Abstract
Plant-associated endophytes are recognized as sources of novel bioactive molecules having diverse applications. In this study, an endophytic yeast-like fungal strain was isolated from the fruit of eggplant (Solanum melongena) and identified as Geotrichum candidum through phenotypic and genotypic characterizations. This endophytic G. candidum isolate PF005 was found to emit fruity scented volatiles. The compositional profiling of volatile organic compounds (VOCs) revealed the presence of 3-methyl-1-butanol, ethyl 3-methylbutanoate, 2-phenylethanol, isopentyl acetate, naphthalene, and isobutyl acetate in significant proportion when analyzed on a time-course basis. The VOCs from G. candidum exhibited significant mycelial growth inhibition (54%) of phytopathogen Rhizoctonia solani, besides having mild antifungal activity against a few other fungi. The source of carbon as a nutrient was found to be an important factor for the enhanced biosynthesis of antifungal VOCs. The antifungal activity against phytopathogen R. solani was improved up to 91% by feeding the G. candidum with selective precursors of alcohol and ester volatiles. Furthermore, the antifungal activity of VOCs was enhanced synergistically up to 92% upon the exogenous addition of naphthalene (1.0 mg/plate). This is the first report of G. candidum as an endophyte emitting antifungal VOCs, wherein 2-penylethanol, isopentyl acetate, and naphthalene were identified as important contributors to its antifungal activity. Possible utilization of G. candidum PF005 as a mycofumigant has been discussed based upon its antifungal activity and the qualified presumption of safety status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants support a suite of microorganisms that do not cause overt damage to the plants in which they live, and these organisms are known as endophytes [1]. Since very little research has been performed on them, there is a prospect of finding untold numbers of novel fungal genera existing as plant-associated microbes [2]. Furthermore, strain variations among the same fungal species make them more diverse and appropriate for the discovery of new metabolites, as well as a known metabolite in high titer [3]. One of the logical arguments behind the production of a range of novel metabolites by endophytic fungi could be due to their habitat in a specialized niche, i.e., inside the tissues of particular plant species. In order to escape from the defense mechanism of their host plant and fight against the competing neighboring microbes, the endophyte turns on pathways that are otherwise “switched off” in the living free strains [3, 4].
Several fungal strains producing volatile organic compounds (VOCs) have been isolated and studied for the last two decades. However, production of volatile antibiotics or antimicrobials is rare among microorganisms and thus has not been heavily investigated [5]. Many fungal species with distinctive obnoxious odor are known for the major emitters of VOCs, and such observations have resulted in the characterization of fungal volatiles [6]. These chemical characterizations subsequently led to the discovery of a few VOCs that are unique to a particular fungal species. These VOCs often possess antimicrobial activities signifying that they might have spontaneously evolved in nature to create microenvironments that inhibit or kill the competing microorganisms [3, 4]. Volatiles of rhizobacteria (Stenotrophomonas, Serratia, and Bacillus species) significantly inhibit growth of many fungi and Arabidopsis thaliana [7]. VOCs from endophytic fungi are known to have a phytotoxic activity [8]. An example to this is Trichoderma species, which efficiently control other wood-decaying fungi through the release of VOCs [9]. Though not reported as an endophyte, Saccharomyces cerevisiae can produce antifungal VOCs against the phytopathogen Guignardia citricarpa [10]. Other examples include Muscodor, which produces volatile antimicrobial agents [11,12,13], and Oidium, an endophyte in Terminalia catappa that synthesizes antimicrobial VOCs [14]. In recent past, bioactive volatile compounds have been reported from two endophytic fungi, Phoma sp. and Nodulisporium sp., which produce biologically active volatile compounds having fuel potential and antifungal activity [15, 16]. Esters, alcohols, acids, and naphthalene/azulene derivatives mainly constitute the bioactive VOCs in fungi [12]. Different components of VOCs act additively and synergistically to show maximum biological activity [14]. An endophytic Gloeosporium sp. in the presence of some exogenously added organic compounds (termed synergistans) is capable of producing combined inhibitory (synergistic) effects with the VOCs [17]. Effectiveness as a mycofumigant is one of the most important applications of these VOCs. The concept of mycofumigation has the potential to replace or minimize the use of otherwise hazardous synthetic chemicals that are currently being applied to crops, soils, and buildings [4].
In the present study, we report the isolation of an endophytic yeast-like fungal strain PF005 from the fruit of eggplant (Indian brinjal) Solanum melongena, and its characterization revealed that it is Geotrichum candidum (the teleomorph of Galactomyces candidus). We have identified through GC-MS analysis the major components of fruity scented VOCs produced by this endophyte, which showed a significant antifungal activity against the rice pathogenic fungus Rhizoctonia solani. The antifungal activity of VOCs was significantly enhanced through metabolite feeding of certain precursors and exogenous supplementation of a selective constituent of VOCs.
Materials and Methods
Isolation and Storage of Endophytic Fungi
The edible fruit of eggplant S. melongena (Indian brinjal) was the source for isolation of endophytic fungi. Isolation procedure as described by Strobel and his group was essentially followed with few modifications [15]. The fruit was washed in tap water and cut into small pieces. The cut pieces were firstly surface sterilized with 4% sodium hypochlorite solution and, thereafter, with 70% ethanol in an aseptic condition. The explants were washed three times with autoclaved distilled water for removing the residual amount of disinfectants and dried using a sterile blotting paper. These explants were further cut into small pieces to expose the inner surface and placed over a potato dextrose agar (PDA, HiMedia) medium supplemented with antibiotic (chloramphenicol, 75 g l−1) for cultivation at 30 °C. A few of the surface-sterilized explants’ surfaces were imprinted over the PDA medium and monitored for 4 days to observe the presence of any visual microbial growth to ascertain their proper surface sterilization. A 4-day timeline was set to identify contaminants. Microbial growth found within day 4 was considered as a contaminant and thereby discarded. Considering the fact that endophytes require time to come out of the plant’s inner tissue space, microbial growth that emerged from the surface-sterilized fruit parts only after 5–10 days were considered as endophytes. Pure plates for axenic culture were prepared onto new PDA plates. One of the pure isolates, termed as PF005 hereafter, was found to produce fruity scented VOCs. The isolate PF005’s liquid culture was stored in 15% glycerol at − 80 °C.
Genomic DNA Extraction, Amplification, and Sequencing of the Partial DNA Fragments of 18S Ribosomal RNA Gene with Adjacent Internal Transcribed Spacer Region and 26S Ribosomal RNA Gene
For genomic DNA extraction, 10 ml potato dextrose broth (PDB, HiMedia) was inoculated with 1% overnight-grown mother culture of PF005. It was incubated for 4 days at 30 °C in shaking condition. Biomass was separated from the culture by centrifugation. The genomic DNA was extracted from the harvested biomass by the reported CTAB method with few minor modifications [18]. Two sets of primers were used for amplifying parts of the ribosomal RNA (rRNA) gene region. A fungus-specific primer set of NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′) was used for amplifying a part of the D1/D2 domain of the 26S rRNA gene. Another primer set of internal transcribed spacer (ITS)1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) was used to amplify a DNA fragment, which encompasses the 18S rRNA gene partial sequence, ITS1, 5.8S rRNA gene, ITS2, and 28S rRNA gene partial sequence. Both the PCR profiles were set with an initial denaturation step of 94 °C/4 min followed by 30 cycles of 94 °C/0.45 min denaturation, 55 °C/1 min annealing, and 72 °C/1 min extension with a final extension of 72 °C/10 min. The amplified products (10 μl) were visualized on 1% (w/v) agarose gel stained with ethidium bromide to confirm the presence of a single amplicon. Amplicons were purified from the agarose gel using Nucleopore Quick Gel Recovery Kit (Genetix Biotech, Asia). The PCR amplicon (named partial 26S) generated from the NL1 and NL4 primer pair was directly sequenced. On the other hand, the PCR amplicon (named as ITS-5.8S) produced by the ITS1 and ITS4 primer set was cloned into a pTZ57R/T vector using InsTAclone PCR Cloning Kit (Thermo Fisher Scientific) and subsequently sequenced. The sequence information from both the amplicons was analyzed through NCBI BLAST search to ascertain the sequence homology with closely related organisms. Finally, the sequences were submitted to GenBank (https://www.ncbi.nlm.nih.gov/genbank/).
Morphological Identification of Isolate PF005
PF005’s morphology was visualized under the phase-contrast light microscope after lactophenol cotton blue staining. Scanning electron microscopy (SEM) was performed for surface morphology visualization following the modified protocol of Olesen and Kier [19]. The PF005 was grown in a modified Czapekdox medium [for 1 l medium: arginine (1.5 g), K2HPO4 (1.0 g), KCl (0.5 g), MgSO4·7H2O (0.5 g), and FeSO4·7H2O (0.01 g), and 1 ml trace solution of 1.0 g ZnSO4·7H2O and 0.5 g CuSO4·5H2O in 100 ml H2O] containing 0.5 and 2% glucose. Cells were harvested after a 1-day growth from a 0.5% glucose medium. From a 2% glucose medium, PF005 cells were collected after 4 days. Cells harvested from a 1-ml culture were washed thrice in phosphate-buffered saline (PBS) (pH 7.2–7.4). Cells were fixed using 2.5% glutaraldehyde in PBS for overnight at 4 °C. Fixatives were washed with PBS and resuspended in 250 μl PBS. Five microliters of cell suspension was spread over a 12-mm cover glass and air-dried inside the laminar air flow. Cells were dehydrated using gradient acetone series (50, 70, 95, and 100%) at 4 °C. Finally, for SEM, the fungal material was gold sputter coated, and images were recorded with a Zeiss EVO 60 scanning electron microscope.
Quantitative and Qualitative Analyses of PF005 VOCs
One percent culture of PF005 (OD600 1.90) was inoculated in nine vacuum filter flasks, each with 100 ml PDB, and incubated at 30 °C in a shaker maintained at 150 rpm. To determine the volatile composition emitted by PF005, these cultures were incubated for 0 to 96 h. During this period of incubation, emitted volatiles were trapped at an interval of 12 h. Trapping of the emitted volatiles in Porapak Type Q polymer (Waters, Milford, MA, USA; 50 mg, mesh size 80/100) was carried out as described in a previous report [20] with some modifications. We have designed a system with a special glass conical flask for the headspace volatile analysis as shown in Fig. 2. A vacuum pump (Rockyvac™ 400; Tarsons, India) was connected to the Teflon tube which dragged the volatile-enriched air (4 l min−1) from the headspace of the glass conical flask through the column for 30 min. The inlet of the conical flask was attached to a compressor (Rockyvac™ 320; Tarsons, India) through an activated charcoal filter so that purified air can enter to the conical flask during the headspace volatile collection process. The trapped volatiles were eluted with 200 μl of dichloromethane (DCM, HPLC grade, 99.5% assay) and stored at − 20 °C.
The growth rate of PF005 was also monitored during this period. In a separate PDB medium, the same amount of inoculum was added and kept for incubation. An aliquot of the culture was removed at specified intervals for monitoring its growth. Optical density was measured at 600 nm in the spectrophotometer. For dry biomass measurement, 1 ml of culture was centrifuged, and the precipitated biomass was dried at 80 °C for 6 h. Due to the yeast-like fungus nature of the PF005, growth could not be monitored through cell count.
GC-MS Analysis of Fungal Volatiles
A gas chromatography mass spectrometry system (GCMS-QP2010SE; Shimadzu Corp.) was used to analyze the headspace fungal volatiles. A ZB-5 column (30 m × 0.25 mm id, film thickness 0.25 μm) was utilized for the separation of volatile compounds with helium as a carrier gas. For each sample analysis, the injection volume was 2 μl with a split ratio 2:2 and the GC-MS program was set as described earlier [20]. A known amount of ethyl hexanoate (internal standard) was added to the sample, and the relative GC peak area percentage of each identified compound was expressed with respect to the internal standard. The compounds were identified by comparing the mass spectrum of the module to that of the mass spectral library from NIST 05 (National Institute of Standards and Technology, Gaithersburg, USA) and Wiley 8.0 (Wiley, New York, NY, USA) and by matching with retention indices of those published in the literature [21,22,23]. Identification of a few compounds was also made by comparing the retention times, retention indices, and mass spectra to that of the authentic standards purchased from Sigma-Aldrich (India).
Test Microorganisms for Antimicrobial Assay
Opportunistic human pathogenic test bacteria Escherichia coli, Staphylococcus aureus, and Mycobacterium smegmatis were maintained, and growth inhibition was assessed in Luria-Bertani (LB) medium. Plant pathogenic bacteria Ralstonia solanacearum and Xanthomonas campestris were also maintained and assessed using the LB medium. The potato dextrose medium was used for the growth of phytopathogenic fungi like R. solani, Cercospora sp., Fusarium oxysporum, Pseudocercospora sp., and Rhizopus sp. Among these, Cercospora sp., Pseudocercospora sp., and Rhizopus sp. were isolated in our laboratory. Kwoniella sp., one of the lab-isolated endophytes from Terminalia bellirica, was used as negative control.
Bioassays for PF005 VOCs Against Microbial Pathogens
Antimicrobial activity of PF005’s VOCs was tested against the different plant and human pathogenic microbes following the methodology reported in the literature [11]. Ten microliters of the overnight-grown culture of PF005 was streaked and grown for 1 day onto PDA on the one side of the half-moon plate (bi-petri plates, HiMedia) to facilitate its growth at the fruity scented volatiles’ synthesizing stage. On the other side of the bi-petri plate, a target test organism was inoculated after 1 day. Cylindrical plugs (3 mm radius × 5 mm height) of test fungal mycelia were inoculated over PDA (HiMedia), whereas 10 μl of overnight-grown test bacterial culture was streaked over Luria-Bertani agar (LBA). Bi-petri plates were tightly wrapped with a double layer of parafilm and then eventually with DuraSeal™ laboratory stretch film (Tarsons) to create an airtight environment and were incubated at 30 °C for 4 days (fast-growing microbes) or 10 days (slow-growing microbes). Control plates were prepared for each experiment with test microbes in the absence of PF005. Extents of mycelial growth inhibition observed under test conditions were expressed as % growth over that occurring with no treatment. Bacterial inhibition was monitored by the absence of their viability. Bioassay with each test organism was repeated for three to four times for statistical validation. After completion (4–10 days) of bioassays, all the test microorganisms were transferred to fresh plates containing their corresponding growth medium for assessing their viability.
Carbon Source Optimization for the Enhanced Production of Antifungal VOCs
Potato agar (infusion of 200 g l−1 potato and 18 g l−1 agar) was supplemented with 20 g l−1 (2%) of seven different carbon substrates, such as four monosaccharides (fructose, galactose, glucose, and xylose), two disaccharides (maltose and sucrose), and one polysaccharide (starch). Glucose, sucrose, and soluble starch were directly added into the potato agar and autoclaved. However, fructose, galactose, maltose, and xylose were filter sterilized and then added into the autoclaved potato agar. On the one side of the bi-petri plate, the potato agar medium containing the particular carbon source was poured, while the adjacent side had the standard PDA (HiMedia). The PF005 was inoculated in different carbon-supplemented potato agar media, and the R. solani’s agar plug was placed on the adjacent side over PDA. The antifungal activity of VOCs produced by PF005 was determined through the same bioassay experiment (described previously) with the test organism phytopathogen R. solani.
Precursor Feeding for the Enhancement of VOC Profile
Based on the VOC profile from the GC-MS analytical experiment, the abundance of a few antimicrobial compounds like 2-phenylethanol and 3-methyl-1-butanol was observed. To increase the antimicrobial efficiency of PF005’s VOCs, the PDB growth media (HiMedia) were supplemented with their respective precursors. Leucine and its precursor α-ketoisocaproic acid (sodium 4-methyl-2-oxovalerate) were fed separately to enhance the quantity of 3-methyl-1-butanol and isopentyl acetate, respectively. Similarly, phenylalanine was used to increase the quantity of 2-phenylethanol. All these experiments were performed in triplicate using a set of three: one PDB control and the other two PDB with 0.0025 and 0.01 mol l−1 precursors supplemented at pH 5.5. The same amount of inoculum was used for all the experiments. Different studies of precursor feeding were conducted on different days. The emitted volatiles were trapped following the previously described method. Further, their effects on the change in antifungal activity against R. solani were measured by the half-moon plate bioassays. These experiments were performed in triplicate.
Testing the Effect of Naphthalene Along with the Volatile Mixture of PF005 as Synergistan
Naphthalene was one of the major volatiles emitted by PF005, and this compound is well known for its antifungal activity. To verify the synergistic effect of VOCs and exogenously added naphthalene, the antifungal activity of PF005 was studied. First, a set of experiments was performed to find out the range of naphthalene quantity to be assessed. Different amounts (0.5, 1.0, 2.0, and 5.0 mg) of external naphthalene were added per plate in half-moon plate bioassay. Naphthalene (dissolved in toluene) solution was added onto the corners of the test plates so that organisms did not come in direct contact with naphthalene. The same experimental plates in the absence of PF005 were used as controls. In this experiment, 1-day pre-incubation was not performed like earlier tests for the PF005’s growth to reach the exponential phase. The entire experiment was set up on the same day, and plates were incubated at 30 °C for 4 days. Measurements of mycelial growth inhibition made under test conditions were expressed as % growth over that occurring with the PF005 absent control plates (but containing the respective concentration of naphthalene).
Statistical Analysis
Every experiment was performed in triplicates, and the average value is expressed as the mean ± standard deviation (SD) unless mentioned otherwise. All of the data were statistically analyzed, and their significance was determined by one-way analysis of variance (ANOVA) and Tukey’s HSD post hoc test. Significant differences between the control and the test measurement were branded as ****P < 0.0001, ***P < 0.001, **P < 0.01, and *P < 0.05.
Results
Molecular, Morphological, and Phenotypic Information Deciphers the Endophytic Yeast-Like Fungal Isolate PF005 as G. candidum
The genomic DNA isolated from the pure culture of yeast-like fungal isolate PF005 obtained from the S. melongena fruit was subjected to PCR using NL1 and NL4 primer set specific for the 26S rRNA gene region, which yielded an amplicon of ~ 500 bp. The sequence of this DNA fragment was analyzed in silico using BLASTN in the NCBI database. The partial 26S rRNA gene sequence (GenBank, KX938344.1) revealed that the endophytic isolate PF005 is phylogenetically related to G. candidus strain feni 105 (GenBank, KP223716.1) with a sequence identity of 100% (100% query coverage). Further, a 372-bp amplicon of the ITS-5.8S rRNA gene region of this PF005 isolate was amplified using the ITS1 and ITS4 primers. The amplicon (ITS-5.8S) was cloned into TA cloning plasmid and sequenced. Following the similar sequence analysis, the ITS-5.8S rRNA gene region (GenBank, KX938343.1) revealed that the PF005 is phylogenetically very close to the fungal species E15020A (GenBank, KM265851.1) with a sequence identity of 99% (100% query coverage). This homology with the fungal sp. E15020A does not give us the actual identity of our endophyte at the genus or species level. However, the PF005’s ITS-5.8S rRNA gene region had the second best sequence homology with the G. candidus isolate UOA/HCPF 9994 (GenBank, GQ376093.1) with a sequence identity of 98% (100% query coverage). From these findings, we predicted that the PF005’s identity is phylogenetically similar to that of G. candidus, which is the anamorph of G. candidum. The isolate PF005 was further morphologically characterized for confirmation of its identity.
The PF005 showed typical fungal growth with creamy white coloration on PDA petri plates (Fig. 1a). However, the morphological pleomorphism was observed under the light microscope (Fig. 1b). We noticed that the PF005 isolate has distinct mycelial hyphae as well as single cellular spore structure, and the spores are mainly cylindrical in different sizes. Both filamentous hyphae and cylindrical spores were clearly observed through SEM analysis of 1-day culture fed with 0.5% glucose (Fig. 1c). On the other hand, the hyphal structure was absent under SEM of 4-day culture fed with 2.0% glucose, and some spores were observed to be drum shaped at higher magnification (Fig. 1d). The isolate PF005 was found to have the capacity for holothallic arthrospore production. These characteristic features are very similar to the fungal strains of G. candidum or G. candidus reported earlier [19, 24].
Morphological identification of the yeast-like fungus isolate PF005. a Mycelial growth of PF005 on the PDA medium. b PF005 stained with lactophenol cotton blue and observed under the light microscope at × 1000 magnification. c Scanning electron micrograph of PF005 grown on 0.5% glucose for 1 day under × 2000 magnification. d Scanning electron micrograph of PF005 grown on 2.0% glucose for 4 days under × 3500 magnification
According to the proposed taxonomic revision of De Hoog and Smith, all G. candidum strains previously included in the Galactomyces geotrichum/G. candidum complex necessitated a validation step based on a few phenotypic tests to differentiate them from the closely related, newly described species [24, 25]. The PF005 strain was grown in a modified Czapekdox medium with different carbon sources (d-glucose, d-galactose, d-xylose, sucrose, maltose, soluble starch, and d-mannitol) and evaluated for the optimal growth of the isolate. The modified Czapekdox medium was prepared without any vitamin supplement. The endophyte PF005 was also grown at different incubation temperatures (25, 30, 35, and 40 °C) in the PDB medium. Almost all results were found to be similar to those with G. candidum (the teleomorph of G. candidus) as per earlier reports, except sucrose utilization (Table 1). The isolate PF005 was found to utilize d-glucose, d-galactose, d-xylose, sucrose, and d-mannitol for its growth. However, it was unable to grow optimally in either maltose- or soluble starch-containing medium. Further, we observed that the PF005 can grow at 25, 30, and 35 °C temperature in the PDB medium, whereas no growth was observed at 40 °C (Table 1). The G. candidum PF005 strain has been submitted in the general deposit of Microbial Type Culture Collection and Gene Bank (MTCC, IMTECH, Chandigarh, India) with an accession number MTCC 12702 and Microbial Culture Collection (MCC-NCCS, Pune, India) with an accession number MCC 1364.
Fruity Scented VOCs of G. candidum Contain Terpenoids, Esters, Hydrocarbons, and Alcohols
The endophytic G. candidum isolate obtained from the fruit tissue of eggplant produces VOCs having an attractive and noticeable fruity aroma. The emitted VOCs of G. candidum in the Porapak Type Q polymer were trapped and finally analyzed with GC-MS (Fig. 2). Preliminary identification of the fungal VOCs was carried out by comparing the MS data of an unknown compound with that of the reference molecule present in NIST and Willey libraries. A total of 36 compounds were detected and identified in the emitted volatile mixture of G. candidum at different incubation periods (Table 2). On the basis of total integrated peak areas of the GC-MS elution profile (Table 2), the major constituents of G. candidum VOCs were categorized into three classes, namely alcohols (3-methyl-1-butanol and 2-phenylethanol), esters (isopentyl acetate, ethyl 3-methylbutanoate, and isobutyl acetate), and hydrocarbons (dodecane, tridecane, tetradecane, pentadecane, hexadecane, and naphthalene). All these metabolites have a very high percentage (70–99%) of similarity of mass spectra with the reference compounds present in the libraries. The predominant compounds behind the G. candidum’s fruity scent are likely to be isopentyl acetate, ethyl 3-methylbutanoate, nonanal, and isobutyl acetates, as these are known compounds for their fruity fragrance. Synthesis of these important compounds in the G. candidum strain PF005 increased considerably after 24 h of growth (Table 2). It was observed that up to 36 h of incubation, the strain produced a good amount of terpenoids (β-ocimene, (−)-(S)-limonene, 1,4-cineole, terpinolene, and β-citronellal as listed in Table 2) and other compounds which were absent (or present in a trace amount) in a 48- to 96-h culture. These terpenoids were identified by matching their MS data, retention time (RT), and retention index (RI) with the authentic standards. The amount percentages of the above-said classes of VOCs followed a rhythmic pattern (Fig. 3). Although with incubation time, the absence of terpenoids and other compounds was observed, but the amount percentages of alcohols and esters were increased. On the other hand, the amount percentages of hydrocarbon followed a particular pattern throughout the incubation period. Moreover, we observed the presence of benzyl acetate, which is usually found in honey bee pheromone and has insecticidal properties (http://www.t3db.ca/toxins/T3D3214). Growth curves of G. candidum were plotted, considering both changes of optical density and change of dry cell biomass in response to variable incubation time. Both curves were of the same pattern (Fig. 3) and indicated the exponential phase of growth up to 90 h. The lag phase of the G. candidum’s growth was found to be completed around 6 h after the incubation (Fig. 3). It is evident from Table 2 that significant proportions of the antimicrobial volatiles (e.g., 3-methyl-1-butanol and 2-phenylethanol) were produced during the exponential phase of the G. candidum’s growth.
Experimental setup for trapping of volatiles emitted by the G. candidum PF005 fungus in the Porapak Type Q polymer, as described in the “Materials and Methods” section
Growth curve of G. candidum PF005 cultured in the PDB medium at 30 °C. Incubation time is indicated on the x-axis and optical density (measured at 600 nm) on the y-axis. Another secondary y-axis is used to depict cell biomass. All data points were put with their mean and standard deviation (±SD) values from three observations. OD600 (filled circle) and biomass (g l−1) (empty circle)
Mycelial Growth of Phytopathogen R. solani Is Inhibited Significantly by the VOCs of G. candidum
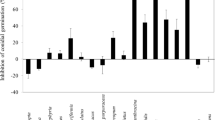
In preliminary experiments, G. candidum’s cell free extract and spent media did not show any antimicrobial activity against a few bacteria and fungi (data not shown); however, the volatile mixture exhibited antifungal activity. The VOCs produced by G. candidum were examined by growth inhibition of test microorganisms by half-moon plate (or bi-petri plate) bioassay. The test microbes were selected on the basis to include a broad taxonomic representation of a few plant fungal pathogens as well as representative Gram-positive and Gram-negative bacteria. To achieve the optimum production of VOCs, the G. candidum was pre-incubated for 1 day in all the test plates to attain its exponential phase. Then, the test microbes were inoculated and incubated for 96 h in the presence of G. candidum and subsequently checked for growth inhibition. However, Pseudocercospora sp. and Cercospora sp. were incubated for 10 days in the presence of G. candidum due to their slower growth rate. Analysis of the results revealed that the growth of E. coli, S. aureus, M. smegmatis, R. solanacearum, X. campestris, and Rhizopus sp. is unaffected in the presence of endophytic G. candidum. Interestingly, the mixture of VOCs produced by G. candidum was found to reduce the growth of R. solani by a significant amount, along with a certain degree of growth retardation for F. oxysporum, Pseudocercospora sp., and Cercospora sp. (Fig. 4a). Growths of R. solani, F. oxysporum, Pseudocercospora sp., and Cercospora sp. were inhibited by ~ 54, 11, 23, and 12%, respectively. All the test microorganisms remained viable after the half-moon plate bioassay (Fig. 4a). One representative picture of R. solani’s growth inhibition by VOCs of G. candidum as revealed by half-moon plate assay is shown in Fig. 4b, c. Further, to confirm R. solani’s mycelial growth inhibition solely due to the presence of G. candidum, we performed a similar experiment against the endophyte Kwoniella sp. as a negative control without G. candidum. No significant change in mycelial growth of R. solani was observed in the presence of Kwoniella sp.
Antimicrobial activity of VOCs produced by G. candidum PF005 against test microorganisms. a Percentage of mycelial growth inhibition of different bacterial colonies or fungal mycelia due to the bioactivity of VOCs from PF005. Dead (D) or alive (A) test microorganisms are also indicated in the table. b Mycelial growth of R. solani on PDA-containing half-moon plate after 96 h. c Mycelial growth inhibition of Rhizoctonia solani in the presence of PF005 after 96 h, each strain grown on either side of PDA-containing half-moon plate
Glucose Is a Suitable Carbon Source for the Optimal Antifungal Activity of G. candidum
The endophyte G. candidum was found to utilize different carbon sources in varying amounts, and the antifungal activity of its VOCs against phytopathogen R. solani changed with each carbon source. The maximum mean growth inhibition of about 62% was observed with glucose as a carbon source, whereas maltose-grown G. candidum had merely 12% growth inhibition capacity (Fig. 5a). The G. candidum grown in fructose, galactose, xylose, sucrose, and soluble starch had about 44, 24, 55, 39, and 21% mean growth inhibition ability, respectively (Fig. 5a). The antifungal activity of G. candidum was found to be significantly different in comparison to the control (the absence of G. candidum) when grown in fructose, glucose, xylose, and sucrose.
Optimization of carbon substrate for the G. candidum PF005 to enhance the bioactivity of VOCs. a Percentage of mycelial growth inhibition of R. solani exposed to VOCs synthesized from PF005 grown on different carbon sources as compared with the control without PF005. Values are represented as means of triplicates (±SD), and a different number of asterisk indicate values that differ significantly from the control (without PF005). Significant differences between the control and the test measurement were branded as ***P < 0.001 and **P < 0.01. b Percentage of mycelial growth inhibition of R. solani exposed to VOCs from PF005 grown on different concentrations of glucose. Values or data points (empty circles) are represented as means of three replicates (±SD)
Potato agar media supplemented with different concentrations of glucose (0.5, 1.0, 2.0, 4.0, and 6.0%) were tested to find out the optimum glucose concentration for G. candidum’s maximum antifungal activity. Enhancement of mycelial growth inhibition of R. solani was observed with the increase in glucose concentration, and the highest mean growth inhibition activity of 81% was observed with 4% glucose, though its bioactivity somewhat decreased to 55% with 6% glucose (Fig. 5b).
Feeding G. candidum with Precursors of 2-Phenylethanol, 3-Methyl-1-Butanol, and Isopentyl Acetate Syntheses Improves Its Antifungal Activity
In order to examine the effect of precursor metabolite feeding on the production of antimicrobial VOCs, the growth medium of G. candidum was supplemented with leucine (Leu), α-ketoisocaproic acid (KIC), and phenylalanine (Phe) separately. The selected precursor feeding experiments changed the VOC profile of G. candidum. Leu and KIC feeding did not have any significant effect on 3-methyl-1-butanol synthesis. Interestingly, a few significant changes in the volatile profile were observed during the critical analysis of the volatile mixture. In both Leu- and KIC-fed G. candidum, syntheses of two important volatile compounds, i.e., ethyl isovalerate (ethyl 3-methylbutanoate) and isopentyl acetate, were boosted. Around three- and fivefold enhancement of ethyl isovalerate production was observed for 0.0025 and 0.01 mol l−1 Leu-fed samples, respectively (Fig. 6a). On the other hand, around threefold increment of isopentyl acetate synthesis was documented for both 0.0025 and 0.01 mol l−1 Leu-fed samples (Fig. 6a). Similarly, two- and threefold boost of ethyl isovalerate production was observed for 0.0025 and 0.01 mol l−1 KIC-fed samples, respectively (Fig. 6a), whereas isopentyl acetate synthesis was improved by two- and fivefold, respectively, with the corresponding concentration of KIC (Fig. 6a). Furthermore, synthesis of 2-phenylethanol was increased by ninefold in the presence of 0.0025 mol l−1 Phe (Fig. 6b). No further improvement of 2-phenylethanol production was observed with an additional amount of Phe; rather its synthesis was reduced to a certain extent.
Precursor feeding into the G. candidum PF005 culture to improve the antifungal activity of VOCs against R. solani. a Percentage of different volatile emissions after the feeding of α-ketoisocaproic acid and leucine. b Percentage of 2-phenylethanol emission after the feeding of phenylalanine. c–e Percentage of mycelial growth inhibition of R. solani exposed to VOCs from PF005 fed with different concentration of c leucine (Leu), d α-ketoisocaproic acid (KIC), and e phenylalanine (Phe). Values are represented as means of triplicates (±SD), and different numbers of asterisk indicate values that differ significantly from the PDB control. Significant differences between the control and the test measurement were branded as ***P < 0.001, **P < 0.01 and *P < 0.05
Additionally, it was found that all three precursors did influence G. candidum’s antifungal activity against the phytopathogen R. solani. Upon feeding of Leu into G. candidum cultures, the control (only PDB without Leu addition), 0.0025 mol l−1 Leu, and 0.01 mol l−1 Leu exhibited the mean growth inhibition ability of ~ 52, 85, and 91%, respectively (Fig. 6c). Moreover, the relative increase in growth inhibition due to Leu feeding was found to be 32% for 0.0025 mol l−1 and 38% for 0.01 mol l−1 more as compared with the PDB control (without any Leu addition). Both these bioactivity data were significantly different from the PDB control in Tukey’s test. However, further improvement of G. candidum’s antifungal activity became stagnant beyond 0.01 mol l−1 Leu (data not shown). During KIC feeding of G. candidum, the PDB control, 0.0025 mol l−1 KIC, and 0.01 mol l−1 KIC showed the mean growth inhibition ability of ~ 45, 54, and 72%, respectively (Fig. 6d). In addition, the relative increase in R. solani’s growth inhibition due to KIC feeding was found to be 9% for 0.0025 mol l−1 and 27% for 0.01 mol l−1 more in comparison with the PDB control (without any KIC addition). These two results had significantly different bioactivities from the PDB control in Tukey’s tests. Similarly, after Phe feeding of G. candidum, the control, 0.0025 mol l−1 Phe, and 0.01 mol l−1 Phe displayed the mean growth inhibition of ~ 52, 82, and 82%, respectively (Fig. 6e). The relative increase in R. solani’s growth inhibition due to Phe feeding was found to be almost same, i.e., 30% for both 0.0025 and 0.01 mol l−1 as compared with the PDB control (without any Phe addition). Both these bioactivity data were found to be significantly different from the PDB control.
Synergistic Effect with Naphthalene Improves Antifungal Activity of G. candidum
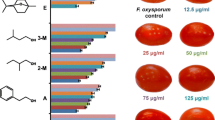
Naphthalene was used in combination with the VOCs of G. candidum to evaluate the synergistic effect on the enhancement of the total antifungal activity against R. solani. From the optimization study, 100 mg naphthalene/plate was found to be inhibiting the mycelial growth of R. solani by ~ 90% and also G. candidum to a little extent. It was also observed that the R. solani’s growth was affected by 10 mg naphthalene/plate. Thus, the upper limit of naphthalene to be assessed was limited to less than 10 mg/plate. After normalization, the growth inhibition of R. solani due to a synergistic effect was observed to be ~ 50, 80, 92, 80, and 23% as compared with their corresponding controls (only naphthalene but without G. candidum) for 0, 0.5, 1.0, 2.0, and 5.0 mg naphthalene/plate, respectively (Fig. 7a). All of these results were found to be significantly different from the test control (without naphthalene but with G. candidum). One representative picture of R. solani’s maximum growth inhibition by VOCs of G. candidum PF005 as a synergistan with 1 mg naphthalene/plate is shown (Fig. 7c). With a further increase of naphthalene concentration, the effective antifungal activity of VOCs produced by G. candidum was decreased, probably indicating that the effect of naphthalene is quite complex in the improvement of total antifungal activity.
Effect of exogenously supplied naphthalene on the antifungal activity of G. candidum PF005’s VOCs against R. solani. a Percentage of mycelial growth inhibition (as compared to their respective controls with corresponding concentrations of naphthalene, and in the absence of PF005) of R. solani exposed to VOCs from PF005 with different quantities of naphthalene per plate. Values are represented as means of triplicates (±SD), and a different number of asterisk indicates values that differ significantly from the PDB control. Significant differences between the control and the test measurement were branded as ***P < 0.001, **P < 0.01 and *P < 0.05. b, c Mycelial growth of R. solani on PDA-containing half-moon plate in the presence of b 1.0 mg naphthalene/plate after 96 h and c both PF005 and 1.0 mg naphthalene/plate after 96 h
Discussion
A yeast-like fungal strain designated as PF005 has been isolated from the fruit of eggplant (S. melongena) as an endophyte. After molecular, morphological, and phenotypic characterization, the PF005 showed a maximum similarity with G. candidum. De Hoog and Smith [25] earlier reported that the G. candidum is unable to utilize sucrose as a carbon source; on the contrary, our strain is capable of using sucrose (Table 1). Therefore, this endophytic yeast-like fungal isolate PF005 (Fig. 1) might be a different subspecies of G. candidum, which belongs to Ascomycota phylum, and their asci are usually produced on undifferentiated hyphae without conspicuous septation, or on the same hypha [25]. For food or feed safety assessment, G. candidum should be differentiated from other pathogenic microorganisms present in the processed food or feed. Although it has been reported to be pathogenic to tomato [26], G. candidum can naturally be found in cheese and fermented milk. Scientists opined that the G. candidum should be proposed for qualified presumption of safety (QPS) status by the European Food Safety Authority [24].
The endophytic isolate G. candidum studied here produces fragrant volatiles. The VOCs emitted from this isolate have a fruity aroma, which consist of terpenoids, alcohols, esters, and hydrocarbons (Table 2). Naphthalene, isopentyl acetate, and 2-phenylethanol have been reported to be antifungal agents [27,28,29]. On the other hand, 3-methyl-1-butanol, tridecane, tetradecane, pentadecane, nonanal, and benzyl acetates are the components of VOCs reported for their antimicrobial property [13, 30,31,32]. The three most abundant VOCs found in G. candidum PF005 are 2-phenylethanol, isopentyl acetate, and 3-methyl-1-butanol. The volatile profile of PF005 exhibits more diverse compounds with a wider spectrum of variations as compared to other strains of G. candidum already reported [33,34,35]. Although there are reports on the presence of naphthalene and its derivatives in VOCs from other endophytic fungi [12], the occurrence of naphthalene in G. candidum’s VOCs has been detected for the first time in our strain PF005. A previous study showed that the alcohols and esters produced in microorganisms are mainly synthesized through amino acid metabolic pathway [36] as depicted in Fig. 8. These amino acids are also involved in the biosynthesis of a large number of fragrant VOCs, including carbonyls, alcohols, esters, and acids [38, 39].
Possible routes of the formation of aroma volatiles via degradation of amino acid(s) in microorganisms. Primary metabolites (amino acids) serve as the precursors for the production of several aroma volatiles (especially esters). The major pathway enzymes catalyzing the reactions are also indicated here. Adapted from El Hadi et al. [37]
Yeasts are known to produce 2-phenylethanol and 3-methyl-1-butanol in their exponential phase of growth [40, 41]. Similarly, we have observed a significant amount of 2-phenylethanol and 3-methyl-1-butanol during the exponential growth phase of G. candidum (Fig. 3). Strobel and his group reported the presence of a few similar compounds like 2-phenylethanol, 3-methyl-1-butanol, and isopentyl acetate in Muscodor albus [11]. Afterward, Ezra and his group detected naphthalene along with other components in different strains of M. albus [12]. The Oidium sp. also synthesizes a few similar molecules like ethyl-3-methyl butanoate, ethyl-3-methyl-2-butenoate, isobutyl-3-methyl butanoate, isopentyl-3-methyl butanoate, and 2-phenylethanol [14].
The G. candidum’s fragrant VOCs have a significant amount of growth inhibitory activity against the phytopathogen R. solani along with some level of antimicrobial activity against F. oxysporum, Pseudocercospora sp., and Cercospora sp. (Fig. 4). Elkahoui and his group reported similar mycelial growth inhibition of the R. solani fungus by VOCs from the endophytic bacteria Pseudomonas sp. P2 strain and observed thickening of the cell wall, vesiculation of protoplasm, and blockage of fungal hypha branching [42]. Tariq and Campbell also documented the hyphal growth inhibition of a few other fungi by volatiles from the non-endophytic G. candidum [43].
The capacity to assimilate and ferment different carbon sources by fungal species has a significant impact on their VOC profiles [44]. Pinotti and his group reported the change in fruity smell from VOCs of non-endophytic G. candidum with a change in culture media [35]. In our study, maximum mycelial growth inhibition of R. solani was exhibited by G. candidum fed with 4% glucose as a substrate among the seven carbon sources tested (Fig. 5). The results suggest the lack of capacity to optimally assimilate maltose and starch as a carbon source by G. candidum, which is reflected on the reduced mycelial growth inhibition of R. solani. In a similar study, Fialho and his team reported glucose as one of the best carbon sources for antifungal VOC production by non-endophyte S. cerevisiae [10]. Interestingly, we have observed a somewhat decrease in the antifungal activity of G. candidum with the higher glucose concentration. The growth reduction of G. candidum due to osmotic stress at high glucose concentration followed by a lesser titer of VOCs might be the reason behind this drop of bioactivity. Although maximum antifungal activity by S. cerevisiae was noticed at around 4% glucose concentration, the authors did not report the effect of much higher concentrations of glucose [10].
In order to boost up the production of major volatiles of G. candidum, we selected 2-phenylethanol and 3-methylbutanol biosynthesis pathways for our precursor feeding experiments. These pathways are well characterized in bacteria and yeasts [36, 45]. The pathway starts with the formation of the corresponding α-ketoisocaproic acid by the deamination or transamination of an amino acid (Fig. 8). Successive decarboxylations of the α-ketoisocaproic acid followed by reductions, oxidations, and/or esterifications lead to the formation of aldehydes, acids, alcohols, and esters, respectively [37, 46]. In fruits, such as banana, strawberry, tomato, and apple, precursors of the branched-chain volatile aldehydes, alcohols, and esters are the branched-chain amino acids, namely leucine, isoleucine, and valine [47,48,49,50]. These amino acids can also serve as the precursors of acyl-coenzyme A (CoA), which are used in alcohol esterification reactions catalyzed by alcohol acetyl transferase (AAT) enzymes.
In general, fungi biosynthesize 3-methyl-1-butanol from either leucine or α-ketoisocaproic acid [40, 51], whereas 2-phenylethanol is produced from phenylalanine in the Ehrlich pathway [41]. Reported elsewhere, feeding of leucine (Leu) and its precursor α-ketoisocaproic acid (KIC) increases the production of 3-methyl-1-butanol and, simultaneously, of isopentyl acetate [51]. On the other hand, phenylalanine (Phe) feeding is expected to improve 2-phenylethanol profile [52]. In our study, we did not observe any significant change in 3-methyl-1-butanol synthesis in any feeding experiments. However, there was a notable improvement in the synthesis of isopentyl acetate and ethyl isovalerate upon both Leu and KIC feedings (Fig. 6). Isopentyl acetate is synthesized in a simple esterification reaction from isoamyl alcohol (3-methyl-1-butanol) and acetyl-coA by the AAT enzyme [53]. As reported earlier, the rate of ester production is dependent on three factors: the concentration of the two co-substrates (the acyl-CoA component and the alcohol), the activity of the enzymes involved in their synthesis, and subsequent hydrolysis [54]. Improvement in ester formation and the absence of significant change in 3-methyl-1-butanol production in the G. candidum PF005 strain could be explained by the presence of a highly active AAT system and weak ester hydrolysis mechanism. Thus, we were unable to detect any improvement in 3-methyl-1-butanol production in Leu and KIC precursor feedings. Furthermore, the highly active AAT system might also be the reason behind the enhanced synthesis of ethyl isovalerate (Fig. 8). The higher percentage of ester synthesis by G. candidum in the stationary phase of growth (Fig. 3b) is another supporting evidence for the presence of a highly active AAT system.
Based on the relative change in the antifungal activity against the phytopathogen R. solani, the maximum improvement of bioactivity by G. candidum was observed with 0.01 mol l−1 Leu feeding among the three precursor feeding tests (Fig. 6). Although in a completely different organism, Leu feeding did not have any significant effect to increase in 3-methylbutanol production by tomato, the KIC-fed tomato plant had a significantly large improvement in the 3-methylbutanol biosynthesis of about tenfold [51]. Leu and KIC feedings in G. candidum enhanced the production of isopentyl acetate, which is a well-documented antifungal agent [29]. Similarly, Phe feeding also significantly improved the synthesis of another antifungal agent 2-phenylethanol. Hence, we have observed a notable improvement in the antifungal activity of G. candidum against R. solani.
The individual component of total VOCs from a specific organism has no or less antimicrobial activity than the total volatile mixture [17]. The additive effect of individual constituents acts synergistically to enhance the total bioactivity. Naphthalene was found to be one of the components in VOCs of G. candidum from the analysis of GC-MS data (Table 2). Since it is very complicated to engineer a naphthalene biosynthesis pathway by metabolite or precursor feeding, naphthalene was directly added at a sublethal dose in the closed system of both the endophyte G. candidum and the phytopathogen R. solani. The maximum improvement of G. candidum’s antifungal activity was observed in the synergistic presence with 1.0 mg naphthalene/plate, whereas the sole presence of naphthalene (in the same dose) did not affect the R. solani’s growth. However, with a further increase in naphthalene concentration, the antifungal activity of G. candidum did not improve proportionally. We have noticed higher effectivity of crude mixture in a synergistic manner than their individual components. An analogous study has reported the enhancement of the total antifungal activity of VOCs from the Oidium sp. by the exogenous addition of isobutyric acid and naphthalene-1,1′-oxybis [14]. In the case of Gloeosporium sp., a few compounds like isoamyl acetate, allyl acetate, and isobutyric acid acted as a synergistan for the improvement of the antifungal activity of VOCs against test phytopathogen: Phytophthora palmivora, R. solani, Ceratocystis ulmi, Botrytis cinerea, and Verticillium dahlia [17].
In conclusion, we have successfully characterized the fruity scented antifungal VOCs produced by the endophyte G. candidum PF005 isolated from the S. melongena fruit and identified some of the components (like 2-penylethanol, isopentyl acetate, and naphthalene) as the contributing antifungal agents. The G. candidum PF005 strain is a potential candidate for metabolic engineering and scale-up processes for the production of sustainable mycofumigant. Owing to its biological origin, VOCs can be easily biodegraded than the chemically synthesized fumigants. The endophytic isolate PF005 has a pleasant fragrance, whereas most of the reported antimicrobial VOCs have an obnoxious smell. Further investigation is required to test the broad-spectrum antimicrobial activity of G. candidum against other microorganisms. Based upon the antifungal activity of the endophytic G. candidum and the qualified presumption of the safety status of G. candidum, we posit that the fruity scented VOCs of the newly isolated strain PF005 will be appropriate to control the phytopathogen R. solani of crop plants.
References
Strobel G, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 67(4):491–502. https://doi.org/10.1128/MMBR.67.4.491-502.2003
Smith SA, Tank DC, Boulanger LA, Bascom-Slack CA, Eisenman K, Kingery D, Babbs B, Fenn K, Greene JS, Hann BD, Keehner J, Kelley-Swift EG, Kembaiyan V, Lee SJ, Li P, Light DY, Lin EH, Ma C, Moore E, Schorn MA, Vekhter D, Nunez PV, Strobel GA, Donoghue MJ, Strobel SA (2008) Bioactive endophytes warrant intensified exploration and conservation. PLoS One 3(8):e3052. https://doi.org/10.1371/journal.pone.0003052
Riyaz-Ul-Hassan S, Strobel G, Geary B, Sears J (2013) An endophytic Nodulisporium sp. from Central America producing volatile organic compounds with both biological and fuel potential. J Microbiol Biotechnol 23(1):29–35
Strobel G (2006) Harnessing endophytes for industrial microbiology. Curr Opin Microbiol 9(3):240–244. https://doi.org/10.1016/j.mib.2006.04.001
Mercier J, Jiménez JI (2004) Control of fungal decay of apples and peaches by the biofumigant fungus Muscodor albus. Postharvest Biol Technol 31(1):1–8. https://doi.org/10.1016/j.postharvbio.2003.08.004
Bjurman J, Kristensson J (1992) Volatile production by Aspergillus versicolor as a possible cause of odor in houses affected by fungi. Mycopathologia 118(3):173–178. https://doi.org/10.1007/bf00437151
Vespermann A, Kai M, Piechulla B (2007) Rhizobacterial volatiles affect the growth of fungi and Arabidopsis thaliana. Appl Environ Microbiol 73(17):5639–5641. https://doi.org/10.1128/AEM.01078-07
Ulloa-Benitez A, Medina-Romero YM, Sanchez-Fernandez RE, Lappe-Oliveras P, Roque-Flores G, Duarte Lisci G, Herrera Suarez T, Macias-Rubalcava ML (2016) Phytotoxic and antimicrobial activity of volatile and semi-volatile organic compounds from the endophyte Hypoxylon anthochroum strain Blaci isolated from Bursera lancifolia (Burseraceae). J Appl Microbiol 121(2):380–400. https://doi.org/10.1111/jam.13174
Wheatley RE, Hackett CA, Bruce A, Kundzewicz A (1997) Effect of substrate composition on production and inhibitory activity against wood decay fungi of volatile organic compounds from Trichoderma spp. Int Biodeterior Biodegrad 39:199–205
Fialho MB, Toffano L, Pedroso MP, Augusto F, Pascholati SF (2010) Volatile organic compounds produced by Saccharomyces cerevisiae inhibit the in vitro development of Guignardia citricarpa, the causal agent of citrus black spot. World J Microbiol Biotechnol 26(5):925–932. https://doi.org/10.1007/s11274-009-0255-4
Strobel GA, Dirkse E, Sears J, Markworth C (2001) Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology 147(11):2943–2950. https://doi.org/10.1099/00221287-147-11-2943
Ezra D, Hess WM, Strobel GA (2004) New endophytic isolates of Muscodor albus, a volatile-antibiotic-producing fungus. Microbiology 150(Pt 12):4023–4031. https://doi.org/10.1099/mic.0.27334-0
Mitchell AM, Strobel GA, Moore E, Robison R, Sears J (2010) Volatile antimicrobials from Muscodor crispans, a novel endophytic fungus. Microbiology 156(Pt 1):270–277. https://doi.org/10.1099/mic.0.032540-0
Strobel GA, Spang S, Kluck K, Hess WM, Sears J, Livinghouse T (2008) Synergism among volatile organic compounds resulting in increased antibiosis in Oidium sp. FEMS Microbiol Lett 283(2):140–145. https://doi.org/10.1111/j.1574-6968.2008.01137.x
Strobel G, Singh SK, Riyaz-Ul-Hassan S, Mitchell AM, Geary B, Sears J (2011) An endophytic/pathogenic Phoma sp. from creosote bush producing biologically active volatile compounds having fuel potential. FEMS Microbiol Lett 320(2):87–94. https://doi.org/10.1111/j.1574-6968.2011.02297.x
Mends MT, Yu E, Strobel GA, Riyaz-Ul-Hassan S, Booth E, Geary B, Sears J, Taatjes CA, Hadi MZ (2012) An endophytic Nodulisporium sp. producing volatile organic compounds having bioactivity and fuel potential. J Pet Environ Biotechnol 3(3):16. https://doi.org/10.4172/2157-7463.1000117
Schaible GA, Strobel GA, Mends MT, Geary B, Sears J (2015) Characterization of an endophytic Gloeosporium sp. and its novel bioactivity with “synergistans”. Microb Ecol 70(1):41–50. https://doi.org/10.1007/s00248-014-0542-y
Doyle JJ, Doyle JL (1990) CTAB total DNA isolation. Focus 12:13–15
Olesen P, Kier U (1984) Optimal preparation of mycelia and arthrospores of Geotrichum candidum: a SEM, TEM and cytochemical study. Nord J Bot 4(3):365–374. https://doi.org/10.1111/j.1756-1051.1984.tb01510.x
Bera P, Kotamreddy JN, Samanta T, Maiti S, Mitra A (2015) Inter-specific variation in headspace scent volatiles composition of four commercially cultivated jasmine flowers. Nat Prod Res 29(14):1328–1335. https://doi.org/10.1080/14786419.2014.1000319
Sunesson A, Vaes W, Nilsson C, Blomquist G, Andersson B, Carlson R (1995) Identification of volatile metabolites from five fungal species cultivated on two media. Appl Environ Microbiol 61(8):2911–2918
Börjesson T, Stöllman U, Schnürer J (1992) Volatile metabolites produced by six fungal species compared with other indicators of fungal growth on cereal grains. Appl Environ Microbiol 58(8):2599–2605
Karahadian C, Josephson DB, Lindsay RC (1985) Volatile compounds from Penicillium sp. contributing musty-earthy notes to Brie and Camembert cheese flavors. J Agric Food Chem 33(3):339–343. https://doi.org/10.1021/jf00063a005
Pottier I, Gente S, Vernoux JP, Gueguen M (2008) Safety assessment of dairy microorganisms: Geotrichum candidum. Int J Food Microbiol 126(3):327–332. https://doi.org/10.1016/j.ijfoodmicro.2007.08.021
De Hoog GS, Smith MT (2004) Ribosomal gene phylogeny and species delimitation in Geotrichum and its teleomorphs. Stud Mycol 50:489–515
Thornton CR, Slaughter DC, Davis RM (2010) Detection of the sour-rot pathogen Geotrichum candidum in tomato fruit and juice by using a highly specific monoclonal antibody-based ELISA. Int J Food Microbiol. 143(3):166–172. https://doi.org/10.1016/j.ijfoodmicro.2010.08.012
Bhansali SG, Shah U, Kulkarni VM, Li L (2013) Synthesis and antifungal activity of terbinafine analogues. Int J ChemTech Res 5(5):1224–1232
Liu P, Cheng Y, Yang M, Liu Y, Chen K, Long CA, Deng X (2014) Mechanisms of action for 2-phenylethanol isolated from Kloeckera apiculata in control of Penicillium molds of citrus fruits. BMC Microbiol 14:242. https://doi.org/10.1186/s12866-014-0242-2
Ando H, Kurata A, Kishimoto N (2015) Antimicrobial properties and mechanism of volatile isoamyl acetate, a main flavour component of Japanese sake (Ginjo-shu). J Appl Microbiol 118(4):873–880. https://doi.org/10.1111/jam.12764
Aboaba SA, Choudhary IM (2015) Chemical composition and biological activities of the volatile oils of Palisota hirsuta (Thunb) K. Schum and Trema orientalis (L) Blume. Int J Chem 7(2):21–26. https://doi.org/10.5539/ijc.v7n221-26
Bruno F, Castelli G, Migliazzo A, Piazza M, Galante A, Lo Verde V, Calderone S, Nucatolo G, Vitale F (2015) Cytotoxic screening and in vitro evaluation of pentadecane against Leishmania infantum promastigotes and amastigotes. J Parasitol 101(6):701–705. https://doi.org/10.1645/15-736
Ozdemir G, Karabay NU, Dalay MC, Pazarbasi B (2004) Antibacterial activity of volatile component and various extracts of Spirulina platensis. Phytother Res 18(9):754–757. https://doi.org/10.1002/ptr.1541
Mo EK, Kang HJ, Lee CT, BJ X, Kim JH, Wang QJ, Kim JC, Sung CK (2003) Identification of phenylethyl alcohol and other volatile flavor compounds from yeasts, Pichia farinosa SKM-1, Pichia anomala SKM-T, and Galactomyces geotrichum SJM-59. J Microbiol Biotechnol 13(5):800–808
Mdaini N, Gargouri M, Hammami M, Monser L, Hamdi M (2006) Production of natural fruity aroma by Geotrichum candidum. Appl BiochemBiotechnol 128(3):227–235
Pinotti T, Carvalho PMB, Garcia KMG, Silva TR, Hagler AN, Leite SGF (2006) Media components and amino acid supplements influencing the production of fruity aroma by Geotrichum candidum. Braz J Microbiol 37:494–498
Tavaria FK, Dahl S, Carballo FJ, Malcata FX (2002) Amino acid catabolism and generation of volatiles by lactic acid bacteria. J Dairy Sci 85(10):2462–2470. https://doi.org/10.3168/jds.S0022-0302(02)74328-2
El Hadi M, Zhang F, Wu F, Zhou C, Tao J (2013) Advances in fruit aroma volatile research. Molecules 18(7):8200–8229. https://doi.org/10.3390/molecules18078200
Sanz C, Olias JM, Perez AG (1997) Aroma biochemistry of fruits and vegetables. In: Tomás-Barberán FA, Robins RJ (eds) Phytochemistry of fruits and vegetables. Oxford University Press, New York, pp. 125–155
Baldwin IT, Kessler A, Halitschke R (2002) Volatile signaling in plant-plant-herbivore interactions: what is real? Curr Opin Plant Biol 5(4):351–354
Bigelis R, Weir PD, Jones RR, Umbarger HE (1983) Exogenous valine reduces conversion of leucine to 3-methyl-1-butanol in Saccharomyces cerevisiae. Appl Environ Microbiol 45(2):658–664
Etschmann MM, Bluemke W, Sell D, Schrader J (2002) Biotechnological production of 2-phenylethanol. Appl Microbiol Biotechnol 59(1):1–8. https://doi.org/10.1007/s00253-002-0992-x
Elkahoui S, Djebali N, Yaich N, Azaiez S, Hammami M, Essid R, Limam F (2015) Antifungal activity of volatile compounds-producing Pseudomonas P2 strain against Rhizoctonia solani. World J Microbiol Biotechnol 31(1):175–185. https://doi.org/10.1007/s11274-014-1772-3
Tariq VN, Campbell VM (1991) Influence of volatile metabolites from Geotrichum candidum on other fungi. Myco Res 95(7):891–893. https://doi.org/10.1016/S0953-7562(09)80058-0
Nout MJR, Bartelt RJ (1998) Attraction of a flying nitidulid (Carpophilus humeralis) to volatiles produced by yeasts grown on sweet corn and a corn-based medium. J Chem Ecol 24(7):1217–1239. https://doi.org/10.1023/a:1022451020013
Beck HC, Hansen AM, Lauritsen FR (2002) Metabolite production and kinetics of branched-chain aldehyde oxidation in Staphylococcus xylosus. Enzym Microb Technol 31(1–2):94–101. https://doi.org/10.1016/S0141-0229(02)00067-4
Reineccius G (2006) Flavor chemistry and technology. CRC Press, Boca Raton,
Goff SA, Klee HJ (2006) Plant volatile compounds: sensory cues for health and nutritional value? Science (New York, NY) 311(5762):815–819. https://doi.org/10.1126/science.1112614
Perez AG, Olias R, Luaces P, Sanz C (2002) Biosynthesis of strawberry aroma compounds through amino acid metabolism. J Agric Food Chem 50(14):4037–4042
Rowan DD, Lane HP, Allen JM, Fielder S, Hunt MB (1996) Biosynthesis of 2-methylbutyl, 2-methyl-2-butenyl, and 2-methylbutanoate esters in red delicious and Granny Smith apples using deuterium-labeled substrates. J Agric Food Chem 44:3276–3285
Wyllie SG, Fellman JK (2000) Formation of volatile branched chain esters in bananas (Musa sapientum L.). J Agric Food Chem 48(8):3493–3496
Kochevenko A, Araújo WL, Maloney GS, Tieman DM, Do PT, Taylor MG, Klee HJ, Fernie AR (2012) Catabolism of branched chain amino acids supports respiration but not volatile synthesis in tomato fruits. Mol Plant 5(2):366–375. https://doi.org/10.1093/mp/ssr108
Tieman D, Taylor M, Schauer N, Fernie AR, Hanson AD, Klee HJ (2006) Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2-phenylethanol and 2-phenylacetaldehyde. Proc Natl Acad Sci U S A 103(21):8287–8292. https://doi.org/10.1073/pnas.0602469103
Fukuda K, Yamamoto N, Kiyokawa Y, Yanagiuchi T, Wakai Y, Kitamoto K, Inoue Y, Kimura A (1998) Balance of activities of alcohol acetyltransferase and esterase in Saccharomyces cerevisiae is important for production of isoamyl acetate. Appl Environ Microbiol 64(10):4076–4078
Saerens SM, Delvaux FR, Verstrepen KJ, Thevelein JM (2010) Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb Biotechnol 3(2):165–177. https://doi.org/10.1111/j.1751-7915.2009.00106.x
Acknowledgements
We are thankful to Dr. Prabuddha Dey and Mr. Nitai Giri for helping in the isolation of endophytic fungi. Ralstonia solanacearum, Xanthomonas campestris, Fusarium oxysporum, and Rhizoctonia solani were generously provided to us by Prof. B. N. Panja (Bidhan Chandra Krishi Viswavidyalaya, Mohanpur, India). The authors wish to acknowledge Ms. Mohor Mitra for critical reading of the manuscript. AM thanks MHRD, Government of India, for providing the fellowship. Grant supports from the SGIRG-SRIC (IIT/SRIC/BT/GRT/2014) and Food Security Project (F. No. 4-25/2013-TS-1) of IIT Kharagpur for developing the research infrastructure are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mookherjee, A., Bera, P., Mitra, A. et al. Characterization and Synergistic Effect of Antifungal Volatile Organic Compounds Emitted by the Geotrichum candidum PF005, an Endophytic Fungus from the Eggplant. Microb Ecol 75, 647–661 (2018). https://doi.org/10.1007/s00248-017-1065-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-017-1065-0