolar inactivation of sspA-encoded serine protease. Infect Immun 69(1):159–169
Rohde H, Knobloch JK, Horstkotte MA, Mack D (2001) Correlation of Staphylococcus aureus icaADBC genotype and biofilm expression phenotype. J Clin Microbiol 39(12):4595–4596
Sadykov MR, Bayles KW (2012) The control of death and lysis in staphylococcal biofilms: a coordination of physiological signals. Curr Opin Microbiol 15(2):211–215
Shibata S (2000) A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi 120(10):849–862
Smith K, Gould KA, Ramage G, Gemmell CG, Hinds J, Lang S (2010) Influence of tigecycline on expression of virulence factors in biofilm-associated cells of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 54(1):380–387
Utaida S, Dunman P, Macapagal D, Murphy E, Projan S, Singh V, Jayaswal R, Wilkinson B (2003) Genome-wide transcriptional profiling of the response of Staphylococcus aureus to cell-wall-active antibiotics reveals a cell-wall-stress stimulon. Microbiology 149(10):2719–2732
Wang D, Yu L, Xiang H, Fan J, He L, Guo N, Feng H, Deng X (2008) Global transcriptional profiles of Staphylococcus aureus treated with berberine chloride. FEMS Microbiol Lett 279(2):217–225
Wang D, Jin Q, Xiang H, Wang W, Guo N, Zhang K, Tang X, Meng R, Feng H, Liu L, Wang X, Liang J, Shen F, Xing M, Deng X, Yu L (2011) Transcriptional and functional analysis of the effects of magnolol: inhibition of autolysis and biofilms in Staphylococcus aureus. PLoS ONE 6(10):e26833
Wann ER, Gurusiddappa S, Höök M (2000) The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J Biol Chem 275(18):13863–13871
Wu T-Y, Khor T, Saw C, Loh S, Chen A, Lim S, Park J, Cai L, Kong A-N (2011) Anti-inflammatory/anti-oxidative stress activities and differential regulation of Nrf2-mediated genes by non-polar fractions of tea Chrysanthemum zawadskii and licorice Glycyrrhiza uralensis. AAPS J 13(1):1–13
Xing M, Shen F, Liu L, Chen Z, Guo N, Wang X, Wang W, Zhang K, Wu X, Wang X, Li Y, Sun S, Yu L (2012) Antimicrobial efficacy of the alkaloid harmaline alone and in combination with chlorhexidine digluconate against clinical isolates of Staphylococcus aureus grown in planktonic and biofilm cultures. Lett Appl Microbiol 54(5):475–482
You YO, Choi NY, Kang SY, Kim KJ (2013) Antibacterial activity of Rhus javanica against methicillin-resistant Staphylococcus aureus. Evid Based Complement Alternat Med 2013:549207
Yu L, Xiang H, Fan J, Wang D, Yang F, Guo N, Jin Q, Deng X (2008) Global transcriptional response of Staphylococcus aureus to rhein, a natural plant product. J Biotechnol 135(3):304–308
Acknowledgments
This work was supported by Important National Science and Technology Specific Projects (2012ZX10003002), the National Nature Science Foundation of China (No. 31172364; No. 31271951; No. 31000822), Program for New Century Excellent Talents in University (NCET-09-0434; NCET-13-0245), Fundamental Research Program of Shen Zhen (JCYJ20130401172016183; JCYJ20120616142424467), and Shenzhen Promotion Plan Basic Research Laboratory in 2012 (ZDSY20120616141302982).
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Fengge Shen and Xudong Tang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 904 kb)
Rights and permissions
About this article
Cite this article
Shen, F., Tang, X., Wang, Y. et al. Phenotype and expression profile analysis of Staphylococcus aureus biofilms and planktonic cells in response to licochalcone A. Appl Microbiol Biotechnol 99, 359–373 (2015). https://doi.org/10.1007/s00253-014-6076-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6076-x