Abstract
Diatoms (Bacillariophyceae) are important primary producers in a wide range of hydro-terrestrial habitats in polar regions that are characterized by many extreme environmental conditions. Nevertheless, how they survive periods of drought and/or freeze remains unknown. A general strategy of microorganisms to overcome adverse conditions is dormancy, but morphologically distinct diatom resting stages are rare. This study aimed to evaluate the annual cycle of freshwater diatoms in the High Arctic (Central Spitsbergen) and provide an insight into their physiological cell status variability. The diversity and viability of diatom cells were studied in samples collected five times at four study sites, tracing the key events for survival (summer vegetative season, autumn dry-freezing, winter freezing, spring melting, summer vegetative season [again]). For viability evaluation, a multiparameter fluorescent staining was used in combination with light microscopy and allowed to reveal the physiological status at a single-cell level. The proportions of the cell categories were seasonally and locality dependent. The results suggested that a significant portion of vegetative cells survive winter and provide an inoculum for the following vegetative season. The ice thickness significantly influenced spring survival. The thicker the ice layer was, the more dead cells and fewer other stages were observed. The influence of the average week max–min temperature differences in autumn and winter was not proven.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural conditions in polar regions are characterized by many extremes that are challenging for any organism. Despite this fact, diatoms (Bacillariophyceae) apparently adapted well and became one of the most abundant algal groups in many polar freshwater and terrestrial habitats [1, 2] where they have to face inhospitable and stressful polar conditions represented by low light and nutrient supply, as well as low temperatures causing recurrent freezing events and irregular liquid water availability [3, 4]. These patterns differ in periodicity, amplitude, and synchronicity, and they initiate several different ecological and physiological acclimation and adaptation responses. For example, annual solar irradiance is over the latitude from 30° to 80° reduced by about 50%, which, together with snow and ice layers, limits the total annual production and strongly influences the seasonality of photosynthesis [3, 4]. Polar phytoplankton experiences temperatures around zero for most of the year, which is usually less than the optimal range for physiological processes [5, 6]. Moreover, at temperatures below − 0.6 °C, biological water becomes thermodynamically unstable under isotonic conditions and tends to crystallize [7]. Intracellular freezing is almost always associated with cell injuries, often resulting in irreversible or lethal cell damages [7, 8]. Furthermore, the climatic conditions in the Arctic are changing; long-term increases in winter air temperatures are being observed and are predicted to be faster than those in the rest of the world and more obvious than in summer [9, 10]. Changes in the amount of precipitation and the proportion that falls as rain or snow, as well as its duration, have important consequences for sub- and supernivian biological activity [10, 11].
Microalgae have had to evolve various universal strategies for successful survival in polar habitats. To maintain an aqueous external environment, microalgae produce numerous extracellular macromolecular substances that act via lowering the freezing point of a solution below the thawing point, changing the viscosity of the brine and increasing the volume of the inhabitable liquid phase and the inner ice–liquid surface area [12, 13]. Equally important effects of these substances are the ability to bind to ice, influence its growth, and inhibit the recrystallization process (growth of large crystals at the expenses of small ones) [12,13,14,15]. Most of these mechanisms have been found only in algae from low-temperature environments, which suggest their cryoprotective function [16]. The intracellular responses to temperature downshift include the synthesis of enzyme stabilizing molecules, which are protecting different cellular compartments from freezing injuries [17, 18], production of compatible solutes as sucrose, prolin [19], betaines [20], and dimethylsulfide (DMS) [20, 21], and increase in the proportion of polyunsaturated fatty that helps to keep fluidity of phospholipid bilayer [22, 23].
Another general strategy to survive unfavorable conditions is to create a resistant long-lasting stage. Diatoms are known to form two types of such stages: resting cells and resting spores. Both stages are characterized by reduced metabolic activity and different structure, changes in cellular components leading to a resting state that requires the consumption of small amounts of cellular carbon to survive [24, 25]. Resting cells are physiologically adapted vegetative-looking cells [26,27,28]. They have dense and dark cytoplasmic matter, and rounder plastids are observed in spores [29], as well as less pigmentation and contracted chloroplasts [24]. Resting cells were observed in many pennate diatoms [30], including freshwater species [31]. It is suggested that resting cells are important for the freezing and desiccation survival of terrestrial diatoms in their variable habitats [31]. Resting spores are morphologically different from vegetative cells. They are characterized by their rounder shape, thicker cell wall, and different patterns [30, 32]. Their formation is usually associated with limited nutrition supplies and winter survival [30, 32, 33]. Resting spores are the most common in centric marine diatoms from temperate neritic habitats [30, 33]. They are generally relatively rare in freshwater and are mostly observed in centric species [25, 30, 34].
Despite the fact that diatoms are a well-studied group of microorganisms, the strategy that enables them to survive polar winters remains unknown. Even though they do not form any morphologically different resting stages, they dominate in many polar habitats. A recent field study of the overwintering strategy of the filamentous cyanobacteria Phormidium sp. revealed the high survival of vegetative cells as filaments enclosed in thick polysaccharide sheaths [35]. Another study on filamentous green microalgae Zygnema spp. presented their survival as modified vegetative cells called “pre-akinetes” [36]. In this study, we decided to study the annual cycle of freshwater diatom populations at four study sites in the High Arctic using fluorescent staining, which enables us to distinguish the physiological status at the single-cell level [37] and show its seasonal and spatial variability.
Materials and Methods
Study Sites and Experimental Setup
The field study was conducted in two valleys in the central part of Spitsbergen (High Arctic). Four sites (shallow hydro-terrestrial tundra habitats) with high abundances of diatoms were selected (Figs. 1 and S1): Bjørndalen 1 (78.217129° N, 15.333050° E, 52 m a.s.l.), Bjørndalen 2 (78.218626° N, 15.339754° E, 93 m a.s.l.), Endalen 1 (78.183551° N, 15.763844° E, 96 m a.s.l.), and Endalen 2 (78.186742° N, 15.791057° E, 60 m a.s.l.). Both Bjørndalen and Endalen are U-shaped valleys cut through the subhorizontal succession of Cretaceous and Tertiary sedimentary rocks. The bedrock is mostly composed of sandstone with siltstone and shale intercalations and, locally, coal seams close to the Cretaceous-Tertiary boundary [39, 40]. Bjørndalen 1 is a stony snow-fed stream, partly moss and grass covered along the banks with an occurrence of cotton grass (Eriophorum sp.); Bjørndalen 2 is a seepage in the middle of a moss- and grass-dominated meadow, with frequent occurrence of cotton grass (Eriophorum sp.). Endalen 1 is a snow-fed stream situated under steep slopes of the valley and Endalen 2 a seepage/wetland, both covered by mosses and grass with occurrence of polar willow (Salix polaris), mountain sorrel (Oxyria digyna), alpine bistort (Bistorta vivipara), Arctic mouse-ear (Cerastium arcticum), whitlow grass (Draba sp.), and buttercup (Ranunculus sp.). All the study sites have an oligotrophic character.
The Arctic region, with Svalbard highlighted. A detailed map illustrating the study sites situated in two valleys in Central Spitsbergen. Map source: Svalbardkartet, Norwegian Polar Institute [38]
Climatic conditions in Spitsbergen are according to Köppen-Geiger climate types classified as semiarid polar tundra [41, 42]. Average year precipitation based on data provided by the Norwegian Meteorological Institute reported during 2005–2015 at Svalbard Airport meteorological station was 221.5 mm. The hottest month was July with an average temperature of 7 °C and the coldest was March with a temperature of − 11 °C. The month with the highest mean precipitation was December (35.9 mm) and lowest was June (6.6 mm). Recent measurements of meteorological parameters in this area (Adventdalen) showed that the mean air temperature (2 m above the ground) exceeded 0 °C for about 5 months, from the beginning of May until the end of September (using data provided by the UNIS weather stations [43]). Daylight was unavailable from the end of October to the middle of February (10/26/2017–2/16/2018).
Two metal poles, three Petri dishes, and two or three plastic boxes were installed per location in the summer of 2017 (Figs. S1 and S2). The dishes and boxes were perforated to ensure water flow inside. Several stones were placed inside the boxes and the installations were fixed by sticks to avoid their loss. Diatoms were caught in the boxes and Petri dishes to ensure multiple samplings of the same population. The poles were installed to find the exact location of installations during the winter season when the surface was snow-covered. To provide temperature characteristics of the studied habitats, three Minikin Tie dataloggers (EMS Brno, Brno, Czech Republic) were installed in each site and measured the temperature at a 1-h interval during the whole period of study (8/8/2017–7/26/2018). In each site, two of the dataloggers were installed in the water body and one in a perforated box for temperature comparison. The average, minimal, and maximal temperatures and max–min temperature difference per week per study site were determined. Data were processed with the program Mini32 (EMS Brno, Brno, Czech Republic).
Sampling
The diatom communities were sampled five times during the period of August 2017–July 2018 to record key events for algal survival: summer vegetative season (8/8/2017), autumn dry-freezing (10/20/2017), frozen state in winter (4/12, 14/2018), spring melting (7/6/2018), and the summer season again (7/26/2018). Three to five samples were collected per study site from the inside of the perforated boxes and Petri dishes (three different spots from each dish/box together made one sample). Material was collected into 15-ml plastic tubes using a Pasteur pipette, knife, or ax, depending on the seasonal conditions. The sampling equipment was cleaned to avoid cross-contamination between samplings. The biomass was immediately used for viability analyses. In total, 79 samples were collected (summer 2017, 17; autumn, 12; winter, 13; spring, 19; and summer 2018, 18). Field samples were transported and processed in the lab at the Czech Arctic Research Station of Josef Svoboda in Longyearbyen (Svalbard). In addition, aliquots of samples were fixed with ethanol for further microscopy investigation.
Viability Evaluation and Sample Processing
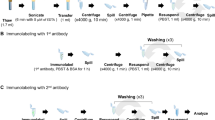
Upon arrival at the lab, the samples were left in subdued light at 8 °C for 30 min or in darkness at 5 °C for 10 h (winter samples) to thaw slowly if necessary. For the viability evaluation, three to five samples were used per site as replicates. Viability was assessed using a multiparameter fluorescent staining protocol originally developed for filamentous cyanobacteria [37]. The combination of this method and light microscopy enabled us to evaluate the exact physiological state of single cells. The staining protocol combines three fluorescent stains: SYTOX Green Nucleic Acid Stain (S7020, Molecular Probes, Eugene, OR, USA), CTC (5-cyano-2,3-ditolyl tetrazolium chloride, Polysciences Europe GmbH, Eppelheim, Germany), and DAPI (4′,6-diamidin-2-fenylindol, Molecular Probes, Eugene, OR, USA). SYTOX Green is a nucleic acid stain detecting membrane integrity and serving as a useful indicator of dead cells because it does not penetrate living cells. It is expressed as green fluorescence of the cell nucleus. CTC shows respiration activity by accumulation of formazan crystals in mitochondria of active cells, which appears as red fluorescence. DAPI stains dsDNA in both living and dead cells and is detected by white-blue fluorescence of the nuclei.
The homogenized suspension of diatoms and sediment was stained by three fluorescent stains consecutively using 1.5-ml Eppendorf tubes. SYTOX Green Dye was used first, at a concentration of 10 μM. Stained samples were incubated for 15 min in cold (8 °C) and protected from light by two layers of aluminum foil. Samples were washed three times afterward with ambient water. To estimate the respiratory activity, the samples were stained with 5 mM CTC and kept for an additional 120 min under dark and cold conditions. The dye solutions were removed afterward; the samples were rinsed once and stained with DAPI at a concentration of 5 μg/ml, and incubated for 30 min under dark and cool conditions. The samples were then washed three times, after which the suspension was transferred onto a slide and studied using the fluorescent microscope Olympus BX53 equipped with a 100-W ultra-high pressure mercury arc lamp (Olympus Corporation, Tokyo, Japan) at × 200 magnification [37]. To assess the proportion of cell physiological states, at least 400 cells per sample were counted on a minimum of 10 random transects. In total, 31,789 cells were studied. According to the staining results, cells were classified in five groups: active healthy cells, inactive but intact dormant cells, injured but active cells, injured and inactive dead cells, and dead cells performing no fluorescence. Cells with no fluorescence but the presence of visible protoplast remains observed under the light microscope were determined to be dead (Table 1; Fig. 2). In addition, a lot of empty silica diatom frustules (50–90%) were observed in samples from all microhabitats. These cells were not included in counting because of the unknown degradation time of the cell content, which means that the real survival could be lower than suggested in this study.
Species Composition
Additional dominant species were determined, and the relative abundances (percentage of total diatom valves per replica and in all five samplings together) were expressed for each study site using stained samples. Only species present at 1% in any single sampling were included. For detailed taxonomical analysis, subsamples were oxidized by adding 37% hydrogen peroxide and incubated overnight. Samples were then rinsed three times with distilled water alternated with centrifugation (10 min at 1200×g). The material was dried on microscope cover slips and embedded in Naphrax (Brunel Microscopes Ltd., Chippenham, UK). Microscopic slides were observed using an Olympus BX43 microscope (Olympus Corporation, Tokyo, Japan) equipped with differential interference contrast (Nomarski) optics under oil immersion at × 1000 magnification. Samples and slides were stored at the Department of Ecology, Charles University, Prague, Czech Republic.
Data Analyses
All statistical analyses were performed in R 3.6.1 (R Core Team, Vienna, Austria) using multivariate methods in the “vegan” package [44]. Prior to the analyses, data were standardized across species (mean variance standardization). To analyze the variability within the dataset, principal component analysis (PCA) and redundancy analysis (RDA) were run on the whole dataset. In RDA, the effect of locality and season was tested using the permutation test for RDA under a reduced model. Furthermore, the potential influence of ice thickness, depth of snow layer, and temperature oscillations (average weekly max–min temperature differences in autumn 10/2/2017–11/26/2017 and winter 1/1/2018–3/4/2018) on the cell viabilities in spring was tested using the permutation test for RDA under a reduced model. As the snow depth was highly correlated with winter temperature oscillation, it was not used in further analyses. Therefore, only temperature data were used for statistical analyses. For graphical visualization, R, ArcGIS (Esri, Redlands, CA, USA), Zoner Photo Studio 16 (Zoner Software, Brno, Czech Republic), InkScape 0.92.3 (Software Freedom Conservancy, New York, NY, USA), and GraphPad Prism 5.03 (GraphPad Software, La Jolla, CA, USA) were used.
Results
Environmental Conditions
According to the measurements provided by dataloggers each hour, the temperature reached zero in mid-September in all the studied sites. The temperature courses corresponded with average ambient air temperature fluctuations (Fig. 3a). With the onset of the polar night (October 26, 2017), the study sites became frozen and remained frozen until the end of May, when the snow and ice melted. The minimum temperature of all sampling sites reached − 18.6 °C at Endalen 1 site and the maximum temperature reached was 13.8 °C at Endalen 2 site. The lowest ambient air temperature was − 20.5 °C and the highest was 11.9 °C. During winter, three warm periods were noticeable (in the middle of January and in the beginning and end of February), when the air temperature increased above 0 °C and the temperatures at some of the study sites reached 0 °C. The minimal and maximal temperatures and environmental conditions per study site are given in Tables 2 and S3. The average max–min temperature differences per week measured in “critical” survival periods, when temperatures fluctuated around freezing point, were as follows: in autumn (10/2/2017–11/26/2017)/winter (1/1/2018–3/4/2018) were 1.06/0.86 °C (Endalen 1), 1.11/0.40 °C (Endalen 2), 0.32/0.38 °C (Bjørndalen 1), 0.39/1.29 °C (Bjørndalen 2) (Fig. 3b).
a Average daily temperatures and b average weekly difference in the maximum and minimum temperatures per study site measured in 8/8/2017–7/26/2018 with sampling events (arrows) and freezing/thawing periods indicated (shading). The source of ambient air temperature data was the UNIS weather stations (2018)
Seasonal Changes of the Habitats
The sampling started in the summer vegetative season, in the beginning of August 2017. Liquid water was available in all of the sampled sites. In autumn 2017, the sediment with diatoms in both the stream and seepage in the Bjørndalen valley was not frozen solid yet; it was slightly moist and easily deformable, but very close to the frozen state. In Endalen, most of the sampled microhabitats were desiccated and frozen (Figs. 4 and 5). A thin layer of ice was visible above the sampled material, where the water level usually occurs, giving evidence of a desiccation event. In the winter season of 2018, all of the localities were frozen and covered by snow. The average thickness of layers observed in the study sites is shown in Table 2. In spring, the sampling sites appeared to be similar to those in both summer vegetative seasons (Figs. 4 and 5). Most of the ice and snow melted, and liquid water was available in all of the localities. The study sites in summer 2018 were very similar to those in spring 2018 and summer 2017.
Species Composition and Diversity
A total of 36 taxa (including species, varieties, and forms) belonging to 22 genera were found in all of the analyzed samples. Five taxa were found outside of the counts when scanning the slides for additional species. Several taxa could not be identified up to the species or genus level; additional morphological investigations are necessary to clarify their taxonomic position.
Each of the study sites was dominated by two to four species (Table 3). In Bjørndalen 1, Hannaea arcus (50%) and Meridion circulare (35%) predominated. M. circulare (40%) together with Diatoma cf. problematica (23%), Fragilaria tenera (17%), and Fragilaria fragilarioides (11%) occurred most frequently in Bjørndalen 2. Despite the close position of the two Endalen sites, the dominant species were different. In Endalen site 1, H. arcus (61%) and M. circulare (36%) prevailed, while Cymbella hantzschiana (34%), Pinnularia frequentis (33%), and F. tenera (11%) dominated in Endalen site 2. The determined diatom taxa are shown in Fig. S4. Filaments of green algae were occasionally observed in most samples as well; cyanobacteria were rare.
Seasonal Changes of Diatom Viability
During the first sampling in August 2017, diatoms were proven to be highly viable and active. In all the observed sites, the majority of evaluated cells were active with percentages between 50 and 90%, depending on the study site (Figs. 6). A low proportion of cells were shown as dormant because there were no respiration activity and undamaged membranes. Some injured active or inactive cells were observed at all sites. Dead cells were in the minority (less than 3%). Autumn sampling in October 2017 showed a much lower diatom survival in the active stage (1–15%). As expected, most of the cells were dormant (19%–61%) or dead (19%–70%). Injured and inactive cells were detected in all sites with percentages between 8 and 14%. Dead cells prevailed in winter samples from April 2018, with percentages of between 54 and 88%. Dormant cells were present at 9–33%. Remarkably, a relatively high proportion of cells were active after winter sampling in both Bjørndalen sites. Hardly any injured active cells were observed, with the exception of those in Bjørndalen site 1. The June 2018 spring sampling showed a high proportion of dead cells (27–83%), which was, in the case of the Endalen site 1, even higher than in winter. A significant ratio of cells in dormant stage was detected (14–49%), and the proportion of active cells increased (3–19%) when compared with the winter sampling results. The last sampling in the end of July 2018 indicated that most of the cells were active (61–66%) and a minority was dead (8–19%), with the exception of Endalen site 2, where most of the cells were dead (36%) and only 26% of cells were active. Dormancy was detected in 9–30% of cells, 4–7% were shown as injured but still active, and 1–15% were injured but inactive.
The effect of season and locality on the proportion of cell types was highly significant (permutation test for RDA under reduced model, P < 0.001). Correlations between the physiological cell status, sampling site, and season using standardized data visualized by principal component analysis (PCA) and redundancy analysis (RDA) are shown in Fig. 7. From the tested factors potentially influencing cell viability in spring, only ice thickness had a significant effect (permutation test for RDA under reduced model, P < 0.007). The thicker ice layer, the more dead cells and fewer other stages were observed (Fig. 8). The influence of average weekly max–min temperature differences was not significant in autumn and winter.
Principal component analysis (PCA) showing the correlation between sampled sites, sampling season, and the physiological status of cells. Sampled sites: star, Bjørndalen 1; triangle, Bjørndalen 2; dot, Endalen 1; and square, Endalen 2. Sampling season: summer 2017 (red contour), autumn (yellow), winter (blue contour), spring (green), summer 2018 (brown)
Redundancy analysis (RDA) showing the influence of ice thickness (ice), and average weekly max–min temperature differences in autumn 10/2/2017–11/26/2017 (temp1) and winter 1/1/2018–3/4/2018 (temp2) on the physiological cell status in spring and sampled sites (star, Bjørndalen 1; triangle, Bjørndalen 2; dot, Endalen 1; and square, Endalen 2)
Discussion
Physical stresses common in polar environments, such as freezing and desiccation related to reduced water availability, play an important role in the microbial species composition and their biological activity [45, 46]. During the winter season especially, snow cover regulates terrestrial habitats by insulating them, which decouples the fluctuations of the surface layer soil temperature from air temperature and affects many thermally sensitive processes [10, 47, 48]. In our study, the lowest minimal temperature (− 17.2 °C) was documented at Bjørndalen site 2, where the thinnest snow cover (2 cm) was observed during winter sampling. In contrast, the highest minimal temperature (− 7.5 °C) occurred at Bjørndalen site 1, where the snow depth was 50 cm, which was the highest of all the sites (Table S3). According to temperature measurements, the freezing began in the mid-September after which the temperature fluctuated around zero. The study sites were frozen until the end of May which corresponds to previously published data from similar freshwater bodies (slow-flowing shallow stream and a shallow pool) in Svalbard [35].
In recent winters, short warm periods (in order of days) occur more frequently when a higher proportion of precipitation falls as rain rather than snow [10]. Rain-on-snow events are mostly common in the maritime Arctic and are associated with water penetration through the snowpack and subsequent ice layer creation on the soil surface [49]. Three warmer periods were documented during this study by temperature measurements. The occurrence of some warm spells was noticeable from the thick ice layer/s present under the snow cover (Table 2). The consequences of such ice crusts could last for years and heavily affect the ecosystem by changing the soil nutrient composition, respiration, and winter gas efflux [10, 49,50,51]. Repeated spring freeze–thaw events were found to act as a bottleneck for the survival of microalgae, suggesting the importance of a few stress-resistant cells under adverse conditions [52,53,54]. The increased occurrence of freeze–thaw cycles by winter warm spells could have fatal consequences for their persistence.
Microalgae inhabiting terrestrial and hydro-terrestrial polar environments form annual or perennial communities [46, 55]. A common strategy for eukaryotic microorganisms, e.g., green algae, is a reestablishment of communities every year from a few resistant cells capable of surviving winter, while the majority are not [55, 56]. Beyond this, snow algae from the order Chlamydomonadales (Chlorophyta) represent an example of a group successfully applying the strategy that involves the formation of specialized metabolically active cyst-like stages with a resistant cell wall, which are believed to be resistant to freezing and other unfavorable environmental conditions [57, 58].
Some filamentous centric diatoms from freshwater lakes evidenced resting stages formation (resting cells or spores) to overcome unfavorable conditions. After the spring high cell abundance on the ice bottom, ice breaks up and the diatom filaments are destroyed by water currents to free-floating flakes, which sink to the bottom [59, 60]. The cells could be able to continue division [61] and create resting stages that survive in cool, intermediate depths (50–150 m) during summer stratification [62] or they remain in the bottom sediment until an autumn overturn [59,60,61].
In contrast, a filamentous green alga from alpine and polar environments, Klebsormidium sp., was proven to be able to survive freezing stress in a vegetative state without forming any specialized cells [63, 64], and it was suggested that some other microalgae and cyanobacteria from the Arctic can survive as vegetative cells with thick cell walls and accumulated storage products [65]. This annual study of diatom survival in the High Arctic showed a remarkable viability of diatom cells during winter season. More than 20% of cells were found in the inactive resting cell stage, and nearly 5% were active after thawing, suggesting that a significant portion of cells survive winter. It is assumed that the inactive cells represent resting cells which could re-activate in the beginning of vegetative season and provide an inoculum for the following vegetative season. No morphologically distinct stages were found. Even a higher winter survival of morphologically and/or ultrastructurally non-modified (vegetative) cells was observed in a similar recent field study that focused on Arctic filamentous cyanobacteria of the genus Phormidium [35]. Although, cyanobacterial dormant cells, akinetes, are thought to be more resistant to desiccation stress, freezing, and prolonged dark periods than vegetative cells [66]. The proportion of viable cells from the frozen samples was more than 80% and resumed respiration within minutes after thawing, suggesting that most of the vegetative cells acquired resistance to winter stress [35]. However, polar cyanobacteria were generally shown to have a higher resistance to freezing and desiccation stress than green algae from similar habitats [45, 67]. Nevertheless, the production of thick polysaccharide sheaths was observed as a protecting mechanism from freeze damage to avoid the contact of the cell surface with ice crystals. Only a small percentage of cells among trichomes without polysaccharide sheaths survived freezing [35]. The formation of dense mucilaginous mats is known to enable stream and soil communities to withstand temperature fluctuations, desiccation, and short summer freeze/thaw cycles, and to maintain metabolic activity over harsh environmental conditions [46, 68].
Another field study of Arctic filamentous algae Zygnema spp. also showed the ability of vegetative cells to survive an entire annual cycle without forming any stress-resistant cells (zygospores or stationary-phase cells filled with storage products [akinetes]). However, harsh periods were survived as modified stationary-phase-like vegetative cells, called pre-akinetes (cells filled with storage material, characterized by reduced chloroplast lobes and thickened cell walls but still forming a filament). These cells are able to quickly recover under favorable conditions and are physiologically active immediately after thawing [36]. Though, a high mortality of newly produced vegetative cells because of frequent freeze–thaw cycles was observed in the early spring [36]. Recent experimental study revealed that the formation of pre-akinetes in polar Zygnema spp. and Zygnemopsis sp. is induced by nitrogen limitation at the end of the summer season and can be hardened by naturally slow desiccation stress to survive rapid drying. Naturally hardened pre-akinetes play a key role in stress tolerance and dispersal under the extreme conditions of polar regions [53, 54].The results of our field study are supported by several laboratory experiments. Some terrestrial diatoms also showed their ability to survive adverse conditions as vegetative cells [31]. A terrestrial diatom, Pinnularia sp., was even able to maintain near-maximum growth rates over a wider range of stress conditions (freezing and desiccation). After repeated freeze–thaw cycles down to − 3 and − 10 °C, survival rates between 80 and 100% were observed [56]. In contrast, in freshwater benthic diatoms, both vegetative and laboratory-induced resting cells were shown to be sensitive to desiccation, abrupt heating, or freezing, suggesting their habitat dependency [31, 69, 70]. In − 20 °C treatments, the tolerance of freshwater polar strains was higher than that of temperate ones [70], and diatoms from aquatic habitats were less tolerant than terrestrial strains [69]. In addition, cyanobacteria isolated from Antarctic seepages were demonstrated to be less tolerant to freezing and desiccation than cyanobacteria from wetland habitats. One possible explanation is that water levels in freshwater seepages are more stable and provide protection against freezing and desiccation than in other habitats, so the communities of microorganisms may not be acclimated to these stresses [67]. Acclimation is known to increase the tolerance to unfavorable conditions in various organisms, including diatoms [71]. Resting cells of terrestrial diatoms induced under laboratory conditions by hardening under lower temperatures, darkness, and nitrogen limitation showed higher survival of stress tolerance, especially desiccation [31]. In addition, the importance of resting stages for freezing tolerance was emphasized for the survival of mild freezing (− 4 °C treatment), which could indicate its relevancy for diatoms from temperate regions [70]. Furthermore, even without nutrient deficiency, changes in the physical and chemical environmental factors changing during the year (e.g., temperature, light intensity, conductivity) can result in reserve accumulation and resistant cell formation [72].
Generally, a similar species composition to those in other studies from non-marine habitats in the High Arctic was found in this study [2, 73, 74]. Hannaea arcus, as a typical representative of streams [73, 75], dominated in two of the study sites, each from a different valley. Meridion circulare was highly common in the entire study area and was also found in many other Arctic lotic ecosystems [73, 74]. Diatoma cf. problematica, a principal species in one of the study sites, was found as a common main component in many Arctic habitats [2, 74]. In addition, Cymbella hantzschiana, which dominated in one of the study sites, is also mentioned as a common species occurring in polar areas [2, 73, 74]. Differences in the species composition could have been caused by the character of each study site (e.g., flowing vs low flow). In any case, the results obtained within this study give an evidence of the survival only for the most abundant species. Other species were represented by a low number of cells, which prevented from making any clear conclusion concerning their physiological performance during the annual cycle.
Conclusion
In conclusion, this study provided a first detailed insight into the physiological status of diatom cells throughout the annual cycle in the extreme conditions of the High Arctic. The adaptation of vegetative cells is crucial for the winter survival of polar freshwater diatoms; a significant proportion of cells were able to survive winter without forming any morphologically distinct stages and served as the inoculum for the following vegetative season. Remarkably, a relatively high number of cells were active immediately after thawing in the winter season. It was shown that the survival is seasonally and locality dependent. The influence of ice thickness to spring survival was also important. The average max–min weekly temperature differences in autumn and winter were not significant.
References
Kim GH, Klochkova TA, Kang SH (2008) Notes on freshwater and terrestrial algae from Ny-Ålesund, Svalbard (high Arctic Sea area). J. Environ. Biol. 29:485–491
Pinseel E, Van de Vijver B, Kavan J et al (2017) Diversity, ecology and community structure of the freshwater littoral diatom flora from Petuniabukta (Spitsbergen). Polar Biol. 40:533–551. https://doi.org/10.1007/s00300-016-1976-0
Douglas MSV, Smol JP (1995) Periphytic diatom assemblages from High Arctic ponds. J. Phycol. 31:60–69. https://doi.org/10.1111/j.0022-3646.1995.00060.x
Vincent WF, Hobbie JE, Laybourn-Parry J (2008) Introduction to the limnology of high-latitude lake and river ecosystems. In: Vincent WF, Laybourn-Parry J (eds) Polar Lakes and Rivers. Oxford University Press, New York, pp 1–23
Lizotte MP (2008) Phytoplankton and primary production. In: Vincent WF, Laybourn-Parry J (eds) Polar Lakes and Rivers. Oxford University Press, New York, pp 157–178
Mellor CJ (2012) Arctic water source dynamics, stream habitat and biodiversity in a changing climate: a field-based investigation in Swedish Lapland. University of Birmingham
Karlsson JOM, Toner M (1996) Long-term storage of tissues by cryopreservation: critical issues. Biomaterials 17:243–256. https://doi.org/10.1016/0142-9612(96)85562-1
Meryman HT (1974) Freezing injury and its prevention in living cells. Annu. Rev. Biophys. Bioeng. 3:341–363. https://doi.org/10.1146/annurev.bb.03.060174.002013
Humlum O, Instanes A, Sollid JL (2003) Permafrost in Svalbard: a review of research history, climatic background and engineering challenges. Polar Res. 22:191–215. https://doi.org/10.3402/polar.v22i2.6455
Cooper EJ (2014) Warmer shorter winters disrupt Arctic terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 45:271–295. https://doi.org/10.1146/annurev-ecolsys-120213-091620
Serreze MC, Walsh JE, Chapin FS et al (2000) Observational evidence of recent change in the northern high-latitude environment. Clim. Chang. 46:159–207. https://doi.org/10.1023/A:1005504031923
Krembs C, Eicken H, Junge K, Deming JW (2002) High concentrations of exopolymeric substances in Arctic winter sea ice: implications for the polar ocean carbon cycle and cryoprotection of diatoms. Deep Sea Res. Part I Oceanogr. Res. Pap. 49:2163–2181. https://doi.org/10.1016/S0967-0637(02)00122-X
Bayer-Giraldi M, Weikusat I, Besir H, Dieckmann G (2011) Characterization of an antifreeze protein from the polar diatom Fragilariopsis cylindrus and its relevance in sea ice. Cryobiology 63:210–219. https://doi.org/10.1016/j.cryobiol.2011.08.006
Raymond JA, Fritsen CH (2001) Semipurification and ice recrystallization inhibition activity of ice-active substances associated with Antarctic photosynthetic organisms. Cryobiology 43:63–70. https://doi.org/10.1006/cryo.2001.2341
Gwak IG, Sic Jung W, Kim HJ et al (2010) Antifreeze protein in Antarctic marine diatom, Chaetoceros neogracile. Mar. Biotechnol. 12:630–639. https://doi.org/10.1007/s10126-009-9250-x
Raymond JA, Morgan-Kiss R (2013) Separate origins of ice-binding proteins in Antarctic Chlamydomonas species. PLoS One 8:6–11. https://doi.org/10.1371/journal.pone.0059186
Phadtare S (2004) Recent developments in bacterial cold-shock response. Curr. Issues Mol. Biol. 6:125–136. https://doi.org/10.21775/cimb.006.125
Liu X, Wang Y, Gao H, Xu X (2011) Identification and characterization of genes encoding two novel LEA proteins in Antarctic and temperate strains of Chlorella vulgaris. Gene 482:51–58. https://doi.org/10.1016/j.gene.2011.05.006
Greenway H, Setter TL (1979) Accumulation of proline and sucrose during the first hours after transfer of Chlorella emersonii to high NaCl. Aust. J. Plant Physiol. 6:69–79. https://doi.org/10.1071/PP9790069
Blunden G, Smith BE, Irons MW, Yang MH, Roch OG, Patel AV (1992) Betaines and tertiary sulphonium compounds from 62 species of marine algae. Biochem. Syst. Ecol. 20:373–388. https://doi.org/10.1016/0305-1978(92)90050-N
Keller MD, Bellows WK, Guillard RRL (1989) Dimethyl sulfide production in marine phytoplankton. In: Cooper WJ (ed) Saltzman, E. American Chemical Society, Biogenic Sulfur in the Environment, pp 167–182
Henderson RJ, Hegseth EN, Park MT (1998) Seasonal variation in lipid and fatty acid composition of ice algae from the Barents Sea. Polar Biol. 20:48–55. https://doi.org/10.1007/s003000050275
Teoh M-L, Chu W-L, Marchant H, Phang S-M (2004) Influence of culture temperature on the growth, biochemical composition and fatty acid profiles of six Antarctic microalgae. J. Appl. Phycol. 16:421–430. https://doi.org/10.1007/s10811-004-5502-3
Kuwata A, Hama T, Takahashi M (1993) Ecophysiological characterization of two life forms, resting spores and resting cells, of a marine planktonic diatom, Chaetoceros pseudocurvisetus, formed under nutrient depletion. Mar. Ecol. Prog. Ser. 102:245–256. https://doi.org/10.3354/meps102245
Jewson DH, Granin NG, Zhdanov AA, Gorbunova LA, Bondarenko NA, Gnatovsky RY (2008) Resting stages and ecology of the planktonic diatom Aulacoseira skvortzowii in Lake Baikal. Limnol. Oceanogr. 53:1125–1136. https://doi.org/10.4319/lo.2008.53.3.1125
Lund JWG (1954) The seasonal cycle of the plankton diatom, Melosira italica (Ehr.) Kutz. subsp. subarctica O. Müll. J. Ecol. 42:151–179. https://doi.org/10.2307/2256984
Anderson OR (1975) Ultrastructure and cytochemistry of resting cell formation in Amphora coffeaeformis (Bacillariophyceae). J. Phycol. 11:272–281. https://doi.org/10.1111/j.1529-8817.1975.tb02778.x
Sicko-Goad L, Stoermer EF, Fahnenstiel G (1986) Rejuvenation of Melosira granulata (Bacillariophyceae) resting cells from the anoxic sediments of Douglas Lake, Michigan. I. Light microscopy and 14 C uptake. J. Phycol. 22:22–28. https://doi.org/10.1111/j.1529-8817.1986.tb02510.x
Round FE, Crawford RM, Mann DG (1990) Diatoms: biology and morphology of the genera. Cambridge University Press, Cambridge
McQuoid MR, Hobson LA (1996) Diatom resting stages. J. Phycol. 32:889–902. https://doi.org/10.1111/j.0022-3646.1996.00889.x
Souffreau C, Vanormelingen P, Sabbe K, Vyverman W (2013) Tolerance of resting cells of freshwater and terrestrial benthic diatoms to experimental desiccation and freezing is habitat-dependent. Phycologia 52:14–24. https://doi.org/10.2216/12-087.1
McQuoid MR, Hobson LA (1995) Importance of resting stages in diatom seasonal succession. J. Phycol. 31:44–50. https://doi.org/10.1111/j.0022-3646.1995.00044.x
Kuwata A, Takahashi M (1999) Survival and recovery of resting spores and resting cells of the marine planktonic diatom Chaetoceros pseudocurvisetus under fluctuating nitrate conditions. Mar. Biol. 134:471–478. https://doi.org/10.1007/s002270050563
Edlund MB, Stoermer EF, Taylor CM (1996) Aulacoseira skvortzowii sp. nov. (Bacillariophyta), a poorly understood diatom from Lake Baikal, Russia 1. J. Phycol. 32:165–175. https://doi.org/10.1111/j.0022-3646.1996.00165.x
Tashyreva D, Elster J (2016) Annual cycles of two cyanobacterial mat communities in hydro-terrestrial habitats of the High Arctic. Microb. Ecol. 71:887–900. https://doi.org/10.1007/s00248-016-0732-x
Pichrtová M, Hájek T, Elster J (2016) Annual development of mat-forming conjugating green algae Zygnema spp. in hydro-terrestrial habitats in the Arctic. Polar Biol. 39:1653–1662. https://doi.org/10.1007/s00300-016-1889-y
Tashyreva D, Elster J, Billi D (2013) A novel staining protocol for multiparameter assessment of cell heterogeneity in Phormidium populations (cyanobacteria) employing fluorescent dyes. PLoS One 8:1–12. https://doi.org/10.1371/journal.pone.0055283
Norwegian Polar Institute Map data Svalbard 1:1 000 000. https://data.npolar.no. Accessed 10 Oct 2019
Major H, Nagy J (1972) Geology of the Adventdalen map area
Piepjohn K, Stange R, Jochmann M, Hübner C (2012) The geology of Longyearbyen. Longyearbyen feltbiologiske forening
Peel MC, Finlayson BL, McMahon TA (2007) Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 4:439–473. https://doi.org/10.5194/hess-11-1633-2007
Førland EJ, Hanssen-Bauer I, Nordli PØ (1997) Climate statistics & longterm series of temperature and precipitation at Svalbard and Jan Mayen. DNMI Klima 21:73
UNIS weather stations Adventdalen (hour dataset) 2016–now. https://www.unis.no/resources/weather-stations/. Accessed 10 Dec 2019
Oksanen J, Blanchet GF, Friendly M, et al. CRAN - Package vegan. In: Vegan Community Ecol. Packag. R Packag. version 2.5–6. https://cran.r-project.org/web/packages/vegan/index.html. Accessed 9 Sep 2019
Davey MC (1989) The effects of freezing and desiccation on photosynthesis and survival of terrestrial Antarctic algae and cyanobacteria. Polar Biol. 10:29–36. https://doi.org/10.1007/BF00238287
Elster J, Benson EE (2004) Life in the polar terrestrial environment with a focus on algae and cyanobacteria. In: Lane N, Benson EE (eds) Fuller B. Taylor and Francis, Life in the Frozen State, pp 111–150
Morgner E, Elberling B, Strebel D, Cooper EJ (2010) The importance of winter in annual ecosystem respiration in the High Arctic: effects of snow depth in two vegetation types. Polar Res. 29:58–74. https://doi.org/10.1111/j.1751-8369.2010.00151.x
Gouttevin I, Menegoz M, Dominé F, Krinner G, Koven C, Ciais P, Tarnocai C, Boike J (2012) How the insulating properties of snow affect soil carbon distribution in the continental pan-Arctic area. J. Geophys. Res. Biogeosci. 117:1–11. https://doi.org/10.1029/2011JG001916
Coulson SJ, Leinaas HP, Ims RA, Søvik G (2000) Experimental manipulation of the winter surface ice layer: the effects on a High Arctic soil microarthropod community. Ecography 23:299–306. https://doi.org/10.1111/j.1600-0587.2000.tb00285.x
Putkonen J, Roe G (2003) Rain-on-snow events impact soil temperatures and affect ungulate survival. Geophys. Res. Lett. 30:1–4. https://doi.org/10.1029/2002GL016326
Rennert KJ, Roe G, Putkonen J, Bitz CM (2009) Soil thermal and ecological impacts of rain on snow events in the circumpolar arctic. J. Clim. 22:2302–2315. https://doi.org/10.1175/2008JCLI2117.1
Tashyreva D, Elster J (2012) Production of dormant stages and stress resistance of polar cyanobacteria. In: Hanslmeier A (ed) Life on Earth and Other Planetary Bodies. Springer Science, Dordrecht, pp 367–386
Pichrtová M, Kulichová J, Holzinger A (2014) Nitrogen limitation and slow drying induce desiccation tolerance in conjugating green algae (Zygnematophyceae, Streptophyta) from polar habitats. PLoS One 9:e113137. https://doi.org/10.1371/journal.pone.0113137
Pichrtová M, Hájek T, Elster J (2014) Osmotic stress and recovery in field populations of Zygnema sp. (Zygnematophyceae, Streptophyta) on Svalbard (High Arctic) subjected to natural desiccation. FEMS Microbiol. Ecol. 89:270–280. https://doi.org/10.1111/1574-6941.12288
Hawes I (1990) Effects of freezing and thawing on a species of Zygnema (Chlorophyta) from the Antarctic. Phycologia 29:326–331. https://doi.org/10.2216/i0031-8884-29-3-326.1
Davey MC (1991) Effects of physical factors on the survival and growth of Antarctic terrestrial algae. Br. Phycol. J. 26:315–325. https://doi.org/10.1080/00071619100650281
Remias D, Karsten U, Lütz C, Leya T (2010) Physiological and morphological processes in the Alpine snow alga Chloromonas nivalis (Chlorophyceae) during cyst formation. Protoplasma 243:73–86. https://doi.org/10.1007/s00709-010-0123-y
Remias D (2012) Cell structure and physiology of alpine snow and ice algae. In: Lütz C (ed) Plants in Alpine Regions. Springer-Verlag, Vienna, pp 175–185
Jewson DH, Granin NG (2015) Cyclical size change and population dynamics of a planktonic diatom, Aulacoseira baicalensis, in Lake Baikal. Eur. J. Phycol. 50:1–19. https://doi.org/10.1080/09670262.2014.979450
Vehmaa A, Salonen K (2009) Development of phytoplankton in Lake Pääjärvi (Finland) during under-ice convective mixing period. Aquat. Ecol. 43:693–705. https://doi.org/10.1007/s10452-009-9273-4
Bondarenko NA, Timoshkin OA, Röpstorf P, Melnik NG (2006) The under-ice and bottom periods in the life cycle of Aulacoseira baicalensis (K. Meyer) Simonsen, a principal Lake Baikal alga. Hydrobiologia 568:107–109. https://doi.org/10.1007/s10750-006-0325-7
Jewson DH, Granin NG, Zhdarnov AA, Gorbunova LA, Gnatovsky RY (2010) Vertical mixing, size change and resting stage formation of the planktonic diatom Aulacoseira baicalensis. Eur. J. Phycol. 45:354–364. https://doi.org/10.1080/09670262.2010.492915
Elster J, Degma P, Kováčik L, Valentová L, Šramková K, Batista Pereira A (2008) Freezing and desiccation injury resistance in the filamentous green alga Klebsormidium from the Antarctic, Arctic and Slovakia. Biologia 63:843–851. https://doi.org/10.2478/s11756-008-0111-2
Karsten U, Lütz C, Holzinger A (2010) Ecophysiological performance of the aeroterrestrial green alga Klebsormidium crenulatum (Klebsormidiophyceae, Streptophyta) isolated from an alpine soil crust with an emphasis on desiccation stress. J. Phycol. 46:1187–1197. https://doi.org/10.1111/j.1529-8817.2010.00921.x
Sheath RG, Vis ML, Hambrook JA, Cole KM (1996) Tundra stream macroalgae of North America: composition, distribution and physiological adaptations. Hydrobiologia 336:67–82. https://doi.org/10.1007/BF00010820
Sutherland JM, Herdman M, Stewart WDP (1979) Akinetes of the cyanobacterium Nostoc PCC 7524: macromolecular composition, structure and control of differentiation. J. Gen. Microbiol. 115:273–287. https://doi.org/10.1099/00221287-115-2-273
Šabacká M, Elster J (2006) Response of cyanobacteria and algae from Antarctic wetland habitats to freezing and desiccation stress. Polar Biol. 30:31–37. https://doi.org/10.1007/s00300-006-0156-z
Hawes I, Howard-Williams C, Vincent WF (1992) Desiccation and recovery of Antarctic cyanobacterial mats. Polar Biol. 12:587–594. https://doi.org/10.1007/BF00236981
Souffreau C, Vanormelingen P, Verleyen E, Sabbe K, Vyverman W (2010) Tolerance of benthic diatoms from temperate aquatic and terrestrial habitats to experimental desiccation and temperature stress. Phycologia 49:309–324. https://doi.org/10.2216/09-30.1
Hejduková E, Pinseel E, Vanormelingen P, Nedbalová L, Elster J, Vyverman W, Sabbe K (2019) Tolerance of pennate diatoms (Bacillariophyceae) to experimental freezing: comparison of polar and temperate strains. Phycologia 58:1–11. https://doi.org/10.1080/00318884.2019.1591835
Mock T, Valentin K (2004) Photosynthesis and cold acclimation: molecular evidence from a polar diatom. J. Phycol. 40:732–741. https://doi.org/10.1111/j.1529-8817.2004.03224.x
Olofsson M, Lamela T, Nilsson E, Bergé JP, del Pino V, Uronen P, Legrand C (2012) Seasonal variation of lipids and fatty acids of the microalgae Nannochloropsis oculata grown in outdoor large-scale photobioreactors. Energies 5:1577–1592. https://doi.org/10.3390/en5051577
Antoniades D, Douglas MSV (2002) Characterization of high arctic stream diatom assemblages from Cornwallis Island, Nunavut, Canada. Can. J. Bot. 80:50–58. https://doi.org/10.1139/b01-133
Pla-Rabés S, Hamilton PB, Ballesteros E, Gavrilo M, Friedlander AM, Sala E (2016) The structure and diversity of freshwater diatom assemblages from Franz Josef Land archipelago: a northern outpost for freshwater diatoms. PeerJ 2016:1–22. https://doi.org/10.7717/peerj.1705
Passy SI (2007) Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquat. Bot. 86:171–178. https://doi.org/10.1016/j.aquabot.2006.09.018
Acknowledgments
The authors wish to thank the Czech Arctic Scientific Infrastructure of the University of South Bohemia, Josef Svoboda Station, Svalbard (CzechPolar2 Project LM2015078 supported by the Ministry of Education, Youth and Sports of the Czech Republic), and the institutional long-term research plan RVO67985939 of the Institute of Botany of the Czech Academy of Sciences. Eveline Pinseel and Bart Van de Vijver are acknowledged for identification of diatoms and Jana Duchoslavová for a statistical help. The authors wish to thank Paul Hamilton and three anonymous reviewers for their constructive comments on an earlier version of the manuscript.
Funding
The research was supported by the Grant Agency of Charles University (project no. 20217).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOCX 92.0 mb)
Rights and permissions
About this article
Cite this article
Hejduková, E., Elster, J. & Nedbalová, L. Annual Cycle of Freshwater Diatoms in the High Arctic Revealed by Multiparameter Fluorescent Staining. Microb Ecol 80, 559–572 (2020). https://doi.org/10.1007/s00248-020-01521-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-020-01521-w