Abstract
Strong spatiotemporal variation in population size often leads to reduced genetic diversity limiting the adaptive potential of individual populations. Key genes of adaptive variation are encoded by the immune genes of the major histocompatibility complex (MHC) playing an essential role in parasite resistance. How MHC variation persists in rodent populations that regularly experience population bottlenecks remains an important topic in evolutionary genetics. We analysed the consequences of strong population fluctuations on MHC class II DRB exon 2 diversity in two distant common vole (Microtus arvalis) populations in three consecutive years using a high-throughput sequencing approach. In 143 individuals, we detected 25 nucleotide alleles translating into 14 unique amino acid MHC alleles belonging to at least three loci. Thus, the overall allelic diversity and amino acid distance among the remaining MHC alleles, used as a surrogate for the range of pathogenic antigens that can be presented to T-cells, are still remarkably high. Both study populations did not show significant population differentiation between years, but significant differences were found between sites. We concluded that selection processes seem to be strong enough to maintain moderate levels of MHC diversity in our study populations outcompeting genetic drift, as the same MHC alleles were conserved between years. Differences in allele frequencies between populations might be the outcome of different local parasite pressures and/or genetic drift. Further understanding of how pathogens vary across space and time will be crucial to further elucidate the mechanisms maintaining MHC diversity in cyclic populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many species frequently display cyclic fluctuations in population size driven by internal or external drivers. In Holarctic regions, cycles of microtine rodents are affecting the entire ecosystems. The factors that drive microtine cycles remain unresolved and are widely debated in the literature (Andreassen et al. 2013; Eccard et al. 2011; Stenseth 1999; Tkadlec and Stenseth 2001; Yan et al. 2013). Suggested factors include internal density or phase dependency (Andreassen et al. 2013; Eccard et al. 2011; Stenseth 1999) and/or external factors such as seasonality or climate (Tkadlec and Stenseth 2001; Yan et al. 2013). Parasites and diseases may also represent strong drivers of rodent cycles (Pedersen and Greives 2008; Smith et al. 2008; Soveri et al. 2000), as they influence individual fitness and survival. Independent of the mechanism, population cycles often result in changes in population genetic structure including a loss of genetic diversity due to genetic drift. This may further lead to reduced individual fitness and a reduction in the adaptive potential of the population (Radwan et al. 2010).
Key genes of adaptive variation with high fitness relevance are encoded by the major histocompatibility complex (MHC) involved in the adaptive immune response. MHC genes encode molecules that bind selectively to antigens and present them to T-cells, which induce further immune reactions (Brown et al. 1993). The large diversity of MHC alleles detected in most vertebrate species is thought to be maintained mainly by pathogen-mediated selection, which operates on the MHC by heterozygote advantage (Doherty and Zinkernagel 1975; Penn et al. 2002) and/or can vary at the local scale in space and time (‘fluctuating selection’) and/or exhibit cyclical selection between host and pathogen (‘negative frequency-dependent selection’) (Sommer 2005, Spurgin and Richardson 2010). Moreover, MHC alleles usually are very divergent and show a high genetic distance at the amino acid level in the parts of the sequence which encode for the functionally important antigen-binding sites (ABS) (Piertney and Oliver 2006; Sommer 2005). Changes at these amino acids of the ABS may thereby result in changes in antigen-binding capability and subsequently in the recognition abilities of the MHC molecule (Brown et al. 1993). However, how this functionally important MHC variation persists in vertebrate populations that regularly experience population bottlenecks remains an important topic in evolutionary genetics and conservation (Winternitz et al. 2014). Studies so far provided controversial results. Whereas several studies revealed reduced MHC allele diversity in populations with decreased population sizes (Radwan et al. 2010), others reported high MHC diversity compared to neutral markers even in bottlenecked populations probably due to the stronger effect of selection processes than genetic drift (Sommer 2005; Hedrick and Hurt 2012; Oliver and Piertney 2012).
Microtine rodents provide an ideal system to investigate genetic diversity and selection processes in natural cyclic populations. Evidence for selection mechanisms acting on MHC variability in microtine rodents has been controversial. Recent studies detected a MHC heterozygote superiority against multiple parasites in a natural population of water voles (Arvicola terrestris; Oliver et al. 2009), but reported no strong evidence for parasite-mediated selection in cyclic populations of montane voles (Microtus montanus) despite relatively high neutral and MHC diversity (Winternitz et al. 2014). A comparison between cyclic and stable rodent species suggested that species that frequently undergo population bottlenecks had lower allelic richness than stable species (Winternitz and Wares 2013).
In this study, we investigate the power of selection on MHC variability using common voles (Microtus arvalis) as study organisms because they are well known for their pronounced natural annual density fluctuations (Boyce and Boyce 1988; Eccard and Herde 2013). Due to their short generation time and high number of offspring, population sizes can rise rapidly and population cycles are commonly observed (e.g. Delattre et al. 1999; Imholt et al. 2014). Thus, it is a highly suitable organism to investigate the variability of genes throughout natural population crashes. The only previous MHC study in this species focussed on MHC class II DQA (Bryja et al. 2006), a locus usually less variable than DRB genes (Sommer 2005).
Specifically, we analysed the MHC diversity of two distinct Microtus arvalis populations in three consecutive years. We (1) characterised the MHC class II DRB exon 2 for the first time in this species using a next-generation sequencing approach and (2) analysed allele diversity and frequency pattern in space and time. We hypothesised that in naturally fluctuating populations, genetic diversity is reduced, due to regular crashes in population size and associated neutral forces. The resulting consequences mainly depend on the power of selection versus genetic drift (Sommer 2005; Oliver and Piertney 2012). (A) If genetic drift dominates selection, different MHC alleles will remain in different populations over time. On the other hand, (B) if pathogen-driven selection is strong enough, the same alleles will be conserved among populations with varying allele frequencies across space and time due to distinct local parasite pressures.
Methods
Study species and trapping sites
Our study species, the common vole (Microtus arvalis, Pallas 1779), occurs all over Europe (Baláž 2010) and is believed to be the most abundant mammal in Europe (Gauffre et al. 2007). The common vole inhabits a wide range of open habitats including agricultural landscape, subalpine zones, diffuse wood vegetation and grasslands (Baláž 2010). It is a small mammal with maximum body weight of 51 g in males and 42 g for non-pregnant females (Stein 1958). The species is promiscuous and iteroparous, and multiple paternities in one litter are common (Borkowska and Ratkiewicz 2010).
In 2010, 2011 and 2012, we caught Microtus arvalis in the proximity of two field stations run by the University of Potsdam/Germany. Both study sites consist of meadows and shrubs and harbour distinct vole populations, which are located within the same geographical region, but at 80-km distance from each other. One field site (‘Potsdam’) is located in Potsdam-Bornim, Brandenburg, Germany (N 52° 26′ 22″, E 13° 0′ 44″). The other field site (‘Guelpe’) in Guelpe, Brandenburg, Germany (N 52° 44′ 01″, E 12° 12′ 41″), is situated in a floodplain (ca. 2 km long and 200–500 m wide) surrounded by arms of the river Havel with regular flooding. It includes a 1-ha dwelling mound around the research station and a sand dune ridge serving as a refuge for voles during flooding. At the Potsdam site, populations may crash due to internal or external biotic reasons (such as diseases, parasites, food shortage, predation pressure, e.g. Andreassen et al. 2013), while in Guelpe population, declines might primarily be due to flooding as an external, abiotic factor.
Common vole densities are commonly indexed in captures per 100 trap nights (TN). Common voles in grassland can reach densities of >1000 individuals per hectare (e.g. Boyce and Boyce 1988; Hušek et al. 2013); however, trapping indices from different literature sources are difficult to compare, since they vary with trap type and trap spacing. We used multiple capture life traps in lines with 10-m spacing. Since we started monitoring in 2009, the index at both sites has ranged between 0 and 150 voles/100 TN (2009–2015), reaching a bottleneck of 0 voles/100 TN after a major flood event at the Guelpe site in 2013. A comparable flood was recorded in 2002. This indicates that bottlenecks due to flooding are common in this population. In 2010, 2011 and 2012, during years of tissue sampling for this study, trapping indices were >13, 93 and 96 voles/100 TN at the Guelpe site and 24, 12 and 70 voles/100 TN at the Potsdam site, respectively.
We used metal life traps (Ugglan special No. 2, Grahnab, Sweden, with shrew exit to prevent the capture of shrews, see Eccard and Klemme 2013). Traps were baited with oat flakes and apples to provide sufficient food and water resources. Traps were checked at least twice a day, around 8 a.m. in the morning and between 6 and 8 p.m. in the evening. Animals were sexed, weighed, sampled and either released directly at the trapping location or brought to the laboratory for further behavioural experiments (Eccard and Herde 2013; Herde and Eccard 2013). Small ear punches (max. 1 × 3 mm) for genetic analyses were taken and stored in 100 % ethanol at −20 °C. Voles were captured under permission of the Landesumweltamt Brandenburg (reference number RW-7.1 24.01.01.10).
DNA sampling
Genomic DNA from samples collected in 2010 was extracted by using the NucleoSpin® Tissue Kit (Machery-Nagel, Düren, Germany). We followed the manufacturer’s protocol and eluted DNA in two steps by 80–130 μl of BE buffer depending on the size of the tissue sample. Genomic DNA from samples collected in 2011 and 2012 was isolated using the First-DNA all-tissue DNA extraction Kit (Genial GmbH, Troisdorf, Germany). By following the manufacturer’s instructions, tissue samples were dissolved for at least 3 h at 65 °C until samples were lysed completely. The DNA was eluted in 50–250 μl sterile water depending on the size of the DNA pellet. Extracted DNA was stored at −20 °C.
Primers and 454 pyrosequencing
We established species- and target-specific primers for the amplification of a 149-bp fragment of the MHC class II DRB exon 2 of common voles based on sequences derived from Sanger sequencing and an initial first 454 pyrosequencing test run (unpublished data). Fusion primers (see Supplemental Table 1) for the 454 pyrosequencing runs consisted of adaptor sequences at the 5′ end (adapter A, forward: CGTATCGCCTCCCTCGCGCCA and adapter B, reverse: CTATGCGCCTTGCCAGCCCGC) and a key sequence (TCAG). We created 18 forward and 18 reverse individual sequence combinations called multiplex identifiers (MIDs) to identify each PCR amplicon. The fusion primers ended with the target-specific primers at the 3′ end (forward: ACGCAGCGCGTGCGGTTTCTGG (MarvJA3), reverse: CYGGTAGTTGTRYCTGCAG (MarvAS2)).
To genotype the MHC of our samples, we conducted two further 454 pyrosequencing runs. For the second run, two independent PCR amplifications, i.e. replicates, were performed for each individual (N = 156 voles) using a definite MID combination to increase the reliability of our data (Sommer et al. 2013). We included the same PCR amplifications in a third run together with a repeated subsample, i.e. one 96-well plate (N = 95 animals), to check for consistency in library preparation, amplification efficiency and allele calling. All 50-μl amplification mixes contained 7–20 ng DNA, 5.0 μl 10× FastStart Buffer with 18 mM MgCl2 (Roche Applied Science, Mannheim, Germany), 0.4 μM of each primer, 0.2 mM of each dNTP and 1.25 U FastStart High Fidelity Taq Polymerase (Roche Applied Science). The thermal profile was set to 3 min at 94 °C followed by 35 cycles of 15 s at 94 °C, 45 s at 55 °C and 1 min at 72 °C. PCR ended with a final elongation at 72 °C for 8 min. Per PCR plate, we used one negative control with an individual MID combination to ensure no occurrence of any contaminations. The amplicons were purified using Agencourt AMPure beads (Agencourt Bioscience, Beverly, USA). We followed the manufacturer’s manual by using 30 μl of PCR product with 30 μl AMPure beads. Purified PCR products were quantified by the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Karlsruhe, Germany). We diluted each amplicon separately to 1 × 107 molecules per microlitre and pooled them for further analysis. For the emulsion PCR (emPCR) of the pooled amplicons, we used the GS FLX Titanium SV emPCR Kit (Lib-A) with GS FLX Titanium emPCR Filters (Roche Diagnostics GmbH) (0.1 cpb) and the GS FLX Titanium emPCR Breaking Kit (Roche Diagnostics GmbH) following the manufacturer’s protocol. The enriched library amplicons were sequenced on a Roche 454 GS Junior instrument (Roche Diagnostic GmbH).
454 read analysis
454 read quality and chimera filtering, downstream analysis and subsequent MHC allele calling were conducted as described in detail by Sommer et al. (2013). We applied the quality check implemented in the Roche 454 Genome Sequencer FLX Software (package 2.0.00.22), which is based on the sequencing results of DNA control beads and only keeps sequences with a Phred quality score < 20. After these filtering steps, 134,352 reads in the first 454 run and 182,165 reads in the second 454 run remained and passed this key quality control step. Next, we removed all reads shorter than 200 bp and assigned them to individual PCR replicates. We removed all unique sequences (singletons), as they are probably a result of sequencing errors (see Sommer et al. 2013). The remaining 111,020 and 88,763 reads (454 runs 1 and 2, respectively) were assembled into clusters with 100 % identity of the reads. As our reads appeared very similar, we adjusted the pipeline by Sommer et al. (2013) by dividing clusters into ‘clusters with 1 nucleotide difference from most frequent cluster’ and into ‘>1 nucleotide differences’ (step I in Fig. 2, Sommer et al. 2013). In total, 661 different variants occurring more than once were identified. These were applied to our strict and very conservative pipeline (steps II, III) to identify true MHC-DRB alleles, which were used for subsequent analyses. We analysed all reads of each individual replicate and run (up to four independent PCRs per individual) separately to compare the performance and check for consistency. Animals were excluded from further analysis when conflicts between PCRs appeared.
Data analysis and statistics
In order to test whether the amplified MHC DRB exon 2 region has been under selection over long periods of time, the rates of non-synonymous (dN) and synonymous (dS) base pair substitutions were calculated separately for non-antigen (non-ABS) and antigen-binding sites (ABS, i.e. residues with side chains in the binding groove plus the residues, which are involved in hydrogen bonding) as defined for house mice by Bondinas et al. (2007) using MEGA 6.06 (Tamura et al. 2013). To be able to compare our results with previous studies, we repeated the analyses using the human ABS annotated by Brown et al. (1988, 1993). We utilised the method according to Nei and Gojobori (1986) and applied the Jukes-Cantor correction for multiple hits (Jukes and Cantor 1969). The dN/dS rates were tested for significant differences from neutrality (dN = dS) using the implemented Z-test. MEGA 6 was also used to calculate pairwise allelic distances between the MHC alleles of each individual based on the number of differences between the nucleotide and amino acid sequences, respectively, applying the pairwise deletion option. The extent of population differentiation and changes in allele frequency across years was examined by pairwise F ST (10,000 permutations (Wright 1965) and the Global test (Markov chain length: 10,000 steps) using ARLEQUIN 3.5 (Excoffier et al. 2005). Allele frequencies were calculated on the amino acid level; i.e. nucleotide allele numbers translating into the same amino acid allele were summed up. Furthermore, MEGA 6 was applied to perform model selection to construct a phylogenetic tree of MHC class II DRB exon 2 alleles based on maximum likelihood. Phylogenetic relationships of MHC alleles were estimated based on 1000 bootstraps using the Whelan and Goldman (WAG) model with partial deletion and uniform rates among sites. Each terminal (nucleotide allele) was labelled by its occurrence in each year and location.
Results
MHC characteristics of common vole
In total, we analysed 156 individuals using 454 pyrosequencing (Roche Diagnostics GmbH, Mannheim, Germany). One hundred forty-three animals remained after applying the strict criteria of our bioinformatics pipeline (Sommer et al. 2013). On average, 677 reads were obtained per individual (min 173, max 2207 reads). Table 1 shows the distribution of individuals across study sites and years.
We detected 25 alleles at the nucleotide level translating into 14 unique amino acid MHC class II DRB exon 2 alleles (Supplementary Fig. 1). No stop codons were detected. Two nucleotide alleles (Miar-DRB*01f, Miar-DRB*03c) revealed a codon deletion leading to the loss of an amino acid but not to a frame shift of the subsequent amino acid sequence (Supplementary Fig. 1). MHC alleles were named Miar-DRB*01 to Miar-DRB*14 based on the standard nomenclature given by Klein et al. (1990) (Supplementary Fig. 1). Nucleotide alleles translating into the same amino acid allele were indicated by additional letters (a–f). A BLAST search confirmed the identity of Miar-DRB alleles to other rodent and microtine DRB sequences with >97 % for the top 10 hits. The allele frequencies at the amino acid level ranged from 0.004 to 0.550 (Table 1).
The number of variable nucleotide position was 51 out of 149 (24 out of 49 amino acids) (Supplementary Fig. 1). The mean number of pairwise nucleotide differences was 18.91 ± 2.38 (0 to 34). The mean number of pairwise amino acid differences was 9.18 ± 1.58 (0 to 17). The number of non-synonymous substitutions was 4.4 and 5.1 times higher in the ABS (according to Bondinas et al. (2007) and Brown et al. (1988); Brown et al. (1993), respectively) than in the non-ABS sites, a sign of strong positive selection. Accordingly, ABS showed a higher mutation rate than non-ABS, but non-synonymous and synonymous substitutions were not significantly different. In non-ABS, synonymous substitutions were 2.4 to 3.3 times more frequent than non-synonymous substitutions (p < 0.05, Table 2).
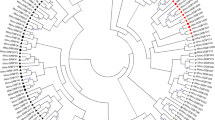
The phylogenetic relationships of amino acid MHC alleles suggested that the Miar alleles form at least three distinguished clusters with high bootstrap support (Fig. 1). All three clustered well with DRB alleles from closely related microtine species (Microtus oeconomus and Microtus montanus, see Fig. 1 for accession numbers). The expression of these alleles was partly proven by cDNA-based sequencing, which supports the functionality of our detected alleles. Individuals exhibited one to four alleles both at the nucleotide as well as at the amino acid level per individual (mean number of alleles ± standard deviation: nucleotide = 1.94 ± 0.84, amino acid = 1.69 ± 0.74). Thirty-six out of 143 individuals (25.2 %) harboured two nucleotide alleles, which translated into the same amino acid allele. Each but one individual (99.3 %, 142 out of 143) harboured an allele from cluster 1, and most individuals (86.0 %, 123 out of 143) carried Miar-DRB*01. Twelve individuals harboured alleles from cluster 1 and cluster 2 (8.4 %), ten individuals from clusters 1 and 3 (7.0 %), and four individuals (2.8 %) from all three clusters.
Phylogenetic relationship of Microtus arvalis MHC class II alleles (this study, Miar-DRB*01 to Miar-DRB*14) in relation to sequences of closely related microtine species (Mioe Microtus oeconomus, Mimo Microtus montanus). GenBank accession numbers are provided in brackets. *Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.h04hr, Winternitz and Wares 2013. Presence of Miar alleles is labelled per study site Guelpe (triangle) and Potsdam (circle). The study year is indicated by open (2010), grey (2011) and black (2012) symbols. The tree is rooted by a sequence of striped mice (Rhpu Rhabdomys pumilio)
Population differentiation in space and time
The overall genetic diversity, i.e. expected heterozygosity, was higher in the Guelpe (0.69 ± 0.03) than in the Potsdam population (0.52 ± 0.04), as well as the mean amino acid divergence ± standard error within an individual (Guelpe: AAdist = 4.13 ± 0.81, Potsdam AAdist = 2.55 ± 0.57, MWU test: n.s.). However, the mean number of alleles per individual did not differ between the two populations (Guelpe: nucleotide = 1.89 ± 0.88, amino acid = 1.76 ± 0.78, Potsdam: nucleotide = 1.99 ± 0.82, amino acid = 1.64 ± 0.72, MWU test: n.s.).
The most frequent MHC alleles (>10 % Miar-DRB*01 and 03) were found in both populations and within all years (Table 1). Guelpe voles harboured nine and Potsdam 11 different MHC class II DRB amino acid alleles. Six alleles occurred at both study sites. Both study populations did not show significant population differentiation between years (all pairwise F ST P values > 0.29). However, they showed a significant population differentiation between sites (F ST = 0.06, P < 0.001).
Discussion
In this study, we analysed for the first time the MHC class II DRB exon 2 allelic variation in common voles, a rodent well known for its frequent population cycles. Microtus arvalis exhibited a moderate variability of MHC alleles in our two populations studied. In 143 individuals, we detected 25 alleles on the nucleotide level translating into 14 unique amino acid alleles but distributed across at least three MHC class II DRB loci. Only two alleles (Miar-DRB*01, Miar-DRB*03) occurred in high frequencies (> 25 %), whereas the 12 others occurred in very low frequencies (< 5 %, Table 1). The number of detected alleles in common voles is considered remarkably high for a species with very regular fluctuations in population size. Our results are in agreement with a previous study in another cyclic population of a Microtus vole species: Winternitz et al. (2014) reported moderate levels of diversity with 21 MHC DRB alleles belonging to at least two loci in 123 individuals of Microtus montanus, considered as remarkably high for a species with very regular density fluctuations. Furthermore, Bryja et al. (2006) found eight MHC alleles in 16 investigated individuals of common voles at the duplicated DQA locus. The number of DQA alleles was within the same range in other vole species (water vole Arvicola terrestris: N = 96, seven MHC-DQA alleles, two loci; bank vole Myodes glareolus: N = 16, seven MHC-DQA, two loci, see Bryja et al. 2006). This was interpreted as a generally high DQA diversity in voles (Bryja et al. 2006). In comparison, in non-cyclic rodent species such as wood mice (Apodemus sylvaticus), 38 unique DRB alleles (N = 119) and in yellow-necked mice (Apodemus flavicollis) 15 DRB alleles (N = 60) were detected (Musolf et al. 2004), respectively. In 36 Eastern woodchucks (Marmota monax), Moreno-Cugnon et al. (2015) identified 15 MHC alleles, which were expressed in two independent loci. In Alpine marmots (Marmota marmota), 11 MHC DRB alleles were detected at two loci in 38 individuals (Kuduk et al. 2012).
In our vole populations, not all loci were present in all individuals though all loci seem to be functional. Individuals exhibited one to four alleles (mean number of alleles = 1.94) indicating the presence of at least two loci at the individual level. Since many MHC alleles at the nucleotide level (up to six) translated into the same amino acid allele, most gene duplications seemed to have occurred recently. Almost all individuals carried at least one allele from cluster 1 (99.3 %), 12 individuals (8.4 %) carried at least one allele from clusters 1 and 2, and 10 individuals (7.0 %) harboured an allele from clusters 1 and 3, but in only 4 individuals (2.8 %), an allele from each cluster was detected. We consider a potential loci amplification bias as unlikely since we used optimal fitting species- and target-specific primers during intensive preliminary analyses though this can never be fully excluded without sequencing whole genes. Our primers have been used in three separate next-generation sequencing runs including independent individual replicates. As we obtained equal results and allele sequences, variations in number of alleles per individual seem to reflect the individual copy number variation. Moreover, copy number variations within populations are common in mammals, and especially within Microtus species, gene duplications are a common phenomenon (Winternitz and Wares 2013). For instance, Microtus oeconomus (root vole) and Microtus montanus (montane vole) inhabit at least two DRB loci (Winternitz and Wares 2013). Furthermore, the MHC class II DQA locus also seems to appear duplicated within Arvicolinae (e.g. Microtus arvalis, Bryja et al. 2006; Myodes glareolus, Axtner and Sommer 2007).
The mean number of pairwise amino acid differences was 9.18 ± 1.58 (0 to 17), which is considered high for a species subject to high amplitude fluctuations and in comparison to other rodent species. For example, in montane voles (Microtus montanus), on average, 6.5 amino acid differences were detected among 21 MHC-DRB alleles (Winternitz and Wares 2013). In wood mice (Apodemus sylvaticus), on average, 5.8 amino acid differences were observed between 13 MHC DQA alleles (de Bellocq et al. 2008). Further, in striped mice (Rhabdomys pumilio), a rodent with a large geographic range ranging from South Africa, across Namibia to the border of Angola, Froeschke and Sommer (2005) revealed a mean number of 7.8 amino acid differences between 20 DRB alleles (N = 58 individuals). In accordance with the observed elevated substitution rate in ABS, this suggests strong positive selection maintaining functional important MHC diversity.
A recent meta-analysis revealed that MHC and neutral genetic diversity are lost during bottleneck events in most vertebrate populations (Sutton et al. 2011). A study in voles (Microtus arvalis) using neutral markers detected, however, that vole migration increased with population density, and the authors assumed that this counteracts the local loss of genetic variety during the low-density phases (Gauffre et al. 2014). Also, in water voles (Arvicola terrestris), high variance in population size did not negatively affect genetic diversity in both neutral (Berthier et al. 2006) and adaptive MHC markers (Bryja et al. 2007).
In our study populations, we did not observe a strong impact of genetic drift on MHC diversity. We found no differences in MHC allele composition and frequencies in both populations between the years. Though the study sites differ in the rhythm and main causes of population crashes, the population dynamics at both study sites seem to be similar. At the Potsdam site, populations may crash due to internal or external biotic reasons (such as diseases, parasites, food shortage, predation pressure, e.g. Andreassen et al. 2013), while in the Guelpe population, declines might primarily be due to flooding as an external, abiotic factor. Interestingly, the mean number of alleles per individual did not differ between the two populations and the overall genetic diversity, and the mean amino acid divergence within an individual was even higher in Guelpe than in Potsdam. This suggests that occasionally occurring wipeouts of the Guelpe population by flooding are not complete. Some local voles might survive a population crash and serve as founders of a new population, sufficient to maintain the observed MHC diversity. There are refugia during flooding, such as a dwelling mount of ca. 1 ha around the research station that was not flooded since the house was build some 150 years ago and a sand dune ridge. Contrarily to the Potsdam population, however, which is connected to a larger continuous population, it is unlikely that the Guelpe population is colonised from neighbouring populations, since the study site is situated on an island surrounded by arms of the river Havel.
Though the two study populations did not differ to a large extent in their MHC diversity, we observed a strong overall differentiation between the populations. The significant F ST value between the two populations could be due to many different factors, including divergent selection, neutral drift with reduced migration or bottlenecks after previous recolonisation, and can thus not be interpreted without comparing with similar analyses using a neutral marker. Evidence for parasite-driven selection and the association of specific MHC alleles, MHC heterozygosity and MHC expression with parasite load have been stated for many other rodent species (Axtner and Sommer 2007; Meyer-Lucht et al. 2008). Vole individuals in our study populations did not show any visible health problems. As already detected in other bottlenecked species like cheetahs and beavers (Babik et al. 2005; Castro-Prieto et al. 2011), a reduced number of MHC alleles do not necessarily lead to more susceptible populations in terms of infections and pathogens (Radwan et al. 2010). It might, however, involve a risk once new pathogens emerge since the adaptation ability might be reduced (Sommer 2005). In Apodemus flavicollis, it was shown that a lower number of MHC-DRB alleles per population were associated with an elevated overall helminth infection and vice versa (Meyer-Lucht and Sommer 2009).
To conclude, our results highlight the key role of selection in maintaining MHC diversity even in species with high population size fluctuations. Detailed studies of how pathogens vary across space and time will be crucial to further elucidate the counteracting forces of pathogen-driven selection versus genetic drift affecting MHC and neutral marker diversity in cyclic populations.
References
Andreassen HP, Glorvigen P, Remy A, Ims RA (2013) New views on how population-intrinsic and community-extrinsic processes interact during the vole population cycles. Oikos 122:507–515. doi:10.1111/j.1600-0706.2012.00238.x
Axtner J, Sommer S (2007) Gene duplication, allelic diversity, selection processes and adaptive value of MHC class II DRB genes of the bank vole. Clethrionomys Glareolus Immunogenet 59:417–426. doi:10.1007/s00251-007-0205-y
Babik W, Durka W, Radwan J (2005) Sequence diversity of the MHC DRB gene in the Eurasian beaver (Castor fiber). Mol Ecol 14:4249–4257. doi:10.1111/j.1365-294X.2005.02751.x
Baláž I (2010) Somatic characteristics and reproduction of common vole, Microtus arvalis. Biologia 65:1064–1071
Berthier K, Charbonnel N, Galan M, Chaval Y, Cosson JF (2006) Migration and recovery of the genetic diversity during the increasing density phase in cyclic vole populations. Mol Ecol 15:2665–2676
Bondinas GP, Moustakas AK, Papadopoulos GK (2007) The spectrum of HLA-DQ and HLA-DR alleles, 2006: a listing correlating sequence and structure with function. Immunogenetics 59:539–553. doi:10.1007/s00251-007-0224-8
Borkowska A, Ratkiewicz M (2010) Promiscuity, male reproductive success and mate relatedness in a natural population of the common vole. J Zool 280:195–201
Boyce CC, Boyce JL (1988) Population biology of Microtus arvalis. III. Regulation of numbers and breeding dispersion of females. J Anim Ecol 737–754
Brown JH, Jardetzky T, Saper MA, Samraoui B, Bjorkman PJ, Wiley DC (1988) A hypothetical model of the foreign antigen-binding site of class II histocompatibility molecules. Nature 332:845–850. doi:10.1038/332845a0
Brown JH, Jardetzky TS, Gorga JC, Stern LJ, Urban RG, Strominger JL, Wiley DC (1993) 3-Dimensional structure of the human class-II histocompatibility antigen HLA-DR1. Nature 364:33–39. doi:10.1038/364033a0
Bryja J, Galan M, Charbonnel N, Cosson JF (2006) Duplication, balancing selection and trans-species evolution explain the high levels of polymorphism of the DQA MHC class II gene in voles (Arvicolinae). Immunogenetics 58:191–202. doi:10.1007/s00251-006-0085-6
Bryja J, Charbonnel N, Berthier K, Galan M, Cosson JF (2007) Density-related changes in selection pattern for major histocompatibility complex genes in fluctuating populations of voles. Mol Ecol 16:5084–5097. doi:10.1111/j.1365-294X.2007.03584.x
Castro-Prieto A, Wachter B, Sommer S (2011) Cheetah paradigm revisited: MHC diversity in the world’s largest free-ranging population. Mol Biol Evol 28:1455–1468. doi:10.1093/molbev/msq330
de Bellocq JG, Charbonnel N, Morand S (2008) Coevolutionary relationship between helminth diversity and MHC class II polymorphism in rodents. J Evol Biol 21:1144–1150. doi:10.1111/j.1420-9101.2008.01538.x
Delattre P, De Sousa B, Fichet-Calvet E, Quere JP, Giraudoux P (1999) Vole outbreaks in a landscape context: evidence from a six year study of Microtus arvalis. Landsc Ecol 14:401–412. doi:10.1023/a:1008022727025
Doherty PC, Zinkernagel RM (1975) Enhanced immunological surveillance in mice heterozygous at H-2 gene complex. Nature 256:50–52. doi:10.1038/256050a0
Eccard JA, Herde H (2013) Seasonal variation in the behaviour of a short-lived rodent. BMC Ecol 13
Eccard JA, Klemme I (2013) Reducing mortality of shrews in rodent live trapping—a method increasing live-trap selectivity with shrew exits. Ann Zool Fenn 50:371–376
Eccard JA, Jokinen I, Ylonen H (2011) Loss of density-dependence and incomplete control by dominant breeders in a territorial species with density outbreaks. BMC Ecol 11:8pp
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinforma 1:47–50
Froeschke G, Sommer S (2005) MHC class II DRB variability and parasite load in the striped mouse (Rhabdomys pumilio) in the Southern Kalahari. Mol Biol Evol 22:1254–1259. doi:10.1093/molbev/msi112
Gauffre B, Berthier K, Inchausti P, Chaval Y, Bretagnolle V, Cosson J-F (2014) Short-term variations in gene flow related to cyclic density fluctuations in the common vole. Mol Ecol 23:3214–3225. doi:10.1111/mec.12818
Gauffre B, Galan M, Bretagnolle V, Cosson JF (2007) Polymorphic microsatellite loci and PCR multiplexing in the common vole, Microtus arvalis. Mol Ecol Notes 7:830–832
Hedrick PW, Hurt CR (2012) Conservation genetics and evolution in an endangered species: research in Sonoran topminnows. Evol Appl 5:806–819. doi:10.1111/j.1752-4571.2012.00259.x
Herde A, Eccard JA (2013) Consistency in boldness, activity and exploration at different stages of life. BMC Ecol 13
Hušek J et al (2013) Cyclicity and variability in prey dynamics strengthens predator numerical response: the effects of vole fluctuations on white stork productivity. Popul Ecol 55:363–375
Imholt C, Reil D, Eccard JA, Jacob D, Hempelmann N, Jacob J (2014) Quantifying the past and future impact of climate on outbreak patterns of bank voles (Myodes glareolus). Pest Manag Sci 71:166–172. doi:10.1002/ps.3838
Jukes TH, Cantor CR (1969) Evolution of protein molecules. Mamm Protein Metab 3:132
Klein J, Bontrop RE, Dawkins RL, Erlich HA, Gyllensten UB, Heise ER, Jones PP, Parham P, Wakeland EK, Watkins DI (1990) Nomenclature for the major histocompatibility complexes of different species: a proposal. Immunogenetics 31:217–219
Kuduk K, Johanet A, Allaine D, Cohas A, Radwan J (2012) Contrasting patterns of selection acting on MHC class I and class II DRB genes in the Alpine marmot (Marmota marmota). J Evol Biol 25:1686–1693. doi:10.1111/j.1420-9101.2012.02537.x
Meyer-Lucht Y, Sommer S (2009) Number of MHC alleles is related to parasite loads in natural populations of yellow necked mice, Apodemus flavicollis. Evol Ecol Res 11:1085–1097
Meyer-Lucht Y, Otten C, Puettker T, Sommer S (2008) Selection, diversity and evolutionary patterns of the MHC class II DAB in free-ranging Neotropical marsupials.cxx BMC Genet 9 doi:10.1186/1471-2156-9-39
Moreno-Cugnon L, Esparza-Baquer A, Larruskain A, Garcia-Etxebarria K, Menne S, Gonzalez-Aseguinolaza G, Jugo BM (2015) Characterization and genotyping of the DRB1 gene of the major histocompatibility complex (MHC) in the Marmota monax, animal model of hepatitis B. Mol Immunol 63:505–512. doi:10.1016/j.molimm.2014.10.011
Musolf K, Meyer-Lucht Y, Sommer S (2004) Evolution of MHC-DRB class II polymorphism in the genus Apodemus and a comparison of DRB sequences within the family Muridae (Mammalia: Rodentia). Immunogenetics 56:420–426. doi:10.1007/s00251-004-0715-9
Nei M, Gojobori T (1986) Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol 3:418–426
Oliver MK, Piertney SB (2012) Selection maintains MHC diversity through a natural population bottleneck. Mol Biol Evol 29:1713–1720. doi:10.1093/molbev/mss063
Oliver MK, Telfer S, Piertney SB (2009) Major histocompatibility complex (MHC) heterozygote superiority to natural multi-parasite infections in the water vole (Arvicola terrestris). Proc R Soc B-Biol Sci 276:1119–1128. doi:10.1098/rspb.2008.1525
Pedersen AB, Greives TJ (2008) The interaction of parasites and resources cause crashes in a wild mouse population. J Anim Ecol 77:370–377. doi:10.1111/j.1365-2656.2007.01321.x
Penn DJ, Damjanovich K, Potts WK (2002) MHC heterozygosity confers a selective advantage against multiple-strain infections. Proc Natl Acad Sci U S A 99:11260–11264. doi:10.1073/pnas.162006499
Piertney SB, Oliver MK (2006) The evolutionary ecology of the major histocompatibility complex. Heredity 96:7–21. doi:10.1038/sj.hdy.6800724
Radwan J, Biedrzycka A, Babik W (2010) Does reduced MHC diversity decrease viability of vertebrate populations? Biol Conserv 143:537–544. doi:10.1016/j.biocon.2009.07.026
Smith MJ, White A, Sherratt JA, Telfer S, Begon M, Lambin X (2008) Disease effects on reproduction can cause population cycles in seasonal environments. J Anim Ecol 77:378–389. doi:10.1111/j.1365-2656.2007.01328.x
Sommer S (2005) The importance of immune gene variability (MHC) in evolutionary ecology and conservation. Front Zool 2 doi:10.1186/1742-9994-2-16
Sommer S, Courtiol A, Mazzoni CJ (2013) MHC genotyping of non-model organisms using next-generation sequencing: a new methodology to deal with artefacts and allelic dropout. BMC Genomic 14 doi:10.1186/1471-2164-14-542
Soveri T et al (2000) Disease patterns in field and bank vole populations during a cyclic decline in central Finland. Comp Immunol Microbiol Infect Dis 23:73–89. doi:10.1016/s0147-9571(99)00057-0
Spurgin LG, Richardson DS (2010) How pathogens drive genetic diversity: MHC, mechanisms and misunderstandings. Proc R Soc B-Biol Sci 277:979–988
Stein GH (1958) Die Feldmaus (Microtus arvalis Pallas). A. Ziemsen
Stenseth NC (1999) Population cycles in voles and lemmings: density dependence and phase dependence in a stochastic world. Oikos 87:427–461. doi:10.2307/3546809
Sutton JT, Nakagawa S, Robertson BC, Jamieson IG (2011) Disentangling the roles of natural selection and genetic drift in shaping variation at MHC immunity genes. Mol Ecol 20:4408–4420. doi:10.1111/j.1365-294X.2011.05292.x
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
Tkadlec E, Stenseth NC (2001) A new geographical gradient in vole population dynamics. Proc R Soc B-Biol Sci 268:1547–1552. doi:10.1098/rspb.2001.1694
Winternitz JC, Wares JP (2013) Duplication and population dynamics shape historic patterns of selection and genetic variation at the major histocompatibility complex in rodents. Ecol Evol 3:1552–1568. doi:10.1002/ece3.567
Winternitz JC, Wares JP, Yabsley MJ, Altizer S (2014) Wild cyclic voles maintain high neutral and MHC diversity without strong evidence for parasite-mediated selection. Evol Ecol 28:957–975. doi:10.1007/s10682-014-9709-8
Wright S (1965) The interpretation of population structure by F-statistics with special regard to systems of mating. Evolution 395–420
Yan C, Stenseth NC, Krebs CJ, Zhang Z (2013) Linking climate change to population cycles of hares and lynx. Glob Chang Biol 19:3263–3271. doi:10.1111/gcb.12321
Acknowledgments
We would like to thank all students and field assistants who trapped common voles over the 3 years of this study. We appreciate the technical support provided by Anke Schmidt und Susan Mbedi and thank Mark Gillingham for language corrections. We are grateful to two anonymous referees for providing very helpful comments on a previous draft of this MS.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 76 kb)
Rights and permissions
About this article
Cite this article
Schuster, A.C., Herde, A., Mazzoni, C.J. et al. Evidence for selection maintaining MHC diversity in a rodent species despite strong density fluctuations. Immunogenetics 68, 429–437 (2016). https://doi.org/10.1007/s00251-016-0916-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-016-0916-z