Abstract
Syndecan-1 (Sdc-1), a transmembrane heparan sulfate protein, is implicated in several pathophysiological processes including rheumatoid arthritis (RA). The exact role of Syndican-1 in this autoimmune disease is still undetermined. This study explores the involvement level of Sdc-1 in the development of RA in a collagen II–induced arthritis mice model. RA was induced in two mice strains (wild-type BALB/c group and Sdc-1 knockout) by collagen II. Mice underwent regular clinical observations and scoring. After sacrifice, leg biopsies were taken from mice for histological examination, using a variety of stains. In addition, proteins were extracted, and molecular assessment of TNF-α was performed using the western blot technique. In the Sdc-1 knockout group, clinical scoring results showed a significantly more severe experimental RA; histology showed a significant increase in bone erosion, cartilage destruction, inflammation, and less granulated mast cells than the wild-type. In addition, molecular assessment of TNF-α showed more increase in expression in the Sdc-1 knockout models compared to the wild-type. Data suggest that lack of Sdc-1 enhances the inflammatory characteristics in RA. However, more molecular studies and investigations are needed to determine its exact role and possible mechanisms involved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation as a process can be both beneficial and detrimental depending on its duration and localization (Furman et al. 2019). It has been documented that acute inflammation after injury or infection is necessary as a defense mechanism for the body; however, a chronic inflammation that occurs in a healthy tissue can lead to the occurrence of many inflammatory diseases contributing to more than half of deaths worldwide (Furman et al. 2019). Rheumatoid arthritis (RA), the most common inflammatory arthropathy, falls in this category of inflammatory diseases, where several molecules, cells, and tissues are known to be involved in the pathophysiologic process. RA is an autoimmune systemic disorder of unknown etiology, characterized by chronic inflammation and synovial infiltration of immune cells (Shen et al. 2021). The RA pathogenic process is quite complex involving synovial cell proliferation, fibrosis, and pannus formation, which is a large abnormal fibrovascular tissue, along with cartilage and bone erosion (Lui et al. 2022). Several inflammatory factors are involved in the pathogenesis. TNF-α, a potent cytokine involved in normal immune response, is one of the major factors present in RA, where its high levels in the synovial fluid enhance the inflammation and cause joint destruction (Vasanthi et al. 2007).
On the other hand, Syndecan-1 (Sdc-1) belongs to a family of transmembrane proteoglycans found on the surface of many cells, predominantly expressed on epithelial cells and endothelial cells, among others (Agere et al. 2018). It has been shown to play a role in numerous cell processes including inflammation, whereby it acts as an inhibitor of inflammation, in non-infectious inflammatory diseases, leading to a reduction in the expression and the activity of pro-inflammatory factors (Götte 2003). Previous studies conducted by our team have demonstrated the involvement of Sdc-1 in wound healing and regeneration, whereby fibroblasts lacking Sdc-1 migrate faster than usual and fail to interact appropriately with the extracellular matrix to initiate healing (Stepp et al. 2010). Other studies showed that leukocytes lacking Sdc-1 interact more with endothelial cells, leading to an increase in the inflammatory response, thus potentially enhancing the incidence of RA (Teng et al. 2012). However, the effect of Sdc-1 on inflammatory factors is not well understood.
This study explored the involvement of Sdc-1 in the development of rheumatoid arthritis with collagen-induced arthritis in BALB/c normal mice and BALB/c Sdc-1 knockout mice. Such investigations shed light on the role of Sdc-1 in modulating and downregulating the inflammatory pathogenic process in RA and its possible mechanism of action.
Materials and methods
Animals
The BALB/c Sdc-1 knockout mice were obtained from the Department of Anatomy and Regenerative Biology, George Washington University, DC, USA. Breeding was performed at the Animal Care Facility of the American University of Beirut, Beirut, Lebanon. The entire experiment was approved by the Institutional Animal Care and Use Committee (IACUC# 19–09-552) of the American University of Beirut.
Rheumatoid arthritis: collagen II model
A total of 24 mice (12 BALB/c wild-type and 12 Sdc-1 KO), 8–10 weeks old, were divided into 8 groups according to Table 1. They were tail-injected with different concentrations of collagen II (CII) and complete Freund’s adjuvant (CFA) to induce arthritis (Williams 2004): Complete Freund’s adjuvant (CFA) (7009, Lot 190,447) and bovine type II collagen (20,022, Lot 190,494) were purchased from Chondrex (Woodinville, WA 98072, USA). A two-dose regimen, 3 weeks apart, was followed according to Table 1. The first injection was intravenous and consisted for most groups of a mixture of CII and complete Freund’s adjuvant (CFA), which is a solution containing inactivated mycobacteria used as an adjuvant to stimulate the immune system. An intraperitoneal booster injection was delivered on day 21, replacing CFA with phosphate-buffered saline (PBS). The controls in each category were injected only with CFA or PBS, without collagen II. Mice lived on normal diet.
Clinical signs and symptoms
Mice were monitored three times a week for 70 days, and clinical signs of redness and swelling were recorded according to the scale in Table 2 (Gaballah et al. 2022).
Histological analysis
After sacrifice, peripheral joints were taken as biopsies. Part of each joint was frozen for molecular study, while the other part was used for histological examination after 10% formalin fixation.
Histological examination was conducted, according to standard routine procedures, to explore the joint alterations in mice. Five-micrometer-thickness tissue sections were used and four different staining methods were performed: the routine hematoxylin and eosin stain (Fischer et al. 2018), Masson Trichrome stain for the detection of connective tissue (collagen) (Rieppo et al. 2019), Safranin O stain for the identification of cartilage and bones (Zu et al. 2019), and toluidine blue stain used to stain mast cells, proteoglycans, and glycosaminoglycans in tissues such as cartilage (Raja et al. 2021). The assessment was done using Microscope Olympus CX41 for slide imaging, and selected slides were photographed.
Molecular assessment of TNF-α
Protein extraction
Mice paws were crushed into powder and put in Laemmeli lysis buffer containing protease and phosphatase inhibitors. The mix was centrifuged for 10 min at 4 °C and at 9000 rpm. The supernatant was collected and stored in − 80 °C freezers.
Western blotting
The Folin-Lowry assay was used for protein quantification. Proteins were resolved by 12% polyacrylamide gel electrophoresis and were transferred to nitrocellulose membranes. The membranes were blocked in non-fat milk (5%) for 1 h at room temperature and incubated for 24 h at 4 °C with primary antibodies against β-actin (1:500; human/mouse; Santa Cruz Biotechnology, Dallas, TX, USA) and TNF-α (1:500; human/goat; R&D, Minneapolis, MN, USA). After washing three times for 10 min each with TBST, the membranes were incubated with the corresponding secondary antibodies at room temperature for 1 h and washed three times with TBST. Protein levels were determined by enhanced chemiluminescence (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions. Band intensities were quantified using ImageJ software (https://imagej.net/ij/).
Results
Severity of the inflammation in the RA mice
Clinical signs and symptoms
To explore further the involvement of Sdc-1 in the development of rheumatoid arthritis, we established a collagen-induced arthritis mice model in 2 groups: wild-type and Sdc-1 knockout. These mice were monitored 3 times per week for 70 days and clinical scores were recorded. No significant inflammatory signs and swelling were observed in the wild-type group. However, paw thickening, pronounced swelling, and erythema were seen in the Sdc-1 null group at day 50 (Fig. 1). Moreover, a significant increase in clinical scores was found in the knockout group, compared to the wild-type (WT) group where the score was close to 0. We can deduce that redness and swelling of joints appeared stronger in the Sdc-1 null group injected with 75 µl CII + 75 µl CFA compared to the wild-type BALB/c group with similar treatment (Fig. 2).
Arthritis clinical score. Arthritis score of four hind paws per mouse of the groups injected with 75 µl CII + 75 µl CFA and 100 µl CII + 100 µl CFA was evaluated every other day for 23 days after the second injection. Each paw of each mouse is scored from 0 to 3. Then, the scoring of all paws of each mouse is added to obtain a total score varying from 0 to 12 for each mouse. The highest score obtained was 6 for the Sdc-1 knockout mice
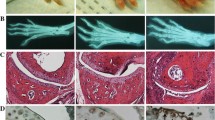
Photos of forefeet and hindfeet of Sdc-1 knockout and wild-type mice on day 21 (booster injection day) and day 50 after the start of the experiment. Sdc-1 null mice developed redness and swelling on day 50 while wild-type mice did not show gross inflammatory signs by then. Significant difference can be seen both in the forelimbs and hindlimbs: the fingers are more curved and swollen in the Sdc-1 knockout mice
Histological assessment
Histological assessment showed the structural differences between the various groups in bone, cartilage, and mast cells and inflammatory cells in general of the knee-joint and hind paw sections.
Hematoxylin–eosin staining showed more alterations in the Sdc-1 null mice group compared to the WT group, especially in the group injected with 100 µl CII + 100 µl CFA: bone erosion and fusion, cartilage destruction, synovial hyperplasia, inflammatory cell infiltration, subchondral bone porosity as well as replacement of bone tissue by connective tissue (Fig. 3; Table 3).
H&E staining for CII model. Magnification × 200. Hematoxylin–eosin staining of joints for CII model mice, wild-type, and Sdc-1 knockout. Note the similarities among the control non-injected mice of both strains (a, b). On the other hand, there are significant differences between the 2 strains in the experimental groups. There exist more cartilaginous erosions in the Sdc-1 knockout mice compared to wild-type and such alteration increases with increasing concentration c–h where the joint is fully fibrotic and fused
In contrast, none of these RA characteristics was observed in the control group.
Safranin O staining confirmed these results by showing an increase in articular cartilage destruction in the Sdc-1 null group compared to the WT group, especially in the group injected with 100 µl CII + 100 µl CFA where a total loss of cartilage is detected as well as bone deterioration, while no cartilage destruction was observed in the control group (Fig. 4; Table 4).
Safranin O staining for CII model. Magnification × 200. Safranin O stain of joints for CII model mice, wild-type, and Sdc-1 knockout. Note the significant similarities between the 2 non-injected groups (a–d). On the other hand, a thinner cartilage is seen in the Sdc-1 knockout group compared to wild-type (e, f). There is a complete cartilage degradation in the group with increased concentration of CII (g, h)
However, more granulated mast cells can be seen in the WT group compared to the Sdc knockout group after CII and CFA injection. This can be explained by the fact that the mast cells reacted and responded to the inflammation in the RA-WT group, while in the RA-Sdc knockout group, they may had an earlier response, degranulated, and released their cytokines, and no more mast cells are produced (Fig. 5; Table 5).
TB staining for CII model. Magnification × 200. Toluidine blue stain of joints for CII model mice, wild-type, and Sdc-1 knockout. Note the higher number of larger mast cells in the wild-type compared to smaller size and relatively lower numbers in the knockout mice (degranulating mast cells in Sdc-1 knockout mice) (a–h)
TNF-α expression
TNF-α expression was also studied. We investigated its expression in normal control groups (untreated), CFA-PBS control groups, and treated groups with 150 µl and 200 µl CFA + CII.
Figure 6 shows a slight increase in TNF-α expression can be detected in the Sdc-1 knockout models compared to WT models among all groups (treated and untreated). In the Sdc-1 knockout group treated with 200 µl CFA + CII, we can notice an important increase in TNF-α expression compared to the WT group treated with 200 µl CFA + CII and compared to other groups. However, no significant differences were noted across all groups. More experimentation with a panel of cytokines is warranted before any conclusion.
Tumor necrosis factor alpha (TNF-α) immunoblot analysis derived from bones. TNF-α of molecular weight 17 kDa shows an important increase in expression in the Sdc-1 knockout group treated with 200 µl CFA + CII compared to WT. However, no significant increase in TNF-α expression can be detected among all groups
Discussion
Emanating data seem to support the fact that Sdc-1 is implicated in RA pathophysiological processes when its expression is altered (Teixeira and Götte 2020). Based on several studies, the Syndecans family, a protein family, was involved in acute and chronic inflammatory diseases (Koliakou et al. 2022). However, their role differs between the types of tissues they are expressed in. The literature shows that Sdc-1 has a controversial role: it can exert either an anti-inflammatory or a pro-inflammatory activity depending on the type of the tissue and whether it is shed from the membrane or not (Koliakou et al. 2022). Little is known about its involvement rheumatoid arthritis. Therefore, we performed a Sdc-1 knockout mice model induced with collagen II, to assess and explore the effect of the loss of this protein on inflammation and the inflammatory process in the development of rheumatoid arthritis disease.
Previous studies found that Sdc-1 expression has increased in chondrocytes isolated from damaged cartilage in early stages of osteoarthritis, suggesting that this molecule is involved in the repair mechanism of the damaged joints (Salminen-Mankonen et al. 2005). However, its role in RA was still undetermined. It is only known that antirheumatic drugs decrease the serum level of Sdc-1, which might reflect a decrease in Sdc-1 shedding (Deyab et al. 2021). In our work, we noticed more histological alterations and damage with higher inflammation in joints lacking the presence of Sdc-1. Moreover, less mast cells were present in the Sdc-1 knockout group, which can be explained by 2 mechanisms: either they exerted an early response, released their components, degranulated and no more mast cells were produced, or the expression of mast cells was downregulated because of the deletion of Sdc-1 protein whereby its loss is known to have an impact on different leucocyte cell types (Gilfillan et al. 2011; Gopal et al. 2021). Mast cells strikingly increased in number in the wild-type, while it was noted that many degranulated in the Sdc-1 knockout, leaving the so-called phantom mast cells. Mast cell granules released into the tissue in response to stimuli in the microenvironment (CFA, CII) via multiple receptors can contribute to the ongoing process of inflammation via multiple mechanisms. In addition, there are numerous important mediators constitutively expressed and stored, or newly synthetized on stimulation of mast cells including cytokines like TNF-α, chemokines, growth factors, lipids, proteases, heparin, and histamine (Mukai et al. 2018).
In the initial stages of the pathogenic process, mast cells being preferentially present around blood vessels, where injection of CFA and CII took place, could have contributed via dependence on their stimulated microenvironment, early in the process, through the multiple receptors and release of a panoply of mediators. As such, the mast cells we encountered in Sdc-1 knockout mice were mostly degranulated phantom cells.
Western blot was performed to explore the effect of Sdc-1 knockout on TNF-α expression in the CII-induced arthritis mice model. As a result, no significant increase in TNF-α expression was seen among all groups. Only a slight increase was detected in the Sdc-1 knockout mice compared to WT mice, in all groups, suggesting that the absence of Sdc-1 did not significantly affect the expression of the pro-inflammatory cytokine TNF-α. Other pro-inflammatory (IL-6 or IL-1β) or anti-inflammatory (IL-4, IL-10, or IL-11) molecules could have been affected; they will be explored in a future study. It is also likely that this pathologic process may act through other inflammatory pathways, which were not explored. A previous study explored the regulation of Sdc-1 expression by inflammatory cytokines in IBD. Results showed that stimulation of colonic epithelial cells with TNF-α or IL-1β downregulated the expression of Sdc-1 at both protein and mRNA levels. However, this effect was not detected when the colonic cells were stimulated with IL-6 (Day et al. 2003), proving that, even in chronic inflammatory diseases, only selective pro-inflammatory cytokines could be affected (Day et al. 2003).
Conclusion
This study proved that the absence of Sdc-1 could lead to increase in inflammatory response. Moreover, no previous data are available showing the effect of Sdc-1 knockout on TNF-α expression in rheumatoid arthritis disease. This study is presenting preliminary data suggesting that Sdc-1 may have anti-inflammatory properties in RA arthritis disease; however, more studies at the molecular level should be conducted to confirm such a role and possible mechanistic pathways involved.
The Sdc-1 null mice developed a faster and more severe experimental RA by collagen-CFA injections; however, more characterization at the macroscopic, microscopic, and molecular levels is needed. Uncovering the role of Sdc-1 in RA paves the way for future investigations that can lead to the emergence of new therapeutic modalities that could be adopted along with conventional treatments.
Data availability
Not applicable.
References
Agere SA, Kim EY, Akhtar N, Ahmed A (2018) Syndecans in chronic inflammatory and autoimmune diseases: pathological insights and therapeutic opportunities. J cell physiol. https://doi.org/10.1002/jcp.26388
Day RM, Mitchell TJ, Knight SC, Forbes A (2003) Regulation of epithelial syndecan-1 expression by inflammatory cytokines. Cytokine 21:224–233. https://doi.org/10.1016/s1043-4666(03)00091-7
Deyab G, Reine TM, Vuong TT et al (2021) Antirheumatic treatment is associated with reduced serum Syndecan-1 in rheumatoid arthritis. PLoS ONE 16:e0253247. https://doi.org/10.1371/journal.pone.0253247
Fischer AH, Jacobson KA, Rose J, Zeller R (2018) CSH Protoc 2008:pdb-rot4986. https://doi.org/10.1101/pdb.prot4986
Furman D, Campisi J, Verdin E et al (2019) Chronic inflammation in the etiology of disease across the life span. Nat Med 25:1822–1832. https://doi.org/10.1038/s41591-019-0675-0
Gaballah EM, Morita K, Shimizu S et al (2022) Non-lethal rodent malarial infection prevents collagen-induced arthritis in mice via anti-arthritic immunomodulation. Parasite Immunol 44:e12901. https://doi.org/10.1111/pim.12901
Gilfillan AM, Austin SJ, Metcalfe DD (2011) Mast cell biology: introduction and overview. Adv Exp Med Biol 716:2–12. https://doi.org/10.1007/978-1-4419-9533-9_1
Gopal S, Arokiasamy S, Pataki C et al (2021) Syndecan receptors: pericellular regulators in development and inflammatory disease. Open Biol 11:200377. https://doi.org/10.1098/rsob.200377
Götte M (2003) Syndecans in inflammation. FASEB J 17:575–591. https://doi.org/10.1096/fj.02-0739rev
Koliakou E, Eleni MM, Koumentakou I et al (2022) Altered distribution and expression of Syndecan-1 and -4 as an additional hallmark in psoriasis. Int J Mol Sci 23:6511. https://doi.org/10.3390/ijms23126511
Lui H, Zhao J, Su M et al (2022) Recombinant CD300c-Ig fusion protein attenuates collagen-induced arthritis in mice. Rheumatology (Oxford, England). https://doi.org/10.1093/rheumatology/keab450
Mukai K, Tsai M, Saito H, Galli SJ (2018) Mast cells as sources of cytokines, chemokines and growth factors. Immunol Rev 282:121–150. https://doi.org/10.1111/imr.12634
Raja N, Naikodi S, Govindarajan A, Palanisamy K (2021) Toluidine blue staining of murine mast cells and quantitation by a novel, automated image analysis method using whole slide skin images. J Histotechnol 44:190–195. https://doi.org/10.1080/01478885.2021.1915934
Rieppo L, Janssen L, Rahunen K et al (2019) Histochemical quantification of collagen content in articular cartilage. PLoS ONE 14:e0224839. https://doi.org/10.1371/journal.pone.0224839
Salminen-Mankonen H, Säämänen A-M, Jalkanen M et al (2005) Syndecan-1 expression is upregulated in degenerating articular cartilage in a transgenic mouse model for osteoarthritis. Scand J Rheumatol 34:469–474. https://doi.org/10.1080/03009740500304338
Shen P, Lin W, Ba X et al (2021) Quercetin-mediated SIRT1 activation attenuates collagen-induced mice arthritis. J ethnopharmacol. https://doi.org/10.1016/j.jep.2021.114213
Stepp MA, Daley WP, Bernstein AM et al (2010) Syndecan-1 regulates cell migration and fibronectin fibril assembly. Exp Cell Res 316:2322–2339. https://doi.org/10.1016/j.yexcr.2010.05.020
Teixeira FCOB, Götte M (2020) Involvement of Syndecan-1 and heparanase in cancer and inflammation. Adv Exp Med Biol 1221:97–135. https://doi.org/10.1007/978-3-030-34521-1_4
Teng YH-F, Aquino RS, Park PW (2012) Molecular functions of syndecan-1 in disease. Matrix Biol 31:3–16. https://doi.org/10.1016/j.matbio.2011.10.001
Vasanthi P, Nalini G, Rajasekhar G (2007) Role of tumor necrosis factor-alpha in rheumatoid arthritis: a review. APLAR J Rheumatol 10:270–274. https://doi.org/10.1111/j.1479-8077.2007.00305.x
Williams RO (2004) Collagen-induced arthritis as a model for rheumatoid arthritis. Methods Mol Med 98:207–216. https://doi.org/10.1385/1-59259-771-8:207
Zu Y, Mu Y, Li Q et al (2019) Icariin alleviates osteoarthritis by inhibiting NLRP3-mediated pyroptosis. J Orthop Surg Res 14:307. https://doi.org/10.1186/s13018-019-1307-6
Acknowledgements
The Syndecan-1 knockout mice were gifted from Professor Marie Anne Stepp, from the Department of Anatomy and Cell Biology, George Washington University, Washington DC, USA.
Funding
Grant from the Medical Practice Plan of the American University of Beirut Medical Center in 2019 (MPP-2019/20).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the Institutional Animal Care and Use Committee of the American University of Beirut, Lebanon (IACUC# 19-09-552).
Consent to participate
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jurjus, R., Dosh, L., Farhat, R. et al. Lack of Syndecan-1 promotes the pathogenesis of experimental rheumatoid arthritis. Immunogenetics 76, 145–154 (2024). https://doi.org/10.1007/s00251-024-01337-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-024-01337-9