Abstract
Osteonecrosis of the femoral head (ONFH) usually occurs in young people and is closely associated with autoimmune reactions. Follistatin-like 1 (FSTL1) was recently proven to participate in several inflammation-related diseases. The role of FSTL1 in ONFH is still unclear. Serum levels of FSTL1 were not significantly different in ONFH patients and healthy individuals. In contrast, elevated expression levels of FSTL1 were observed in degraded cartilage and synovial fluid in ONFH patients and in a cultured human primary chondrocyte model treated with interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α). Suppression of FSTL1 by FSTL1-siRNA downregulated the inflammatory response mediated by IL-1β or TNF-α in cultured human chondrocytes. In a human cartilage culture model, FSTL1 promoted the production of inflammatory cytokines and cartilage degradation enzymes. The activation of NFκB signaling pathway was detected in degenerated cartilage from ONFH patients and in FSTL1-treated chondrocytes. Additionally, administration of an NFκB inhibitor (JSH-23) significantly reduced the overexpression of inflammatory cytokines and protein degradation enzymes induced by FSTL1 and maintained the level of major cartilage matrix components (aggrecan and collagen II). In summary, FSTL1 was involved in the degeneration progression of the ONFH and might provide a novel direction for treating and curing ONFH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Osteonecrosis of the femoral head (ONFH), also known as avascular necrosis (AVN), occurs mostly in young people between the ages of 30 and 50 [1], and the average age of onset is 35 years, which is earlier than that of osteoarthritis (OA). The etiology of ONFH involves many factors, including radioactive factors, trauma, blood system diseases, systemic steroid hormone application, and viral infection [1]. In addition, recent studies have also found that the incidence of ONFH is also related to genetic predisposition [2].
Early ONFH can be alleviated by reducing joint burden and applying nonsteroidal drugs. Advanced osteonecrosis with severe joint disease usually requires surgical prosthesis implantation, including femoral resurfacing arthroplasty, hemiarthroplasty, and total hip arthroplasty (THA) [3, 4]. Surgical prosthesis implantation can provide good clinical benefits in relieving pain and in improving exercise quality. However, postoperative complications are not negligible, such as infections in patients with immune dysfunction and high risk of dislocation and loosening of the implant, especially in young patients [1, 4]. Once a complication occurs, the patient will have a poor prognosis. Therefore, the effect of surgical treatment does not meet the expectations of doctors and patients; it is necessary to explore new ways to improve the treatment status of ONFH.
Previous studies have found that the production of interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), and IL-6 are significantly elevated in ONFH [5,6,7], indicating that inflammation may play an essential role in the occurrence and development of ONFH and in cartilage degeneration, destruction, and repair. Follistatin-like 1 (FSTL1), a secreting glycoprotein, has been known for approximately 20 years [8]. Early studies found that FSTL1 has an anti-inflammation effect in a mouse arthritis model [9], but recent studies have found that FSTL1 promotes the occurrence and progression of inflammatory reactions, exhibiting a pro-inflammatory role by activating several signaling pathways in osteoarthritis and rheumatoid arthritis (RA) models [10, 11]. However, the regulation of FSTL1 in ONFH and its potential role in inflammation in this condition is still unknown. In this study, the systemic and local production of FSTL1 in ONFH patients was evaluated, and human primary chondrocytes and cartilage culture models were used to investigate the potential role of FSTL1 in the inflammation and metabolism of ONFH.
MATERIALS AND METHODS
Ethics Statement
Our research protocol was approved by the Medical Ethics Committee of the Second Hospital of Jilin University (Changchun, China). Written informed consent was obtained from all enrolled patients.
Sample Collection
According to Ficat classification [2], a total of 14 patients with ONFH grades I–II (median age, 52 years; range, 44 to 64 years) and 14 patients with ONFH grades III–IV (median age, 54 years; range, 48 to 67 years) who intended to accept surgical treatment from the Second Hospital of Jilin University were enrolled in this study. Additionally, ten patients with a femoral head fracture (FNF) (median age, 57.5 years; range, 54 to 67 years) who intended to accept total medullary arthroplasty or femoral head replacement were enrolled in this study as a control. The details of all enrolled patients are shown in Table 1. All patients were diagnosed by their clinical manifestation and by magnetic resonance imaging (MRI) analysis without pulmonary tuberculosis, rheumatism, and glucocorticoid application. Next, peripheral blood was collected from every ONFH patient and from healthy individuals (median age, 45 years; range, 30 to 56 years); synovial fluid and articular cartilage were obtained from ONFH and FNF patients. Peripheral blood and synovial fluid samples were centrifuged, and the supernatant was stored at − 80 °C for further testing.
Primary Chondrocyte Extraction and Culture
Cartilage tissue specimens were obtained from the FNF patients, who acted as the control group, and the specimens were repeatedly washed in sterile PBS to remove the residual blood; then, the specimens were cut into 1-mm3 pieces. After the specimens were digested with 0.25% trypsin at 37 °C for 20 min, the digests were centrifuged, and the sediment was digested with 0.2% type II collagenase at 37 °C overnight and was filtered using a sterile 200 mesh screen. After the filtrate was centrifuged, the substrate was dispersed and purified with DMEM/F12 media (HyClone, Thermo Co., USA) including 10% FBS and 0.1% penicillin–streptomycin. After that, the cells were seeded and cultured in DMEM/F12 media in an incubator at 37 °C with 5% CO2. The cell culture medium was changed twice a week. After the cells were subcultured for two to three passages, the chondrocytes were seeded into six-well plates to be used in the experiments.
The Culture of Human Cartilage Explants
Human cartilage explants obtained from FNF patients who accepted total medullary arthroplasty or femoral head replacement were isolated and cultured as previously reported [12]. The cartilage samples were cut into small pieces with a diameter of 1 mm and a thickness of 1–2 mm. Then, the cartilage specimens were cultured in serum-free DMEM medium containing 25 mM HEPES, 2 mM glutamine, 100 mg/ml streptomycin, 100 IU/ml penicillin, and 2.5 mg/ml gentamicin in tissue-culture flasks at 37 °C with 5% CO2. After that, the cultured cartilage specimens were stimulated by recombinant human FSTL1 protein at a concentration of 300 ng/ml (R&D Systems Inc., MN, USA) for 5 days.
Immunohistochemistry Staining
Immunohistochemical (IHC) analyses in this study were performed as previously reported [7]. In brief, the cartilage tissues or cultured cartilage explants were fixed, dehydrated, and embedded in paraffin to prepare 5-μm-thick slices. The sections were dewaxed, rehydrated, and blocked with 5% normal donkey serum. Thereafter, the serial sections were incubated with the following primary antibodies at 4 °C overnight: goat anti-FSTL1 (1:100 dilution; Santa Cruz Biotechnology Co., USA), rabbit anti-IL-1β, rabbit anti-TNF-α, rabbit anti-matrix metalloproteinase 13 (MMP-13), rabbit anti-nitric oxide synthases 2 (NOS-2) (1:200 dilution; Abcam Biotechnology Co., USA), and rabbit anti-IL-6 (1:100 dilution; Zhong Shan Golden Bridge Biotechnology Co., Ltd., Beijing, China). After the serial sections were washed, they were incubated with donkey anti-rabbit immunoglobulin (IgG)–horseradish peroxidase (HRP) and donkey anti-goat IgG–HRP secondary antibodies (1:500 dilution; Jackson ImmunoResearch Laboratories, Inc., PA, USA) at 37 °C for 30 min. The images were captured by an electron microscope (Olympus, Tokyo, Japan) at × 200 magnification and were quantified by Image-Pro Plus software (Media Cybernetics, Inc., USA). Each part was examined independently by two researchers blinded to the sample identities.
Western Blot Analysis
Total proteins were extracted from primary chondrocytes following standard protocols as previously reported [12]. The nuclear and cytoplasmic protein were separated and extracted by the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, USA) according to the instruction of the manufacturer and the protein concentrations were tested by the BCA assay kits (Beyotime Biotechnology, Shanghai, China). After the proteins were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, they were transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA). Then, the membranes were blocked with 5% bovine serum albumin in Tris buffer–saline Tween 20 (10 mM Tris–HCl, pH 8.0; 150 mM NaCl; and 0.5% Tween 20) at room temperature for 30 min followed by incubation with the following primary antibodies at 4 °C for 12 h: goat anti-FSTL1, rabbit anti-laminB, rabbit anti-aggrecan, rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:1000 dilution; Santa Cruz, USA), rabbit anti-IκBα, rabbit anti-pIκBα, rabbit anti-NFκB-p65 (1:1000 dilution; Cell Signaling Technology Inc., MA, USA), rabbit anti-cyclooxygenase-2 (COX-2), rabbit anti-NOS-2, rabbit anti-MMP-13, and rabbit anti-collagen II (Col-2) (1:1000 dilution; Abcam, USA). The GAPDH and laminB antibodies were used as the internal protein loading controls for data analysis. After the membranes were incubated with the HRP-conjugated secondary antibodies (Jackson Laboratory, USA), the protein signals were detected using the enhanced chemiluminescence (ECL) kit (Pierce Biotechnology, USA). All experiments were repeated in triplicate.
Enzyme-Linked Immunosorbent Assay (ELISA)
The supernatants of sera, synovial fluid, and stimulated cultured chondrocytes were collected, centrifuged, and stored separately at − 80 °C. The protein levels of FSTL1 in human sera and synovial fluid were determined by human FSTL1 ELISA kits (Abcam, USA) using protocols provided by the manufacturers. The secretion of TNF-α, IL-1β, prostaglandin E2 (PGE-2), and IL-6 in cell culture supernatants were determined by human ELISA kits (Abcam, USA) according to the protocols provided by the manufacturers. The absorbance values (OD values) at 450 nm were detected by an absorbance microplate reader (Bio-Rad) according to the instructions of the manufacturer.
Real-Time PCR
Total RNA was extracted from cartilage tissue, primary chondrocytes, or cultured human cartilage explants from each indicated group using the RNAeasy kit (Beyotine Biotechnology Corporation, Shanghai, China) according to the instructions of the manufacturer. SYBR Green I dye was used to perform real-time PCR and to monitor DNA synthesis. Sequence-specific primers in this study are listed in Table 2 (synthesized by Sangon Company, Shanghai, China). Each single PCR product was verified by a melting curve analysis and was normalized to GAPDH. The experiments were performed in triplicate. The relative expression level of each target gene was calculated using the 2−△△Ct method.
FSTL1-siRNA Transfection
The small interfering RNA (siRNA) targeting human FSTL1 mRNA (siRNA group) and the negative mismatched control siRNA (NC group) were designed and synthesized by RiboBio (Guangzhou, China). Primary chondrocytes were transfected with 50 nmol/L FSTL1-siRNA and negative control for 48 h as the manufacturer’s instructions. The total mRNA of the transfected chondrocytes was extracted to detect the mRNA content of FSTL1, and the cell supernatant was collected to identify the levels of TNF-α, IL-6, and IL-1β by ELISA assay. In addition, chondrocytes pretreated with FSTL1-siRNA for 24 h were stimulated with IL-1β and TNF-α, and mRNA expression of related inflammatory cytokines was measured.
Statistical Analysis
The data were presented as the mean ± standard deviation (SD) from three independent experiments. The statistical analysis between the two groups was performed with the paired T test, and the statistical analysis of three or more groups used one-way analysis of variance (ANOVA) by GraphPad Prism 5.0. Statistical significance was considered if the value of P was < 0.05.
RESULTS
Production of FSTL1 in Peripheral Blood and Synovial Fluid of ONFH Patients
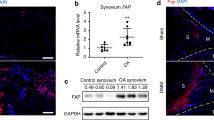
Previous studies reported that the FSTL1 concentration in peripheral blood and synovial fluid was upregulated both in OA and RA patients [10]. To investigate the FSTL1 levels in ONFH patients, we collected serum from ONFH patients and healthy individuals to perform ELISAs. Serum FSTL1 levels in healthy controls (4.738 ± 1.482 ng/ml) were not different from those in ONFN patients (group A, 5.157 ± 1.464 ng/ml; group B, 5.164 ± 1.409 ng/ml) (Fig. 1a). There was no statistical difference in serum FSTL1 levels between the mild ONFH group (group A) and the severe ONFH group (group B). To determine the production of FSTL1 in ONFH patients, we collected synovial fluid and cartilage in ONFH patients to assess the protein and mRNA levels of FSTL1. Here, FNF patients were used as a control group. As shown in Fig. 1b, the FSTL1 concentrations in synovial fluid from the ONFH groups (group A, 22.19 ± 3.767 ng/ml; group B, 21.06 ± 5.044 ng/ml) were significantly higher than that in the FNF group (12.05 ± 2.298 ng/ml) (Fig. 1b). IHC results showed a poor expression of FSTL1 in the FNF group (Fig. 1c). In contrast, the secretion of FSTL1 was greatly increased in cartilage in ONFH patients (Fig. 1d). When compared to the FNF group, the mRNA levels were upregulated by approximately 3.1-fold in group A and 3.8-fold in group B (Fig. 1e). However, mRNA and protein levels showed no statistically significant difference between the mild ONFH group (group A) and the severe ONFH group (group B) (Fig. 1b, d, and e). The data suggest that serum FSTL1 cannot be used as a systemic indicator for evaluating the progression of ONFH. However, the obvious changes of FSTL1 in local joints suggest that FSTL1 may be closely related to the progression of ONFH.
Expression levels of FSTL1 in peripheral blood, synovial fluid, and articular cartilage in enrolled patients. (a) Detection of FSTL1 levels in the serum of healthy people, patients with ONFH grades I–II (group A) and patients with ONFH grades III–IV (group B), as determined by ELISA. (b) The FSTL1 levels in the synovial fluid of patients in the FNF group, group A, and group B were detected, and the synovial fluid of patients in the three groups was collected and measured by ELISA. (c) Articular cartilage of patients in the FNF group, group A, and group B were collected, and the levels of FSTL1 in each group were tested by IHC, which was analyzed in (d). (e) The FSTL1 expression levels in articular cartilage of patients in the FNF group, group A, and group B were detected by real-time PCR. *P < 0.05, **P < 0.05. Scale bar, 50 μm.
Pro-Inflammatory Cytokines (IL-1β and TNF-α) Induced FSTL1 Level in Primary Chondrocytes
Many studies have shown that pro-inflammatory cytokines are involved in regulating the secretion of FSTL1, and IL-1β, TNF-α, IFN-gamma can induce the overexpression of FSTL1 in nucleus pulposus cells, MT3T3 cells, stromal cells, and stroma cells [13,14,15]. To test whether IL-1β and TNF-α regulate FSTL1 secretion, IL-1β or TNF-α, respectively, was added in the cultured human primary chondrocyte for 48 h. Real-time PCR and ELISA results showed that both IL-1β and TNF-α promote the level of FSTL1 in a chondrocyte culture model (Fig. 2a, b). Given that both IL-1β and TNF-α played a central role in the degeneration of ONFH, we hypothesized that locally elevated FSTL1 in the joints might arise from the continued stimulation of peripheral IL-1β and TNF-α and the inflammatory mediators associated with them.
IL-1β and TNF-α could promote the expression of FSTL1 in chondrocytes. (a) The FSTL1 mRNA expression levels in chondrocytes stimulated by IL-1β and TNF-α were detected by real-time PCR. (a) Human chondrocytes were cultured with 10 ng/ml IL-1β or 10 ng/ml TNF-α for 48 h, and the total mRNA of each group was extracted. The PBS group was used as a control. (b) Human chondrocytes were cultured with PBS, 10 ng/ml IL-1β, or 10 ng/ml TNF-α for 48 h, and the supernatant of every group was collected for the detection of FSTL1 expression level by ELISA. *P < 0.05, **P < 0.01, ***P < 0.001.
FSTL1 Downregulation by FSTL1-siRNA Reduced the IL-1β and TNFα-Mediated Inflammatory Reaction in Primary Chondrocytes
Previous studies reported using short hairpin RNA to downregulate the FSTL1 level could decrease the pro-inflammatory cytokine-mediated inflammatory response in stromal cells [14, 16]. In the present study, we used siRNA targeting human FSTL1 (FSTL1-siRNA) to transfect human primary chondrocytes, and real-time PCR and western blot were performed to test the effect of FSTL1-siRNA on the production of FSTL1. Figure 3a shows that the expression of FSTL1 was significantly decreased by siRNA. The western blot result confirmed the efficiency of FSTL1-siRNA, as the protein level of FSTL1 was inhibited by the administration of FSTL1-siRNA (Fig. 3b–c). Here, the production of inflammation-associated biomarkers (IL-1β, TNF-α, and IL-6) was evaluated in a cell culture model. Administration of FSTL1-siRNA alone did not significantly affect the levels of IL-1β, TNF-α, and IL-6 (Fig. 3d–f), as assayed by ELISA.
Interference of the expression of FSTL1 can inhibit IL-1β and TNF-α-induced inflammation. (a) FSTL1 expression levels in human primary chondrocytes transfected with FSTL1-siRNA were detected by real-time PCR (a) and western blot assay (b–c). Primary chondrocytes were transfected with FSTL1-siRNA for 48 h, and the total mRNA and protein were collected (d–f). The supernatants of primary chondrocytes were obtained after 48 h of transfection, and the levels of IL-1β (d), IL-6 (e), and TNF-α (f) were measured by ELISA. The IL-6 (g) and TNF-α (h) expression levels in transfected primary chondrocytes, which were further stimulated by 10 ng/ml IL-1β for 48 h, were tested by real-time PCR. The IL-6 (i) and TNF-α (j) expression levels in transfected primary chondrocytes, which were further stimulated by 10 ng/ml TNF-α for 48 h, were also measured. *P < 0.05, **P < 0.01, ***P < 0.001.
To test the effect of FSTL1-siRNA in inflammation condition, IL-1β and TNF-α were used to establish a chondrocyte inflammatory model separately to assess the effect of FSTL1 by adding FSTL1-siRNA in the culture medium. As Fig. 3g and h demonstrate, the treatment of chondrocytes with FSTL1-siRNA downregulated the IL-1β-induced overexpression of TNF-α and IL-6 mRNA levels. TNF-α-mediated increases in IL-1β and IL-6 levels were inhibited by FSTL1-siRNA treatment (Fig. 3i, k). The data suggested that blocking the expression of FSTL1 can inhibit IL-1β or TNF-α-induced inflammatory processes in chondrocytes.
FSTL1 Enhanced the Secretion of Inflammatory Cytokines in Cultured Cartilage Explants
To assess the effect of FSTL1 in cartilage inflammation and metabolism, the cartilage explants from five FNF patients were collected, and a cartilage explant culture model was constructed in vitro. Because FSTL1 was highly increased in ONFH patient synovial fluid, the present study used a high dosage of FSTL1 (300 ng/ml) cocultured with cartilage explant in vitro for 5 days. After they were stimulated with FSTL1, the cultured cartilage samples were harvested to evaluate the production of inflammatory cytokines (IL-1β, IL-6, TNF-α, and NOS-2) and the primary cartilage catabolic enzyme (MMP-13), as measured by real-time PCR and IHC. Real-time PCR revealed a significant increase in the expression of IL-1β (2.7-fold), IL-6 (1.9-fold), TNF-α (3.7-fold), NOS-2 (2.5-fold), and MMP-13 (4.9-fold) in the FSTL1 group relative to the control group (Fig. 4a–e). There was a significantly lower protein expression of inflammatory cytokines and catabolic enzyme in the control group; in contrast, these biomarkers were highly increased in the group treated with FSTL1 (Fig. 4f–j). The result indicated increased FSTL1 could accelerate inflammation and degeneration of cartilage in vitro.
Inflammatory cytokine levels in FSTL1-stimulated human cartilage were detected. The secretion of IL-1β (a), IL-6 (b), TNF-α (c), NOS-2 (d), and MMP-13 (e) in cultured human cartilage in vitro was measured by real-time PCR. Human cartilage was cultured and stimulated by 300 ng/ml FSTL1 in vitro for 5 days, and the total mRNA, including that of the control group, was collected. IHC was used to verify the levels of IL-1β (f), IL-6 (g), TNF-α (h), NOS-2 (i), and MMP-13 (j) in cultured human cartilage stimulated by FSTL1 for 5 days. *P < 0.05, **P < 0.01. Scale bar, 50 μm.
FSTL1 Activated the NFκB Signaling Pathway In Vitro
The NFκB signaling pathway is closely associated with the degeneration and necrosis of articular cartilage in ONFH models [17, 18]. Many groups have reported that FSTL1 promotes inflammatory progression in OA, RA, obesity, and lumbar disc herniation (LDH) by activating NFκB signaling [11]. To investigate the activation of the NFκB pathway, three FNF patients and three ONFH patients were randomly selected to donate cartilage to test the activation of the NFκB pathway. Cartilage sections were stained with phospho-IκBα (pIκBα), and RNA extracts were collected to detect the NFκB2 level by real-time PCR. As indicated in Fig. 5a, pIκBα expression was higher in the ONFH group than in the FNF group. Moreover, the NFκB2 level was increased approximately 3-fold in the ONFH group relative to the control group (Fig. 5b). To further test the effect of FSTL1 on the activation of NFκB signaling, human primary chondrocytes were treated with different concentrations of FSTL1 (0, 100, and 300 ng/ml) for 1 h. FSTL1 dramatically induced the production of pIκBα in the cytoplasm and NFκBp65 in the nucleus (Fig. 5c, d), as assayed by western blot. RNA extracts were harvested from chondrocytes from the three groups. The result of real-time PCR indicated that FSTL1 significantly upregulated chondrocyte NFκB2 levels relative to the levels of the untreated cultured chondrocytes, and the high-dose group (300 ng/ml) exhibited significantly increased production of NFκB2 relative to that of the low-dose group (100 ng/ml) (Fig. 5e). The data suggest that FSTL1 enhances the activity of the NFκB signaling pathway in a dose-dependent manner.
The NFκB signaling pathway was activated by FSTL1 in vitro. (a) The expression level of pIκBα in the human cartilage of patients with ONFH was higher than that of the FNF group, as detected by IHC. (b) The NFκB2 level in the cartilage of patients with ONFH was significantly increased compared with that of the control group. The total mRNA in the human cartilage of patients with FNF or ONFH was isolated and assayed by real-time PCR. (c) The IκBα and pIκBα expression in primary cells stimulated by FSTL1 were detected by western blot assay. Primary cells were treated with 0, 100, or 300 ng/ml FSTL1 for 1 h and were lysed by cell lysis buffer to obtain the total protein. (d) The NFκB-p65 levels in primary cells stimulated by FSTL1 were also detected by western blot. Primary chondrocytes were cultured with 0, 100, or 300 ng/ml FSTL1 for 1 h, and the nuclear and cytoplasmic protein were collected for each group. (e) The NFκB2 expression in human primary chondrocytes stimulated by FSTL1 was also detected by real-time PCR. *P < 0.05. Scale bar, 50 μm.
Inhibitors of NFκB Reduced Inflammation and Catabolism in Human Primary Chondrocytes
To investigate whether the pro-inflammatory effect of FSTL1 is dependent on the NFκB signaling pathway, primary chondrocytes were treated with FSTL1 (300 ng/ml) in the presence or absence of NFκB signaling inhibitor-JSH-23 (20 μmol). To test the role of JSH-23 on the expression of FSTL1-inducible cytokines and catabolic enzymes was analyzed by ELISA and real-time PCR. As shown in Fig. 6a–d, administration of JSH-23 reduced the secretion of IL-1β, IL-6, TNF-α, and PGE-2, which are induced by FSTL1. Western blot results showed the protein overexpression of COX-2, NOS-2, and MMP-13 was suppressed with the application of JSH-23 (Fig. 6e). Increasing the production of inflammation mediators and catabolism-related enzymes cause the degeneration and destruction of the cartilage of the femoral head and promote the progression of ONFH. The present study detected the expression of the critical components of cartilage matrices, such as aggrecan and Col-2, using real-time PCR and western blot. Figure 6f–h shows that the high dose of FSTL1 destroyed the balance of synthesis and catabolism for the cartilage matrix and speeds up the catabolism of cartilage, as indicated by the loss of the production of aggrecan and Col-2. The application of JSH-23 blocked the suppression of aggrecan and Col-2 induced by FSTL1. These data suggest inhibition of the NFκB signaling pathway can downregulate the inflammatory response and the cartilage destruction caused by FSTL1.
Inhibition of the NF-κB signaling pathway could reduce the FSTL1-mediated inflammatory reaction and catabolism in chondrocytes. The secretion of IL-1β (a), IL-6 (b), TNF-α (c), and PGE-2 (d) in FSTL1-treated primary cells with or without JSH-23 were determined by ELISA. Human primary chondrocytes were treated with 300 ng/ml FSTL1 with or without 20 μmol JSH-23 for 48 h. The control group was added to the same concentration of DMSO without FSTL1 or JSH-23. (e) Total protein of each treated group was also collected, and COX-2, NOS-2, and MMP-13 expression levels were detected by western blot. (e–h) The expression levels of aggrecan and Col-2 were assessed by real-time PCR and western blot. Col-2 and aggrecan expression levels were determined by western blot. *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
ONFH is characterized by increasing joint destruction, cartilage collapse and loss of joint function, and persistent pain. Although the exact cause of ONFH is still unknown, the consensus is that ONFH is caused by a variety of exogenous factors, such as trauma and hormonal use, and by endogenous factors, such as immune disorders, metabolic disorders, and even genetic changes [1]. Recent studies have shown that a variety of treatments such as nonsteroidal anti-inflammatory drugs, cytokines such as hepatocyte growth factor (HGF), transforming growth factor-β (TGF-β), and vascular endothelial growth factor (VEGF), and stem cell therapy and viral vector gene therapy can block the inflammatory manifestations and alleviate the symptoms of ONFH [4, 9, 19]. However, none of these treatments can change the outcome of ONFH-joint replacement. Loss of motor function and various complications caused by joint replacement also bring great physical and mental pain to patients. The extensive incidence of ONFH and the difficulty of treatment suggest that we should thoroughly explore the pathogenesis of ONFH and seek better treatment strategies for ONFH.
Many studies have found that several inflammatory factors or enzymes in the serum, synovial fluid, and degenerative cartilage of ONFH patients have changed significantly and participate in the pathological process of ONFH [5,6,7]. A previous study showed that certain genotypes of IL-1β, TGF-β, IL-10, and TNF-α associated with the pathogenesis of ONFH [5]. TNF-α and IL-1β are major systemic pro-inflammatory factors that play an essential role in the pathogenesis of ONFH. Importantly, TNF-α has been reported to promote the expression of other cytokines, such as IL-3, IL-6, IL-12, MMP-13, and ADAMTS-7, to accelerate the progression of ONFH [20, 21]. In addition, human and animal studies have shown that the loss of bone related to menopause may be caused by the activation of osteoclasts promoted by IL-6 [22] and that inhibiting IL-6 expression could reduce the inflammatory response and reduce necrosis to promote bone reconstruction [23, 24]. Moreover, a previous study reported that the levels of TNF-α, COX-2, ADAMTS-4, and MMP-13 were significantly higher in ONFH patients than in FNF patients, while the aggrecan and collagen levels were decreased in ONFH patients [6]. These data indicated that finding and inhibiting the appropriate targets during the ONFH inflammation process may provide new treatment strategies for ONFH.
Since most inflammatory factors are released by inflammatory cells such as macrophages, somatic cells, and TH cells in the body, it has been thought that the occurrence of joint necrosis is caused by local or systemic or local immune attacks on the joints. However, present research shows that cartilage degeneration and necrosis destruction may be induced by the joint itself. FSTL1 can be produced by a variety of cells in nonhematopoietic cell lines, especially mesenchymal cells. In the joint system, FSTL1 is expressed in both synovial and cartilage tissues [25]. In patients with OA and RA, the expression level of FSTL1 in synovial tissue was significantly higher than that in cartilage tissue [10, 11]. In addition, FSTL1 had high levels in mouse models or human rheumatoid arthritis patients and was found to aggravate RA progression by promoting the expression of MMP-1, MMP-3, and MMP-13 through activating NFκB, JAK/STAT3, and MAPK pathways [26]. In lumbar disc degeneration patients, FSTL1 was overexpressed in human serum and lumbar discs, and it has a positive correlation with visual analog scale; FSTL1 can also be upregulated by the administration of TNF-α and can be enhanced the production of inflammatory cytokines and MMP-13 by activation of the MAPK and NFκB signaling pathways [27].
Although the role of FSTL1 in the regulation of inflammation is still controversial, most studies have shown that FSTL1 may promote the occurrence and progression of inflammatory reactions in bone and joint degeneration-related diseases such as OA, RA, and LDH [11]. Herein, we explored the role of FSTL1 in human ONFH for the first time. We found that the expression levels of FSTL1 in the human peripheral blood did not display a difference between healthy individuals and ONFH patients. In a further test, the effect of FSTL1 in local joints in the production of synovial fluid and articular cartilage was examined and showed significantly elevated in ONFH patients compared with that in FNF patients, testing by ELISA and IHC staining. To test the regulatory effect of FSTL1 in IL-1β and TNF-mediated inflammatory responses in the chondrocyte culture model, downregulation of FSTL1 by adding FSTL1-siRNA inhibited the level of inflammatory cytokines induced by IL-1β and TNF. FSTL1 promoted the expression of IL-1β, IL-6, TNF-α, NOS-2, and MMP-13 in primary chondrocyte and cartilage explants in vitro. Strikingly, inhibiting the NFκB pathway can prevent the pro-inflammatory effect of FSTL1 and rescue the depression of collagen II and aggrecan.
In conclusion, FSTL1 had high expression in the synovial fluid and joint cartilage tissues of ONFH patients and significantly enhanced the appearances of IL-1β, IL-6, TNF-α, NOS-2, and MMP-13 in ONFH models in vitro, mainly through activating the NFκB signaling pathway. These findings suggest that FSTL1 may act as an endogenous trigger or factor in the regulation of human ONFH and can be considered as a promising target for human ONFH therapy in the future.
References
Kaushik, A.P., A. Das, and Q. Cui. 2012. Osteonecrosis of the femoral head: an update in year 2012. World Journal of Orthopedics 3 (5): 49–57. https://doi.org/10.5312/wjo.v3.i5.49.
Moya-Angeler, J., A.L. Gianakos, J.C. Villa, A. Ni, and J.M. Lane. 2015. Current concepts on osteonecrosis of the femoral head. World Journal of Orthopedics 6 (8): 590–601. https://doi.org/10.5312/wjo.v6.i8.590.
Mont, M.A., J.J. Cherian, R.J. Sierra, L.C. Jones, and J.R. Lieberman. 2015. Nontraumatic osteonecrosis of the femoral head: where do we stand today? A ten-year update. The Journal of Bone and Joint Surgery. American Volume 97 (19): 1604–1627. https://doi.org/10.2106/jbjs.o.00071.
Arbab, D., and D.P. Konig. 2016. Atraumatic femoral head necrosis in adults. Deutsches Ärzteblatt International 113 (3): 31–38. https://doi.org/10.3238/arztebl.2016.0031.
Samara, S., P. Kollia, Z. Dailiana, C. Chassanidis, L. Papatheodorou, T. Koromila, and K.N. Malizos. 2012. Predictive role of cytokine gene polymorphisms for the development of femoral head osteonecrosis. Disease Markers 33 (4): 215–221. https://doi.org/10.3233/dma-2012-0928.
Chen, B., Y. Liu, and L. Cheng. 2018. IL-21 enhances the degradation of cartilage through the JAK-STAT signaling pathway during osteonecrosis of femoral head cartilage. Inflammation 41 (2): 595–605. https://doi.org/10.1007/s10753-017-0715-1.
Li, J.K., L. Cheng, Y.P. Zhao, Y.J. Guo, Y. Liu, W. Zhang, S.S. Wang, Y.Q. Zhang, X. Pan, and L. Nie. 2015. ADAMTS-7 exhibits elevated expression in cartilage of osteonecrosis of femoral head and has a positive correlation with TNF-alpha and NF-kappa B P65. Mediators of Inflammation 2015: 196702. https://doi.org/10.1155/2015/196702.
Tanaka, M., S. Ozaki, F. Osakada, K. Mori, M. Okubo, and K. Nakao. 1998. Cloning of follistatin-related protein as a novel autoantigen in systemic rheumatic diseases. International Immunology 10 (9): 1305–1314.
Tanaka, M., S. Ozaki, D. Kawabata, M. Kishimura, F. Osakada, M. Okubo, M. Murakami, K. Nakao, and T. Mimori. 2003. Potential preventive effects of follistatin-related protein/TSC-36 on joint destruction and antagonistic modulation of its autoantibodies in rheumatoid arthritis. International Immunology 15 (1): 71–77.
Chaly, Y., B. Hostager, S. Smith, and R. Hirsch. 2014. Follistatin-like protein 1 and its role in inflammation and inflammatory diseases. Immunologic Research 59 (1–3): 266–272. https://doi.org/10.1007/s12026-014-8526-z.
Mattiotti, A., S. Prakash, P. Barnett, and M.J.B. van den Hoff. 2018. Follistatin-like 1 in development and human diseases. Cellular and Molecular Life Sciences 75 (13): 2339–2354. https://doi.org/10.1007/s00018-018-2805-0.
Qu, R., X. Chen, W. Wang, C. Qiu, M. Ban, L. Guo, K. Vasilev, J. Chen, W. Li, and Y. Zhao. 2018. Ghrelin protects against osteoarthritis through interplay with Akt and NF-kappaB signaling pathways. The FASEB Journal 32 (2): 1044–1058. https://doi.org/10.1096/fj.201700265R.
Clutter, S.D., D.C. Wilson, A.D. Marinov, and R. Hirsch. 2009. Follistatin-like protein 1 promotes arthritis by up-regulating IFN-gamma. Journal of Immunology 182 (1): 234–239.
Chaly, Y., A.D. Marinov, L. Oxburgh, D.S. Bushnell, and R. Hirsch. 2012. FSTL1 promotes arthritis in mice by enhancing inflammatory cytokine/chemokine expression. Arthritis and Rheumatism 64 (4): 1082–1088. https://doi.org/10.1002/art.33422.
Fan, N., H. Sun, Y. Wang, Y. Wang, L. Zhang, Z. Xia, L. Peng, et al. 2013. Follistatin-like 1: a potential mediator of inflammation in obesity. Mediators Inflamm 2013: 752519. https://doi.org/10.1155/2013/752519.
Campfield, B.T., T. Eddens, M. Henkel, M. Majewski, W. Horne, Y. Chaly, S.L. Gaffen, R. Hirsch, and J.K. Kolls. 2017. Follistatin-like protein 1 modulates IL-17 signaling via IL-17RC regulation in stromal cells. Immunology and Cell Biology 95 (8): 656–665. https://doi.org/10.1038/icb.2017.26.
Okazaki, S., S. Nagoya, K. Tateda, R. Katada, K. Mizuo, S. Watanabe, T. Yamashita, and H. Matsumoto. 2013. Experimental rat model for alcohol-induced osteonecrosis of the femoral head. International Journal of Experimental Pathology 94 (5): 312–319. https://doi.org/10.1111/iep.12035.
Okazaki, S., S. Nagoya, H. Matsumoto, K. Mizuo, M. Sasaki, S. Watanabe, T. Yamashita, and H. Inoue. 2015. Development of non-traumatic osteonecrosis of the femoral head requires toll-like receptor 7 and 9 stimulations and is boosted by repression on nuclear factor kappa B in rats. Laboratory Investigation 95 (1): 92–99. https://doi.org/10.1038/labinvest.2014.134.
Rackwitz, L., L. Eden, S. Reppenhagen, J.C. Reichert, F. Jakob, H. Walles, O. Pullig, R.S. Tuan, M. Rudert, and U. Noth. 2012. Stem cell- and growth factor-based regenerative therapies for avascular necrosis of the femoral head. Stem Cell Research & Therapy 3 (1): 7. https://doi.org/10.1186/scrt98.
Dai, C.Y., W.L. Chuang, L.P. Lee, S.C. Chen, N.J. Hou, Z.Y. Lin, M.Y. Hsieh, M.Y. Hsieh, L.Y. Wang, W.Y. Chang, and M.L. Yu. 2006. Associations of tumour necrosis factor alpha promoter polymorphisms at position −308 and −238 with clinical characteristics of chronic hepatitis C. Journal of Viral Hepatitis 13 (11): 770–774. https://doi.org/10.1111/j.1365-2893.2006.00767.x.
Liacini, A., J. Sylvester, W.Q. Li, W. Huang, F. Dehnade, M. Ahmad, and M. Zafarullah. 2003. Induction of matrix metalloproteinase-13 gene expression by TNF-alpha is mediated by MAP kinases, AP-1, and NF-kappaB transcription factors in articular chondrocytes. Experimental Cell Research 288 (1): 208–217.
Woo, J.T., T. Yonezawa, B.Y. Cha, T. Teruya, and K. Nagai. 2008. Pharmacological topics of bone metabolism: antiresorptive microbial compounds that inhibit osteoclast differentiation, function, and survival. Journal of Pharmacological Sciences 106 (4): 547–554.
Kuroyanagi, G., N.S. Adapala, R. Yamaguchi, N. Kamiya, Z. Deng, O. Aruwajoye, M. Kutschke, E. Chen, C. Jo, Y. Ren, and H.K.W. Kim. 2018. Interleukin-6 deletion stimulates revascularization and new bone formation following ischemic osteonecrosis in a murine model. Bone 116: 221–231. https://doi.org/10.1016/j.bone.2018.08.011.
Yamaguchi, R., N. Kamiya, N.S. Adapala, H. Drissi, and H.K. Kim. 2016. HIF-1-dependent IL-6 activation in articular chondrocytes initiating synovitis in femoral head ischemic osteonecrosis. The Journal of Bone and Joint Surgery. American Volume 98 (13): 1122–1131. https://doi.org/10.2106/jbjs.15.01209.
Wang, Y., D. Li, N. Xu, W. Tao, R. Zhu, R. Sun, W. Fan, P. Zhang, T. Dong, and L. Yu. 2011. Follistatin-like protein 1: a serum biochemical marker reflecting the severity of joint damage in patients with osteoarthritis. Arthritis Research & Therapy 13 (6): R193. https://doi.org/10.1186/ar3522.
Ni, S., C. Li, N. Xu, X. Liu, W. Wang, W. Chen, Y. Wang, and A.J. van Wijnen. 2018. Follistatin-like protein 1 induction of matrix metalloproteinase 1, 3 and 13 gene expression in rheumatoid arthritis synoviocytes requires MAPK, JAK/STAT3 and NF-kappaB pathways. Journal of Cellular Physiology 234 (1): 454–463. https://doi.org/10.1002/jcp.26580.
Liu, Y., J. Wei, Y. Zhao, Y. Zhang, Y. Han, B. Chen, K. Cheng, J. Jia, L. Nie, and L. Cheng. 2017. Follistatin-like protein 1 promotes inflammatory reactions in nucleus pulposus cells by interacting with the MAPK and NFkappaB signaling pathways. Oncotarget 8 (26): 43023–43034. https://doi.org/10.18632/oncotarget.17400.
Funding
This work was not supported by any grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All the authors hereby state that they do not possess financial interests and they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qu, Y., Liu, Y. & Li, R. FSTL1 Promotes Inflammatory Reaction and Cartilage Catabolism through Interplay with NFκB Signaling Pathways in an In Vitro ONFH Model. Inflammation 42, 1491–1503 (2019). https://doi.org/10.1007/s10753-019-01012-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01012-2