Abstract
Rivers are known to be major contributors to eutrophication in marine coastal waters, but little is known on the short-term impact of freshwater surges on the structure and functioning of the marine plankton community. The effect of adding river water, reducing the salinity by 15 and 30%, on an autumn plankton community in a Mediterranean coastal lagoon (Thau Lagoon, France) was determined during a 6-day mesocosm experiment. Adding river water brought not only nutrients but also chlorophyceans that did not survive in the brackish mesocosm waters. The addition of water led to initial increases (days 1–2) in bacterial production as well as increases in the abundances of bacterioplankton and picoeukaryotes. After day 3, the increases were more significant for diatoms and dinoflagellates that were already present in the Thau Lagoon water (mainly Pseudo-nitzschia spp. group delicatissima and Prorocentrum triestinum) and other larger organisms (tintinnids, rotifers). At the same time, the abundances of bacterioplankton, cyanobacteria, and picoeukaryote fell, some nutrients (NH4 +, SiO4 3−) returned to pre-input levels, and the plankton structure moved from a trophic food web based on secondary production to the accumulation of primary producers in the mesocosms with added river water. Our results also show that, after freshwater inputs, there is rapid emergence of plankton species that are potentially harmful to living organisms. This suggests that flash flood events may lead to sanitary issues, other than pathogens, in exploited marine areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Streams and rivers provide the main inputs of anthropogenic pollutants into estuarine and coastal marine environments. They are the main contributors of nitrogen and phosphorus to coastal ecosystems with potentially serious consequences because they influence the N and P limitation of algal production found in many estuarine and marine systems [1]. In the Mediterranean climate, flash floods have a rapid, intense effect on coastal productivity in restricted exchange areas such as lagoons [2, 3] and in open areas such as bays [4, 5]. Floodwaters bring freshwater that is highly enriched with nutrients, particles, organic and metal contaminants, and microbial components into marine waters within a short period of time (days or a few weeks; [2, 6]). The main consequence for marine waters of this sudden increased supply of nutrients is a marked increase in phytoplankton biomass and production within days after the flood surge [2, 4]. Both the occurrence of potentially harmful phytoplankton species [2] and high concentrations of nitrogen [7] have been reported in coastal Mediterranean waters after a flood event. However, owing to the unpredictability of such rapid, episodic events, little research has been carried out to determine the consequences of these flash floods on lagoon water. The mixing of freshwater and marine microbial communities may result in a more diverse, active community similar to communities observed along a permanent salinity gradient in estuaries [8]. The highest levels of phytoplankton and bacterial biomass and production have often been observed in more saline waters (salinity 25–35) along a salinity gradient, where there is a balance between salinity tolerance and nutrient availability [2, 9, 10]. Moreover, mixing zones may allow the emergence of new assemblages that favor rare species in the source populations [11] or the coexistence of plankton from different sources [12].
The short-term (over days or weeks) effect of an autumn Mediterranean flash flood on the abundance, biomass, diversity, and production of the coastal microbial food web has already been studied along a salinity gradient in the Thau Lagoon (France). The substantial loading of nutrients in the river encouraged the development of marine diatoms [3]. However, this previous experiment also showed that the large inputs of river organic matter did not appear to cause an immediate increase in marine heterotrophic bacterial production [2]. This suggests that bacteria and phytoplankton respond to flood events over different timescales. The consequences for the higher trophic levels remain unclear. Although flash floods cause changes in plankton diversity by importing freshwater organisms such as heterotrophic bacteria and ciliates into the lagoon, they also lead to a reduction in the number of more sensitive lagoon species, such as tintinnids. This may result in a predator/prey mismatch [3] when the timing between the occurrence of predators and their prey is not synchronized [13].
An experimental in situ mesocosm approach was used to study the direct effects of different volumes of river discharge on the responses of bacteria, phytoplankton and predator diversity, biomass, and production of the Thau Lagoon plankton community as well as the timescales of these responses. Experimental mesocosms were used to exclude rapidly shifting hydrodynamic effects and assess the short-term (over hours) and medium-term (over days) effects of the addition of different volumes of river water to 2 m3 mesocosms containing water from the Thau Lagoon, resulting in moderate reductions in salinity (down to 30 and 25). Our results highlight the rapid response (1–2 days) of the whole microbial food web after the river water addition, followed by the growth of potentially harmful diatoms and dinoflagellates, reaching a very high abundance of up to 107 cells L−1.
Materials and Method
Experiment Design
A mesocosm experiment was performed in autumn 2009 at the MEDiterranean platform for Marine Ecosystem Experimental Research (MEDIMEER, http://www.medimeer.univ-montp2.fr/) located on the shores of the Thau Lagoon in the south of France (43° 24′ 49″ N, 3° 41′ 19″ E). Water from the Véne River (the main tributary of the lagoon) was collected on September 28 using a pump and screened (1 mm) to remove very large living and non-living particles. The water was then stored in a 3000-L cooling tank (4 °C) stirred continuously using a submersible pump (25 L min−1) to homogenize and preserve the river water for the 2-day period required to set up the mesocosm experiment. The temperature of the river water was 15 °C when collected and was 13 °C just before it was added to the mesocosms. We assumed that this 2 °C change did not modify the river community and chemistry during the 2-day storage period.
Water was pumped from the subsurface (1 m) of Thau Lagoon, close to the MEDIMEER Pontoon, on September 30 into six moored mesocosms before nightfall. The experiment started the following morning (October 1) in duplicate with two mesocosms having 30% v/v freshwater (1400 L lagoon water and 600 L river water, HIGH treatment), two mesocosms having 20% v/v freshwater (1600 L lagoon water and 400 L river water, LOW treatment), and two mesocosms having no added freshwater (2000 L lagoon water only, CONT treatment). The volume of water added to each mesocosm was measured using a water meter mounted on the hose used for filling the mesocosms with water from the Thau lagoon and with river water successively. The water column in each mesocosm was mixed continuously using a pump (Tunze) with a flow rate of 30 L min−1.
Samples of the river water were taken just before the mesocosms were topped up (October 1) to measure the concentrations of dissolved and particulate matter, plankton abundance (except mesozooplankton), chlorophyll a concentration, and bacterial production. In each mesocosm, the salinity, temperature, and pH were monitored daily using a multiparameter probe (multi350i, WTW) between October 1 (day 0) and October 7 (day 6). After the addition of the river water, samples from each mesocosm were taken daily using the pump to fill a 20-L carboy to determine the nutrient concentrations and dissolved and particulate organic carbon and nitrogen, chlorophyll a, and bacterio- and phytoplankton abundance and production. Several samples were collected every 4 h during the first 36 h of the experiment to study (i) nitrate, phosphate, and silicate concentrations; (ii) abundance of heterotrophic bacterioplankton, cyanobacteria, and picoeukaryote, and (iii) bacterial production. Samples for estimating the microplankton abundance and for identification were collected on days 0, 2, 4, and 6, and samples for mesozooplankton quantification and identification were collected on days 0 and 6.
Concentrations of Nutrients, Dissolved Organic Carbon, and Nitrogen
Samples (80 mL) were collected in triplicate. Ammonium (NH4) concentrations were measured in unfiltered samples immediately after sampling (50 mL) using a spectrophotometer (Hitachi U-3000) and the indophenol blue method [14]. Samples for the concentrations of dissolved nitrate (NO3 and NO2), nitrite, soluble reactive phosphate (PO4), and silicic acid (Si(OH)4) were filtered onto 0.2-μm nuclepore filters and stored frozen (−20 °C) until analysis using an automated colorimeter (Skalar Autoanalyzer) using standard analysis methods [15]. Samples for dissolved organic carbon (DOC) analyses were preserved with phosphoric acid, filtered using precombusted Whatman GF/F filters, and analyzed using a Shimadzu TOC VCPH as described in Fouilland et al. [2]. The concentration of dissolved organic nitrogen (DON) was measured using the wet oxidation procedure described by Raimbault et al. [16].
Concentrations of Chlorophyll A, Particulate Organic Carbon, and Nitrogen
Samples for measuring the chlorophyll a (chl a) concentrations were filtered (0.1 to 1 L) onto glass fiber filters (Whatman GF/F, 25 mm, 0.7 μm nominal pore size), frozen in liquid nitrogen, and then stored at −80 °C until analysis. Chl a was measured by HPLC following the method described by Zapata et al. [17] with some modifications for the HPLC system used [18].
The concentrations of particulate organic carbon and nitrogen were measured using the samples for the assessment of primary production (see section below). After 4 h incubation with a 13C tracer (sodium bicarbonate-13C at a final concentration of 100 μmol L−1), the samples (0.5 L) were filtered onto precombusted Whatman GF/F filters and stored at −80 °C until analysis. The filters were dried at 60 °C for 24 h, pelleted, and analyzed for particulate organic carbon (POC) and nitrogen (PON) using an ANCA mass spectrometer (Europa Scientific).
Abundance of Prokaryotes and Picoeukaryotes
To determine the prokaryote and picoeukaryote abundance, samples (1.6 mL) were fixed with prefiltered (0.02 μm) buffered formaldehyde (2% final concentration) and stored at −80 °C until analysis. The samples were thawed, and the bacterioplankton, cyanobacteria, and picoeukaryote abundances were determined using a FACSCalibur flow cytometer (Becton Dickinson) as described by Pecqueur et al. [3]. The abundances of cyanobacteria and picoeukaryotes were converted into carbon biomass using a conversion factor of 100 and 3000 fg C cell−1, respectively [19]. The bacterial carbon biomass was estimated from the bacterioplankton abundance using a conversion factor of 20 fg C cell−1 [20].
Abundance, Biomass, and Diversity of Nanoplankton, Microplankton, and Mesozooplankton
Phytoplankton cells were enumerated using 125 mL samples fixed with 2% Lugol’s solution. Quantifications were performed using the Utermöhl sedimentation method [35] using an inverted microscope (Nikon Eclipse TS100). Units (cells or colonies) were counted and sized in random fields [21] under ×20 magnification until at least 100 units of the dominant species had been enumerated [22]. The biovolume was calculated for each species according to Hillebrand et al. [23] and Sun and Liu [24] and converted into biomass carbon using a conversion factor of 220 fg C μm3 [25].
Heterotrophic flagellates (HF) were enumerated using an epifluorescent microscope while naked ciliates and tintinnids were identified, quantified, and sized using an inverted microscope as described by Pecqueur et al. [3]. Heterotrophic flagellates were classified into two size classes of <3 and 3–5 μm. To calculate the biovolume, each cell was considered as a sphere with a median diameter of 1.5 and 4 μm for each of the two size classes and was then converted to biomass carbon using a conversion factor of 0.22 pg C μm3 [26]. The biovolume of naked ciliates and tintinnids was estimated considering their cells as spheres and then converted to biomass carbon using a conversion factor of 0.19 pg C μm3 [27].
For mesozooplankton abundance and identification, samples (60 L) were collected using three 20-L polycarbonate containers. The water was then filtered through a 60-μm screen to collect and concentrate the zooplankton, which were then stored in neutralized formalin (4% final concentration). Metazooplankton taxa were identified as described by Rose [31] and Razouls et al. [28] and enumerated using a Leica MZ6 dissecting microscope. The mesozooplankton biomass carbon was determined from the biovolumes using the Alcaraz et al. [29] relationship for the northwestern Mediterranean.
Heterotrophic Bacterial Production and Primary Production
The net bacterial production (BP) was estimated from the DNA synthesis rates measured by [3H-methyl] thymidine (3H-TdR) incorporation as described by Bouvy et al. [30]. The BP was calculated using a conversion factor of 2 × 1018 cells mol−1 TdR [31] and 20 fg C cell−1 [20]. The net carbon uptake rates were determined using a 13C stable isotope tracer (sodium bicarbonate-13C at a final concentration of 100 μmol L−1) as described in Fouilland et al. [2]. This method was used to assess the net particulate primary production (PPp): the net uptake of CO2 and its accumulation in the particulate matter after 4 h incubation of samples around midday under mesocosm in situ natural light and temperature conditions. The PPp was also expressed as the daily rate by multiplying the hourly rate by 10, assuming 10 h of effective daylight at that time of year.
Index of Dissimilarity Calculated from the Plankton Species Composition
The index of dissimilarity is used to compare the degree of dissimilarity of the plankton community structure between the treatments (adapted from Whittaker’s index of association [32]). This index of dissimilarity was used because it is responsive to the less abundant species or groups, which is particularly relevant when addressing diversity at community level. The species abundance weighted by the biovolume was used rather than the species abundance alone [32]) in order to take into account the difference in cell size between the plankton species or groups. For this experiment, the index of dissimilarity was calculated between two different conditions on day 0 and day 6 (i.e., River vs CONT, River vs LOW, River vs HIGH, CONT vs LOW, CONT vs HIGH, LOW vs HIGH) and between 2 days (i.e., day 0 vs day 6). The index was calculated for the various species of microphytoplankton and zooplankton identified using a microscope and enumerated in all treatments at the beginning (day 0) and end of the experiment (day 7). All the biovolumes (the species abundance multiplied by the cell biovolume) were then converted into a percentage of total plankton biovolume (microphytoplankton + zooplankton) to normalize the data for comparison between days and treatments. The index of dissimilarity D was calculated by summing the differences between each species of a pair of corresponding values and dividing by 2 to give an index between 0 (similar) and 100 (dissimilar), according to the following equation:
with
ai the biovolume of the “i” species under the “a” condition, A: the total plankton biovolume under the “a” condition
bi the biovolume of the “i” species under the “b” condition, B: the total plankton biovolume under the “b” condition
Statistical Tests
Repeated measure multivariate analysis of variance (MANOVA, Systatv.11.0, SPSS) was used to test for significant differences in the various chemical and biological variables between the CONT, LOW, and HIGH treatments. In this statistical method, the repeated measures of a given variable are considered as dependent variables [33]. When no significant difference between LOW and HIGH treatments was observed with repeated measure MANOVA, a one-way analysis of variance was performed on the variables for each sampling day followed by an a posteriori Fisher’s least significant difference (LSD) test [34]. This test was used to identify means that were significantly different between the LOW and HIGH treatments. The significance threshold was set at p < 0.05.
Results
Initial Characteristics of the Water from the River and Thau Lagoon
The temperature of the water collected from the Vène River was 15 °C and decreased to 13 °C during storage in a cooling tank before being added to the mesocosms. The concentrations of PO4 3−, NH4 +, and NO3 − (0.8–3.7 mg L−1) in the river water were two or three orders of magnitude greater than those measured in Thau Lagoon (0.003–0.02 mg L−1) during the study period (Table 1). The abundances of bacterioplankton and picoeucaryotes (50 × 106 and 0.5 × 106 cells mL−1, respectively) in the river water just before being added to the mesocosms were one order of magnitude greater (ANOVA and a posteriori Fisher’s LSD test, p < 0.001) than those sampled from the Thau Lagoon at the beginning of the mesocosm experiment (7 × 106 and 0.02 × 106 cells mL−1, respectively). The chlorophyll a concentrations (7 μg chl a L−1) and bacterial production (14 μg C L−1 h−1) were also much higher (ANOVA and a posteriori Fisher’s LSD test, p = 0.005 and 0.001, respectively) in the river water than in the lagoon water at the beginning of the experiment (2 μg chl a L−1 and 1.8 μg C L−1 h−1, respectively). Of the two species of ciliates observed in the river water, Coleps sp. was dominant and the phytoplankton community was largely dominated by freshwater chlorophyceans of the order of Chlamydomonadales.
Effect of Adding River Water on Nutrient Concentrations
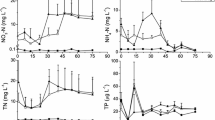
The addition of 20% v/v of river water to the LOW treatment reduced the salinity from 36 to 29, and the addition of 30% v/v to the HIGH treatment reduced the salinity to 26 (Table S1). Adding river water immediately reduced the temperature (by 0.7 °C in the HIGH treatment) and pH (by 0.1 in the HIGH treatment) (Table S1). The short-term variations in nitrate, phosphate, and silicate concentrations were measured during the first 36 h of the experiment, showing significant increases in concentration within the first 4 h after adding river water for both LOW and HIGH treatments (Fig. 1). Thereafter, these concentrations remained stable for several hours and, 24 h after adding the river water, the concentrations of organic matter (Fig. 2) and SiO4 3− were two to six times greater than in the CONT treatment and the concentrations of NO3 −, NO2, NH4 +, and PO4 3− were 40 to 400 times greater (Fig. 1). After a few days, the concentrations of NO3 −, NO2 −, PO4 3−, SiO4 3− (Fig. 1), and DON decreased whereas the DOC concentrations (Fig. 2) increased substantially from the middle of the experiment, reaching 6 and 7 mg C L−1 in the LOW and HIGH treatments, respectively. The pH also increased from 8 to 8.8 in the LOW treatment and 8.9 in the HIGH treatment by the end of the experiment (Table S1).
Concentrations (mean and range) of a nitrate, b nitrite, c ammonium, d phosphate, and e silicate during the experiment in mesocosms without added river water (CONT) and in mesocosms with the addition of 20% (LOW) and 30% (HIGH) river water. The p values are for the repeated measure multivariate analysis of variance (rm MANOVA) performed between the CONT, LOW and HIGH treatments (CLH) and LOW and HIGH (LH). Asterisks denote a significant difference between the LOW and HIGH treatments when an a posteriori Fisher’s LSD test was performed for each sampling day
Concentrations (mean and range) of a dissolved organic carbon (DOC), b dissolved organic nitrogen (DON), c particulate organic carbon (POC), and d particulate organic nitrogen (PON) measured during the experiment in mesocosms without added river water (CONT) and in mesocosms with 20% (LOW) and 30% (HIGH) river water. The p values are for the repeated measure multivariate analysis of variance (rm MANOVA) performed between the CONT, LOW, and HIGH treatments (CLH) and LOW and HIGH (LH). Asterisks denote a significant difference between the LOW and HIGH treatments when an a posteriori Fisher’s LSD test was performed for each sampling day
Effect of Adding River Water on Plankton Abundance, Activity, and Diversity
The addition of river water clearly increased the abundance of bacterioplankton through the introduction of river organisms (Fig. 3a). The abundances of bacterioplankton increased rapidly during the first 2 days of the experiment in the LOW and HIGH treatments, but suddenly decreased with the lowest abundances on days 3 and 4 (Fig. 3a). At the end of the experiment, the bacterioplankton abundance increased in both the LOW and HIGH treatments (Fig. 3a). There were three peaks of bacterial production after the addition of river water (Fig. 3b): one during the first 12 h of the experiment, the second on the second day of the experiment, and the last after 5 days. Although not statistically different, the bacterial production rates were generally lower in the LOW treatment than in the HIGH, reaching a maximum of 8 μg C L−1 h−1, more than twice the maximum rates measured in the CONT treatment.
Abundance (mean and range) of bacterioplankton (a) and rates (mean and range) of bacterial production (b) measured during the experiment in mesocosms without added river water (CONT) and in mesocosms with 20% (LOW) and 30% (HIGH). The p values are for the repeated measure multivariate analysis of variance (rm MANOVA) performed between the CONT, LOW, and HIGH treatments (CLH) and LOW and HIGH (LH). Asterisks denote a significant difference between the LOW and HIGH treatments when an a posteriori Fisher’s LSD test was performed for each sampling day
The sudden addition of river water did not significantly change the initial concentration of autotrophic biomass (i.e., chl a concentrations). However, there was a significant increase in concentration from day 3, reaching about 50 μg chl a L−1 in the LOW treatment and almost double this in the HIGH treatment (Fig. 4a). For the first day of the experiment, the particulate primary production PPp rates were lower in the LOW and HIGH treatments than in the CONT treatment (Fig. 4b). Thereafter, the rates substantially increased up to 235 μg C L−1 h−1 in the LOW treatment and 495 μg C L−1 h−1 in the HIGH treatment, which were about an order of magnitude higher than the rates in the CONT treatment (maximum of 41 μg C L−1 h−1).
Concentrations (mean and range) of chlorophyll a (a) and rates (mean and range) of primary production (b), measured during the experiment in mesocosms without added river water (CONT) and in mesocosms with 20% (LOW) and 30% (HIGH) river water. The p values are for the repeated measure multivariate analysis of variance (rm MANOVA) performed between the CONT, LOW, and HIGH treatments (CLH) and LOW and HIGH (LH). Asterisks denote a significant difference between the LOW and HIGH treatments when an a posteriori Fisher’s LSD test was performed for each sampling day
The addition of river water also increased the abundance of picoeukaryotes and chlorophyceans through the introduction of river organisms (Fig. 5). However, after this initial increase, the chlorophycean abundance fell significantly throughout the experiment in both HIGH and LOW treatments (Fig. 5c). The abundance of picoeukaryotes increased rapidly after adding the river water, followed by much greater increases in diatoms (Fig. 5e) and dinoflagellates (Fig. 5d) in the LOW and HIGH treatments to levels significantly higher than in CONT. The addition of river water significantly increased the microphytoplankton richness especially during the first days of the experiment (Fig. S2). The diatom Pseudo-nitzschia spp. group delicatissima and the dinoflagellate Prorocentrum triestinum were dominant in both the LOW and HIGH treatments (45–73% of total abundance) while the diatom Ceratoneis closterium was dominant (65% of total abundance) in the CONT treatment.
Abundance (mean and range) of a cyanobacteria, b picoeukaryotes, c chlorophyceans, d dinoflagellates, and e diatoms measured during the experiment in mesocosms without added river water (CONT) and in mesocosms with 20% (LOW) and 30% (HIGH). The p values are for the repeated measure multivariate analysis of variance (rm MANOVA) performed between the CONT, LOW, and HIGH treatments (CLH) and LOW and HIGH (LH). Asterisks denote a significant difference between the LOW and HIGH treatments when an a posteriori Fisher’s LSD test was performed for each sampling day
However, the addition of river water, which had very low abundances of cyanobacteria and ciliates, reduced the abundance of these organisms originally present in marine lagoon waters in the LOW and HIGH treatments (Fig. 5a and Fig. S3). Nevertheless, the abundance of tintinnids increased in the HIGH treatment, resulting in a very high final abundance (Fig. S3). In general, tintinnids dominated the ciliate community, with a high abundance of Tiarina fusus in the CONT treatment and Tintinnopsis beroidea in the HIGH treatment at the end of the experiment. At the end of the experiment, rotifers largely dominated the mesozooplankton in the LOW and HIGH treatments while copepod nauplii, together with rotifers, were dominant (76–83% of total mesozooplankton abundance) in the CONT treatment (Table S4). The ciliate richness (just after adding the river water) and mesozooplankton (on the last day of the experiment) was lower in the LOW and HIGH treatments (Fig. S2) than in CONT, although there was no significant difference between the treatments and CONT over the whole experiment period. Overall, there was no significant difference in abundance of cyanobacteria, picoeukaryotes, diatoms, dinoflagellates, chlorophyceans, heterotrophic flagellates, naked ciliates, rotifers, and copepods between LOW and HIGH treatments (rm MANOVA p > 0.05).
Effect of Adding River Water on the Plankton Community Structure and Production
The index of dissimilarity was calculated on day 0 and day 6 in order to compare the degree of dissimilarity of the plankton community structure between the experimental conditions at the beginning and end of the experiment (Table S5). The plankton community structure of the river water was clearly dissimilar to that of the mesocosm waters at the beginning and end of the experiment (index of dissimilarity >60). The plankton community structure in the LOW and HIGH treatments changed significantly between the beginning and end of the experiment with an index of dissimilarity of 75 and 78, respectively, and also differed significantly from the CONT treatment (index of dissimilarity >60) at the end of experiment.
The net carbon biomass production of the various plankton communities, the net DOC production, and the net POC production were estimated for the CONT, LOW, and HIGH treatments as the difference between the values measured for the carbon biomass of each community and the DOC and POC concentrations at the end (day 6) and at the beginning of the experiment (day 0). In LOW and HIGH, the phytoplankton carbon biomass and DOC were higher and the biomass of large predators (ciliates, mesozooplankton) was lower than in CONT (Fig. 6). In CONT, the net plankton production (= sum of all the components of the plankton community) was much lower than the net POC production (Table 2) (ANOVA and a posteriori Fisher’s LSD test, p = 0.03). Furthermore, in CONT, the particulate primary production (PPp) converted into daily rate by multiplying the hourly rates by 10 (photoperiod) and cumulated over the whole experimental period was higher (ANOVA and a posteriori Fisher’s LSD test, p = 0.08) than the net POC production (Table 2). Such discrepancies were not found in the LOW and HIGH treatments where no significant differences (ANOVA and a posteriori Fisher’s LSD test, p > 0.2) were found between the net plankton and POC production and cumulative particulate primary production (Table 2).
Estimated (mean and range) contributions (%) of the net organic carbon production of the various plankton groups and dissolved organic carbon (DOC) obtained from the difference of values between the end and the beginning of the experiment and averaged for the mesocosms without added river water (CONT) and for mesocosms with 20% (LOW) and 30% (HIGH) river water
Discussion
Experimental Design
During flash floods, Mediterranean lagoon systems are characterized by high particle loads and rapidly changing hydrodynamics that can result in rapid advection and a change in sedimentation. It is, therefore, difficult to distinguish between the direct and indirect effects of flash floods and to draw conclusions on the direct effect of river discharge on coastal plankton communities from in situ observations. The experimental procedure used in this study simulated a moderate input of river waters into a marine coastal ecosystem, leading to a reduction in salinity and a substantial increase in dissolved matter, comparable to a sudden flash flood event as normally observed in coastal waters, particularly in autumn in the Mediterranean Thau Lagoon [2, 3]. Fouilland et al. [2] described in situ changes following a flash flood event similar to that studied in this experiment, with the exception of PO4 3− and POC concentrations. The PO4 3− concentrations were higher and the POC concentrations were lower in the mesocosms with added river water than those reported in situ at similar salinity by Fouilland et al. These differences are probably due to the source of the freshwater used in this experiment as the water was pumped from the Vène during a period of baseflow when the water tends to be highly loaded in PO4 − and not sampled during a flash flood event when it is highly loaded with particles.
In the present study, the dynamics of the nutrient concentrations sampled at frequent intervals (every 4 h during the first 36 h of the experiment) showed that a period of at least 4 h hours was required to mix the water in each mesocosm after adding the river water. The concentrations of PO4 3−, NO3 −, NO2 −, and SiO4 3− increased for 4 h after adding the river water and only started to decrease afterwards. This also explains the reduction in salinity (from 26.15 on day 0 to 24.55 on day 1) in the HIGH treatment. The concentrations of dissolved and particulate matter 4 h after adding the river water were, therefore, considered to be representative of the entire mesocosm water column. The use of different volumes of added river water showed that marine primary production responded proportionally and positively to the addition of river water, with no apparent strong stress due to the reduction in salinity for the range tested here (25–30).
Differences in the Immediate Effects of Adding River Water on Plankton Richness and Production
One day after adding the river water, NO3 − and PO4 3− concentrations were one to three orders of magnitude greater than those in the CONT treatment. Similarly, the abundances of bacterioplankton, picoeukaryotes, and chlorophyceans were much higher at the beginning of the experiment in the mesocosms with added river water. This clearly shows that the river water brought nutrients (NO3 − and PO4 3−) and living organisms (e.g., chlorophyceans) that were not initially present in the lagoon water. However, the abundances of cyanobacteria and ciliates (naked ciliates and tintinnids) in the river water were much lower than in the Thau Lagoon water at the beginning of the experiment and adding river water to the mesocosms diluted the organisms initially present. Similarly, as the microbial community differed between the river and the Thau Lagoon, the sudden introduction of river water into lagoon marine water significantly increased the richness of the resulting community of phytoplankton but reduced the richness of ciliates. However, some of the river phytoplankton groups detected by microscope disappeared rapidly after being introduced into the mesocosm, suggesting that these river communities did not survive in the brackish waters of the mesocosms. This is consistent with the reduced PPp rates and reduced PPp per unit of chl a, (data not shown) measured in the LOW and HIGH treatments at the beginning of the experiment, suggesting that there was a significant disruption of autotrophic activity. The river community dominated by Chlorophyceans (Scenedesmus spp., and Chlorococcales species) was, therefore, probably affected by the osmotic shock that occurred when the river water was added to the saline water. In a similar study, a significant reduction in phytoplankton growth was observed when phytoplankton communities from the Schelde River were mixed with water (salinity 20) from the brackish Schelde estuary [35]. Flaming and Kromkamp [36] pointed out the importance of osmoregulatory processes causing respiration to increase while the maximum photosynthetic capacity decreased, when cultivated freshwater phytoplankton (Scenedesmus protuberans) is exposed to a sharp increase in salinity. This may well explain the reduction of primary production that was observed immediately following a flash flood in the Thau Lagoon in Autumn 2008 [3] and also supports the substantial reduction in nanophytoplankton observed during the same flash flood in 2008 in Thau Lagoon [3]. Nevertheless, the decrease in the abundance and richness of ciliates that was also observed in Thau Lagoon during the flash flood in 2008 [3] was probably due to their dilution with ciliate-poor river water, as was also observed here.
Substantial Change in the Marine Plankton Community Structure After Adding River Water
The small plankton community (bacterioplankton and picoeukaryotes) rapidly benefited from the addition of nutrients from the river water (i.e., bottom-up control). This suggests that the autochthonous Thau Lagoon microbial communities can increase rapidly with the introduction of allochthonous organic and inorganic nutrients. The resident microbial community in the Thau Lagoon responded within 2 days after the addition of a soil extract during a mesocosm experiment performed in the same area in June 2011 [37]. There was a strong top-down control from the second day of the experiment, resulting in a decrease in the abundance of small plankton cells. This is consistent with the increase of the abundance of heterotrophic flagellates, tintinnids, and rotifers observed in the LOW and HIGH treatments. After the addition of river water, the abundance of rotifers increased by almost two orders of magnitude (up to about 2 × 103 ind. L−1) in the LOW and HIGH treatments. Rotifers appeared to be the first to benefit from the substantial increase in phytoplankton and tintinnids, accounting for 66–91% of total mesozooplankton abundance.
After day 3, diatoms and dinoflagellates (potentially toxic or otherwise harmful species: Pseudo-nitzschia spp. group delicatissima and P. triestinum) that were already present in the Thau Lagoon water increased substantially (up to 28 × 106 and 13 × 106 cells L−1, respectively) in the LOW and HIGH treatments. This clearly demonstrates the ability of some potentially toxic or otherwise harmful phytoplankton species to increase rapidly in the Thau Lagoon during an intense, episodic event such as a river flash flood in autumn. This corroborates the findings that nutrient pollution promotes the development of many harmful estuarine/coastal algal blooms [38]. The occurrence of the third peak of bacterioplankton biomass and production observed in the LOW and HIGH treatments was probably a direct consequence of the substantial production of DOC observed at the middle of the experiment. The production of such high amounts of DOC is probably due to the very high PPp rates measured after adding the river water. This supports the strong dependence of heterotrophic bacteria on freshly released phytoplankton-derived carbon as has been observed during phytoplankton blooms in these coastal waters [39, 40] and others Mediterranean areas [41].
For mesozooplankton, the pattern is complex and not so clear probably due to the timescale of the experiment. Indeed, copepods’ development time from egg to adult is around 1 month and a 6-day experiment is not expected to modify substantially the abundances of communities within a treatment other than egg production and hatching. Indeed, the egg production depends on copepod recent past feeding (2 last days) [42]. Copepod nauplii have more than doubled in the CONT treatment, they slightly increased in the LOW treatment, and they did not change in the HIGH treatment while their initial occurrence was similar (Table S4). The difference observed between treatments may be due to the direct effect of a change in the seawater characteristics such as increased pH and/or lower salinity, which have been shown to impact the hatching success and the naupliar development [43, 44]. The prey type and size might also be of importance on copepods’ reproduction and growth [45]. Dietary diversity is a nutritional requirement, which changes ontogenetically from copepodid to adult stage. Mixed food sources are recognized to be more efficient than a single food source in terms of egg production and viability in omnivorous copepods [45]. As the main copepod species occurring in the mesocosms were omnivorous (Oithona sp. and Acartia sp.), we suggest that the sudden increase in abundance of some phytoplankton species only, which in addition might be potentially toxic or otherwise harmful species, might not favor the egg production and hatching and consequently nauplii abundance.
The addition of river water had a generally substantial effect on the change in plankton structure after the 6 days of the experiment compared to the CONT treatment, with plankton structures differing by about 70%. This suggests that the short-term dynamics of the Thau Lagoon plankton community when subjected to a moderate flood event are species-dependent, implying rapid responses of trophic interactions. Such a change in plankton community structure would have also significant consequences on carbon production.
Consequences of the Addition of River Water on the Carbon Production
The main consequence of the addition of a substantial volume of river water on the production of the planktonic and dissolved compartments (zooplankton, phytoplankton, bacterioplankton, DOC) was the large accumulation of microphytoplankton carbon biomass and DOC in the LOW and HIGH treatments, accounting for 67 and 25% of total (plankton + DOC) carbon production (Fig. 6). In the CONT treatment, however, zooplankton biomass accounted for about 27% of the total carbon production (Fig. 6). The large discrepancy between the net carbon production of plankton and POC and the cumulative particulate primary production measured over the experiment period in CONT (Table 2) suggests an intense production of detrital material which is probably due to the mortality of phytoplankton and bacterioplankton through predation. The insignificant differences between all these carbon production estimates in LOW and HIGH treatments over the period of the experiment (Table 2) suggest that the strong increase in primary production resulted in the accumulation of particulate organic matter as plankton living organisms. However, observations during a previous study in Thau Lagoon showed a substantial increase in in situ primary production 12 days after a flash flood event without accumulation of particulate organic matter in the water column during or after the flash flood [3]. It is, therefore, suggested that most of the locally produced microbial biomass production during the flash flood event was rapidly filtered by oysters reared in the lagoon or advected outside the study area. We therefore conclude that the sudden discharge of nutrients into coastal lagoon waters may lead to the substantial growth of phytoplankton coupled with an intense production of dissolved organic matter within a week that may not immediately benefit the autochthonous trophic food web. The rapid emergence of potentially harmful phytoplankton species after the addition of river waters observed during the present study reinforces the need to monitor areas exploited for shellfish and fish farming during or just after flash flood events.
References
Smith VH, Tilman GD, Nekola JC (1999) Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 100:179–196. doi:10.1016/S0269-7491(99)00091-3
Fouilland E, Trottet A, Bancon-Montigny C, et al (2012) Impact of a river flash flood on microbial carbon and nitrogen production in a Mediterranean Lagoon (Thau Lagoon, France). Estuar. Coast. Shelf Sci. 113:192–204
Pecqueur D, Vidussi F, Fouilland E, et al (2011) Dynamics of microbial planktonic food web components during a river flash flood in a Mediterranean coastal lagoon. Hydrobiologia 673:13–27. doi:10.1007/s10750-011-0745-x
Guadayol Ò, Peters F, Marrasé C, et al (2009) Episodic meteorological and nutrient-load events as drivers of coastal planktonic ecosystem dynamics: a time-series analysis. Mar. Ecol. Prog. Ser. 381:139–155. doi:10.3354/meps07939
Guizien K, Charles F, Lantoine F, Naudin J-J (2007) Nearshore dynamics of nutrients and chlorophyll during Mediterranean-type flash-floods. Aquat. Living Resour. 20:3–14. doi:10.1051/alr:2007011
Chu Y, Salles C, Tournoud M-G, et al (2011) Faecal bacterial loads during flood events in Northwestern Mediterranean coastal rivers. J. Hydrol. 405:501–511. doi:10.1016/j.jhydrol.2011.05.047
López-Flores R, Garcés E, Boix D, et al (2006) Comparative composition and dynamics of harmful dinoflagellates in Mediterranean salt marshes and nearby external marine waters. Harmful Algae 5:637–648. doi:10.1016/j.hal.2006.01.001
Brown JM, Felice NR, Scalfone NB, Hewson I (2012) Influence of habitat confluence on aquatic microbial assemblages in experimental mesocosms. Aquat. Microb. Ecol. 66:33–40
Naudin J-J, Cauwet G, Fajon C, et al (2001) Effect of mixing on microbial communities in the Rhone River plume. J. Mar. Syst. 28:203–227. doi:10.1016/S0924-7963(01)00004-5
Pujo-Pay M, Conan P, Joux F, et al (2006) Impact of phytoplankton and bacterial production on nutrient and DOM uptake in the Rhône River plume (NW Mediterranean). Mar. Ecol. Prog. Ser. 315:43–54. doi:10.3354/meps315043
Remane A (1934) Die Brackwasserfauna. Zool. Anz. 7:34–74
Attrill M, Rundle S (2002) Ecotone or ecocline: ecological boundaries in estuaries. Estuar. Coast. Shelf Sci. 55:929–936. doi:10.1006/ecss.2002.1036
Cushing DH (1990) Plankton production and year-class strength in fish populations: an update of the match/mismatch hypothesis. Adv. Mar. Biol. 26:249–293. doi:10.1016/S0065-2881(08)60202-3
Koroleff F (1983) Determination of ammonia. In: Grasshoff K, Ehrhardt M, Kremling K (eds) Methods of seawater analysis, 2nd ef. Verlag Chemie, Weinheim, p 150–157
Tréguer P, Le Corre P (1975) Handbook of seawater nutrient analyses. Autoanalyser II Technicon user guide, 2nd edn. Univ. Bretagne Occidentale, Laboratoire de Chimie Marine, Brest, France
Raimbault P, Pouvesle W, Diaz F, et al (1999) Wet-oxidation and automated colorimetry for simultaneous determination of organic carbon, nitrogen and phosphorus dissolved in seawater. Mar. Chem. 66:161–169. doi:10.1016/S0304-4203(99)00038-9
Zapata M, Rodriguez F, Garrido JL (2000) Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar Ecol Progr Ser 195:29–45
Vidussi F, Marty JC, Chiaverini J (2000) Phytoplankton pigments during the transition from spring bloom to oligotrophy in the northwestern Mediterranean Sea. Deep Sea Res 47:423–445
DuRand MD, Olson RJ (1996) Contributions of phytoplankton light scattering and cell concentration change diel variation in beam attenuation in equatorial Pacific from flow cytometry measurements of pico-, ultra and nanoplankton. Deep Sea Res 43:891–906
Lee S, Fuhrman JA (1987) Relationships between biovolume and biomass of naturally derived marine bacterioplankton. Appl Environ Microb 53:1298–1303
Utermöhl H (1958) Zur Vervollkommung der quantitativen Phytoplankton-Methodik. Mitt Int Ver Theor Angew Limnol 9:1–38
Lund JWG, Kipling C, Lecren ED (1958) The inverted microscope method of estimating algal number and the statistical basis of estimating by counting. Hydrobiologia 11:143–170
Hillebrand H, Dürselen CD, Kirschtel D, Pollingher D, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 35:403–424
Sun J, Liu D (2003) Geometric models for calculating cell biovolume and surface area for phytoplankton. J. Plankton Res. 25:1331–1346
Mullin MM, Sloan PR, Eppley RW (1966) Relationship between carbon content, cell volume and area in phytoplankton. Limnol. Oceanogr. 11:307–311
Booth CB (1993) Estimating cell concentration and biomass of autotrophic plankton using microscopy. In: Kemp PF, Sherr BF, Sherr EB, Cole JJ (eds). Handbook of methods in aquatic microbial ecology. Lewis. pp 199–205
Putt M, Stoecker DK (1989) An experimentally determined carbon: volume ratio for marine oligotrichous’ ciliates from estuarine and coastal waters. Limnol Oceangr 34:1097–1103
Rose M (1933) Copepodes pelagiques, Faune de France 26. Paul Lechevalier, Paris
Alcaraz M, Saiz E, Calbet A, Trepat I, Broglio E (2003) Estimating zooplankton biomass through image analysis. Mar. Biol. 143:307–315
Bouvy M, Bettarel Y, Bouvier C, Domaizon I, Jacquet S, Le Floc’h E, Montanié H, Mostajir B, Sime-Ngando T, Torréton J-P, Vidussi F, Bouvier T (2011) Trophic interactions between viruses, bacteria and nanoflagellates under various nutrient conditions and simulated climate change. Environ. Microbiol. 13:1842–1857
Bell RT (1990) An explanation for the variability in the conversion factor deriving bacterial cell production from incorporation of (3H)-thymidine. Limnol. Oceanogr. 35:910–915
Whittaker RH (1952) A study of summer foliage insect communities in the Great Smoky Mountains. Ecol. Monogr. 22:1–44
Von Ende CN (1993) Repeated-measures analysis: growth and other time-dependent measures. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Chapman and Hall. pp 113–137
Zar JH (1984) Biostatistical analysis, 2nd edn. Prentice-Hall Inc., Englewood Cliffs
Lionard M, Muylaert K, Van Gansbeke D, Vyverman W (2005) Influence of changes in salinity and light intensity on growth of phytoplankton communities from the Schelde river and estuary (Belgium/The Netherlands). Hydrobiologia 540:105–115
Flaming IA, Kromkamp J (1994) Responses of respiration and photosynthesis of Scenedesmus protuberans Fritsch to gradual and steep salinity increases. J. Plankton Res. 16:1781–1791
Liess A, Rowe O, Francoeur SN, Guo J, Lange K, Shröder A, Reichstein B, Lefèbure R, Deininger A, Mathisen P, Faithfull CL (2016) Terrestrial runoff boots phytoplankton in a Mediterranean coastal lagoon, but these effects do not propagate to higher trophic level. Hydrobiologia. doi:10.1007/s10750-015-2461-4
Heisler J, Glibert PM, Burkholder JM, Anderson DM, Cochlan W, Dennison WC, Dortch Q, Gobler CJ, Heil CA, Humphries E, Lewitus A, Magnien R, Marshall HG, Sellner K, Stockwell DA, Stoecker DK, Suddleson M (2008) Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8:3–13
Fouilland E, Tolosa T, Bonnet D, Bouvier C, Bouvier T, Bouvy M, Got P, Le Floc’h E, Mostajir B, Roques C, Sempéré R, Sime-Ngando T, Vidussi F (2014) Bacterial carbon dependence on freshly produced phytoplankton exudates under different nutrient availability and grazing pressure conditions in coastal marine waters. FEMS Microbiol. Ecol. 87:757–769
Trottet A, Leboulanger C, Vidussi F, Pete R, Bouvy M, Fouilland E (2015) Heterotrophic bacteria show weak competition for nitrogen in coastal waters (Thau Lagon) in autumn. Microb. Ecol. doi:10.1007/s00248-015-0658-8
López-Flores R, Boix D, Badosa A, Brucet S, Quintana XD (2009) Environmental factors affecting bacterioplankton and phytoplankton dynamics in confined Mediterranean salt marshes (NE Spain). J. Exp. Mar. Biol. Ecol. 369:118–126
Bonnet D, Carlotti F (2001) Development and egg production in Centropages typicus (Copepoda: Calanoida) fed different food types: a laboratory study. Mar. Ecol. Prog. Ser. 224:133–148
Hessen DO, Nilssen JP (1983) High pH and the abundances of two commonly co-occurring freshwater copepods (Copepoda, Cyclopoida). Inter J Limnol 19:195–201
Chinnery FE, Williams JA (2004) The influence of temperature and salinity on Acartia (Copepoda: Calanoida) nauplii survival. Mar. Biol. 145:733–738
Brucet S, Compte J, Boix D, et al (2008) Feeding of nauplii, copepodites and adults of Calanipeda aquaedulcis (Calanoida) in Mediterranean salt marshes. Mar. Ecol. Prog. Ser. 355:183–191
Acknowledgments
This study was part of the RESTHAU project (2007-2010) “Impact of river loadings on microbial communities from Thau Lagoon” funded by the French national EC2CO program and coordinated by E. Fouilland. A. Trottet received a postdoctoral fellowship from University of Montpellier 2. D. Pecqueur received a national PhD fellowship provided by the French Ministry of Education and Research. We should like to thank Louise Oriol (UMR 7621 LOMIC, Banyuls/Mer) and Thibault Dinet for the nutrient analyses and P. Raimbault (UMR Institut Méditerranéen d’Océanologie, Marseille) for the stable isotope analyses. This project used the facilities of the Mediterranean Center of Marine Ecosystem Experimental Research MEDIMEER funded by UMR 5119 ECOSYM “Ecologie des Systèmes Marins Côtiers,” CNRS Institute of Ecology and Environment (InEE), University of Montpellier 2, IFR 129 Armand Sabatier, CNRS-GDR2476 Réseaux Trophiques Aquatiques, and Région Languedoc-Roussillon. The authors thank the two anonymous reviewers for their insightful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fouilland, E., Trottet, A., Alves-de-Souza, C. et al. Significant Change in Marine Plankton Structure and Carbon Production After the Addition of River Water in a Mesocosm Experiment. Microb Ecol 74, 289–301 (2017). https://doi.org/10.1007/s00248-017-0962-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-017-0962-6