Abstract
Submerged macrophytes play an important role in aquatic ecosystems, which has led to an increase in studies on vegetation recovery in polluted lakes from which submerged macrophytes have disappeared. The comparison of microbial communities in sediment cloned with planted and naturally growing submerged macrophytes is an interesting but rarely studied topic. In this investigation, Maojiabu and Xilihu, two adjacent sublakes of West Lake (Hangzhou, China), were selected as aquatic areas with planted and naturally growing macrophytes, respectively. Sediment samples from sites with/without Vallisneria natans were collected from both sublakes. The results showed that sediment total nitrogen and organic matter were significantly lower in the plant-covered sites than that in the non-plant sites in Maojiabu. Additionally, the sediment microbial community characterized by 16S ribosomal RNA (rRNA) sequencing differed more significantly for Maojiabu than for Xilihu. The relative abundances of microbes involved in C, N, and S elemental cycling were significantly higher in the sediments with plants than in those without. Results from both fatty acid methyl ester analysis and 16S rRNA sequencing indicated that vegetation significantly influenced the sulfate-reducing bacteria (SRB). Thus, the gene copies and composition of SRB were explored further. The relative gene abundance of SRB was 66% higher with natural vegetation colonization but was not influenced by artificial colonization. An increase in dominant SRB members from the families Syntrophobacteraceae and Thermodesulfovibrionaceae contributed to the increase of total SRB. Thus, macrophyte planting influences sediment nutrient levels and microbial community more than natural growth does, whereas the latter is more beneficial to sediment SRB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The recovery of submerged macrophytes in freshwater environments is an indicator for a clear waterbody and healthy ecosystem, in which aquatic vascular plants are the dominant primary producers [1, 2]. The maintenance of clear water state by aquatic vascular plants is attributed to their abilities of reducing nutrients in the water column, protecting phytoplankton grazers such as Daphnia against fish predation, and preventing sediment resuspension [3]. Due to the resistance of aquatic ecosystems, it often takes decades for successful macrophyte recolonization [4, 5]. Summer TP concentrations of the investigated 58 lakes in northeastern Germany were less than 100 μg L−1 but recolonization or expansion was recorded only in six lakes, indicating a resilience against recolonization [6]. The macrophyte coverage remained unchanged 8 years after nutrient loading reductions in 17 Danish lakes [7]. There are many factors influencing the recovery delay, such as high internal nutrient loading, grazing by herbivorous birds, disturbance by fish, and lack of viable propagules in the sediment [8, 9].
Due to the demand for urban landscape and living environment, measurements for accelerating the recovery process are exploring continually. Artificial support of macrophyte development is one of the measurements [8]. After a reduction in external nutrient loading and sediment dredging, multiple approaches including aquatic macrophyte planting resulted in an increase of vegetation coverage from 0 to 45.7% [10]. Charophytes, one of the groups that usually growing in oligotrophic waterbodies, appeared spontaneously after several species of submerged macrophytes were artificially planted [1]. On one hand, planting can increase the viable propagation units and seed bank of submerged vegetation. On the other hand, the optimal light climate, which initially acts as a trigger to stimulate macrophyte growth and development, is prolonged by the presence of macrophytes [11].
The purpose of both natural development and artificial planting of submerged vegetation is constructing a clear water stable state. Except for recovery periods, studies on the ecological influence of the two measurements are lacking. We can only find the related examples in terrestrial ecosystems where many studies have been carried out comparing natural and planted growth. Compared with naturally growing vegetation, planted vegetation usually has greater impact on the soil chemical characteristics and microbial community. For example, the soil C and N concentrations from plantations were lower by 36.0 and 26.5%, respectively, when compared with natural forests in a forest ecosystem [12]. Bacterial and fungal communities from the forest plantation, compared to those from natural forest soils, had different community structure and alpha diversity (α-diversity) values [13]. For special microbes, the relative abundance of dominant genera belonging to the phylum Acidobacteria was significantly different between a natural hardwood forest and plantations of conifers [14]. However, scarce research could be found on this topic in terms of aquatic ecosystems, and how naturally growing and planted aquatic vegetations influence their surrounding sediment is still unknown.
Similar to terrestrial forests, nutrient acquisition by aquatic vegetation is mainly from the sediment and the decomposition products of plant litter go back to the sediment. However, terrestrial and aquatic plants are different in life form and ecological function. Trees in the forest are perennial woody plants and support timber export [15], whereas submerged macrophytes are mainly annual herbaceous plants that provide primary production for aquatic ecosystems [16]. Sediments act as repositories for waterbody nutrients [17] and as release sources for internal phosphorus and nitrogen [18, 19]. Microorganisms embedded in sediments are important participants for carrying out biogeochemical processes that are essential in natural nutrient cycling [20]. Thus, the purpose of this study is exploring the impacts of naturally growing and planted submerged vegetation colonization in aquatic ecosystem from the aspect of sediment microbial ecology.
Materials and Methods
Study Sites and Sampling
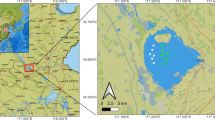
The study area included two adjacent sublakes (Maojiabu and Xilihu) of West Lake, in the city of Hangzhou, China (Fig. 1, created in Photoshop 7.0). MP and MN represent the sampling sites covered densely with plants (Vallisneria natans) and lacking plants in Maojiabu, respectively, whereas XP and XN represent the corresponding sampling sites in Xilihu. The submerged macrophyte V. natans was planted in May 2015 in Maojiabu. This investigation was carried out in October 2015 when V. natans reached its maximum biomass for the year. The coverage of V. natans reached 100% in the sampling sites MP and XP. The upper layer of sediment (0–10 cm) was collected using a Peterson sediment collector and divided into three subsamples. One subsample was air dried at room temperature for nutrient-level analysis (results shown in Table 1), and the other two were stored at −20 and −80 °C for the analysis of sediment microbial community.
Location of the sampling sites in Maojiabu and Xilihu, two sublakes of West Lake, Hangzhou, China. MP and MN represent the sampling sites with and without plants, respectively, in Maojiabu, where submerged macrophytes were planted; XP and XN are the corresponding sampling sites in Xilihu, where submerged macrophytes grew naturally
Measurement of Ester-Linked Fatty Acid Methyl Esters
The mild alkaline methanolysis technique [21] was used to extract ester-linked fatty acid methyl esters (EL-FAMEs) from the sediment samples stored at −20 °C. The extracted EL-FAMEs were analyzed by Agilent 6890N gas chromatograph (Agilent Technologies, USA) equipped with an Agilent 5793 inert MSD.
16S rRNA Sequencing of the Sediment Microbial Community
The microbial DNA was extracted in triplicate from the sediment stored at −80 °C using the E.Z.N.A.™ Soil DNA Kit (Omega, USA), according to the manufacturer’s instructions. After combining the DNA extracts of the triplicates for each sampling site, the bacterial hypervariable regions V4–V5 of the 16S ribosomal RNA (rRNA) gene were PCR-amplified using the individually barcoded forward primer 515F (5′-GTGCCAGCMGCCGCGG-3′) and reverse primer 907R (5′-CCGTCAATTCMTTTRAGTTT-3′). PCR was performed using TransStart® FastPfu DNA Polymerase. Briefly, the reaction mix (20 μl) contained 10 ng of template DNA, 250 μM of dNTPs, 0.2 μM of each primer, 0.4 μl of FastPfu DNA Polymerase, and 4 μl of 5× TransStart® FastPfu Buffer. PCR included 27 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s in a thermal cycler (ABI GeneAmp® 9700, USA). After purification and quantification of the PCR product, a DNA library was constructed. Subsequently, 250 bp paired-end (PE) sequencing was carried out on the Illumina HiSeq 2500 platform to generate the raw reads.
The raw PE reads were assigned to a sample by their unique barcode, and then, the barcode and primer sequence were truncated. After assembly of the PE reads, quality filtering, and chimeric sequence deletion, 35,493 effective tags, on average, were generated. Operational taxonomic units (OTUs) were defined at 97% sequence similarity. Relative abundance for each taxonomic level was constructed using R software, following OTU assignment and contaminative OTU deletion.
Analysis of Sulfate-Reducing Bacteria in the Surface Sediment
The copy numbers of the 16S rRNA gene and dsrB gene were determined in each sample replicate using 2 μl of extracted DNA (approximately 20 ng). Quantification was performed in a real-time PCR cycler (Roche LightCycler 480®, Switzerland) using SYBR Green as the binding dye. The reactions for the two genes were performed in 20 μl containing 10 μl SG Fast qPCR Master Mix (BBI Life Sciences, China) and 0.2 μM of the specific primers for each gene. The 16S rRNA gene was quantified using universal primers for bacteria, 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 518R (5′-ATTACCGCGGCTGCTGG-3′). The gene dsrB was quantified using the primers DSRp2060F (5′-CAACATCGTYCAYACCCAGGG-3′) and DSR4R (5′-GTGTAGCAGTTACCGCA-3′) [22]. Both amplification reactions were initially denatured at 95 °C for 3 min followed by 45 cycles of 95 °C for 15 s, 57 °C for 20 s, and 72 °C for 30 s. To verify the specificity of amplification, a denaturation curve was performed at the end of each reaction at temperatures ranging from 60 to 100 °C. Standard curves were calculated based on purified PCR products of known copy number. The copy numbers of the 16S rRNA and dsrB genes per gram of sediment were determined via standard curves. Each qPCR measurement was done in triplicate. The amplification efficiency (E) was calculated from the slope of the standard curves using the formula \( E={10}^{\raisebox{1ex}{$-1$}\!\left/ \!\raisebox{-1ex}{$\mathrm{slope}$}\right.}-1 \).
Data Analysis
Two-way analysis of variance (ANOVA) supported by the software package PASW Statistics 18.0 was used to analyze any significant effects of the sublake regions and vegetation colonization on the ratios and groups of characteristic fatty acids (FAs). Subsequently, one-way ANOVA was applied to analyze any significant differences, either by factor region or by vegetation, in the indices. The Student-Newman-Keuls method was used for multiple comparisons.
The multivariate analysis methods of principal coordinate analysis (PCoA) and principal component analysis (PCA) were applied to analyze the dissimilarity of the microbial communities at different sampling sites [23]. PCoA, based on Bray-Curtis distance, and PCA, based on the relative abundance of OTUs, were carried out using R software. A heat map of species relative abundance for the top 35 genera was constructed using R software.
Statistical analysis for the α-diversity indices, relative abundance differences at the genus level, number of sulfate-reducing bacteria (SRB) gene copies, and relative abundances of SRB genera were all carried out among MP, MN, XP, and XN. In this study, effective comparisons only existed in the following four pairs: XN-MN, MN-MP, XN-XP, and XP-MP. Thus, an independent-samples t test was used to identify the significant difference between each pair. The figures were created by program Origin 8.0.
Results
Sediment Microbial Community Analyzed by EL-FAMEs
The characteristic ratio, monounsaturated fatty acid (MUFA)/Branched, which represents the comparative advantage of aerobic over anaerobic bacteria, was significantly different based on the existence of vegetation, and groups II, III, and IV were significantly different based either on region or on vegetation (two-way ANOVA, Table 2). Therefore, the differences in these four indices were tested via one-way ANOVA. The other three indices including Fungal/bact, trans/cis, and group I are shown in Fig. S1. MUFA/Branched was higher at the planted sites in both sublakes and was more significant in Maojiabu (Fig. 2a) than in Xilihu. Groups II and III were lower for the planted sites in Xilihu, whereas they did not differ in Maojiabu (Fig. 2b, c). Group IV, representing the SRB and other anaerobic bacteria, showed the same pattern in both sublakes (Fig. 2d) and was significantly higher at the non-covered sites than at the plant-covered sites.
Sediment Microbial Community Analyzed by 16S rRNA Sequencing
We obtained 38,509 high-quality 16S rRNA sequence reads from the Illumina HiSeq platform. After equalizing the high-quality reads, the equalized reads were assigned to 2381 OTUs at 97% similarity. These OTUs were clustered into 23 phyla or unclassified (relative abundance >0.1%), with 37.99% classified as Proteobacteria (Fig. S2), followed by Chloroflexi (21.48%), Acidobacteria (12.27%), and Actinobacteria (7.60%).
The α-diversity indices of observed species number, Shannon diversity index, and Chao1 index are listed in Table 3. Although the MN region was highest for each index value when compared with the other three regions, no significant differences were found among any of the sampling regions. A cluster tree of the top 35 genera showed that the distance between XN and XP was closer than that between MN and MP (Fig. S3). The dissimilarities of the sediment microbial communities for each sampling region were visualized using two multivariate analysis methods: PCoA and PCA. The first two axes explained more than 70% of the data variability through both PCoA and PCA, with the microbial communities differing distinctly from each other, as shown in the two-dimensional plots in Fig. 3. The sites from sublake Maojiabu and Xilihu, in which submerged macrophytes were planted and naturally growing, respectively, were separated along axis 1. On the other hand, the sites from the regions with and without plants were separated along axis 2. Although community diversity did not change, the distinct structure of the overall sediment microbial community suggested that specific microbes might be different for each region.
We used independent-samples t test to identify which genera were significantly different between each pair. Eight genera belonging to the phyla Proteobacteria, Chloroflexi, Firmicutes, and Planctomycetes were significantly different between the sites of Maojiabu and Xilihu without plants (Fig. 4a). The relative abundances of the genera Methylocaldum and Carnobacterium, belonging to the phyla Proteobacteria and Firmicutes, respectively, were higher for MN than for XN, whereas the other six genera showed an opposite trend. This may reflect the basic difference between the two sublake regions. The difference between the sites with and without plants in the same aquatic area was larger for Maojiabu than for Xilihu, because the number of genera and relative abundance of the significant different microbes were higher in the MN-MP pair than in the XN-XP pair (Fig. 4b, c). Eleven genera belonging to the phyla Proteobacteria, Nitrospirae, and Chloroflexi were significantly different between MN and MP. In the pair MN-MP, Dechloromonas, Caldilinea, and Desulfococcus were the top 3 abundant genera that were higher for MP, whereas Nitrospira, GOUTA19, and 4–29, belonging to the phylum Nitrospirae, were higher for MN. Only five genera, which also belong to the phyla Proteobacteria, Nitrospirae, and Chloroflexi, were significantly different between XN and XP. Except for Rubrivivax, the abundances of the other four genera were higher for XP than for XN. The difference in the pair XP-MP was more complex than it was in the other pairs’ comparisons (Fig. 4d). Relative abundances of genera belonging to Nitrospirae and Firmicutes were higher for XP, whereas genera belonging to Planctomycetes, Chloroflexi, and Bacteroidetes were higher for MP. Both Desulfococcus and Desulfobacca, belonging to Proteobacteria, are SRB, the relative abundances of which were also higher in MP.
Qualitative and Quantitative Analysis for the Sediment SRB
The gene copies for 16S rRNA and dsrB analyzed by qPCR were tested for relative abundances of sediment SRB for the different regions. The efficiencies of qPCR for 16S rRNA and dsrB were 99.5 and 93.1%, and the standard curves for both genes were linear over six orders of magnitude (R 2 > 0.998). As shown in Table 4, the gene abundance of 16S rRNA, which characterizes total bacteria, reached 109 copies g−1 sediment (dry weight), and the gene abundance of dsrB, which characterizes SRB, was three orders of magnitude less than that of 16S rRNA. Considering the relative abundances of the dsrB gene, the ratio of dsrB to 16S rRNA ranged from 0.048 to 0.083%. There were no statistical differences of dsrB to 16S rRNA between MN and XN or between MP and MN, whereas this ratio for XP was 66% higher than that for XN, according to the independent-samples t test.
The SRB genera, separately picked out from high-throughput sequencing results of the whole microbial community, were analyzed further to determine which SRB genera contributed to the quantitative difference between each paired region. Seven specific genera and four unclassified genera of SRB were found in the sediment of Maojiabu and Xilihu (Table 5). Similar to the qPCR results, the total relative abundance of SRB was highest for XP. Except for Thermoprotei_unclassified belonging to Archaea, the other genera all belonged to Bacteria. The three unclassified genera of class Deltaproteobacteria and Thermodesulfovibrionaceae_unclassified of phylum Nitrospirae were assigned at the family level. Microbes belonging to family Syntrophobacteraceae were the most abundant SRB in our sediment samples, accounting for 42.62 and 2.21% of the SRB and total microbes on average. Although the relative gene abundance of dsrB did not differ between MN and XN, the relative abundances of Syntrophobacteraceae_unclassified, Syntrophobacter, and Desulfobacca were significantly different (P = 0.007, 0.037, and 0.002, respectively). Similarly, the relative abundances of Desulfococcus, Syntrophobacter, and Desulfomonile were significantly different (P < 0.001, P = 0.004 and 0.004, respectively) between MP and MN. We found that the relative gene abundance of dsrB was higher for XP than for XN, and Syntrophobacter and Desulfobulbaceae_unclassified (P = 0.013 and 0.032) were the two special SRB with higher abundances for XP. In the MP-XP pair, only Syntrophobacteraceae_unclassified and Thermoprotei_unclassified (P = 0.001 and 0.037) were higher for XP; the relative abundances of Desulfococcus, Desulfobacca, Syntrophobacter, and Desulfomonile (P < 0.001, P = 0.010, 0.004, and 0.001, respectively) were significantly lower for XP.
Discussion
Effects of V. natans on Sediment Nutrient Levels and Microbial Community
Microbial community characteristics in the sediment cloned with planted and natural growing submerged vegetation were explored in this study. Samples of sediment from regions with and without submerged macrophytes in Maojiabu and Xilihu, which represented aquatic areas with planted and naturally growing macrophytes, respectively, were collected for analysis. Sediment nutrient loading was lower for MP than that for MN, whereas it was not significantly influenced in Xilihu, according to the significance analysis of the sediment total nitrogen (TN) and percentage of organic matter (OM%). Studies on the comparison between planted and naturally growing vegetation in aquatic ecosystems are scarce; however, numerous examples exist for terrestrial ecosystems. In forest ecosystems, the soil C and N concentrations from plantations are lower by 36.0 and 26.5%, respectively, when compared to natural forests [12]. Due to faster decomposition, natural forests also show the lowest mean residence time of nutrients and the highest nutrient return to the soil [24]. This might be related to the higher soil microbial biomass and microbial activity in the soil of natural forests than in plantations [25, 26]. The sediment microbial community in natural ecosystems is an adaption to plants’ growth and litter input; therefore, planting, as a human intervention, would break the intrinsic adaptability between the environment and organisms. Thus, similar to the sediment nutrients, submerged macrophyte colonization had a stronger influence on the sediment microbial community in Maojiabu than in Xilihu.
Microbes involved in the C, N, and S elemental cycles were significantly higher in the sediment from the sites covered with submerged macrophytes than in that from sites without macrophytes. For example, Dechloromonas and Caldilinea, which are related to N2 generation [27, 28], the SRB Desulfococcus and Syntrophobacter, the carbon flow contributor to the grazer food chain Limnohabitans [29], and Rhodobacter for chloralkane and phenol degradation [30, 31] were more abundant for MP than for MN. The genera of HB118, Methylocaldum, and Caldilinea, which are involved in sulfate reduction, methane oxidation, and anaerobic ammonium oxidation, respectively, were higher for XP than for XN. The relative abundances of 11 genera were significantly different between MP and MN, whereas this number was only 5 between XP and XN (Fig. 4b, c). Similarly, the difference in the MP-XP pair showed 13 genera more abundant in MP, whereas only 5 genera were more abundant in XP (Fig. 4d). These results indicate that the elemental cycles and material transformation were more active in the vegetation-covered sediment.
Effects of V. natans on Sediment SRB
We found that the recovery of submerged macrophytes significantly influenced the relative abundance of SRB through both FAME analysis and 16S rRNA sequencing. Meanwhile, the highest distribution of SRB and the most active sulfate reduction were usually in the sediment at a depth less than 10 cm [32–34]. Thus, the upper layer sediment samples in this study were analyzed further for the number of gene copies and composition of SRB.
SRB are anaerobic microorganisms that use sulfate as a terminal electron acceptor in the degradation of organic compounds, and they have an important role in both the S and C cycles [35]. Although named after their ability to reduce sulfate, they are also capable of nitrate reduction, nitrogen fixation, and removal of heavy metals [36–38]. The copy number of the gene dsrB, which encodes a subunit of dissimilatory sulfate reductase enzyme, was 1.0–1.7 × 106 copies g−1, which comprised 0.048–0.083% of the total copies of 16S rRNA found in this study. The number of dsrB gene copies was similar to the ranges previously reported in the literature for fishpond sediment [39], mangrove soils [40], and river estuary sediment [41], whereas it was one or two orders of magnitude less than that in marine sediment [42, 43], because the sulfate concentration in marine sediment is much higher than that in freshwater sediment [44].
Special genera of SRB were significantly higher in the sediment from sites covered with submerged macrophytes when compared with that from sites without macrophytes, for example, Desulfococcus, Syntrophobacter, and Desulfomonile for Maojiabu and Syntrophobacter and Desulfobulbaceae_unclassified for Xilihu. The cell number or relative abundance of SRB could be promoted by the growth of plants including emergent macrophyte [44], floating macrophyte [36], and marine submerged macrophyte [45]. Fermentation of cellulose from plant litter produces organic acids and alcohols, which can be then utilized as growth substrates for SRB [46]. The diverse SRB in salt marshes are also closely related to the decomposition of soil organic matter produced by Spartina alterniflora [47]. Oxygen leakage from the root system is a possible yet unambiguous factor that influences the SRB community in the rhizosphere [44], because SRB do not live strictly in anaerobic habitats and can even respire with oxygen [48].
Considering the relative gene abundance of dsrB/16S rRNA, the effect of submerged macrophyte colonization on the SRB community was more obvious in the natural growth region of Xilihu. The abundance of the total SRB community was higher because of the natural vegetation colonization but was not influenced by the artificial colonization. Although the relative abundances of several SRB genera were significantly different within Xilihu, the families Syntrophobacteraceae and Thermodesulfovibrionaceae, which were the dominant SRB members, had higher relative abundances of total SRB in the sites covered with plants. Members of the family Syntrophobacteraceae are flexible in their substrate metabolism, as they can reduce sulfate and have the capability to switch from a sulfate-reducing lifestyle to a syntrophic lifestyle in the absence of sulfate [49]. Their metabolic versatility likely represents a selective advantage in the undisturbed environment.
Planting Versus Natural Growth: Which Is Better?
The recovery delay of submerged macrophytes in lakes refers to an unstable phenomenon that clear state and turbid state may coexist alternatively [50]. Submerged macrophytes play an important role in stabilizing clear state in multiple ways by which the ecosystem resilience increases in a clear water state [51]. Macrophyte rehabilitation by planting can enlarge their coverage and therefore enhance the ecosystem resilience. However, ecosystem behavior is unpredictable accurately [52] after a human intervention, such as planting. A greater influence on the sediment nutrients and microbial community was observed under planting than with natural growth of submerged macrophytes in this study. Planting may result in a decrease in soil fertility [12], soil exchangeable K and Mg contents [53], fungal diversity [54], and regional bird diversity [55], as reported for forest ecosystems. Submerged macrophytes are mainly annual herbaceous plants, and as r-strategists in aquatic ecosystems, their population density may easily experience great fluctuation [50]. Accompanying this fluctuation, sediment TN (mainly Org-N) changes drastically, which, in turn, influences the regeneration of submerged macrophytes in the next growing season (unpublished data). These potential negative impacts should be of concern when considering aquatic ecosystems. We recommend that planting be a preliminary measure for the recovery of submerged macrophytes, for example, providing seed bank and modifying sediment conditions and epiphyte community. However, natural growth is more important for long-term ecosystem stability.
References
Rodrigo MA, Rojo C, Alonso-Guillen JL, Vera P (2013) Restoration of two small Mediterranean lagoons: the dynamics of submerged macrophytes and factors that affect the success of revegetation. Ecol. Eng. 54:1–15. doi:10.1016/j.ecoleng.2013.01.022
Sondergaard M, Johansson LS, Lauridsen TL, Jorgensen TB, Liboriussen L, Jeppesen E (2010) Submerged macrophytes as indicators of the ecological quality of lakes. Freshw. Biol. 55(4):893–908. doi:10.1111/j.1365-2427.2009.02331.x
Scheffer M, Carpenter S, Foley JA, Folke C, Walker B (2001) Catastrophic shifts in ecosystems. Nature 413(6856):591–596. doi:10.1038/35098000
Sand-Jensen K, Pedersen NL, Thorsgaard I, Moeslund B, Borum J, Brodersen KP (2008) 100 years of vegetation decline and recovery in Lake Fure, Denmark. J. Ecol. 96(2):260–271. doi:10.1111/j.1365-2745.2007.01339.x
Hilt S, Van de Weyer K, Koehler A, Chorus I (2010) Submerged macrophyte responses to reduced phosphorus concentrations in two peri-urban lakes. Restor. Ecol. 18:452–461. doi:10.1111/j.1526-100X.2009.00577.x
Korner S (2002) Loss of submerged macrophytes in shallow lakes in north-eastern Germany. Int. Rev. Hydrobiol. 87(4):375–384. doi:10.1002/1522-2632(200207)87:4<375::aid-iroh375>3.0.co;2-7
Lauridsen TL, Jensen JP, Jeppesen E, Sondergaard M (2003) Response of submerged macrophytes in Danish lakes to nutrient loading reductions and biomanipulation. Hydrobiologia 506(1–3):641–649. doi:10.1023/b:hydr.0000008633.17385.70
Hilt S, Gross EM, Hupfer M, Morscheid H, Mahlmann J, Melzer A, Poltz J, Sandrock S, Scharf EM, Schneider S, de Weyer KV (2006) Restoration of submerged vegetation in shallow eutrophic lakes—a guideline and state of the art in Germany. Limnologica 36(3):155–171. doi:10.1016/j.limno.2006.06.001
Sondergaard M, Jeppesen E, Lauridsen TL, Skov C, Van Nes EH, Roijackers R, Lammens E, Portielje R (2007) Lake restoration: successes, failures and long-term effects. J. Appl. Ecol. 44(6):1095–1105. doi:10.1111/j.1365-2664.2007.01363.x
Chen K-N, Bao C-H, Zhou W-P (2009) Ecological restoration in eutrophic Lake Wuli: a large enclosure experiment. Ecol. Eng. 35(11):1646–1655. doi:10.1016/j.ecoleng.2008.10.009
Gulati RD, Pires LMD, Van Donk E (2008) Lake restoration studies: failures, bottlenecks and prospects of new ecotechnological measures. Limnologica 38(3–4):233–247. doi:10.1016/j.limno.2008.05.008
Liao CZ, Luo YQ, Fang CM, Chen JK, Li B (2012) The effects of plantation practice on soil properties based on the comparison between natural and planted forests: a meta-analysis. Glob. Ecol. Biogeogr. 21(3):318–327. doi:10.1111/j.1466-8238.2011.00690.x
Vitali F, Mastromei G, Senatore G, Caroppo C, Casalone E (2016) Long lasting effects of the conversion from natural forest to poplar plantation on soil microbial communities. Microbiol. Res. 182:89–98. doi:10.1016/j.micres.2015.10.002
Lin YT, Hu HW, Whitman WB, Coleman DC, Chiu CY (2014) Comparison of soil bacterial communities in a natural hardwood forest and coniferous plantations in perhumid subtropical low mountains. Bot. Stud. 55:9. doi:10.1186/s40529-014-0050-x
Heilmayr R (2014) Conservation through intensification? The effects of plantations on natural forests. Ecol. Econ. 105:204–210. doi:10.1016/j.ecolecon.2014.06.008
Espinar JL (2006) Sample size and the detection of a hump-shaped relationship between biomass and species richness in Mediterranean wetlands. J. Veg. Sci. 17(2):227–232. doi:10.1111/j.1654-1103.2006.tb02441.x
Costantini ML, Rossi L, Fazi S, Rossi D (2009) Detritus accumulation and decomposition in a coastal lake (Acquatina-southern Italy). Aquat. Conserv. 19(5):566–574. doi:10.1002/aqc.1004
Cornwell JC, Owens MS (2011) Quantifying sediment nitrogen releases associated with estuarine dredging. Aquat. Geochem. 17(4–5):499–517. doi:10.1007/s10498-011-9139-y
Li H, Song CL, Cao XY, Zhou YY (2016) The phosphorus release pathways and their mechanisms driven by organic carbon and nitrogen in sediments of eutrophic shallow lakes. Sci. Total Environ. 572:280–288. doi:10.1016/j.scitotenv.2016.07.221
Obi CC, Adebusoye SA, Ugoji EO, Ilori MO, Amund OO, Hickey WJ (2016) Microbial communities in sediments of Lagos lagoon, Nigeria: elucidation of community structure and potential impacts of contamination by municipal and industrial wastes. Front. Microbiol. 7:16. doi:10.3389/fmicb.2016.01213
Schutter ME, Dick RP (2000) Comparison of fatty acid methyl ester (FAME) methods for characterizing microbial communities. Soil Sci. Soc. Am. J. 64(5):1659–1668
Geets J, Borrernans B, Diels L, Springael D, Vangronsveld J, van der Lelie D, Vanbroekhoven K (2006) DsrB gene-based DGGE for community and diversity surveys of sulfate-reducing bacteria. J. Microbiol. Methods 66(2):194–205. doi:10.1016/j.mimet.2005.11.002
Chaparro JM, Badri DV, Vivanco JM (2014) Rhizosphere microbiome assemblage is affected by plant development. ISME J 8(4):790–803. doi:10.1038/ismej.2013.196
Leon JD, Gonzalez MI, Gallardo JF (2011) Biogeochemical cycles in natural forest and conifer plantations in the high mountains of Colombia. Rev. Biol. Trop. 59(4):1883–1894
Yang K, Zhu JJ, Zhang M, Yan QL, Sun OJ (2010) Soil microbial biomass carbon and nitrogen in forest ecosystems of Northeast China: a comparison between natural secondary forest and larch plantation. J. Plant Ecol. 3(3):175–182. doi:10.1093/jpe/rtq022
da Silva DKA, Freitas ND, de Souza RG, da Silva FSB, de Araujo ASF, Maia LC (2012) Soil microbial biomass and activity under natural and regenerated forests and conventional sugarcane plantations in Brazil. Geoderma 189:257–261. doi:10.1016/j.geoderma.2012.06.014
Song K, Harper WF, Hori T, Riya S, Hosomi M, Terada A (2015) Impact of carbon sources on nitrous oxide emission and microbial community structure in an anoxic/oxic activated sludge system. Clean Techn. Environ. Policy 17(8):2375–2385. doi:10.1007/s10098-015-0979-9
Kindaichi T, Yuri S, Ozaki N, Ohashi A (2012) Ecophysiological role and function of uncultured Chloroflexi in an anammox reactor. Water Sci. Technol. 66(12):2556–2561. doi:10.2166/wst.2012.479
Simek K, Nedoma J, Znachor P, Kasalicky V, Jezbera J, Hornak K, Sed’a J (2014) A finely tuned symphony of factors modulates the microbial food web of a freshwater reservoir in spring. Limnol. Oceanogr. 59(5):1477–1492. doi:10.4319/lo.2014.59.5.1477
Wang Z, Yang YY, Sun WM, Xie SG, Liu Y (2014) Nonylphenol biodegradation in river sediment and associated shifts in community structures of bacteria and ammonia-oxidizing microorganisms. Ecotox Environ Safe 106:1–5. doi:10.1016/j.ecoenv.2014.04.019
Liang SH, Liu JK, Lee KH, Kuo YC, Kao CM (2011) Use of specific gene analysis to assess the effectiveness of surfactant-enhanced trichloroethylene cometabolism. J. Hazard. Mater. 198:323–330. doi:10.1016/j.jhazmat.2011.10.050
Holmer M, Storkholm P (2001) Sulphate reduction and sulphur cycling in lake sediments: a review. Freshw. Biol. 46(4):431–451. doi:10.1046/j.1365-2427.2001.00687.x
Zhang W, Zhang L (2016) Vertical and temporal distributions of sulfate-reducing bacteria in sediments of Lake Erhai, Yunnan Province, China. Earth and Environment (in Chinese) 44(2):177–184
Wang MY, Liang XB, Yuan XY, Zhang W, Zeng J (2008) Analyses of the vertical and temporal distribution of sulfate-reducing bacteria in Lake Aha (China). Environ. Geol. 54(1):1–6. doi:10.1007/s00254-007-0787-6
Muyzer G, Stams AJM (2008) The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol 6(6):441–454. doi:10.1038/nrmicro1892
Acha D, Iniguez V, Roulet M, Guimaraes JRD, Luna R, Alanoca L, Sanchez S (2005) Sulfate-reducing bacteria in floating macrophyte rhizospheres from an Amazonian floodplain lake in Bolivia and their association with Hg methylation. Appl. Environ. Microbiol. 71(11):7531–7535. doi:10.1128/aem.71.11.7531-7535.2005
Marietou A (2016) Nitrate reduction in sulfate-reducing bacteria. FEMS Microbiol. Lett. 363(15). doi:10.1093/femsle/fnw155
Blumenberg M, Hoppert M, Krueger M, Dreier A, Thiel V (2012) Novel findings on hopanoid occurrences among sulfate reducing bacteria: is there a direct link to nitrogen fixation? Org. Geochem. 49:1–5. doi:10.1016/j.orggeochem.2012.05.003
Fernandez ML, Granados-Chinchilla F, Rodriguez C (2015) A single exposure of sediment sulphate-reducing bacteria to oxytetracycline concentrations relevant to aquaculture enduringly disturbed their activity, abundance and community structure. J. Appl. Microbiol. 119(2):354–364. doi:10.1111/jam.12846
Varon-Lopez M, Franco Dias AC, Fasanella CC, Durrer A, Melo IS, Kuramae EE, Andreote FD (2014) Sulphur-oxidizing and sulphate-reducing communities in Brazilian mangrove sediments. Environ. Microbiol. 16(3):845–855. doi:10.1111/1462-2920.12237
Zeleke J, Lu S-L, Wang J-G, Huang J-X, Li B, Ogram AV, Quan Z-X (2013) Methyl coenzyme M reductase a (mcrA) gene-based investigation of methanogens in the mudflat sediments of Yangtze River estuary, China. Microb. Ecol. 66(2):257–267. doi:10.1007/s00248-012-0155-2
Besaury L, Ouddane B, Pavissich JP, Dubrulle-Brunaud C, Gonzalez B, Quillet L (2012) Impact of copper on the abundance and diversity of sulfate-reducing prokaryotes in two chilean marine sediments. Mar. Pollut. Bull. 64(10):2135–2145. doi:10.1016/j.marpolbul.2012.07.042
He H, Zhen Y, Mi T, Xu B, Wang G, Zhang Y, Yu Z (2015) Community composition and distribution of sulfate- and sulfite-reducing prokaryotes in sediments from the Changjiang estuary and adjacent East China Sea. Coast Shelf Sci 165:75–85. doi:10.1016/j.ecss.2015.09.005
Vladar P, Rusznyak A, Marialigeti K, Borsodi AK (2008) Diversity of sulfate-reducing bacteria inhabiting the rhizosphere of Phragmites australis in lake velencei (Hungary) revealed by a combined cultivation-based and molecular approach. Microb. Ecol. 56(1):64–75. doi:10.1007/s00248-007-9324-0
Cifuentes A, Anton J, de Wit R, Rodriguez-Valera F (2003) Diversity of Bacteria and Archaea in sulphate-reducing enrichment cultures inoculated from serial dilution of Zostera noltii rhizosphere samples. Environ. Microbiol. 5(9):754–764. doi:10.1046/j.1470-2920.2003.00470.x
Muyodi FJ, Rubindamayugi MST, Semesi AK (2004) Effect of water hyacinth on distribution of sulphate-reducing bacteria in sediments of Lake Victoria. Water SA 30(3):421–425
Nie M, Wang M, Li B (2009) Effects of salt marsh invasion by Spartina alterniflora on sulfate-reducing bacteria in the Yangtze River estuary, China. Ecol. Eng. 35(12):1804–1808. doi:10.1016/j.ecoleng.2009.08.002
Cypionka H (2000) Oxygen respiration by Desulfovibrio species. Annu. Rev. Microbiol. 54:827–848. doi:10.1146/annurev.micro.54.1.827
Plugge CM, Zhang W, Scholten JCM, Stams AJM (2011) Metabolic flexibility of sulfate-reducing bacteria. Front. Microbiol. 2. doi:10.3389/fmicb.2011.00081
Ibelings BW, Portielje R, Lammens E, Noordhuis R, van den Berg MS, Joosse W, Meijer ML (2007) Resilience of alternative stable states during the recovery of shallow lakes from eutrophication: Lake Veluwe as a case study. Ecosystems 10(1):4–16. doi:10.1007/s10021-006-9009-4
van Altena C, Bakker ES, Kuiper JJ, Mooij WM (2016) The impact of bird herbivory on macrophytes and the resilience of the clear-water state in shallow lakes: a model study. Hydrobiologia 777(1):197–207. doi:10.1007/s10750-016-2779-6
Gunderson LH (2000) Ecological resilience—in theory and application. Ann Rev Ecol Syst 31:425–439. doi:10.1146/annurev.ecolsys.31.1.425
Hou EQ, Wen DZ, Li JL, Zuo WD, Zhang LL, Kuang YW, Li J (2012) Soil acidity and exchangeable cations in remnant natural and plantation forests in the urbanised Pearl River Delta, China. Soil Res 50(3):207–215. doi:10.1071/sr11344
Sharma G, Pandey RR, Singh MS (2011) Microfungi associated with surface soil and decaying leaf litter of Quercus serrata in a subtropical natural oak forest and managed plantation in Northeastern India. Afr. J. Microbiol. Res. 5(7):777–787. doi:10.5897/AJMR10.621
Martinez-Jauregui M, Diaz M, de Ron DS, Solino M (2016) Plantation or natural recovery? Relative contribution of planted and natural pine forests to the maintenance of regional bird diversity along ecological gradients in Southern Europe. For. Ecol. Manag. 376:183–192. doi:10.1016/j.foreco.2016.06.021
Acknowledgements
We thank Zhigang Dai, Jian Sun, and Fenli Min for the sediment sample collection on the lake. This study was supported by the Major Science and Technology Program for Water Pollution Control and Treatment of China 12th Five-Year Plan (No. 2012ZX07101007-005) and the Key Research Program of the Chinese Academy of Sciences (No. KFZD-SW-302-02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Chuan Wang and Shuangyuan Liu are co-first authors of this paper.
Electronic Supplementary Material
ESM 1
(DOCX 353 kb)
Rights and permissions
About this article
Cite this article
Wang, C., Liu, S., Zhang, Y. et al. Effects of Planted Versus Naturally Growing Vallisneria natans on the Sediment Microbial Community in West Lake, China. Microb Ecol 74, 278–288 (2017). https://doi.org/10.1007/s00248-017-0951-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-017-0951-9