Abstract
The global climate change may lead to more extreme climate events such as severe flooding creating excessive pulse-loading of nutrients, including nitrogen (N), to freshwaters. We conducted a 3-month mesocosm study to investigate the responses of phytoplankton, zooplankton and Vallisneria spinulosa to different N loading patterns using weekly and monthly additions of in total 14 g N m−2 month−1 during the first 2 months. The monthly additions led to higher phytoplankton chlorophyll a and total phytoplankton biomass than at ambient conditions as well as lower leaf biomass and a smaller ramet number of V. spinulosa. Moreover, the biomass of cyanobacteria was higher during summer (August) in the monthly treatments than those with weekly or no additions. However, the biomass of plankton and macrophytes did not differ among the N treatments at the end of the experiment, 1 month after the termination of N addition. We conclude that by stimulating the growth of phytoplankton (cyanobacteria) and reducing the growth of submerged macrophytes, short-term extreme N loading may have significant effects on shallow nutrient-rich lakes and that the lakes may show fast recovery if they are not close to the threshold of a regime shift from a clear to a turbid state.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the Intergovernmental Panel on Climate Change (IPCC) 2014 report, extreme weather and climate events, including extreme rainfall, will increase in frequency during the 21th century (Field et al., 2014) and led to higher inputs of fine sediment and nutrients to lakes, streams and other surface waterbodies (Jeppesen et al., 2009; Higashino & Stefan, 2014; Leigh et al., 2014).
Phosphorus (P) is a key limiting nutrient for primary production in lakes (Vollenweider, 1976; Schindler, 1977). Recent studies in shallow lakes have, however, revealed that also nitrogen (N) may play a role in the temperate zone, leading to biomass reduction and species richness decline of submerged macrophytes when P concentrations are moderate to high. (González Sagrario et al., 2005; James et al., 2005; Moss et al., 2013). However, the results obtained so far are somewhat ambiguous when considering other climate zones. Özkan et al. (2010) found that the biomass of three dominant species of submerged macrophytes in eutrophic lakes was not affected by variation in nitrogen concentrations in a three-month mesocosm study. The authors attributed this to a concurrent decline in water level (evaporation) counteracting light limitation due to an N-induced increase in periphyton on the plants. Also Li et al. (2008) found that high N concentration (up to 10 mg l−1) did not negatively affect the growth of Vallisneria spinulosa Yan in a 4-month mesocosm study in a subtropical zone, while Olsen et al. (2015), conversely, found severely negative effects of higher N concentration (5 mg l−1) on the biomass of V. spinulosa in a 1-year mesocosm study in the same climate zone. Whether submerged macrophytes are differently affected by the N loading in warmer than in colder climates is therefore still not fully resolved.
With climate change more extreme, rainfall is expected in many regions of the world (Field et al., 2014), which may lead to pulse dosing of nutrients to streams and then lakes, and higher productivity thereafter (Leigh et al., 2014). A study of eutrophic ponds showed that nutrient pulses induced short-term blooming of algae, but quick recovery to the clear state followed after an increase in the abundance of large cladocerans (Strauss et al., 1994). A study conducted in a river system found flood pulses of N to stimulate phytoplankton growth (Hein et al., 1999), with larger pulses having stronger effects on the river ecosystem than smaller pulses (Biggs & Thomsen, 1995; Shivoga, 2001). Extreme N loading, induced by heavy rainfall, may therefore affect the vulnerability of freshwater ecosystems and lead to a more unstable clear water status, but so far only few studies have been conducted in ponds and lakes (Leigh et al., 2014; Löfgren et al., 2014).
We aimed to investigate the effects of extreme N loading on plankton and submerged macrophytes at moderate P concentrations under subtropical climate conditions. Our hypothesis was that extreme N loading would stimulate the growth of phytoplankton more than a gradual supply of the same loading. This, in turn, would induce shading and reduce macrophyte growth, which would make the lake more vulnerable to a shift from a clear to a turbid state.
Materials and methods
The submerged macrophyte Vallisneria spinulosa, grown in a concrete pool (length 30 m, width 15 m and depth 1.5 m) in Wuhan Botanical Garden, China (originally collected from Lake Xiliang), was selected for the experiment. Five individuals of similar size were planted in plastic pots (top diameter 27 cm, bottom diameter 22 cm, height 14 cm, two-thirds filled with washed river sand) and placed in a large (ca. 0.3 ha) pond about 1 month prior to the experiment. In total, 360 pots were used. Nine (in triplicates) concrete mesocosms (length 2 m, width 2 m, depth 1 m and 4000 l) were used in the experiment, and 40 pots with macrophytes were placed in each mesocosm to obtain 100% coverage, after which the mesocosms were filled with water from the same pond where the macrophytes had been kept. Mesocosms were randomly selected for each treatment.
The experiment ran from 18th July to 16th Oct 2007, and pond water (total nitrogen = 0.887 ± 0.052 mg l−1) was used as control (hereafter called no addition (NA) treatment). Total N addition was set to 14 g N m−2 month−1 (320 g NH4NO3 in total) based on a previous study conducted in a similar mesocosm set up (Li et al., 2008). To simulate two different loading scenarios, we added N weekly (40 g NH4NO3 per week) to mimic a frequent loading scenario and monthly (160 g NH4NO3 per month) to mimic an extreme loading scenario (hereafter weekly addition (WA) treatment and monthly addition (MA) treatment). During the first two months, N was added twice in the MA treatment and eight times in the WA treatment, while no N was added during the last month to simulate a recovery phase. We added the chemical salt 1 day prior to the sampling. Concentrations of total nitrogen (TN), total phosphorus (TP), NH4-N and NO3-N in the mesocosms were measured weekly, and NH4-N was determined using the Nessler’s reagent colorimetric method. NO3-N was determined with phenol disulphonic acid, and TN and TP were recorded using spectrophotometric methods after digestion with K2S2O8 solution (Huang et al., 1999).
Samples for water chlorophyll a (Chla) analysis were taken weekly and determined by filtering 1 l of water on a Whatman GF/C filter following ethanol extraction (Huang et al., 1999). The water samples represented the entire water column and were taken with a tube sampler and thoroughly mixed before analysis. After sampling, tap water was added to each mesocosm to maintain a water level of 1 m. The tap water added during the experiment had relatively constant nutrient concentrations (TP: 0.036 ± 0.01 mg l−1 TN: 1.65 ± 0.18 mg l−1). Due to the high total water volume in the mesocosms, the amount of nutrients added with tap water was negligible.
Every month 1 l water samples were collected from each mesocosm and fixed with Lugol’s solution before being condensed to 30 ml for algae identification and counting. Meanwhile 20 l water samples from each mesocosm were filtered with 0.064 mm filters before conservation with formaldehyde for further zooplankton identification and counting. Plankton biomass was calculated from the geometry volume (Huang et al., 1999). Phytoplankton was identified to genus level and dominant zooplankton to species level, except for copepods that were separated into cyclopoids, calanoids and nauplii. Every month one pot from each mesocosm was randomly selected for plant ramet counting, and macrophytes were dried at 80°C for 48 h to determine total biomass and the individual biomasses of roots, leaves and stolons.
The average individual biomass of phytoplankton was calculated using total phytoplankton biomass divided by total phytoplankton abundance. The zooplankton:phytoplankton biomass ratio was calculated using zooplankton biomass (wet weight) divided by phytoplankton biomass (wet weight). Average ramet biomass of submerged macrophytes was calculated by dividing total plant biomass by ramet number.
Data analysis
For weekly sampled data (e.g. water chemistry and phytoplankton Chla), linear mixed models (LMM) were used to test the differences between three N addition treatments for the data obtained from the 2-month N addition period using mesocosm number as the random factor in R 3.1.1 (Zuur et al., 2009), and one-way ANOVA was used to separately test the differences between the three treatments at each sampling after the termination of the N addition. For monthly sampled data (e.g. phytoplankton biomass, zooplankton biomass and macrophyte biomass), one-way ANOVA was used to separately test the differences between the three treatments in each month as the data were too scarce for an RM-ANOVA. To test for significant differences between treatments, post hoc comparisons were made using Tukey’s method in the ‘multcomp’ package. Residual plots were used to check for normality and homogeneity of variance by visual inspection. When the data did not meet assumptions of the Gaussian distribution or homogeneity of variance, log(1 + x) or sqrt transformation was performed. Data were given as mean ± SD.
Results
Water chemistry
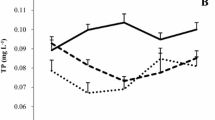
The NO3-N concentrations did not differ between the three treatments during the first 3 weeks but were higher in two N addition treatments than in the NA treatment during the weeks that followed (Fig. 1; Table 1). The NH4-N concentrations were higher in the two N addition treatments during the first 2 months but did not differ between the three treatments in the 10th week. The TN concentrations were higher in the two N addition treatments throughout the whole experiment. The MA treatment recorded a peak in TN (19.5 ± 0.6 mg l−1) and NH4-N (9.2 ± 0.1 mg l−1) in the beginning of the second month. With a few exceptions, TP was generally relatively stable, averaging 0.024 ± 0.011 mg l−1, and did not differ between the three treatments.
Phytoplankton Chla and biomass
Phytoplankton Chla did not differ significantly between the treatments for the first 3 weeks (Fig. 2; Table 1). During the following 6 weeks, it was higher in the MA treatment than in the NA treatment, whereas phytoplankton Chla in the WA treatment did not differ from the other two treatments. No significant differences were detected in the last 2 weeks. Maximum phytoplankton Chla was 37.7 ± 24.2 μg l−1 in the MA treatment. During the N addition period, the phytoplankton Chla:TP ratio averaged 0.71 ± 0.34 in the WA treatment and 0.84 ± 0.24 in the MA treatment, both being significantly higher than the 0.52 ± 0.18 recorded in the NA treatment. However, the Chla:TP ratio was 0.93 ± 0.26 in the MA treatment in the 10th week and higher than that in the NA treatment but did not differ between the WA treatment and the other two treatments (Fig. 3).
Total phytoplankton biomass peaked at 74.7 ± 24.2 mg l−1 in the MA treatment in August, being significantly higher than in the NA but not higher than in the WA treatment (Table 2). Total phytoplankton biomass did not differ among the three treatments in the other months. The average biomass of individual phytoplankton groups in the three treatments showed similar trends over the season, which did not differ among the three treatments. Cyanophyta biomass was significantly higher in the MA treatment than in the other two treatments in August. Similarly, Chlorophyta biomass was marginally higher in the MA treatment than in the NA treatment in August, whereas Bacillariophyta was negligibly higher in the NA treatment than in the two N addition treatments in September. The dominant genera by numbers during the experiment were Microcystis followed by Melosira, Merismopedia and Kirchneriella.
Zooplankton biomass
Total zooplankton biomass did not differ significantly between treatments during the whole experiment (Fig. 3; Table 2). In October, rotifer biomass was higher in the NA treatment than in the two N addition treatments, and copepod biomass was higher in the MA treatment than in the NA treatment but did not differ significantly between the WA treatment and the other two treatments. The cladoceran community mainly consisted of four taxa, Daphnia magna Straus, Scapholeberis mucronata (O. F. Müller), Chydorus sphaericus (O. F. Müller) and Alona sp., whose levels were marginally higher in September in the WA treatment than in the MA treatment. The zooplankton:phytoplankton biomass ratio did not differ significantly between the three treatments during the 4 months.
Vallisneria spinulosa biomass and ramet number
In the beginning of the experiment, the submerged macrophyte V. spinulosa had an average biomass of 5.20 ± 2.19 g and an average ramet number of 18 ± 7 per pot (Fig. 4; Table 2). Root, stolon, total and average ramet biomass did not differ among the three treatments during the experimental period. However, leaf biomass was marginally lower in the MA than in the WA treatment in August, and ramet number was significantly lower in the MA than in the WA treatment in September. Yet, leaf biomass and ramet number did not differ among the three treatments in the other months of the experiment.
Discussion
Inorganic N concentrations were higher in the two N addition treatments than in control (NA treatment) throughout the experiment except that the NH4-N concentrations decreased after the termination of N addition and did not differ between the three treatments by the end of the experiment. We found that monthly addition of N resulted in a higher phytoplankton Chla than at ambient conditions even though TP did not differ between the treatments. Monthly N loading also led to higher cyanobacteria, Chlorophyta and total phytoplankton biomass in August than at ambient conditions. These results support the hypothesis that in addition to P (Vollenweider, 1976; Schindler, 1977), nitrogen can affect primary production (e.g. Elser et al., 1990; González Sagrario et al., 2005). Furthermore, the Chla:TP ratio recorded at ambient conditions was close to the value (ca. 0.42–0.44) found in macrophyte-dominated systems within the same climate region (Li et al., 2008; Wang et al., 2014), but both weekly and monthly N loading induced a significantly higher Chla:TP ratio during the first 2 months when N was added, with ratios that are more typical for lakes with low plant abundance. Differences in grazing pressure may also alter the Chla:TP ratio (Jeppesen et al., 2003; Jackson et al., 2007), but since zooplankton biomass and the zooplankton:phytoplankton biomass ratio were not higher at ambient conditions, the higher N loading seems to be of key importance for the higher Chla:TP ratio and higher Chla levels.
High N (especially of ammonia) can impede the growth of submerged macrophytes (Cao et al., 2004; Moss et al., 2013). However, earlier studies have shown that Vallisneria spinulosa tolerates high N concentrations. Li et al. (2008) recorded limited negative and only temporal effects on V. spinulosa growth of high inorganic nitrogen (10 mg l−1 NH4-N or NO3-N). In fact, weekly N loading was found to stimulate the growth of V. spinulosa in a former experiment conducted at N concentrations up to 10 mg l−1 (Li et al., 2008), which perhaps also was the case in our study (Fig. 3), though not significantly so. Moreover, in a recent 1-month mesocosm experiment, Zhao et al. (2015) found no adverse physiological changes in V. spinulosa exposed to relatively high N concentrations (about 5 mg l−1). In our study, macrophyte biomass was not adversely affected by the N loading. This indicates that the higher N concentrations (ca. 10–20 mg l−1), occurring in both the weekly and monthly loaded mesocosms, had no direct (toxic) effect on macrophyte growth. Therefore, it is reasonable to assume that the lower leaf biomass and smaller ramet number of the macrophytes in the monthly N loading treatment in August and September, respectively, than in the weekly treatments can be attributed to higher phytoplankton shading (higher phytoplankton Chla) rather than to the toxic effect of high N concentrations. Enhanced growth of periphyton (although not quantified in the present study) as seen in other studies (Özkan et al., 2010; Olsen et al., 2015) may also have contributed to the decline of macrophyte biomass. Our study was run without fish, and the effects would presumably have been even stronger in the presence of fish due to reduced zooplankton grazing on phytoplankton (Jeppesen et al., 2011).
In our study, the highest spiking of N (monthly N addition)-promoted phytoplankton growth (Chla), especially of cyanobacteria, in high summer (August) to a larger extent than did the gradual (weekly) loading. However, by the end of the experiment, phytoplankton biomass and Chla were similar irrespective of treatment, suggesting fast recovery after the cease of N loading; an effect of seasonality cannot be excluded, though, as the last sampling was in October. In a longer term experiment with N addition, Olsen et al. (2015) found resilience of the submerged macrophytes (V. spinulosa) in the short term, but a complete loss of plants at high N additions in the long term. Pulses related to extreme weather events are typical of short-term duration and recovery may therefore be possible after such events. However, if the lakes are close to the threshold for a shift from a clear to a turbid state (Scheffer et al., 1993), such pulses may be the trigger of a general shift. We argue that a future with more extreme climate events as predicted by IPCC (Field et al., 2014), including extreme rainfall and thus enhanced nutrient runoff, potentially may have severe effects on the ecological state of shallow lakes in summer. The risk would be highest in shallow lakes with short retention time (days to weeks) as the pulse effect on the N concentration will be only minor in lakes with long retention time (years) unless the extreme events result in a generally higher loading of nitrogen.
Our results also provide some suggestions for freshwater management. Agricultural landscape buffer structures in head-water catchments and wetland buffer zones around shallow lakes are considered effective to reduce the total amount of nutrient loading to freshwaters (Wang et al., 2005), and since these structures might also reduce the size of nutrient pulses under the extreme rainfall scenarios, we argue that more buffer zones should be constructed, not only to reduce total nutrient loading but also to create a more gradual nutrient loading pattern.
References
Biggs, B. J. F. & H. A. Thomsen, 1995. Disturbance of stream periphyton by perturbations in shear stress: time to structural failure and differences in community resistance. Journal of Phycology 31: 233–241.
Cao, T., L. Ni & P. Xie, 2004. Acute biochemical responses of a submersed macrophyte, Potamogeton crispus L., to high ammonium in an aquarium experiment. Journal of Freshwater Ecology 19: 279–284.
Elser, J. J., E. R. Marzolf & C. R. Goldman, 1990. Phosphorus and nitrogen limitation of phytoplankton growth in the freshwaters of North America: a review and critique of experimental enrichments. Canadian Journal of Fisheries and Aquatic Sciences 47: 1468–1477.
Field, C. B., V. R. Barros, K. Mach, & M. Mastrandrea, 2014. Climate change 2014: impacts, adaptation, and vulnerability. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York.
González Sagrario, M. A., E. Jeppesen, J. Gomà, M. Søndergaard, J. P. Jensen, T. Lauridsen & F. Landkildehus, 2005. Does high nitrogen loading prevent clear-water conditions in shallow lakes at moderately high phosphorus concentrations? Freshwater Biology 50: 27–41.
Hein, T., G. Heiler, D. Pennetzdorfer, P. Riedler, M. Schagerl & F. Schiemer, 1999. The Danube restoration project: functional aspects and planktonic productivity in the floodplain system. Regulated River Research & Management 15: 259–270.
Higashino, M. & H. G. Stefan, 2014. Modeling the effect of rainfall intensity on soil-water nutrient exchange in flooded rice paddies and implications for nitrate fertilizer runoff to the Oita River in Japan. Water Resources Research 50: 8611–8624.
Huang, X. F., W. M. Chen & Q. M. Cai, 1999. Survey, observation and analysis of lake ecology. Standard methods for observation and analysis in Chinese Ecosystem Research Network, Series V. Standards Press of China, Beijing. (in Chinese).
Jackson, L. J., M. Søndergaard, T. L. Lauridsen & E. Jeppesen, 2007. Patterns, processes, and contrast of macrophyte-dominated and turbid Danish and Canadian shallow lakes, and implications of climate change. Freshwater Biology 52: 1782–1792.
James, C., J. Fisher, V. Russell, S. Collings & B. Moss, 2005. Nitrate availability and hydrophyte species richness in shallow lakes. Freshwater Biology 50: 1049–1063.
Jeppesen, E., J. P. Jensen, C. Jensen, B. Faafeng, D. O. Hessen, M. Søndergaard, T. Lauridsen, P. Brettum & K. Christoffersen, 2003. The impact of nutrient state and lake depth on top-down control in the pelagic zone of lakes: a study of 466 lakes from the temperate zone to the arctic. Ecosystems 6: 313–325.
Jeppesen, E., B. Kronvang, M. Meerhoff, M. Søndergaard, K. M. Hansen, H. E. Andersen, T. L. Lauridsen, L. Liboriussen, M. Beklioglu & A. Özen, 2009. Climate change effects on runoff, catchment phosphorus loading and lake ecological state, and potential adaptations. Journal of Environmental Quality American Society of Agronomy 38: 1930–1941.
Jeppesen, E., P. Nõges, T. A. Davidson, J. Haberman, T. Nõges, K. Blank, T. L. Lauridsen, M. Søndergaard, C. Sayer & R. Laugaste, 2011. Zooplankton as indicators in lakes: a scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD). Hydrobiologia 676: 279–297.
Leigh, C., A. Bush, E. T. Harrison, S. S. Ho, L. Luke, R. J. Rolls & M. E. Ledger, 2014. Ecological effects of extreme climatic events on riverine ecosystems: insights from Australia. Freshwater Biology. doi:10.1111/fwb.12515.
Li, W., Z. Zhang & E. Jeppesen, 2008. The response of Vallisneria spinulosa (Hydrocharitaceae) to different loadings of ammonia and nitrate at moderate phosphorus concentration: a mesocosm approach. Freshwater Biology 53: 2321–2330.
Löfgren, S., U. Grandin & S. Stendera, 2014. Long-term effects on nitrogen and benthic fauna of extreme weather events: examples from two Swedish headwater streams. Ambio 43: 58–76.
Moss, B., E. Jeppesen, M. Søndergaard, T. Lauridsen & Z. Liu, 2013. Nitrogen, macrophytes, shallow lakes and nutrient limitation: resolution of a current controversy? Hydrobiologia 710: 3–21.
Olsen, S., F. Chan, W. Li, S. Zhao, M. Søndergaard & E. Jeppesen, 2015. Strong impact of nitrogen loading on submerged macrophytes and algae: a long-term mesocosm experiment in a shallow Chinese lake. Freshwater Biology 60: 1525–1536.
Özkan, K., E. Jeppesen, L. S. Johansson & M. Beklioglu, 2010. The response of periphyton and submerged macrophytes to nitrogen and phosphorus loading in shallow warm lakes: a mesocosm experiment. Freshwater Biology 55: 463–475.
Scheffer, M., S. H. Hosper, M.-L. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology and Evolution 8: 275–279.
Schindler, D. W., 1977. Evolution of phosphorus limitation in lakes. Science 195: 260–262.
Shivoga, W. A., 2001. The influence of hydrology on the structure of invertebrate communities in two streams flowing into Lake Nakuru, Kenya. Hydrobiologia 458: 121–130.
Strauss, E. A., W. K. Dodds & C. C. Edler, 1994. The impact of nutrient pulses on trophic interactions in a farm pond. Journal of Freshwater Ecology 9: 217–228.
Vollenweider, R. A., 1976. Advances in defining critical loading levels for phosphorus in lake eutrophication. Memorie dell’Istituto italiano di idrobiologia 33: 53–83.
Wang, X. H., C. Q. Yin & B. Q. Shan, 2005. The role of diversified landscape buffer structures for water quality improvement in an agricultural watershed, North China. Agriculture, Ecosystems & Environment 107: 381–396.
Wang, H. J., H. Z. Wang, X. M. Liang & S. K. Wu, 2014. Total phosphorus thresholds for regime shifts are nearly equal in subtropical and temperate shallow lakes with moderate depths and areas. Freshwater Biology 59: 1659–1671.
Zhao S., L. Yin, F. Chang, S. Olsen, M. Søndergaard, E. Jeppesen. & W. Li, 2015. Response of Vallisneria spinulosa (Hydrocharitaceae) to contrasting nitrogen loadings in controlled lake mesocosms. Hydrobiologia. doi: 10.1007/s10750-015-2456-1.
Zuur, A. F., E. N. Ieno, N. J. Walker, A. A. Saveliev & G. M. Smith, 2009. Mixed effects models and extensions in ecology with R. Statistics for biology and health. Springer, New York.
Acknowledgments
This study was supported by National Natural Science Foundation of China (31170340), CRES (Danish Strategic Research Council), CLEAR (a Villum Kann Rasmussen Centre of Excellence project) and the MARS project (Managing Aquatic ecosystems and water Resources under multiple Stress) funded under the 7th EU Framework Programme, Theme 6 (Environment including Climate Change), Contract No.: 603378 (http://www.mars-project.eu). Mrs. Xie is greatly acknowledged for water sample analyses and Anne Mette Poulsen for valuable editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Sidinei M. Thomaz
Rights and permissions
About this article
Cite this article
Zhang, Z., Cao, Y., Jeppesen, E. et al. The response of Vallisneria spinulosa (Hydrocharitaceae) and plankton to pulse addition of inorganic nitrogen with different loading patterns. Hydrobiologia 767, 175–184 (2016). https://doi.org/10.1007/s10750-015-2494-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2494-8