Abstract
Studies on testate amoeba species distribution at small scales (i.e., single peatland sites) are rare and mostly focus on bogs or mineral-poor Sphagnum fens, leaving spatial patterns within mineral-rich fens completely unexplored. In this study, two mineral-rich fen sites of contrasting groundwater chemistry and moss layer composition were selected for the analysis of testate amoeba compositional variance within a single site. At each study site, samples from 20 randomly chosen moss-dominated plots were collected with several environmental variables being measured at each sampling spot. We also distinguished between empty shells and living individuals to evaluate the effect of empty shell inclusion on recorded species distribution. At the heterogeneous-rich Sphagnum-fen, a clear composition turnover in testate amoebae between Sphagnum-dominated and brown moss-dominated samples was closely related to water pH, temperature and redox potential. We also found notable species composition variance within the homogeneous calcareous fen, yet it was not as high as for the former site and the likely drivers of community assembly remained unidentified. The exclusion of empty shells provided more accurate data on species distribution as well as their relationship with some environmental variables, particularly moisture. Small-scale variability in species composition of communities seems to be a worthwhile aspect in testate amoeba research and should be considered in future sampling strategies along with a possible empty shell bias for more precise understanding of testate amoeba ecology and paleoecology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a growing body of literature on the ecology of testate amoebae in peatlands, covering broad range of habitat types worldwide and exploring diversity patterns at spatial scales from 40 × 60 cm Sphagnum carpet [1] to whole mountain ranges [2]. The majority of studies, however, explored testate amoeba communities on regional scales [3,4,5,6,7,8]. Conversely, spatial variability of testate amoebae within single sites is poorly known and studies dealing with species distribution and composition variation in this context are focused primarily on the Sphagnum microforms in bogs [9,10,11]. However, such knowledge might be of consequence for both paleoecological interpretation and overall understanding of the ecology of individual species and communities over larger scales. Also, apart from being scarce, existing studies on small-scale variability in testate amoeba assemblages remain limited to Sphagnum-dominated habitats such as bogs and acidic (mineral poor) fens [1, 12], resulting in the complete lack of information on this subject from more mineral-rich fens.
Unlike in bogs with a distinct and stable microtopography, water table in more alkaline fens with tighter relationship to groundwater supply tends to be more dynamic over the season. Combined with local changes in mineral richness and more diversified vegetation [13], these fen types often display remarkable variability of conditions within the surface moss layer. Despite this apparent heterogeneity, studies concerning testate amoebae in alkaline fens were primarily focused on community changes along the poor-rich gradient [5, 6, 8, 14] with little regard for species distribution on finer spatial scales. Nonetheless, more detailed studies on fens might be valuable, as they are known to harbor remarkable testate amoeba diversity [5, 14], but also due to the endangered status of these habitats, which became very rare across the globe [15, 16].
In this study, we selected two model fen sites of contrasting small-scale heterogeneity in terms of mineral richness and vegetation composition to explore the compositional variance of testate amoebae across two 20-m transects. We hypothesized that the variance of testate amoeba communities would reflect small scale environmental variation and we expected a higher species turnover among more environmentally distinct plots. As microorganisms have short generation times and potentially a high dispersal ability, we also decided to take into consideration the proportion of empty shells in our samples to obtain more realistic information on community structure and dynamics within our study sites. Shells without protoplasm accumulate in the uppermost parts of moss layers due to mortality caused by unfavorable change of conditions, predation [17], reproduction [18, 19], and passive dispersal caused by wind [20, 21] or animal/human activity [22]. Despite their likely potential to bias species composition and response to environmental factors, their effect has never been properly addressed in ecological studies. Empty shells have been regularly included into community description [9, 23,24,25] and preferential use of living individuals is applied quite marginally, mostly in experimental studies [26,27,28,29] and less in the field studies focused on the community ecology [12, 17, 30, 31]. Here, we specifically aim to evaluate the effect of empty shells on species composition of the surface samples and to find out whether and to which extent their exclusion modifies species correlation to measured environmental variables. This topic seems to be of unexplored nature but with potential implications for future testate amoeba research.
Material and Methods
Study Site, Sample Collection, and Processing
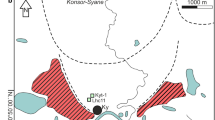
The sites chosen for sample collection were two minerotrophic mires located in the Western Carpathian flysch zone (Fig. 1). The first site was a small calcareous spring fen near the village of Jasenka in Hostýn-Vsetín Mts., Czech Republic (49° 22′ 4″ N, 18° 01′ 2″ E), characterized by a strong tufa (i.e., calcium carbonate) precipitation caused by extreme mineral-rich groundwater supply. The vegetation composition of the site belongs to the Caricion davallianae alliance and the moss layer is formed exclusively by brown mosses with the overall appearance being rather homogeneous. The second sampling site was a mineral-rich Sphagnum fen in the Obidová Nature Reserve (Moravskoslezské Beskydy Mts., Czech Republic; 49° 31′ 02″ N, 18° 31′ 26″ E). Central European rich (Sphagnum-) fens are characterized by vegetation of the Sphagno warnstorfii-Tomentypnion nitensis alliance and have been known to host some of the richest plant and moss fen communities [13, 16]. The moss layer is quite heterogeneous, compared to the first site, consisting of a spatial matrix of brown mosses and calcium-tolerant Sphagnum species which form a variety of microhabitats with diverse biotic and abiotic conditions. The study sites will be referred to as the “homogeneous fen” and the “heterogeneous fen” throughout the text.
To explore the species distribution of testate amoebae at the studied fens, 20 moss tufts were collected at each site. The area for sample collection was chosen to cover most of the microhabitat diversity of the fens and was delimited by two perpendicular (i.e., crossing in the middle) transects of 20 m each. Random selection of sampling plots with minimal subjective influence of the collectors was achieved by using a wooden frame (1 × 1 m) divided into a 10 × 10 cm grid, which was placed in a zigzag manner along each transect using 2 m spacing. The coordinates of a square section on the grid were then randomly generated and at the respective spot living parts of moss stems were sampled. Prior to sample collection, moisture conditions at each sampling spot were evaluated using a semi-quantitative scale (1, dry; 2, moderately wet; 3, wet; and 4, submerged). After sampling pH, conductivity, redox potential, and water temperature were measured using a portable instrument (HACH HQ40d). For further details on the environmental variables and moss species sampled see Online Resource (Table 3).
In the laboratory, the collected moss stems were washed with distilled water, the suspension was filtered through a 250-μm sieve and fixed with formaldehyde. The moss stems were dried and kept for further identification (for full species list see Online Resource, Table 4). For better distinction between empty shells and living individuals at the time of sampling, 1 ml of 0.2% Rose Bengal stain solution was added into 10 ml of sample suspension. Samples were then left to stain for 2 weeks following the FOBIMO protocol [32]. Testate amoebae were identified under × 200 and × 400 magnification and empty and living shells were tallied separately throughout the scanning. For each sample, two species datasets consisting of 150 individuals were obtained. The first dataset labeled as “ALL” included all shells regardless of being empty or alive. The second dataset labeled as “LIVING” included only shells with protoplasm (i.e., active as well as encysted). No more than 50 shells were examined per slide, so that the community description was always based on at least three replicates. For testate amoeba identification, multiple identification keys were used [19, 33,34,35,36,37,38] along with the Microworld web pages [39]. The species-level identification of individuals was based on shell morphology. The nomenclature follows [19, 40], with some critical taxa being pooled and labeled as “type” (see Online Resource, Tables 1 and 2).
Data Analyses
Although 20 samples were taken at each of the studied fens, in case of the heterogeneous fen, only 19 samples were included in the analyses due to the loss of one sample during processing. All data analyses were performed pairwise for the two fen types as well as the “ALL” and “LIVING” species data sets. Samples from the heterogeneous fen were further sorted based on the two functional groups of mosses present. To test the effect of moss type, the Sphagnum/brown moss ratio was assessed from the rinsed moss tufts and scored on the following scale: 0%, 1 ≤ 30%, 31 ≤ 70%, and 71 ≤ 100% of Sphagnum stems. The threshold of 30% Sphagnum proportion was further adopted for the distinction between Sphagnum- and brown moss-dominated samples. For a comparison between groups of samples, Mann-Whitney U test or Wilcoxon signed-rank test were used. All analyses were performed in R software [41] using the “vegan” [42] and “packfor” [43] packages.
To explore the differences in species composition among samples, we employed distance-based multivariate techniques. In order to reduce the influence of dominant taxa, the species data were log transformed and Bray-Curtis dissimilarity index was applied. For both sampled fens, testate amoeba assemblages were displayed using two-dimensional NMDS ordination analysis and environmental variables measured at each sampling spot were projected onto the resulting ordination diagrams. The variables were fitted using the regression-based “envfit” function and the significance was tested with 4999 permutations. To quantify the changes in community composition after the exclusion of empty shells, we opted for the PCoA based “betadisper” method, so that the distances between samples and their group centroids could be compared between the “ALL” and “LIVING” data sets.
Redundancy analysis (RDA) was used to quantify the correlation between environmental variables and species distribution. Water pH, temperature, conductivity, redox potential, and moisture at the sampling spot were included in the analyses at both study sites. In case of the heterogeneous fen, Sjörs’s correction for conductivity was applied due to low pH values [44], and the proportion of Sphagnum stems was added as the sixth variable. All variables were tested together in one model as well as separately. The significance of results was assessed using permutation test with 4999 runs. The explained variability values were always converted into adjusted R2 and the resulting values were further used as the threshold in the forward selection process.
Results
Species Diversity and Live/Empty Shell Ratio
Overall, 64 testate amoeba taxa were identified, with 40 species found at the homogeneous fen and 55 species at the heterogeneous fen (for complete species lists see Online Resource Tables 1 and 2). About a half of all the species (31) were shared. For both sites, the number of species did not differ between “ALL” and “LIVING” data sets, with 40 and 39 species at the homogeneous fen, and 52 at the heterogeneous fen. The number of species per sample was rather variable, ranging between 16 and 27 species at the homogeneous and 9 and 33 species at the heterogeneous fen. The change in number of species after empty shell exclusion was significant mostly at the homogeneous fen where the number of species decreased by two species per sample on average (Table 1 and Fig. 2a). At the heterogeneous site, the average number of species per sample differed substantially between Sphagnum- and brown moss-dominated patches (14 ± 2.8 vs. 25 ± 4.5, see Table 1). In the case of Sphagnum samples, the difference between “ALL” and “LIVING” data sets was also significant (Fig. 2b). In general, brown mosses displayed higher species richness, but the proportion of living individuals in these samples was notably lower as compared to Sphagnum samples (Fig. 3).
Comparison of the number of testate amoeba species recorded in samples based on all shells (i.e. living and empty, white) and living individuals only (gray). a) Overall situation at both fens. b) For the heterogeneous fen, Sphagnum- and brown moss-dominated samples are also compared separately. Circles show values for individual samples while lines indicate the shifts in species richness between pairs of samples based on all or living shells only. The significance of differences was tested by Wilcoxon signed-rank test. In the box plots, box edges represent the 1st and 3rd quartiles, middle lines indicate the median and whiskers contain the values found in the interval of 1.5 × interquartile range. If present, outliers are marked with an asterisk
Number of living individuals recorded among 150 analyzed shells. a) Overall situation at both sampled fens. b) For the heterogeneous fen, Sphagnum- and brown moss (BM)-dominated samples are treated separately. For detailed information on the box plots see the caption for Fig. 2
Community Composition and Environmental Variables
The variability in species composition at both sampling sites was explored by NMDS and PCoA ordination plots (Figs. 4 and 5). Despite the macroscopic homogeneity of the calcareous fen, species distribution at this site was spatially variable and this variance significantly increased after empty shell exclusion (see Figs. 5a, b and 6). However, it was still significantly lower than at the heterogeneous site (MW U test, p < 0.001, not shown in the figures). At the heterogeneous fen, the clear distinction between Sphagnum and brown moss samples was found, although assemblages also varied notably within each of the two moss groups. Unlike in the homogeneous fen, the shifts in species composition between the “ALL” and “LIVING” data sets did not prove significant for this sampling site (Figs. 5c, d and 6).
Two-dimensional NMDS ordination plots comparing species composition of testate amoeba assemblages collected at the homogeneous fen (a, b) and the heterogeneous fen (c, d). The diagrams are given for species data based on all shells (i.e. living and empty; a, c) or living individuals only (b, d). For the heterogeneous fen, symbols indicate the type of sampled microhabitats (brown mosses or Sphagnum) while the shading of symbols reflects the percentage of Sphagnum stems in each moss sample. Vectors represent a passive projection of environmental variables (i.e. water pH, proportion of Sphagnum stems, redox potential, temperature, conductivity, and moisture), which were significantly associated with the sample scores on the NMDS ordination axes (4999 permutations, p < 0.05). For more detailed information on the environmental variables see Tab. 2
PCoA ordination diagrams representing testate amoeba assemblages at the homogeneous fen (a, b) and the heterogeneous fen (c, d). The diagrams are given based on all shells (i.e., living and empty; a, c) and living individuals only (b, d). Empty symbols indicate the type of sampled microhabitat (Sphagnum or brown mosses) and full black circles represent the group centroids in multidimensional space. For more information see also Fig. 6
Variation in sample distance to the group centroids calculated based on the position of samples in PCoA ordination space. For both studied fens, the results are shown pairwise based on all shells (i.e., living and empty, white) and living individuals only (gray). The significance of the differences between data sets was tested by Mann-Whitney U test. For detailed information on the box plots, see the caption for Fig. 2
Although species composition at the homogeneous fen was clearly not uniform among samples, we failed to detect any likely drivers for these compositional changes. While the projection of explanatory variables in the NMDS diagrams suggested a correlation with conductivity and moisture for the “ALL” and “LIVING” data set respectively (Fig. 4a, b; Table 2), RDA results for this site were not significant (Table 3). In contrast, variables tested in the RDA explained approximately 24% of the variance at the heterogeneous fen (Table 3), and when tested individually four variables were significant in “ALL” data set and five variables in “LIVING” data set (Table 4). In the RDA with forward selection, the proportion of Sphagnum in the sampled patches was found to be the principal predictor of testate amoeba community composition (Table 4) and was also strongly correlated with other environmental variables such as pH, redox potential, and temperature (Table 4, Fig. 4c, d). Total variability explained by environmental variables was comparable in “ALL” and “LIVING” data sets (Table 4), however, moisture emerged as an additional significant factor in the “LIVING” data set in both NMDS and RDA analyses (Tables 2 and 4).
Discussion
Species Composition Variance on Small Spatial Scales
Studies using a complex sampling with a higher number of repetitions per site are rare in testate amoeba research, even though they often provide important information concerning these organisms [1, 12] and lead to more precise diversity estimates as compared to traditional sampling of representative micro- or mesohabitats [45]. In this study, a repetitive sampling strategy at small spatial scales proved useful in demonstrating that microhabitats which seem macroscopically alike may harbor different assemblages. Similar results were previously reported from acidic Sphagnum-dominated peatlands [11, 12], yet have never been described from highly alkaline conditions. Our results provide the first evidence for spatially variable testate amoeba composition at a calcareous fen with a topographically uniform brown moss carpet. Nonetheless, as the measured variables expressed only small portion of variation among samples, we seemingly failed to determine the environmental factors responsible for the compositional variance in the testate amoeba assemblages at this study site. At the environmentally heterogeneous mineral-rich Sphagnum-fen, however, the within-site variability in testate amoeba assemblages was notably higher and primarily corresponded with the changes of the moss layer formed by two functional groups of bryophytes, resembling the situation observed along the poor-rich gradient [5, 6, 14]. The dominance of Sphagnum or brown mosses was associated with small-scale changes in abiotic conditions (e.g., water pH, redox potential and temperature) and though the major compositional turnover occurred between Sphagnum and brown mosses, this factor itself accounted for 21–24% of variability in the species data. Variation within the samples from the two moss types at the heterogeneous fen as well as the brown moss-dominated fen points to additional, not detected factors shaping the species composition of testate amoeba assemblages at the microhabitat scale [4].
Surprisingly, moisture only explained a minor fraction of the variance in our analyses, even though it is often reported as one of the principal variables shaping testate amoeba assemblages in peatlands [2, 3]. This might be caused by a minimal topographical variation at our sites and consequent short moisture gradient due to missing waterlogged microhabitats or pools. Also, grade-scale estimates were used to assess the moisture conditions while depth to water table measurements would be more appropriate here. In addition to moisture, there are other potentially significant factors, especially water chemistry characteristics, which were not explored in this study but were reported as significant by some previous studies focused on community changes along the poor-rich gradient. These include concentrations of silica and phosphorus [46], calcium [5, 8], zinc and iron [47], and sodium and magnesium [5]. Sulfate concentration was also reported as an important factor influencing testate amoebae [29, 48] and an indirect impact via modification of the food web was suggested by Payne et al. [29]. Nonetheless, the influence of these factors is not consistent among studies and there is not enough information on the variability in ground-water chemistry on smaller spatial scales, again completely missing from alkaline fens. But, based on the results of Ulanowski and Branfireun [49], who documented a significant variability in sodium, calcium, magnesium, and sulfate concentrations within the range of a 7 × 7 m plot over a 2-week period in a fen of neutral reaction, we can assume that the groundwater properties are possibly quite unstable in fens in general.
Besides the chemical properties of the groundwater, discrete difference in testate amoeba assemblages from brown moss- and Sphagnum-dominated patches can be also attributed to biotic factors such as phenolic compounds released by Sphagnum. Jassey et al. [12] suggested a direct physiological effect of Sphagnum mosses on testate amoebae and also a possible indirect influence via their impact on prey organisms. In our samples, we noticed a strong affiliation of Hyalosphenia papilio to Sphagnum-dominated microsites, occasionally comprising up to 70% of all identified individuals. Tight relationship between H. papilio and other mixotrophic testate amoebae was also confirmed in previous studies, e.g., [11, 50, 51]. However, in two of our Sphagnum samples, this species was missing completely with no obvious differences in abiotic conditions being found between these and our other Sphagnum microsites. One might thus speculate if the effect of phenolic compounds or other factors alternating the food web were responsible. Our alternative explanation here, however, is linked to possibly differing successional age of the sampled microsites. If some of the Sphagnum patches were recently formed, they might not yet had been colonized by all species from the local species pool. This is in line with rather low dispersal potential of H. papilio [50]. Of course, this species belongs to the larger testate amoeba taxa and perhaps the compact structure of Sphagnum may hamper the dispersal of the shells. Nonetheless, being the most abundant species at our heterogeneous fen (accounting for more than 20% of all identified individuals), some sort of mass effect could be expected here. Still, the mobility appears to be quite limited as H. papilio shells were also only present in two of our brown moss samples.
Considering spatial distribution of individual species at the homogeneous fen, the occurrence of Difflugia geosphaira was the most intriguing. This species was found only in five samples located in sequence along one of the transects while forming a dominant component of the community in two of those samples. As this species was not found in any of the remaining 15 samples, not even as empty shells, it seems that it did not recently thrive in other (sampled) parts of the site. However, we were not able to identify possible causes of this limited spatial distribution. Although the reasons behind spatially limited occurrence of species such as D. geosphaira and H. papilio remain unexplained, we learned that the dispersal of shells across both our study sites seems to be much lower than expected over such short distances.
Effect of Empty Shells
Testate amoebae are often considered as good indicator organisms due to their short generation times and quick response to changing environmental conditions [52]. It was shown that some factors may vary considerably within the time of just a few weeks [49] and seasonal shifts in testate amoeba communities from Sphagnum-dominated peatlands were previously reported by several authors [11, 25, 30, 45]. These were often related to changes in hydrology throughout the vegetation season, yet more factors appear to be involved and there is still not enough consistent information available concerning the topic of short-term variability. While changes of conditions seem to be the most common source of empty shells, little is also known about the role of predation, reproduction, and dispersal over small and large spatial scales caused by wind [20, 21], wild animals, and even humans [22]. Moreover, better understanding of the vertical distribution of living/dead individuals within the surface peat layers is also needed [53].
Meanwhile, the use of undifferentiated (i.e., living and dead) shells is quite habitual in the testate amoeba research, e.g., [9, 23, 25, 54], according to a common assumption that the potential bias caused by empty shells is negligible. Undifferentiated or even dead assemblages are, for example, often used in transfer function development for paleoecological reconstructions [55,56,57]. However, the information on species ecology might be skewed when the matter of living and dead individuals is disregarded. Our data showed that the proportion of living and dead individuals can vary substantially among samples, even within a single site. We further documented lower percentages of living individuals in brown mosses (9–53%) as compared to those in Sphagnum (44–82%), suggesting that brown mosses might be more prone to variation in conditions, such as DWT and ground-water chemistry, given their loosened spatial structure and lower buffering capacity. Besides, depending on the conditions, higher growth rates can be expected for Sphagnum mosses [58] and surface layers with brown mosses might thus contain shells accumulated over longer periods of time. It is likely that the differentiation between living and empty shells would be more useful in more alkaline conditions than in Sphagnum-dominated habitats. However, we are not aware of any study that would focus specifically on the effect of empty shells on distributional pattern assessment in any peatland habitat so far. It is common that some species show different optima among studies which is often explained by taxonomical discrepancies. Perhaps, this could also be due to the inclusion of dead shells of species that did not tolerate the conditions recorded at the time of the sampling.
Here, we tested the effect of empty shells exclusion assuming this will lead to some shifts in community composition between the “ALL” and “LIVING” data sets. We further expected an increase in explained variability, as the “LIVING community should correspond better with our single-shot measurements. At the homogeneous fen, the exclusion of empty shells had indeed a significant impact on the species composition of the assemblages, however, the testing of environmental variables showed non-significant relationships in both cases. At the heterogeneous fen, the main compositional changes in testate amoeba communities occurred between Sphagnum and brown moss-dominated patches which formed the major environmental gradient here. Even though the presence of the two bryophyte groups can be considered stable over the vegetation season, the shift in species composition between the “ALL” and “LIVE” datasets might still be caused by mass effect with species (or their empty shells) spreading from source areas to sites with less favorable conditions. However, based on the species distribution among our samples, we learned that passive dispersal at our study sites was not as frequent as we would expect, and no significant changes in species composition occurred after empty shell exclusion. On the other hand, we did observe a difference in relation to moisture which emerged as significant variable for the “LIVING” data set in both NMDS and RDA. In fact, the moisture content of the moss layer was the only factor with a potential for short-term variability during the season that we included here. Unfortunately, only a coarse grade scale was used to assess the moisture conditions at our sampling spots and more precise description such as DWT measurements would possibly yield more robust results. We thus believe that, despite the low r2 values and significance levels, especially the result of forward selection supports our hypothesis that the inclusion of empty shells might indeed lead to biased results when it comes environmental variables that are more prone to spatio-temporal fluctuation.
Conclusion
In this study, we aimed to bring new insights into the ecology of testate amoebae inhabiting minerotrophic mires, as well as to draw attention to some methodological aspects that we believe have not yet been adequately addressed. Our results proved that, although marginally studied, minerotrophic mires represent unique environments in terms of testate amoeba assemblages. Using a small-scale approach, we found that macroscopic variability (or homogeneity) of the sampling sites does not correspond to the situation at the microscopic level and the complexity of testate amoeba spatial patterns within these habitats is greater than assumed. However, our data did not allow us to describe properly the mechanisms and factors shaping the compositional variation of testate amoebae at individual microsites. Still, we can conclude that the heterogeneity of conditions in the moss layer proved to be much higher than recognized by vegetation composition changes observed in the field. We find our results challenging for the traditional sampling methods focused primarily on the most representative micro- or mesohabitats, which might lead to severe loss of information and biased understanding of testate amoebae ecology. On the other hand, the applied approach of randomly selected sampling spots is not thoroughly convenient as some microhabitats might be skipped using this technique. Similar sampling strategies, nonetheless, allow to explore spots that would otherwise be considered unsuitable, redundant or simply unappealing and would probably never get sampled. In this context, Mitchell et al. [59] suggested that sampling at more unconventional spots might perhaps be the key to finding analogous communities for those present in peat cores yet missing in the modern data sets.
Additionally, our results proved that exclusion of empty shells can provide more accurate information on species distribution as well as their relationship with certain environmental variables, especially those with higher spatio-temporal variability (e.g., moisture). Further work elaborating this issue is still needed to fully assess the consequences of different approaches used for community description in ecological studies. Of course, the need for different methods may vary depending on the study objectives and, admittedly, the time cost of sample processing must be considered. In our samples, the proportion of living individuals ranged between 9 and 82%, with lower numbers usually found in brown moss-dominated (micro)habitats. These also tend to yield samples with higher content of inorganic sediments. Thus, aiming for more precise community description based on living individuals can result in a fairly time-consuming process in more alkaline fens. On the other hand, we believe that these habitats, often recognized for their ecological uniqueness, need more thorough exploration when it comes to testate amoebae.
References
Mitchell EAD, Borcard D, Buttler AJ, Grosvernier P, Gilbert D, Gobat J (2000) Horizontal distribution patterns of testate amoebae (Protozoa) in a Sphagnum magellanicum carpet. Microb Ecol 39:290–300
Booth RK, Zygmunt JR (2005) Biogeography and comparative ecology of testate amoebae inhabiting Sphagnum-dominated peatlands in the Great Lakes and Rocky Mountain regions of North America. Divers Distrib 11:577–590. https://doi.org/10.1111/j.1366-9516.2005.00154.x
Bobrov AA, Charman DJ, Warner BG (1999) Ecology of testate amoebae (Protozoa : Rhizopoda) on peatlands in western Russia with special attention to niche separation in closely related taxa. Protist 150:125–136
Jassey VEJ, Lamentowicz L, Robroek BJM, Gąbka M, Rusińska A, Lamentowicz M (2014) Plant functional diversity drives niche-size-structure of dominant microbial consumers along a poor to extremely rich fen gradient. J Ecol 102:1150–1162. https://doi.org/10.1111/1365-2745.12288
Lamentowicz L, Gabka M, Rusinska A et al (2011) Testate amoeba (Arcellinida, Euglyphida) ecology along a poor-rich gradient in fens of western Poland. Int Rev Hydrobiol 96:356–380. https://doi.org/10.1002/iroh.201111357
Lamentowicz M, Lamentowicz L, van der Knaap WO, Gąbka M, Mitchell EAD (2010) Contrasting species-environment relationships in communities of testate amoebae, bryophytes and vascular plants along the fen-bog gradient. Microb Ecol 59:499–510. https://doi.org/10.1007/s00248-009-9617-6
Lamentowicz M, Mitchell EAD (2005) The ecology of testate amoebae (protists) in Sphagnum in North-Western Poland in relation to peatland ecology. Microb Ecol 50:48–63. https://doi.org/10.1007/s00248-004-0105-8
Opravilová V, Hájek M (2006) The variation of testacean assemblages (Rhizopoda) along the complete base-richness gradient in fens: a case study from the Western Carpathians. Acta Protozool 45:191–204
Kishaba K, Mitchell EAD (2005) Changes in testate amoebae (Protists) communities in a small raised bog. A 40-year study. Acta Protozool 44:1–12
Niedźwiecki M, Mieczan T, Adamczuk M (2016) Ecology of testate amoebae (Protists) in a Sphagnum-dominated peat bog and the relationship between species assemblages and environmental parameters. Oceanol Hydrobiol Stud 45(344). https://doi.org/10.1515/ohs-2016-0031
Marcisz K, Lamentowicz L, Slowinska S et al (2014) Seasonal changes in Sphagnum peatland testate amoeba communities along a hydrological gradient. Eur J Protistol 50:445–455. https://doi.org/10.1016/j.ejop.2014.07.001
Jassey VEJ, Chiapusio G, Mitchell EAD, Binet P, Toussaint ML, Gilbert D (2011) Fine-scale horizontal and vertical micro-distribution patterns of testate amoebae along a narrow fen/bog gradient. Microb Ecol 61:374–385. https://doi.org/10.1007/s00248-010-9756-9
Hájek M, Horsák M, Hájková P, Dítě D (2006) Habitat diversity of central European fens in relation to environmental gradients and an effort to standardise fen terminology in ecological studies. Perspect Plant Ecol Evol Syst 8:97–114. https://doi.org/10.1016/j.ppees.2006.08.002
Křoupalová V, Opravilová V, Bojková J, Horsák M (2013) Diversity and assemblage patterns of microorganisms structured by the groundwater chemistry gradient in spring fens. Ann Limnol Int J Limnol 49:207–223. https://doi.org/10.1051/limn/2013056
Lamers LPM, Vile MA, Grootjans AP, Acreman MC, van Diggelen R, Evans MG, Richardson CJ, Rochefort L, Kooijman AM, Roelofs JGM, Smolders AJP (2015) Ecological restoration of rich fens in Europe and North America: from trial and error to an evidence-based approach. Biol Rev 90:182–203. https://doi.org/10.1111/brv.12102
Peterka T, Hájek M, Jiroušek M, Jiménez-Alfaro B, Aunina L, Bergamini A, Dítě D, Felbaba-Klushyna L, Graf U, Hájková P, Hettenbergerová E, Ivchenko TG, Jansen F, Koroleva NE, Lapshina ED, Lazarević PM, Moen A, Napreenko MG, Pawlikowski P, Plesková Z, Sekulová L, Smagin VA, Tahvanainen T, Thiele A, Biţǎ-Nicolae C, Biurrun I, Brisse H, Ćušterevska R, de Bie E, Ewald J, FitzPatrick Ú, Font X, Jandt U, Kącki Z, Kuzemko A, Landucci F, Moeslund JE, Pérez-Haase A, Rašomavičius V, Rodwell JS, Schaminée JHJ, Šilc U, Stančić Z, Chytrý M (2017) Formalized classification of European fen vegetation at the alliance level. Appl Veg Sci 20:124–142. https://doi.org/10.1111/avsc.12271
Lamentowicz M, Bragazza L, Buttler A, Jassey VEJ, Mitchell EAD (2013) Seasonal patterns of testate amoeba diversity, community structure and species-environment relationships in four Sphagnum-dominated peatlands along a 1300 m altitudinal gradient in Switzerland. Soil Biol Biochem 67:1–11. https://doi.org/10.1016/j.soilbio.2013.08.002
Lahr DJG, Parfrey LW, Mitchell EAD, Katz LA, Lara E (2011) The chastity of amoebae: re-evaluating evidence for sex in amoeboid organisms. Proc R Soc B Biol Sci 278:2081–2090. https://doi.org/10.1098/rspb.2011.0289
Mazei YA, Tsyganov AN (2006) Freshwater testate amoebae. KMK, Moscow
Wanner M, Elmer M, Sommer M, Funk R, Puppe D (2015) Testate amoebae colonizing a newly exposed land surface are of airborne origin. Ecol Indic 48:55–62. https://doi.org/10.1016/j.ecolind.2014.07.037
Wilkinson DM, Koumoutsaris S, Mitchell EAD, Bey I (2012) Modelling the effect of size on the aerial dispersal of microorganisms. J Biogeogr 39:89–97. https://doi.org/10.1111/j.1365-2699.2011.02569.x
Wilkinson DM (2010) Have we underestimated the importance of humans in the biogeography of free-living terrestrial microorganisms? J Biogeogr 37:393–397. https://doi.org/10.1111/j.1365-2699.2009.02236.x
Fournier B, Coffey EED, van der Knaap WO, Fernández LD, Bobrov A, Mitchell EAD (2016) A legacy of human-induced ecosystem changes: spatial processes drive the taxonomic and functional diversities of testate amoebae in Sphagnum peatlands of the Galápagos. J Biogeogr 43:533–543. https://doi.org/10.1111/jbi.12655
Mitchell EAD, Buttler A, Grosvernier P et al (2000) Relationships among testate amoebae (Protozoa), vegetation and water chemistry in five Sphagnum-dominated peatlands in Europe. New Phytol 145:95–106. https://doi.org/10.1046/j.1469-8137.2000.00550.x
Sullivan ME, Booth RK (2011) The potential influence of short-term environmental variability on the composition of testate amoeba communities in Sphagnum peatlands. Microb Ecol 62:80–93. https://doi.org/10.1007/s00248-011-9875-y
Jassey VEJ, Gilbert D, Binet P, Toussaint ML, Chiapusio G (2011) Effect of a temperature gradient on Sphagnum fallax and its associated living microbial communities: a study under controlled conditions. Can J Microbiol 57:226–235. https://doi.org/10.1139/w10-116
Krashevska V, Sandmann D, Maraun M, Scheu S (2014) Moderate changes in nutrient input alter tropical microbial and protist communities and belowground linkages. ISME J 8:1126–1134
Marcisz K, Fournier B, Gilbert D, Lamentowicz M, Mitchell EAD (2014) Response of Sphagnum peatland testate amoebae to a 1-year transplantation experiment along an artificial hydrological gradient. Microb Ecol 67:810–818. https://doi.org/10.1007/s00248-014-0367-8
Payne R, Gauci V, Charman DJ (2010) The impact of simulated sulfate deposition on peatland testate amoebae. Microb Ecol 59:76–83. https://doi.org/10.1007/s00248-009-9552-6
Song L, Li H, Wang K, Yan X, Wu D (2018) Seasonal dynamics in the community structure and trophic structure of testate amoebae inhabiting the Sanjiang peatlands, Northeast China. Eur J Protistol 63:51–61. https://doi.org/10.1016/j.ejop.2018.01.005
Swindles GT, Green SM, Brown L, Holden J, Raby CL, Turner TE, Smart R, Peacock M, Baird AJ (2016) Evaluating the use of dominant microbial consumers (testate amoebae) as indicators of blanket peatland restoration. Ecol Indic 69:318–330. https://doi.org/10.1016/j.ecolind.2016.04.038
Schönfeld J, Alve E, Geslin E, Jorissen F, Korsun S, Spezzaferri S (2012) The FOBIMO (FOraminiferal BIo-MOnitoring) initiative - towards a standardised protocol for soft-bottom benthic foraminiferal monitoring studies. Mar Micropaleontol 94–95:1–13. https://doi.org/10.1016/j.marmicro.2012.06.001
Decloitre L (1962) Le genre Euglypha Dujardin. Arch Protistenkd 106:51–100
Mitchell EAD (2002) The identification of Centropyxis, Cyclopyxis, Trigonopyxis and similar Phryganella species living in Sphagnum. International Society for Testate Amoeba Research (ISTAR). http://istar.wikidot.com/id-keys. Accessed May 2012
Mitchell EAD (2002) The identification of Heleopera species living in Sphagnum. International Society for Testate Amoeba Research (ISTAR). http://istar.wikidot.com/id-keys. Accessed May 2012
Mitchell EAD (2003) The identification of Nebela and similar species with indications on their ecology and distribution. International Society for Testate Amoeba Research (ISTAR). http://istar.wikidot.com/id-keys. Accessed May 2012
Ogden CG (1983) Observations on the systematics of the genus Difflugia in Britain (Rhizopoda, Protozoa). Bull Br Mus Nat Hist (Zool) 44:1–73
Ogden CG, Hedley RH (1980) An atlas of freshwater testate amoebae. Oxford University Press [for the] British Museum (Natural History), Oxford
Siemensma FJ (2018) Microworld, world of amoeboid organisms. http://www.arcella.nl. Accessed April 2008
Kosakyan A, Lahr DJG, Mulot M, Meisterfeld R, Mitchell EAD, Lara E (2016) Phylogenetic reconstruction based on COI reshuffles the taxonomy of hyalosphenid shelled (testate) amoebae and reveals the convoluted evolution of shell plate shapes. Cladistics 32:606–623. https://doi.org/10.1111/cla.12167
RCoreTeam (2017) R: a language and environment for statistical computing. R Foundation for statistical computing, Vienna
Oksanen J, Blanchet FG, Friendly M, et al (2017) Vegan: community ecology package. R package version 2.4–3
Dray S, Legendre P, Blanchet G (2013) packfor: Forward Selection with permutation (Canoco p.46). R package version 0.0-8/r109
Sjörs H (1950) On the relation between vegetation and electrolytes in north Swedish mire waters. Oikos 2:241–258. https://doi.org/10.2307/3564795
Warner BG, Asada T, Quinn NP (2007) Seasonal influences on the ecology of testate amoebae (Protozoa) in a small Sphagnum peatland in Southern Ontario, Canada. Microb Ecol 54:91–100. https://doi.org/10.1007/s00248-006-9176-z
Hájková P, Bojková J, Fránková M et al (2011) Disentangling the effects of water chemistry and substratum structure on moss-dwelling unicellular and multicellular micro-organisms in spring-fens. J Limnol 70:54–64. https://doi.org/10.3274/jl11-70-s1-04
Payne RJ (2011) Can testate amoeba-based palaeohydrology be extended to fens? J Quat Sci 26:15–27. https://doi.org/10.1002/jqs.1412
Lamentowicz L, Gabka M, Lamentowicz M (2007) Species composition of testate amoebae (Protists) and environmental parameters in a Sphagnum peatland. Pol J Ecol 55:749–759
Ulanowski TA, Branfireun BA (2013) Small-scale variability in peatland pore-water biogeochemistry, Hudson Bay Lowland, Canada. Sci Total Environ 454:211–218. https://doi.org/10.1016/j.scitotenv.2013.02.087
Marcisz K, Colombaroli D, Jassey VEJ, Tinner W, Kołaczek P, Gałka M, Karpińska-Kołaczek M, Słowiński M, Lamentowicz M (2016) A novel testate amoebae trait-based approach to infer environmental disturbance in Sphagnum peatlands. Sci Rep 6:33907
Jassey VEJ, Signarbieux C, Hättenschwiler S, Bragazza L, Buttler A, Delarue F, Fournier B, Gilbert D, Laggoun-Défarge F, Lara E, T. E. Mills R, Mitchell EAD, Payne RJ, Robroek BJM (2015) An unexpected role for mixotrophs in the response of peatland carbon cycling to climate warming. Sci Rep 5:16931
Payne RJ (2013) Seven reasons why protists make useful bioindicators. Acta Protozool 52:105–113. https://doi.org/10.4467/16890027AP.13.0011.1108
Roe HM, Elliott SM, Patterson RT (2017) Re-assessing the vertical distribution of testate amoeba communities in surface peats: implications for palaeohydrological studies. Eur J Protistol 60:13–27. https://doi.org/10.1016/j.ejop.2017.03.006
Koenig I, Mulot M, Mitchell EAD (2018) Taxonomic and functional traits responses of Sphagnum peatland testate amoebae to experimentally manipulated water table. Ecol Indic 85:342–351. https://doi.org/10.1016/j.ecolind.2017.10.017
Charman DJ (2001) Biostratigraphic and palaeoenvironmental applications of testate amoebae. Quat Sci Rev 20:1753–1764. https://doi.org/10.1016/s0277-3791(01)00036-1
Hájková P, Grootjans AB, Lamentowicz M, Rybníčková E, Madaras M, Opravilová V, Michaelis D, Hájek M, Joosten H, Wołejko L (2012) How a Sphagnum fuscum-dominated bog changed into a calcareous fen: the unique Holocene history of a Slovak spring-fed mire. J Quat Sci 27:233–243. https://doi.org/10.1002/jqs.1534
Mitchell EAD, Payne RJ, van der Knaap WO, Lamentowicz Ł, Gąbka M, Lamentowicz M (2013) The performance of single- and multi-proxy transfer functions (testate amoebae, bryophytes, vascular plants) for reconstructing mire surface wetness and pH. Quat Res 79:6–13. https://doi.org/10.1016/j.yqres.2012.08.004
Vitt DH (1990) Growth and production dynamics of boreal mosses over climatic, chemical and topographic gradients. Bot J Linn Soc 104:35–59. https://doi.org/10.1111/j.1095-8339.1990.tb02210.x
Mitchell EAD, Charman DJ, Warner BG (2008) Testate amoebae analysis in ecological and paleoecological studies of wetlands: past, present and future. Biodivers Conserv 17:2115–2137. https://doi.org/10.1007/s10531-007-9221-3
Acknowledgements
Special thanks goes to Eva Kristová for her help with the field and laboratory work as well as the moss identification, to Anna Šímová for her help with testate amoeba taxonomy, and to Ondřej Hájek for his help with GIS and the study site map. For technical and laboratory support, we would like to thank Marcela Růžičková and Zuzana Formánková. Also, we would like to thank the reviewers for their comments and suggestions that helped to improve the manuscript.
Funding
This study was supported by the institutional support for Ph.D. students at the Masaryk University and the Czech Science Foundation (project no. P505/16-03881S).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Lizoňová, Z., Zhai, M., Bojková, J. et al. Small-scale Variation of Testate Amoeba Assemblages: the Effect of Site Heterogeneity and Empty Shell Inclusion. Microb Ecol 77, 1014–1024 (2019). https://doi.org/10.1007/s00248-018-1292-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-018-1292-z