Abstract
Fungal communities associated with early stages of decomposition of Spartina maritima (Curtis) Fernald were assessed in two geographically distinct salt marshes in Portugal by direct observation of fungal sporulating structures. Twenty-three fungal taxa were identified from 390 plant samples, 11 of which were common to both study sites. Natantispora retorquens, Byssothecium obiones, Phaeosphaeria spartinicola, Phoma sp. 1 and Stagonospora sp. were the most frequent fungal taxa in the studied communities. The fungal species Anthostomella spissitecta, Camarosporium roumeguerii, Coniothyrium obiones, Decorospora gaudefroyi, Halosarpheia trullifera, Leptosphaeria marina and Stagonospora haliclysta were recorded for the first time on S. maritima plants; with the exception of C. roumeguerii and L. marina, all of these species were also new records for Portugal. The differences between species composition of the communities associated with S. maritima were attributed to differences in abiotic conditions of the salt marshes. Although the fungal taxa were distributed differently along the host plants, common species to both fungal communities were found on the same relative position, e.g. B. obiones, Lulworthia sp. and N. retorquens occurred on the basal plant portions, Buergenerula spartinae, Dictyosporium pelagicum and Phoma sp. 1 on the middle plant portions and P. spartinicola and Stagonospora sp. on the top plant portions. The distinct vertical distribution patterns reflected species-specific salinity requirements and flooding tolerance, but specially substrate preferences. The most frequent fungi in both communities also exhibited wider distribution ranges and produced a higher number of fruiting structures, suggesting a more active key role in the decay process of S. maritima.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine fungi constitute an ecological group of fungi that colonize marine environments, ranging from intertidal zones to open ocean areas [35]. The greatest diversity of marine fungi, though, is found in estuarine ecotones, given the higher productivity and availability of substrates for colonization [e.g. 3, 38, 44, 48, 56, 63, 98]. It is, in fact, in salt marshes and mangroves that saprobic marine fungi play a key role in the ecosystem’s ecological balance and dynamics by contributing to the degradation of complex organic matter and recycling of nutrients.
Spartina species, one of the most dominant primary producers in temperate salt marshes [21], represent simultaneously one of the main substrates for saprobic marine fungi. These cordgrasses are highly enriched with lignocellulose (c.a. 75 % of total biomass; [42, 67]) and strictly depend on an active decomposition process to release the nutrients into the surrounding environment. This process is mainly triggered and carried out by ascomycetous fungi [e.g. 9, 71, 77, 81, 104]. Likewise in other grass-like plants, the major involvement of fungal species in the decomposition process occurs during the early stages when the senescent organs are still attached to standing-live plants in natural positions [22, 66, 74, 79, 95]. In fact, senescence and consequent decomposition processes may begin even before these plants have reached physiological maturity, occurring gradually from the outer and lower vegetative structures towards the inner and higher structures [72]. Saprobic ascomycetous fungi were found to dominate the living microbial biomass on standing-decaying shoots of cordgrasses in the form of mycelia and reproductive structures, being the principal secondary producers [71, 72, 77, 79, 80].
The marine fungal colonizers of intertidal cordgrasses exhibit species-specific ecological patterns that determine their distribution on the plants. Barata [6], Cornick et al. [26], Kohlmeyer and Volkmann-Kohlmeyer [60, 61] and Kohlmeyer et al. [62, 64] considered the tidal regime, the vertical distribution of fungi in standing grasses and the definition proposed by Kohlmeyer and Kohlmeyer [58] to distinguish between obligate and facultative marine fungi and set ecological boundaries; obligate marine fungi colonize preferentially lower portions of the plants, halotolerant terrestrial fungi inhabit aerial non-immersed parts and facultative marine fungi occur in between. The differentiation of these ecological groups of fungi based exclusively on this criterion is not easy or reliable in all circumstances since there are other factors interfering in the vertical distribution of fungi on host plants, such as plant tissue type [6, 38, 94] or interspecific competition [12, 72, 77]. The relative subjectivity of the criterion to distinguish facultative from obligate fungi prompted Jones et al. [53] to consider some of the described facultative fungal species as obligate fungal species. Nevertheless, on the dependence of personal opinion of the criterion, it is important to clarify the possible origin and ecological requirements of each fungus in order to better understand its role in functioning of the ecosystem.
According to the classification of Jones et al. [53], the current list of obligate marine fungi associated with Spartina spp. includes 53 fungal species [16]; most of these species were identified on standing-decaying culms of Spartina alterniflora Loisel in North American salt marshes [e.g. 5, 11, 39, 72, 75, 82, 95, 110]. A comparison among studies on different Spartina species in geographically distant salt marshes using different morphological and/or molecular approaches highlighted a core group of marine fungi composed by the same ascomycetous species. Specifically, Phaeosphaeria spartinicola, Mycosphaerella sp. II and Phaeosphaeria halima have been mentioned as ubiquitous and dominant colonizers of Spartina leaf blades [e.g. 11, 12, 39, 58, 66, 72, 75, 78, 82, 110] and Buergenerula spartinae on leaf sheaths [72] and Byssothecium obiones on stems [6, 81]. These mentioned ascomycetous fungi were found to play an important functional role in the degradation of lignocellulosic secondary walls of plant cells [8, 9, 65, 77, 81, 104]. The presence of these fungal species on Spartina plants over a wide geographic range and the absence from other standing plants colonizing the same habitat suggested that these saprobic fungi are host–genus exclusive [5, 105, 110]. Host exclusivity was proposed by Zhou and Hyde [111] to apply in the cases of an exclusive occurrence of a strictly saprobic fungus on a particular or on a restricted range of related host plants, which does not reveal any symbiotic phase during its life cycle.
Although this core group of fungi is considerably well-known, in terms of species composition and general ecological preferences, there are still gaps in understanding the ecology of each fungal species and its specific role on decomposition process.
South European salt marshes are dominated by Spartina maritima (Curtis) Fernald, one of the main primary producers of these ecosystems [20, 102], and the marine mycota associated with this plant has been surprisingly poorly investigated. Barata [6] surveyed S. maritima standing plants from three salt marshes situated in the central west coast of Portugal and identified 20 fungal taxa; in one of these salt marshes, Barata [7] recorded 26 colonizers of S. maritima baits exposed to different submersion conditions. Azevedo et al. [4] also inventoried the saprobic marine mycota associated with S. maritima, but from drift substrates collected in four Portuguese west coast beaches; 31 fungal taxa were recorded on S. maritima stems. Although the fungal community associated with standing plants and drift stems included some common fungal species and belonging to the core group, both substrates were dominated by different fungal species [4, 6].
Therefore, and in a general perspective, the present study intends to be the first comprehensive study of fungi associated with S. maritima in Portugal, providing key information on ecological requirements of fungi inhabiting standing-live plants.
Specifically, this study aims to contribute to: (1) the inventory of higher filamentous marine fungi associated with S. maritima, (2) a better understanding of ecology and functional role of fungi in early stages of decomposition of S. maritima and (3) the evaluation of the effects of seasonality and environmental parameters on fungal community by comparing two Portuguese salt marshes with distinct geographical locations, biophysical structures, anthropogenic pressures and representativeness of this host plant. Fungal species were identified by direct observation of the reproductive structures (traditional microscopy-based methods) and then classified into obligate or facultative marine fungi based on the average vertical position on plants and salt requirements for growth assessed by a culture-dependent assay.
Material and Methods
Study Sites
The study was conducted in two salt marshes: the Guadiana estuary (Castro Marim) situated in the southeastern coast (37.23° N, 7.42° W) in the Mediterranean region [27] and the Ria de Aveiro coastal lagoon located in the northwest of Portugal (40.62° N, 8.74° W) included in Eurosiberian region [27] (Fig. 1).
Both ecosystems are mesotidal, with a mean tidal range of 2.0 m, and have predominantly semi-diurnal tides that dominate the hydrodynamics of the systems [32, 70]. However, the two study sites exhibit a different physical configuration. Lower Guadiana estuary consists of a narrow channel bordered by marsh ecosystems, which is oriented perpendicular to the coast and connects the fluvial channel with the open littoral zone [70]. Ria de Aveiro coastal lagoon runs parallel to the coastline and consists of a complex network of channels surrounded by mud flats and salt marshes; the lagoon is permanently connected to the Atlantic Ocean by a deep and narrow artificial channel [32, 33]. The freshwater flow in both systems is also different; Guadiana estuary receives a high input of freshwater from Guadiana river whereas Mira channel in Ria de Aveiro lagoon receives a lower freshwater discharge from a small system of ponds and rivers [31, 55].
Additional details of study sites are summarized in Table 1.
The conservation state of both study sites is also different as a result of different conservation status and anthropogenic pressure. Guadiana estuary is protected as a Natural Reserve, being subjected to less negative human impacts [14, 15]. In contrast, Ria de Aveiro lagoon was for decades (and until 1994) the main receptor of highly contaminated effluent discharges [84, 89], and a relatively low mercury fraction is still present in the water column, sediment and biota [24, 25, 89].
Host Plant
In addition to being one of the main primary producers, S. maritima represents an important pioneer grass that occupies the first level of emerged vascular vegetation. Given its rhizomatous nature, it assumes a fundamental role in the protection of coastline from erosion by trapping and aggregating sediment within the clumps [18, 36, 97] and in the reduction of eutrophication of the system by sequestering nutrients and metals from sediments [15, 28, 29, 102]. S. maritima communities include distinguishable tall and short growth forms, which have been attributed to genotypic differences [85, 96] and phenotypic plasticity to different environmental conditions [19]. In Castro Marim salt marsh, S. maritima plants are shorter (average plant height 39 ± 5 cm) and with more inrolled and smaller leaf blades (1/3 of total plant height) whereas in Ria de Aveiro salt marsh, plants are taller (average plant height 49 ± 6 cm) and with more expanded and larger leaf blades (1/2 of total plant height). In addition to intraspecific differences in morphology, both communities exhibited different distribution patterns; in Castro Marim salt marsh, S. maritima community forms extensive monotypic beds along the riverside whereas in Ria de Aveiro salt marsh, community is fragmented and disrupted in relatively small and dispersed patches.
Although both communities of S. maritima follow the natural phenological cycle, with a growing season occurring during spring to early summer, plants of different maturation phases are present throughout all seasons.
Sampling Procedure
Mature standing-live plants experiencing the same daily tidal wet–dry cycles (i.e. occupying the same topographic level, with similar height and containing green, senescent and early-decay plant tissues) were randomly collected in Castro Marim and Ria de Aveiro salt marshes (2.45 and 2.37 m above the Portuguese hydrographic zero, respectively), bimonthly over a 2-year period (October 2010 to August 2012). Twenty plants were collected each of the first 3 sampling periods and 15 plants afterwards (a total of 390 plants). Five additional plants were also collected in each period of the last sampling year (February 2012 to August 2012) for isolation of marine fungi (a total of 20 plants). Plant samples were placed in plastic bags and returned to laboratory.
Morphology-Based Species Identification
The collected plants were carefully rinsed with running tap water to remove fine-grained sediments and seaweeds and air-dried. Each air-dried plant was sequentially analysed from the basal to the top portion and from the external vegetative structures (leaf sheaths and blades) towards the more internal structures (stems). Fungal structures (fruit bodies, spores and hyphopodia) observed on each vegetative structure were picked up under a dissecting microscope (Wild M8) mounted into a drop of sterile seawater on a slide examined under a light microscope (Leitz Laborlux S, with Normaski) with detailed morphology recorded. The fungi were identified using the dichotomous keys of Kohlmeyer and Kohlmeyer [58], Kohlmeyer and Volkmann-Kohlmeyer [59], Hyde and Sarma [45] and Jones et al. [53]. The vertical position of identified fungi was also recorded, as well as the density of produced fungal structures; for more than 10 fungal structures per square centimeter of colonized vegetative structure, the density was considered high. The fungal structures were photographed and preserved on microscope slides after replacement of seawater by glycerin and sealed with several layers of nail varnish. Moreover, some of the identified fungal structures were maintained on the original dry plant material and included in the personal collection of M. Barata (Herbarium of the University of Lisbon—LISU).
Isolation of Marine Fungi and Preservation of Pure Cultures
Cultures were obtained by single spore method, according to the conventional procedures of Vrijmoed [109]. Five fruiting structures (ascomata or pycnidia) of a given fungal taxon growing on fresh plant materials were transferred into a drop of sterile seawater on a microscope slide and squashed to force the discharge of the spores. This suspension of spores was then transferred with a Pasteur pipette onto gridded plates containing cornmeal agar made with aged diluted seawater (CMA/sw 50 %) and supplemented with chloramphenicol (0.05 %), one drop per square. Plates were incubated at room temperature for 1–2 days until germination of the spores. Each germinated spore was then transferred onto a new CMA/sw plate.
In order to establish a culture collection, each isolated fungus was subcultured and preserved by three methods: (1) one colony was maintained on CMA/sw plate at 4 °C, (2) plugs removed from the growing margin of four colonies were transferred to McCartney bottles filled with sterile diluted seawater (50 %) and kept at 4 °C and (3) to cryotubes filled with glycerol (10 %) and stored at −80 °C.
Growth Rates
Growth rates of selected fungi were determined in cornmeal agar media made with diluted seawater (CMA/sw 50 %) and with distilled water (CMA/dw) at room temperature (18–25 °C). With this purpose, an agar disc was cut from the growing edge of fungal colonies and inoculated at the intersection point of two perpendicular lines previously drawn on the bottom of CMA/sw 50 % and CMA/dw plates; three replicates were performed for each monospore isolate. The colony growth was assessed every 2 days, for 30 days, by measuring and averaging the colony diameter along the two perpendicular axes.
Data Analyses
Diversity Indices
The following diversity indices were calculated in order to better characterize and compare the fungal communities inhabiting Castro Marim and Ria de Aveiro salt marshes: Shannon diversity index (H′ = − ∑ Si = 1 pi ⋅ ln(pi), where s is the number of fungal taxa in the community and pi is the proportion of occurrences of fungal taxon i relative to total number of occurrences, Shannon’s equitability index (E = H′/H max, where Hmax = ln s ) and Sorenson similarity index (Sl = 2j/(a + b), where j is the number of common fungal taxa to both sites, a is the number of fungal taxa in one site and b is the number of fungal taxa in the other site). The comparison of Shannon diversity indices between study sites was performed based on randomization procedures of bootstrapping using the PAST v2.17c statistical software [41]. P values were estimated by resampling and randomly redistributing the data 1000 times [34]; differences were considered statistically significant for p value <0.05.
The average number of fungal taxa per plant sample (total number of fungal occurrences divided by the total number of plant samples) was also determined for Castro Marim and Ria de Aveiro salt marshes and for the assembly of the two salt marshes.
Frequencies of Occurrence and Vertical Distribution Patterns—Total and in Each Sampling Period
The percent frequency of occurrence for each taxon in the fungal community was assessed (number of plant samples colonized by a specific fungus divided by the total number of plant samples × 100). Fungal taxa were grouped according to the percent frequency of occurrence and the classification proposed by Tan et al. [103] in very frequent (>20 %), frequent (10–20 %) and infrequent (<10 %).
The average vertical distribution data of common fungal taxa in both study sites were compared by Student’s t tests using IBM SPSS v22.0 statistical software (IBM Corporation, Somers, NY).
In an attempt to better discriminate vertical distribution patterns and ecological requirements of fungal taxa in the two salt marshes, three vertical microhabitats were defined by separating the plant samples in three equal portions based on maximum plants height (basal 0–20 cm, middle >20–40 cm, top >40–60 cm). For each plant portion, the same diversity indices (Shannon, Equitability and Sorensen similarity indices) were determined; comparisons among Shannon diversity indices in plant portions were performed adopting the same procedures described above. Additionally, frequencies of occurrence of each fungal taxon, in each sampling period, in each plant portion were calculated. These dataset matrices were used to perform a preliminary Cluster Analysis with Bray-Curtis similarity measure; seven samples were considered outliers, given the atypical and divergent behavior and excluded from the subsequent analyses. The reconstructed dataset matrices were then used to perform another cluster analysis and a detrended correspondence analysis using the PAST v2.17c statistical software.
The effect of seasonality on fungal communities was assessed by analysing the variations of the frequencies of occurrence and vertical positions of all fungal taxa during the two sampling years; the former parameters were interpreted graphically, and the second parameters were tested statistically using IBM SPSS v22.0 software. A two-way analysis of variance (ANOVA) was performed in order to test the effect of sampling periods and fungal taxa on the total variations of vertical distributions in both communities. After this procedure, a new one-way ANOVA was performed for each taxon to evaluate the statistical significance of its vertical distribution variation.
Flooding Regime in Castro Marim and Ria de Aveiro Study Sites
The flooding conditions in both study sites were assessed, given the differences in the physical configuration of intertidal systems and in the morphology of host plant. The percentage of days in each month that Spartina plants were totally submerged, at least once, was determined, considering average plants height, tidal range (high tides height) and the topographic position of the plants on both salt marshes. The average time per day that the plants remained flooded at each sampling site was estimated using a model developed by Serôdio and Catarino [99]. The frequency and time length of flooding were also determined for basal, middle and top plant portions.
Vegetative Growth Rates of Fungal Isolates
The growth rates were extracted from linear regression equation of colony diameter increase over 30 days. The differences between growth rates in the two culture media were assessed with Student’s t tests using IBM SPSS v22.0 statistical software.
Results
Fungal Diversity
Twenty-three sporulating higher filamentous marine fungi were recorded from the total 390 analysed plants, with 20 and 14 fungal taxa occurring in Castro Marim and Ria de Aveiro salt marshes, respectively (Table 2; Fig. S1, Online Resource). The average number of fungi per plant was found to be five in both sites.
The fungal communities of Castro Marim and Ria de Aveiro salt marshes were mostly composed of the Ascomycota, representing 60 % (12) and 71 % (10) of the total number of fungal taxa and 76 % (769) and 82 % (762) of the number of occurrences, respectively. The remaining fungal taxa found in both study sites belong to asexual fungi, mainly coelomycetes (30 % in Castro Marim and 21 % in Ria de Aveiro). Ascomycetous fungi were restricted to Dothideomycetes, Sordariomycetes and Sordariomycetes incertae sedis; Pleosporales, Microascales (i.e. Halosphaeriaceae) and Capnodiales were the most representative orders, with 33, 25 and 17 % in Castro Marim and 20, 30 and 20 % in Ria de Aveiro, respectively.
Although the diversity of fungal community was significantly higher in Castro Marim than Ria de Aveiro (p<0.01), both communities revealed a similar high equitability value.
The results also evidenced a high overlap between fungal communities of Castro Marim and Ria de Aveiro salt marshes regarding species composition and common taxa. From the 23 total fungal taxa associated with S. maritima, 48 % (11) were common between the study sites. Natantispora retorquens, B. obiones, P. spartinicola, Phoma sp. 1 and Stagonospora sp. were very frequent in both communities. Sorensen’s index revealed a similarity of 0.65 between both fungal communities.
The main differences between the two fungal communities were the number of exclusive infrequent fungal taxa, which was higher in Castro Marim than in Ria de Aveiro salt marsh (6 vs 2). Moreover, the former study site included two frequent and one very frequent exclusive fungal taxa, namely Leptosphaeria marina, Decorospora gaudefroyi and Phoma sp. 2, respectively, while Ria de Aveiro harboured only one very frequent exclusive fungal species, Mycosphaerella sp. I. Sphaerulina orae-maris was very frequent in Castro Marim and infrequent in Ria de Aveiro salt marsh.
Vertical Distribution of Fungi
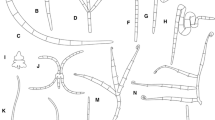
Fungal taxa inhabiting S. maritima in both study sites were distributed vertically along the plant, displaying distinct distribution patterns; some were restricted to the upper or lower portions of the plants while others spanned widely along the plant, showing different extents of substrate occupation (Fig. 2).
Vertical distribution of fungal taxa on standing S. maritima in Castro Marim (a) and Ria de Aveiro (b) salt marshes. The boxplot shows the distribution of the average vertical positions of each fungal taxon on all plant samples: The quartiles include 50 % of the distribution, and the whiskers indicate the spread of the data outside the upper and lower quartiles. The grey circle represents the average of the average vertical positions on all samples. The black marker represents the average vertical position where the density of fruiting structures is higher in all plant samples (for the majority of the rare or infrequent fungal taxa, it was not observed a high number of fruiting structures on the plant samples, and for this reason this information is lacking in the figure). B. spartinae differentiated hyphopodia and ascomata at different vertical levels on the plant, being divided in two groups accordingly with the type of structure
In general, the fungi produced a higher number of reproductive or other fungal structures at the average vertical position of their distribution (Fig. 2).
A comparison between vertical distribution data of common fungal taxa in Castro Marim and Ria de Aveiro salt marshes demonstrated that the differences were statistically significant (p < 0.05), except for Dictyosporium pelagicum. However, the shared fungal taxa appeared to occupy the same relative vertical position on the plants, despite the variations on the absolute vertical distribution.
Fungal subcommunities on basal, middle and top portions of the plants in both study sites were shown to be considerably different by diversity indices (Table 3), cluster analysis (Fig. 3) and Detrended Correspondence Analysis (DCA; Fig. 4).
Cluster dendrogram based on Bray-Curtis similarity of fungal communities colonizing basal, middle and top plant portions in each sampling period and study site (a Castro Marim, b Ria de Aveiro), considering the frequency of occurrence of fungal taxa. The first two letters of the code name indicate the study site (CM Castro Marim, AV Ria de Aveiro), the next three letters and two numbers designate the month and year of the collection, respectively, and the last number indicates the plant portion (1 basal, 2 middle, 3 top)
Two-dimensional DCA plot expressing the fungal taxa and the three vertical plant portions spatial distributions based on frequency of occurrence of fungal taxa in each portion in each sampling period and in each study site (a Castro Marim, b Ria de Aveiro). The black pentagons, dark grey triangles and light grey polygons correspond to basal, middle and top plant portions, respectively. The two-letter code represent fungal taxa: AC Aniptodera chesapeakensis, BO Byssothecium obiones, BS Buergenerula spartinae ascomata, BS(hyp) Buergenerula spartinae hyphopodia, CO Coniothyrium obiones, CR Camarosporium roumeguerii, DG Decorospora gaudefroyi, DP Dictyosporium pelagicum, LM Leptosphaeria marina, Lu Lulworthia sp., My1 Mycosphaerella sp. I, My2 Mycosphaerella sp. 2, NR Natantispora retorquens, Ph1 Phoma sp. 1, Ph2 Phoma sp. 2, PS Phaeosphaeria spartinicola, PV Panorbis viscosus, SH Stagonospora haliclysta, SO Sphaerulina orae-maris, St Stagonospora sp.
Middle portion yielded the highest species richness and diversity than either basal or top portions (Table 3). The differences in the Shannon indices were statistically significant between basal/middle and middle/top portions (p < 0.001), but not between basal/top portions in Castro Marim and Ria de Aveiro salt marshes (p>0.05).
The fungal subcommunities inhabiting basal and middle plant portions revealed higher similarities considering species richness and fungal taxa composition in both study sites than those in basal/top portions and in middle/top portions.
The cluster and DCA analyses, which provided an integrated overview of spatial arrangement of fungal community based on the frequencies of occurrence of fungal taxa in each vertical plant portion corroborated the existence of three distinct microhabitats supporting distinct fungal subcommunities (Figs. 3 and 4).
The cluster analysis (Fig. 3) performed with Castro Marim dataset separated first the top plant portion (ca. 0.29 of similarity) and then basal (ca. 0.31 of similarity) from middle portion, coinciding exactly with the defined microhabitats; with Ria de Aveiro dataset, the analysis only distinguished clearly the basal plant portion from the middle and top portions (ca. 0.42 of similarity).
The DCA reinforced the results from the previous analysis (Fig. 4). Along the axis 1, with the higher eigenvalue (Castro Marim: 0.52; Ria de Aveiro: 0.86) and explanatory power, there was a clear spatial separation of the three plant portions in Castro Marim dataset, which was not so evident between middle and top portions in Ria de Aveiro dataset. The graphical separation of basal, middle and top portions followed the natural vertical sequence of microhabitats, which confirmed the higher similarity of fungal subcommunities between adjacent plant portions.
The DCA analysis also highlighted specific ecological niches by plotting the distribution of fungal taxa across plant portions. Fungal taxa were distributed along the axis 1 following the vertical distribution on the standing plants in Castro Marim and Ria de Aveiro salt marshes, from the top to the basal plant portions. The spatial proximity of each fungal taxon to a certain plant portion in the plot suggested higher affinities to that particular microhabitat. Thus, the results evidenced a subcommunity associated with basal portions, mainly represented by B. obiones, Lulworthia sp. and N. retorquens in both salt marshes, and Panorbis viscosus and L. marina in Castro Marim; a subcommunity colonizing middle portions composed by B. spartinae, D. pelagicum and Phoma sp. 1 in both study sites and Coniothyrium obiones, D. gaudefroyi, Phoma sp. 2 and S. orae-maris in Castro Marim; and a subcommunity associated with upper portions composed by P. spartinicola and Stagonospora sp. in both salt marshes, Mycosphaerella sp. I in Ria de Aveiro, and Mycosphaerella sp. 2 and Stagonospora haliclysta in Castro Marim.
Both axes 2 and 3 presented a lower eigenvalue in Castro Marim (axis 2: 0.12; axis 3: 0.06) and Ria de Aveiro (axis 2: 0.08; axis 3: 0.06) datasets, explaining little variation in the data.
Comparisons of micro-environmental conditions on the three plant portions revealed some differences. Specifically, it was observed a decrease in flooding time (from 8 to 2 daily hours) and frequency (from 100 to 50 % of the days per month) along the vertical axis of the plants, from the basal upwards to the top portions, in both salt marshes; although it was not measured in this study, this vertical gradient of tidal flooding reflected obviously in salinity and water availability levels in each plant portion. Middle and top plant portions in Castro Marim salt marsh remained slightly longer and were more frequently submerged than analogous portions in Ria de Aveiro salt marsh. Furthermore, it was found that the vegetative structures in each plant portion were different: basal portions included mostly a senescent naked stem or a stem enwrapped by leaf sheaths; middle portions included mainly a stem enwrapped by leaf sheaths and leaf blades; top portions included mostly upright-standing leaf blades. Nevertheless, the host plants from the two salt marshes presented some differences in the proportions of vegetative structures included in analogous portions, as a result of differences in the morphology. The main differences were found in the middle plant portions; Castro Marim plants included mostly the stem and leaf sheaths whereas Ria de Aveiro plants also included leaf blades in this portion.
Seasonality
The effects of seasonality on fungal community dynamics and particularly in the frequencies of occurrence of the most frequent fungal taxa in the two study sites (Fig. 5) and for fungi producing a high density of fruiting structures (Fig. 6) were investigated.
Bimonthly variation of the frequency of occurrence (%) of each fungal taxon in Castro Marim (a) and Ria de Aveiro (b) salt marshes and average variation of the frequencies of occurrence in each sampling period: BO Byssothecium obiones, BS Buergenerula spartinae, DG Decorospora gaudefroyi, LM Leptosphaeria marina, Lu Lulworthia sp., MyI Mycosphaerella sp. I, NR Natantispora retorquens, Ph1 Phoma sp. 1, Ph2 Phoma sp. 2, PS Phaeosphaeria spartinicola, SO Sphaerulina orae-maris, St Stagonospora sp.
Bimonthly variation of frequency of occurrence (%) of high density of fungal structures produced by each fungal taxon in Castro Marim (a) and Ria de Aveiro (b) salt marshes and average variation of the frequencies of occurrence in each sampling period: BO Byssothecium obiones, BS Buergenerula spartinae, LM Leptosphaeria marina, Lu Lulworthia sp., My1 Mycosphaerella sp. I, NR Natantispora retorquens, Ph1 Phoma sp. 1, Ph2 Phoma sp. 2, PS Phaeosphaeria spartinicola, SO Sphaerulina orae-maris, St Stagonospora sp. Buergenerula spartinae only differentiated a high density of hyphopodia and not fruiting structures
The results showed that seasonally driven changes in environmental conditions apparently had no significant effect on the presence and life cycle of N. retorquens in Ria de Aveiro salt marsh, but interfered slightly on the presence and production of fruiting structures of the remaining fungi for both communities (Figs. 5 and 6).
Although no obvious species-specific seasonal patterns were detected, the presence of P. spartinicola and Stagonospora sp. on Spartina plants in Castro Marim and Ria de Aveiro salt marshes was generally lower during warmer months than in cooler periods. Similarly, for these mentioned fungal taxa and also for N. retorquens in Castro Marim salt marsh, it was observed a decrease in the production of fruiting structures during the spring–summer seasons.
Despite the seasonal effect on fungal communities, the dominance pattern was maintained in both salt marshes, i.e. the most frequent fungi were the same over time. In addition to being omnipresent in the communities, these fungi were also investing more intensively in sexual or asexual reproduction and/or differentiating other fungal structures in Castro Marim and Ria de Aveiro salt marshes (Figs. 5 and 6). Seasonal variation on the vertical positions of fungal taxa that occurred during the sampling periods on the plants was also investigated (Fig. 7).
Bimonthly variation in the average height of host plants and vertical position of fungal taxa that occurred in most of the sampling periods on the plants in Castro Marim (a) and Ria de Aveiro (b) salt marshes: BO Byssothecium obiones, LM Leptosphaeria marina, Lu Lulworthia sp., My1 Mycosphaerella sp. I, NR Natantispora retorquens, Ph1 Phoma sp. 1, PS Phaeosphaeria spartinicola, SO Sphaerulina orae-maris, St Stagonospora sp.
The results evidenced statistically significant variations on the mean positions of fungal taxa during the study period in both sites (Castro Marim: p < 0.001, F = 1.97; Ria de Aveiro: p < 0.05, F = 1.42). The one-way ANOVA performed for each fungal taxon revealed that the differences were statistically significant (p < 0.05) for all fungal taxa, except for L. marina. However, the relative mean position of each fungus on the plants seemed to be maintained, as well as the spatial pattern of occupancy along the vertical axis of the plant by different fungal taxa.
Salinity Requirements of Fungi
To confirm the salinity requirements for the isolated fungi with vertical distribution pattern on the standing plants in natural environment, a culture experiment was performed. Fifteen strains were randomly selected from the 57 isolated fungi from Castro Marim salt marsh representing 8 fungal taxa, namely B. spartinae (2 strains), B. obiones (2 teleomorph strains), L. marina (2 strains), N. retorquens (2 strains), P. spartinicola (2 strains), Phoma sp. 1 (2 strains), S. orae-maris (1 strain) and Stagonospora sp. (2 strains); 13 strains were selected from the 66 isolated fungi from Ria de Aveiro salt marsh representing 6 fungal taxa, specifically B. obiones (2 teleomorph strains and 2 anamorph strains), Lulworthia sp. (2 strains), N. retorquens (1 strain), P. spartinicola (2 strains), Phoma sp. 1 (2 strains) and Stagonospora sp. (2 strains).
The comparison between mycelia growth rates under two different culture conditions, on media lacking and containing diluted seawater, provided additional information about the ecological preferences of each taxon (Fig. 8).
Daily growth rate of eight fungal taxa isolated from Castro Marim (a) and six fungal taxa isolated from Ria de Aveiro (b) salt marshes under two culture conditions, media with diluted seawater (SW) and with distillate water (DW): BO Byssothecium obiones (teleomorph), BO(an) Byssothecium obiones (anamorph), BS Buergenerula spartinae, LM Leptosphaeria marina, Lu Lulworthia sp., NR Natantispora retorquens, Ph1 Phoma sp. 1, PS Phaeosphaeria spartinicola, SO Sphaerulina orae-maris, St Stagonospora sp. The differences between growth rates were considered statistically significant (*p < 0.05; **p < 0.01; ***p < 0.001) or non-significant (ns, p > 0.05)
The obtained results indicated that all the tested fungal strains grew in both culture conditions. Although some strains representing the same species exhibited contradictory growth results, the majority of strains showed significantly higher growth under saline conditions. An exception was L. marina, which grew faster in media without seawater, and P. spartinicola, which grew equally well under both conditions. Additionally, the results revealed different growth rates among fungal taxa, with Lulworthia sp., Stagonospora sp. and B. spartinae displaying the highest growth rates on both media.
Discussion
Fungal Diversity
In the present study, 23 fungal taxa were identified associated with early stages of decomposition of S. maritima; 20 fungal taxa inhabited Castro Marim salt marsh and 14 occurred in Ria de Aveiro salt marsh (Table 2). Both fungal communities were predominantly represented by the Ascomycota, particularly Dothideomycetes and Sordariomycetes. The clear preference and dominance of these taxonomic groups in intertidal habitats, characterized by alternate cycles of immersion and exposure, have been widely documented in several studies [e.g. 2, 5, 6, 39, 40, 79, 80, 82, 95].
The species richness and diversity of fungal communities colonizing S. maritima in Castro Marim and Ria de Aveiro salt marshes were similar to those found in the same host plant by Barata [6, 7], in S. alterniflora by Gessner [39], Samiaji and Barlocher [95] and Al-Nasrawi and Hughes [5], and in Spartina densiflora Brongn. by Peña and Arambarri [87]. Both fungal communities from Castro Marim and Ria de Aveiro salt marshes were found to be well-balanced, without a clear dominance. These findings corroborated observations by Gessner et al. [38] and Van Ryckegem et al. [108], who denoted that fungal communities associated with Spartina spp. are not particularly complex, with a low diversity and few dominant species.
Fungal species composition of communities associated with Spartina spp.
A comparison between the species composition of the studied fungal communities associated with S. maritima and the list of marine fungi reported from Spartina species [6, 16, 58, 61] revealed nine common fungal species. These species can be categorized into three groups according to their geographical distribution and substrate specificity: (1) host–genus-exclusive fungi that have been described from different Spartina species in different geographical locations, namely Anthostomella spissitecta, B. spartinae, B. obiones, Mycosphaerella sp. I and P. spartinicola [6, 26, 40, 61, 87, 110]; (2) temperate fungi that have a broad substrate preference, such as L. marina and S. orae-maris, which were found on Spartina spp. [6, 23, 40, 58, 88, 101] and also on Juncus roemerianus [23, 58] and driftwood [23, 37, 58, 87]; and (3) cosmopolitan fungal species that have also been recorded on a wide variety of substrates, such as Aniptodera chesapeakensis and D. pelagicum in temperate [7, 37, 52] and tropical climates [3, 46, 54, 68, 90, 94].
In this study, B. spartinae, B. obiones and P. spartinicola, mentioned in the literature as the main colonizers of decaying Spartina plants [6, 11, 12, 26, 39, 66, 72, 75, 78, 82, 110], were also very frequent or frequent in both studied communities.
The high number of common fungal species colonizing different Spartina hosts corroborated the existence of a very stable core group of fungi, mainly dominated by the same host-exclusive fungi. This core group apparently is not much affected by variations in abiotic conditions [40].
Different Spartina species, however, supported some different fungal species. This finding, previously demonstrated by Blum et al. [10] and Lyons et al. [66], was attributed to the higher variation in the morphology and chemical composition between different host species than within the same species. In fact, and as pointed out by several authors [40, 105], the substrate quality appears to be primarily responsible for determining fungal community composition. This reason could explain the absence of some fungal species that have been frequently collected from other Spartina species on S. maritima plants in this study and in a similar study performed by Barata [6], as well as the exclusive presence of other fungal species in these communities.
In concordance with Barata [6] study, N. retorquens was found to be the most frequent and dominant species in the two studied fungal communities, although it has not been reported from other Spartina species. The absence of N. retorquens on these host plants is not easy to explain, considering that this fungal species has been collected from driftwood in temperate regions [4, 37, 52] and from different substrates in tropical climates [3, 92, 94]. However, it could be related with the fact that most of studies that inventoried Spartina spp. focused mainly or exclusively on leaf blades. Similarly, P. viscosus, another cosmopolitan species that has been described from temperate and tropical regions [3, 37, 52, 88, 92], was reported for the first time on standing plants of Spartina by Barata [6], on drift stems of the same host plant by Azevedo et al. [4], and collected again in this study.
Even though the high overlapping of fungal communities associated with the same host species, it were found some variations in the mycota associated with standing plants of S. maritima in different salt marshes in terms of species composition and frequency patterns.
S. maritima was found to be a new host plant for seven fungal species; A. spissitecta, C. obiones, D. gaudefroyi, L. marina and S. haliclysta were exclusively collected from Castro Marim plants, Halosarpheia trullifera was exclusively present in Ria de Aveiro plants and Camarosporium roumeguerii occurred in both study sites (Table 2). From all the mentioned fungal species, only A. spissitecta and L. marina have been previously described from other Spartina species. C. roumeguerii, C. obiones and D. gaudefroyi have been observed inhabiting other salt marsh plants [1, 47]. S. haliclysta and H. trullifera have been found colonizing the seaweed Pelvetia canaliculata [1] and driftwood in temperate regions [52, 88], respectively. With the exception of C. roumeguerii and L. marina, all the other fungal species were also new records for Portugal.
Although the differences in sampling methods applied in this study and in Barata [6] study may explain some differences between surveyed fungal communities, these are more likely to have resulted from different environmental conditions in the study sites. Similarly, this last reason could explain the differences in the fungal communities from Castro Marim and Ria de Aveiro salt marshes. The higher species richness and diversity found in Castro Marim salt marsh may be attributed to suitable environmental conditions given by a more preserved habitat; these conditions may favour the colonization and reproduction of less well-adapted species.
The fact that S. maritima community is more reduced and fragmented in Ria de Aveiro marsh and the vestigial presence of mercury in this study site (not measured in the present study, but mentioned by Coelho et al. [24, 25] and Pereira et al. [89]) might have provided less favourable conditions for colonization by occasional and infrequent species. The total mercury, as demonstrated by Coelho et al. [24], accumulates more in old leaves than in stems, although in lower concentrations than in belowground biomass. These conditions seemed to have no effects on the most frequent fungal taxa in the community. The resistance of dominant saprobic ascomycetous fungi associated with S. alterniflora to several potentially toxic pollutants was already demonstrated by Newell and Wall [76] and Newell et al. [83]; they measured the living fungal biomass and sexual productivities of dominant fungi in standing-decaying leaf blades in the presence of mercury, methylmercury, polychlorinated biphenyls, chlorinated organocyclic insecticide toxaphene, chromium, copper, lead and polycyclic aromatic hydrocarbons and showed that these biological parameters were not affected by the presence of the toxicants. Moreover, the omnipresence of dominant fungal species in different Spartina communities of different states of conservation was also revealed by Cornick et al. [26]—stable versus declining beds of Spartina anglica C. E. Hubbard—and Walker and Campbell [110]—natural versus created S. alterniflora salt marshes.
However, the absence of Mycospharella sp. I in Castro Marim salt marsh, as well as the absence and infrequent occurrence of L. marina and S. orae-maris respectively in Ria de Aveiro salt marsh, was more difficult to interpret under an ecological perspective. The absence of L. marina from three salt marshes highly exposed to anthropogenic pressure surveyed by Barata [6] and the absence of S. orae-maris from the most polluted one [6] suggested that both species may require habitats with favourable conservation status to occur.
On the other hand, the slightly differences in the tidal regime in Castro Marim and Ria de Aveiro salt marshes, which reflected on the flooding patterns and salinity exposure in the study sites, might have limited the colonization by L. marina, S. orae-maris and Mycosphaerella sp. I. Although S. maritima plants occurred at a higher topographic level in Castro Marim salt marsh, they were shorter (±39 cm) and submerged more frequently during the sampling period (79 % days per month); S. maritima plants in Ria de Aveiro salt marsh colonized a lower topographic level but were taller (±49 cm), being totally submerged less frequently (60 % days per month). In addition to different flooding frequency, both study sites presented different salinity ranges, varying more in Castro Marim than in Ria de Aveiro salt marsh (Table 1).
Furthermore, and as pointed out by Torzilli et al. [105] and Lyons et al. [66], the intraspecific morphological variations in host plants, which implied differences in their chemical composition, may have restricted the colonization process to the more well-adapted species. Also, the general smaller size of leaf blades of S. maritima in Castro Marim might have promoted the interspecific competition among fungi, conditioning the colonization by Mycosphaerella sp. I. Newell and Zakel [78] observed that Mycosphaerella sp. II tended to produce more ascospores in larger and thicker leaf blades.
Although these last enumerated hypotheses to explain the presence/absence of fungal species in the communities are merely speculative, this study clearly demonstrated the importance of environmental factors (biotic and abiotic) for the colonization of some fungal taxa, especially less frequent ones.
Vertical Distribution Patterns of Fungi
The fungal taxa colonizing S. maritima plants were found to exhibit vertical distribution patterns on the host plants in Castro Marim and Ria de Aveiro salt marshes (Fig. 2). This finding is in concordance with similar studies that focused on fungal communities inhabiting mangroves trees and shrubs [94] and other standing grasses distributed from brackish [90, 91, 106–108] to more saline tidal marshes [5, 6, 39, 58]. Fungal taxa occupy their own ecological niche, as a consequence of species-specific ecological requirements (i.e. chemical composition of the substrate, and temperature, salinity and moisture of the microhabitat) and interspecific competition [48].
The separation of basal, middle or top portions of the plants in both study sites based on the distribution of fungal taxa (Figs. 3 and 4) emphasized the importance of micro-environmental conditions for the colonization and establishment of ecological niches. An integration of all results highlighted some ecological patterns, particularly of the most representative fungal taxa in the community (Figs. 2 and 4): N. retorquens, B. obiones, Lulworthia sp. and L. marina (in Castro Marim) occurred mostly in the more frequently flooded plant portions (basal portions) associated with stems and/or leaf sheaths; B. spartinae, S. orae-maris and Phoma sp. 1 occupied preferentially the middle portions, colonizing stems and/or sheaths and basal portions of leaf blades; P. spartinicola, Stagonospora sp. and Mycosphaerella sp. I (in Ria de Aveiro) were found in the less inundated top portion of the plants, mainly associated with leaf blades.
The less obvious separation between middle and top portions in Ria de Aveiro plants established by the cluster analysis (Fig. 3) might have resulted from differences in plant heights; the plants were considerable shorter in the first four sampling periods than in the remaining period (Fig. 7). As a consequence, the fungal communities that were mainly found on the top portions during the period June 2011–August 2012 were detected on the middle portion during the period October 2010–April 2011. Therefore, and considering the fact that the relative positions of fungi on the standing plants were maintained, it was not attributed any biological meaning for this result.
The fact that all fungal taxa occurred more frequently on the same plant portion of Castro Marim and Ria de Aveiro plants, i.e. basal, middle or top portion, even though the morphological differences between the host plants suggested a clear preference for the micro-environmental conditions of the colonized microhabitat. However, none of the fungal taxa was exclusively restricted to one particular microhabitat; in fact, the majority was observed in two plant portions, and only P. spartinicola and Phoma sp. 1 were detected in all portions in Castro Marim and Ria de Aveiro salt marshes. This finding, in addition to the differences in the absolute distribution ranges of common fungal taxa in plants from both salt marshes (Fig. 2) and the seasonal variation of vertical position of fungi on the plants (Fig. 7), suggested that the plant substrate might be the major key factor determining distribution boundaries. This reason may also explain the higher similarity between middle and top plant portions in Ria de Aveiro salt marsh based on the frequency of occurrence of fungal taxa harboured in those microhabitats (Fig. 4), which were found to be more similar in terms of the proportion of vegetative structures available for colonization.
The distribution ranges of fungal taxa, determined in this study by the vertical positions of fruiting structures on standing plants, were assumed to be more realistic for the species more frequently collected than for the infrequent ones. This assumption was complemented with the argument that a high density of fruiting structures implies substantial supportive matrix of an active mycelium [77] to infer about the importance of the fungi on the decay process. Thus, it was hypothesized that fungal taxa that were producing more fruiting structures over a larger distribution area, such as B. obiones and N. retorquens on leaf sheaths and P. spartinicola, Phoma sp. 1 and Stagonospora sp. on leaf blades, were presumably assuming a more active role in the decomposition of colonized plant tissues. However, the absence/paucity of fruiting structures does not directly indicate if a fungus is absent/less abundant on the substrate, but probably that the required species-specific biotic and abiotic conditions for reproduction were not achieved.
Some of the ecological niches revealed in the present study have already been documented in similar studies performed with S. maritima and also with other species of Spartina; specifically, the higher occurrence and dominance of P. spartinicola and Mycosphaerella sp. I in the top of the canopy on leaf blades [5, 6, 11, 12, 39, 66, 72, 75, 78, 82, 110]. Newell and Wasowski [75] demonstrated that the extent of occupancy of fruiting structures on S. alterniflora produced by P. spartinicola is not affect by the frequency of flooding, but rather by the colonized vegetative structure, i.e. the lower percentage occupancy was found on the leaf sheaths. B. spartinae observed in present study and Barata [6] study (as ascomata and hyphopodia) on leaf sheaths and stems in the middle portion of S. maritima plants has been mostly recorded on Spartina leaf blades and sheaths in the middle-top portions [5, 26, 72, 75, 82, 110]. In addition to host exclusivity, the general agreement between this study and previous studies indicated that these fungi also presented a high degree of preference for vegetative structures and for particular vertical positions on standing plants.
The sequential vertical positions of Lulworthia sp., N. retorquens, B. obiones, S. orae-maris, D. pelagicum, B. spartinae, Stagonospora sp., P. spartinicola and Phoma sp. 1 along S. maritima plants described by Barata [6] was confirmed in the present study, with slight variations. Even though there are similarities in the relative positions and colonized vegetative structures of common fungal taxa found in this study and Barata [6] study, the absolute positions were different.
Thus, this study demonstrated that although the vertical distribution patterns of fungi resulted from the combined effect of micro-environmental conditions and substrate preference, it is this last biological factor that exerts a greater influence in determining the distribution range of these fungi.
Ecological Characterization of Fungi
Even though most of the fungal species recorded in this study are considered as obligate marine fungi by Jones et al. [53], Barata [6] presented some strong evidences to support the classification into obligate or facultative marine fungi. The higher or lower tolerance of fungi to salinity, air exposure and water submersion conditions that influences their vertical distribution on standing plants may, in fact, be related with their origin and physiological and morphological adaptations. In agreement with Barata [6] observations, both fungal communities from Castro Marim and Ria de Aveiro salt marshes did not included terrestrial or halotolerant fungi since the plants were normally totally submerged twice a day during high tides. Therefore, the results from the present study corroborate the classification of Lulworthia sp., N. retorquens and B. obiones into obligate marine fungi and Stagonospora sp. and P. spartinicola into facultative marine fungi, which were found on basal and top portions of the plants, respectively. Lulworthia sp. and N. retorquens were frequently collected by Barata [7] from S. maritima baits exposed to permanent and temporary submersion conditions, which reinforce the argument that these fungi are highly adapted to marine environments. Moreover, Sadaba et al. [94] also recorded N. retorquens on basal portions of Acanthus ilicifolius, an herbaceous mangrove standing plant.
The average vertical positions and distribution ranges of obligate and facultative fungi were taken into account to establish a virtual threshold value to distinguish from other fungal taxa. The threshold value (22 cm) was found to be situated in the middle plant portion, which means that this microhabitat constituted a vertical transition area for obligate and facultative marine fungi. As a transition zone, this microhabitat was colonized by fungal taxa both of the basal and top plant portions, which led to the greatest fungal richness, number of occurrences and diversity in both study sites (Table 3).
With this assumption, the fungal species more frequently recorded on basal portions, such as P. viscosus and L. marina, and on top portions, such as Mycosphaerella sp. I, Mycosphaerella sp. 2 and S. haliclysta, are likely to be obligate and facultative marine fungi, respectively.
The classification of the fungal taxa located in the middle plant portions was, though, more complicated. Nevertheless, and considering the established threshold value, the present study confirmed the classification of S. orae-maris as an obligate marine fungus and D. pelagicum, B. spartinae and Phoma sp. 1 as facultative marine fungi proposed by Barata [6]. Moreover, the results suggested that C. obiones, D. gaudefroyi and Phoma sp. 2, which occurred in the middle portion of Castro Marim plants, are facultative marine fungi.
Although the low occurrence of some fungal taxa in both salt marshes did not enable to distinguish their real distribution range, the presence of A. chesapeakensis, H. trullifera and A. spissitecta on lower plant portions and of Fusarium sp., Leptosphaeria sp. and C. roumeguerii on top plant portions might indicate that these species are obligate and facultative marine fungi, respectively. A. spissitecta was also found in lower portions of Spartina plants, being classified by Kohlmeyer and Volkmann-Kohlmeyer [61] as an obligate marine fungus. H. trullifera was more frequently recorded by Jones and Kuthubutheen [51] on submerged mangrove wood, which suggested that this species is, indeed, an obligate marine species.
A focus on the morphology of reproductive structures and mechanism of spores dispersal of the fungal taxa present along the vertical axis of the host plants seemed to corroborate the distinction previously made. As pointed out by Hyde and Lee [44], Alias and Jones [2] and Hyde and Sarma [46], the subcommunities inhabiting the basal and top plant portions possessed, in general, morphological characteristics that well adapt them to marine and terrestrial environments respectively. The group of marine fungi that colonized the basal microhabitat included fungal taxa with membranous (e.g. N. retorquens), carbonaceous (e.g. B. obiones) and coriaceous (e.g. Lulworthia sp.) ascomata, whereas the majority of the fungal taxa that occurred on the upper plant portions produced coriaceous ascomata, i.e. more resistant to desiccation imposed by a terrestrial habitat. Regarding ascus morphology and spore-discharge mechanism, the Sordariomycetes with dissolving unitunicate asci and passive spore-discharge dominated the basal portions, while the Dothideomycetes with bitunicate asci and an active spore-discharge inhabited the top portions. These findings are in agreement with Fell and Newell [35], Alias and Jones [2], Barata [6] and Hyde and Sarma [46] studies. According with Kohlmeyer and Kohlmeyer [58], the spore dispersal mechanism through a forceful ejection has probably evolved in terrestrial habitats, whereas a passive release of spores directly in water is more likely to have evolved in aquatic species, given the spores are easily washed away by tidal currents. The hypothesis that the active mechanism for spores discharge has a terrestrial origin was also proposed by Jones and Kuthubutheen [51] referring to some mangrove fungi. No clear correspondence was found between the vertical position of fungal taxa on the plants and the colour, presence/morphology of spore appendages and position of reproductive structures on the plant tissues (i.e. immersed, erumpent, superficial); most of the fungal reproductive structures were immersed on the substrate.
The evidences showed in this study supported the existence of obligate and facultative marine fungi colonizing different positions on intertidal standing plants, with distinct morphological adaptations and possibly distinct origins.
Seasonality
The results revealed that fungal composition of the communities of Castro Marim and Ria de Aveiro salt marshes did not considerably change during the study period, with the most frequent fungi present in all sampling periods. This finding, which is in agreement with previous studies conducted in intertidal ecosystems [12, 105, 110], reinforced the observation of Gessner [39] of a characteristic, resilient and stable community associated with Spartina species.
The occurrences and production of fruiting structures by frequent and very frequent fungal taxa in both communities, though, varied over the sampling time, except for N. retorquens in Ria de Aveiro salt marsh (Figs. 5 and 6). In general, the variations in the frequencies of occurrence of fungal taxa and of high density of fruiting structures produced by the same species did not follow a regular pattern. For this reason, these variations cannot be directly related with the seasonal variations of temperature and humidity or inclusively with the seasonal variation of nitrogen content in decaying vegetative structures of Spartina plants [17, 73].
However, the reduction in the frequency of occurrence of P. spartinicola and Stagonospora sp. in the two communities during the warmer periods suggested an effect of seasonality on the life cycle of these fungal species. The climatic factors also seemed to have affected the production of fruiting structures by the same species and N. retorquens in Castro Marim salt marsh. The interference of seasonality in the life cycle of fungal species and particularly the general decrease of fungal biomass and productivity during the warmest months have been already demonstrated in previous studies [22, 73, 77, 95]. Newell [72] documented higher percentages of released spores for P. spartinicola during cooler seasons. In contrast, Buchan et al. [12] study revealed that the abundance of P. spartinicola did not change with the seasonality. The differences between these two studies could be related with the applied methodologies.
The lack of obvious seasonal patterns pointed to a requirement of longer studies to better discriminate the effect of seasonality in fungal community dynamics and avoid biased conclusions. The variations in the vertical distribution of most frequent fungal taxa during the study period seemed not to be directly related with seasonality but either with the phenological growth patterns of the host plants.
Salinity Requirements of Fungi
The results from the culture experiment demonstrated that B. obiones, B. spartinae, Lulworthia sp., N. retorquens, L. marina, P. spartinicola, Phoma sp. 1, S. orae-maris and Stagonospora sp. grew on media lacking and containing seawater (Fig. 8), which suggests that there is not an absolute requirement of sodium chloride at concentrations found in seawater for growth. However, the growth rates in the two culture media were, in general, statistically different and higher under saline conditions, even for fungal taxa previously classified into facultative marine fungi, such as B. spartinae, Phoma sp. 1 and Stagonospora sp. The results from this experiment are in agreement with reported observations in similar studies [13, 30, 43, 48–50, 69, 86, 100] that marine fungi were capable of growing vegetatively without marine salts, although they generally exhibit an optimal growth under higher concentrations of salinity. The ability to grow without marine salts and tolerate salinity fluctuations likely confers an adaptive and competitive ecological advantage over their terrestrial counterparts in intertidal habitats subjected to intermittent dilution by freshwater inputs [100], i.e. seasonal precipitation and continuous freshwater discharges from adjacent rivers.
The fluctuations in salinity were demonstrated by some studies, though, to interfere in the production of antimicrobial metabolites by fungal species [43, 69] and in their sporulation [49].
Even though the general tendency of fungal taxa to grow better in the presence of marine salts, two fungal species revealed different vegetative growth patterns. P. spartinicola demonstrated a higher physiological plasticity than the other fungal taxa to adapt to different culture media conditions, being able to grow to the same extent on media with and without sea salts; this behavior under culture conditions reinforced its classification into facultative marine fungi. L. marina was the only species showing a better growth on culture media without marine salts, which contradicted the observations made on the field; the interpretation of its response was not straightforward since there is no additional evidence that this fungal species colonizes other less saline habitats.
The fact that the results from the culture experiment did not totally corroborate the field observations, suggests that it is not possible to distinguish obligate from facultative marine fungi based exclusively on vegetative growth responses. This means that is not recommended to apply the current definition of marine fungi in laboratory context, as argued by Kohlmeyer [57], even with the certainty that all tested fungi are active in the community.
However, this experiment was important to demonstrate the high physiological plasticity and versatility of marine fungal taxa to adapt to different abiotic conditions, as well as species-specific salinity requirements. The differences among vegetative growth rates and particularly the faster growth of Lulworthia sp., B. spartinae and Stagonospora sp. (Fig. 8) may indicate that these fungal taxa have, in fact, high growth rates or, alternatively, that they were exploring more efficiently this particular artificial substrate.
Final Remarks
This study, conducted in a less surveyed geographical region, supported the existence of a stable core group of fungi associated with Spartina species. Besides being dominated by the same host-exclusive ascomycetous fungi, the studied fungal communities also included other saprobic fungi exclusive to S. maritima, and seven new records were documented for this host plant and five for Portugal. This study also confirmed the species-specific vertical distribution patterns of fungi along the standing plants, which were attributed mainly to the substrate availability and to a lesser extent, the micro-environmental conditions of the habitat. The most frequent fungal taxa in the two communities revealed a high tolerance to salinity fluctuations and exhibited wide vertical distribution ranges and a high investment in the production of fruiting structures. These findings suggested that these fungal species were well-established and adapted to the intertidal habitat, exploring efficiently the substrate and consequently assuming an important and active key role in the early stages of decomposition of S. maritima.
References
Abdel-Wahab MA, Bahkali AHA (2012) Taxonomy of filamentous anamorphic marine fungi : morphology and molecular evidence. In: Jones EBG, Pang K-L (eds) Marine fungi and fungal-like organisms. De Gruyter, Berlin, pp 65–90
Alias SA, Jones EBG (2000) Vertical distribution of marine fungi on Rhizophora apiculata at Morib mangrove, Selangor, Malaysia. Mycoscience 41:431–436
Alias SA, Zainuddin N, Jones EBG (2010) Biodiversity of marine fungi in Malaysian mangroves. Bot Mar 53:545–554
Azevedo E, Rebelo R, Caeiro MF, Barata M (2012) Use of drift substrates to characterize marine fungal communities from the west coast of Portugal. Mycologia 104:623–632
Al-Nasrawi HG, Hughes AR (2012) Fungal diversity associated with salt marsh plants Spartina alterniflora and Juncus roemerianus in Florida. Jordan J Biol Sci 5:247–254
Barata M (2002) Fungi on the halophyte Spartina maritima in salt marshes. In: Hyde KD (ed) Fungi in marine environments. Fungal Diversity Press, Hong Kong, pp 179–193
Barata M (2006) Marine fungi from Mira river salt marsh in Portugal. Rev Iberoam Micol 23:179–184
Benner R, Newell SY, Maccubbin AE, Hodson RE (1984) Relative contributions of bacteria and fungi to rates of degradation of lignocellulosic detritus in salt-marsh sediments. Appl Environ Microbiol 48:36–40
Bergbauer M, Newell SY (1992) Contribution to lignocellulose degradation and DOC formation from a salt marsh macrophyte by the ascomycete Phaeosphaeria spartinicola. FEMS Microbiol Ecol 86:341–348
Blum LK, Roberts MS, Garland JL, Mills AL (2004) Distribution of microbial communities associated with the dominant high marsh plants and sediments of the United States East Coast. Microb Ecol 48:375–388
Buchan A, Newell SY, Moreta JIL, Moran MA (2002) Analysis of internal transcribed spacer (ITS) regions of rRNA genes in fungal communities in a southeastern U.S. salt marsh. Microb Ecol 43:329–340
Buchan A, Newell SY, Butler M, Biers EJ, Hollibaugh JT, Moran MA (2003) Dynamics of bacterial and fungal communities on decaying salt marsh grass. Appl Environ Microbiol 69:6676–6687
Burgaud G, Woehlke S, Rédou V, Orsi W, Beaudoin D, Barbier G, Biddle JF, Edgcomb VP (2013) Deciphering the presence and activity of fungal communities in marine sediments using a model estuarine system. Aquat Microb Ecol 70:45–62
Caetano M, Vale C, Falcão M (2006) Particulate metal distribution in Guadiana estuary punctuated by flood episodes. Estuar Coast Shelf Sci 70:109–116
Caetano M, Fonseca N, Vale RCC (2007) Mobility of Pb in salt marshes recorded by total content and stable isotopic signature. Sci Total Environ 380:84–92
Calado ML, Barata M (2012) Salt marsh fungi. In: Jones EBG, Pang K-L (eds) Marine fungi and fungal-like organisms. De Gruyter, Berlin, pp 345–381
Cartaxana P, Catarino F (2002) Nitrogen resorption from senescing leaves of three salt marsh plant species. Plant Ecol 159:95–102
Castellanos EM, Heredia C, Figueroa ME, Davy AJ (1998) Tiller dynamics of Spartina maritima in successional and non-successional mediterranean salt marsh. Plant Ecol 137:213–225
Castillo JM, Redondo S, Wharmby C, Figueroa ME, Luque T, Castellanos EM, Davy AJ (2005) Environmental determination of shoot height in populations of the cordgrass Spartina maritima. Estuaries 28:761–766
Castillo JM, Leira-Doce P, Rubio-Casal AE, Figueroa E (2008) Spatial and temporal variations in aboveground and belowground biomass of Spartina maritima (small cordgrass) in created and natural marshes. Estuar Coast Shelf Sci 78:819–826
Castillo JM, Rubio-Casal AE, Figueroa E (2010) Cordgrass biomass in coastal marshes. In: Momba M (ed) Biomass. Sciyo, Rijeka, pp 1–26
Castro P, Freitas H (2000) Fungal biomass and decomposition in Spartina maritima leaves in the Mondego salt marsh (Portugal). Hydrobiologia 428:171–177
Cavaliere AR (1977) Marine flora and fauna of the Northeastern United States higher fungi: Ascomycetes, Deuteromycetes, and Basidiomycetes. National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Washing D.C
Coelho JP, Pereira ME, Duarte AC, Pardal MA (2009) Contribution of primary producers to mercury trophic transfer in estuarine ecosystems: possible effects of eutrophication. Mar Pollut Bull 58:358–365
Coelho JP, Pato P, Henriques B, Picado A, Lillebø AI, Dias JM, Duarte AC, Pereira ME, Pardal MA (2014) Long-term monitoring of a mercury contaminated estuary (Ria de Aveiro, Portugal): the effect of weather events and management in mercury transport. Hydrol Process 28:352–360
Cornick J, Standwerth A, Fisher PJ (2005) A preliminary study of fungal endophyte diversity in a stable and declining bed of Spartina anglica Hubbard. Mycologist 19:24–29
Costa JC, Arsénio P, Monteiro-Henriques T, Neto C, Pereira E, Almeida T, Izco J (2009) Finding the boundary between eurosiberian and mediterranean salt marshes. J Coast Res 1340–1344
Curado G, Rubio-Casal AE, Figueroa E, Grewell BJ, Castillo JM (2013) Native plant restoration combats environmental change: development of carbon and nitrogen sequestration capacity using small cordgrass in European salt marshes. Environ Monit Assess 185:8439–8449
Curado G, Rubio-Casal AE, Figueroa E, Castillo JM (2014) Potential of Spartina maritima in restored salt marshes for phytoremediation of metals in a highly polluted estuary. Int J Phytoremediation 16:1209–1220
Dela Cruz TE, Wagner S, Schulz B (2006) Physiological responses of marine Dendryphiella species from different geographical locations. Mycol Prog 5:108–119
Dias JM, Lopes JF (2006) Implementation and assessment of hydrodynamic, salt and heat transport models: the case of Ria de Aveiro Lagoon (Portugal). Environ Model Softw 21:1–15
Dias JM, Lopes JF, Dekeyser I (1999) Hydrological characterisation of Ria de Aveiro, Portugal, in early summer. Oceanol Acta 22:473–485
Dias JM, Lopes JF, Dekeyser I (2000) Tidal propagation in Ria de Aveiro lagoon, Portugal. Phys Chem Earth, Part B Hydrol Ocean Atmos 25:369–374
Efron B, Tibshirani R (1986) Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci 1:54–75
Fell JW, Newell SY (1998) Biochemical and molecular methods for the study of marine fungi. In: Cooksey KE (ed) Molecular approaches to the study of the ocean. Chapman & Hall, London, pp 259–283
Ferreira de Carvalho J, Chelaifa H, Boutte J, Poulain J, Couloux A, Wincker P, Bellec A, Fourment J, Bergès H, Salmon A, Ainouche M (2013) Exploring the genome of the salt-marsh Spartina maritima (Poaceae, Chloridoideae) through BAC end sequence analysis. Plant Mol Biol 83:591–606
Figueira D, Barata M (2007) Marine fungi from two sandy beaches in Portugal. Mycologia 99:20–23
Gessner MO, Gulis V, Kuehn KA, Chauvet E, Suberkropp K (2007) Fungal decomposers of plant litter in aquatic ecosystems. In: Kubice CP, Druzhinina IS (eds) Environental and microbial relationships - Mycota IV. Springer, Berlin, pp 301–324
Gessner RV (1977) Seasonal occurrence and distribution of fungi associated with Spartina alterniflora from Rhode Island estuary. Mycologia 69:477–491
Gessner RV, Kohlmeyer J (1976) Geographical distribution and taxonomy of fungi from salt marsh Spartina. Can J Bot 54:2023–2037
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Hodson RE, Christian RR, Maccubbin AE (1984) Lignocellulose and lignin in the salt marsh grass Spartina alterniflora: initial concentrations and short-term, post-depositional changes in detrital matter. Mar Biol 81:1–7
Huang J, Lu C, Qian X, Huang Y, Zheng Z, Shen Y (2011) Effect of salinity on the growth, biological activity and secondary metabolites of some marine fungi. Acta Oceanol Sin 30:118–123
Hyde KD, Lee SY (1995) Ecology of mangrove fungi and their role in nutrient cycling: what gaps occur in our knowledge? Hydrobiologia 295:107–118
Hyde KD, Sarma VV (2000) Pictorial key to higher marine fungi. In: Hyde KD, Pointing SB (eds) Marine mycology—a practical approach. Fungal Diversity Press, Hong Kong, pp 205–270
Hyde KD, Sarma VV (2006) Biodiversity and ecological observations on filamentous fungi of mangrove palm Nypa fruticans Wurumb (Liliopsida-Arecales) along the Tutong river, Brunei. Indian J Mar Sci 35:297–307
Inderbitzin P, Kohlmeyer J, Volkmann-Kohlmeyer B, Berbee ML (2002) Decorospora, a new genus for the marine ascomycete Pleospora gaudefroyi. Mycologia 94:651–659
Jones EBG (2000) Marine fungi : some factors influencing biodiversity. Fungal Divers 4:53–73
Jones EBG (2011) Fifty years of marine mycology. Fungal Divers 50:73–112
Jones EBG, Jennings DH (1964) The effect of salinity on the growth of marine fungi in comparison with non-marine species. Trans Br Mycol Soc 47:619–625
Jones EBG, Kuthubutheen AJ (1989) Malaysian mangrove fungi. Sydowia 41:160–169
Jones EBG, Vrijmoed LLP, Alias SA (1998) Intertidal marine fungi from San Juan Island and comments on temperate water species. Bot J Scotl 50:177–184
Jones EBG, Sakayaroj J, Suetrong S, Somrithipol S, Pang KL (2009) Classification of marine Ascomycota, anamorphic taxa and Basidiomycota. Fungal Divers 35:1–187
Khan SS, Manimohan P (2011) Diversity and abundance of marine fungi on driftwood collected from Kerala State and Lakshadweep Islands, India. Mycosphere 2:223–229
Kilsby CG, Tellier SS, Fowler HJ, Howels TR (2007) Hydrological impacts of climate change on the Tejo and Guadiana Rivers. Hydrol Earth Syst Sci 11:1175–1189
Kis-Papo (2005) Marine fungal communities. In: Dighton J, White JF, Oudemans P (eds) The fungal community—its organization and role in the ecosystem. Taylor & Francis, Boca Raton, pp 61–92
Kohlmeyer J (1974) On the definition and taxonomy of higher marine fungi. Veröff Inst Meeresforsch Bremerh 5:263–286
Kohlmeyer J, Kohlmeyer E (1979) Marine mycology—the higher fungi. Academic Press, Inc., New York
Kohlmeyer J, Volkmann-Kohlmeyer B (1991) Illustrated key to the filamentous higher marine fungi. Bot Mar 34:1–61
Kohlmeyer J, Volkmann-Kohlmeyer B (2001) The biodiversity of fungi on Juncus roemerianus. Mycol Res News 105:1411–1412
Kohlmeyer J, Volkmann-Kohlmeyer B (2002) Fungi on Juncus and Spartina: New marine species of Anthostomella, with a list of marine fungi known on Spartina. Mycol Res 106:365–374
Kohlmeyer J, Volkmann-Kohlmeyer B, Eriksson OE (1995) Fungi on Juncus roemerianus. New marine and terrestrial ascomycetes*. Mycol Res 100:393–404
Kohlmeyer J, Volkmann-Kohlmeyer B, Newell SY (2004) Marine and estuarine mycelial eumycota and oomycota. In: Mueller GM, Bills GF, Foster MS (eds) Biodiversity of fungi—inventory and monitoring methods. Elsevier Academic Press, San Diego, pp 533–545
Kohlmeyer J, Volkmann-Kohlmeyer B, Tsui CKM (2005) Fungi on Juncus roemerianus. 17. New ascomycetes and the hyphomycete genus Kolletes gen. nov. Bot Mar 48:306–317
Lyons JI, Newell SY, Buchan A, Moran MA (2003) Diversity of ascomycete laccase gene sequences in a southeastern US salt marsh. Microb Ecol 45:270–281
Lyons JI, Alber M, Hollibaugh JT (2010) Ascomycete fungal communities associated with early decaying leaves of Spartina spp. from central California estuaries. Oecologia 162:435–442
Maccubbin AE, Hodson RE (1980) Mineralization of detrital lignocelluloses by salt marsh sediment microflora. Appl Environ Microbiol 40:735–740
Manimohan P, Amritha M, Sairabanu N (2011) A comparison of diversity of marine fungi on three co-habiting mangrove plants. Mycosphere 2:533–538
Masuma R, Yamaguchi Y, Noumi M, Omura S, Namikoshi M (2001) Effect of sea water concentration on hyphal growth and antimicrobial metabolite production in marine fungi. Mycoscience 42:455–459
Morales JA (1997) Evolution and facies architecture of the mesotidal Guadiana River delta (S.W. Spain-Portugal). Mar Geol 138:127–148
Newell SY (1996) Established and potential impacts of eukaryotic mycelial decomposers in marine/terrestrial ecotones. J Exp Mar Bio Ecol 200:187–206
Newell SY (2001) Spore-expulsion rates and extents of blade occupation by ascomycetes of the smooth-cordgrass standing-decay system. Bot Mar 44:277–285
Newell SY (2001) Multiyear patterns of fungal biomass dynamics and productivity within naturally decaying smooth cordgrass shoots. Limnol Oceanogr 46:573–583
Newell SY, Fallon RD (1989) Litterbags, leaf tags, and decay of nonabscised intertidal leaves. Can J Bot 67:2324–2327
Newell SY, Wasowski J (1995) Sexual productivity and spring intramarsh distribution of a key salt-marsh microbial secondary producer. Estuaries 18:241–249
Newell SY, Wall VD (1998) Response of saltmarsh fungi to presence of mercury and polychlorinated biphenyls at a Superfund Site. Mycologia 90:777–784
Newell SY, Porter D (2000) Microbial secondary production from saltmarsh-grass shoots, and its known and potential fates. In: Weinstein MP, Kreeger DA (eds) Concepts and controversies in tidal marsh ecology. Kluwer Academic Publishers, Dordrecht, pp 159–185
Newell SY, Zakel KL (2000) Measuring summer patterns of ascospore release by saltmarsh fungi. Mycoscience 41:211–215
Newell SY, Fallon RD, Miller JD (1989) Decomposition and microbial dynamics for standing, naturally positioned leaves of the salt-marsh grass Spartina alterniflora. Mar Biol 101:471–481
Newell SY, Arsuffi TL, Palm LA (1996) Misting and nitrogen fertilization of shoots of a saltmarsh grass: effects upon fungal decay of leaf blades. Oecologia 108:495–502
Newell SY, Porter D, Lingle WL (1996) Lignocellulolysis by ascomycetes (fungi) of a saltmarsh grass (smooth cordgrass). Microsc Res Tech 33:32–46
Newell SY, Blum LK, Crawford RE, Dai T, Dionne M (2000) Autumnal biomass and potential productivity of salt marsh fungi from 29° to 43° north latitude along the United States Atlantic Coast. Appl Environ Microbiol 66:180–185
Newell SY, Wall VD, Maruya KA (2000) Fungal biomass in saltmarsh grass blades at two contaminated sites. Arch Environ Contam Toxicol 38:268–273
Oliveira M, Ahmad I, Maria VL, Pacheco M, Santos MA (2010) Monitoring pollution of coastal lagoon using Liza aurata kidney oxidative stress and genetic endpoints: an integrated biomarker approach. Ecotoxicology 19:643–653
Otero XL, Sánchez JM, Macías F (2000) Nutrient status in tall and short forms of Spartina maritima in the salt marshes of ortigueira (NW Iberian Peninsula) as related to physicochemical properties of the soils. Wetlands 20:461–469
Pang K-L, Chow RKK, Chan C-W, Vrijmoed LLP (2011) Diversity and physiology of marine lignicolous fungi in Arctic waters: a preliminary account. Polar Res 30:1–5
Peña NI, Arambarri AM (1996) Hongos marinos lignícolas de Mar del Plata (provincia de Buenos Aires, Argentina) II. Darwiniana 34:293–298
Peña NI, Arambarri AM (1998) Hongos marinos lignícolas de la laguna costera de Mar Chiquita (provincia de Buenos Aires, Argentina) I. Ascomycotina y Deuteromycotina sobre Spartina densiflora. Darwiniana 35:61–67
Pereira ME, Lillebø AI, Pato P, Válega M, Coelho JP, Lopes CB, Rodrigues S, Cachada A, Otero M, Pardal MA, Duarte AC (2009) Mercury pollution in Ria de Aveiro (Portugal): a review of the system assessment. Environ Monit Assess 155:39–49
Poon MOK, Hyde KD (1998) Biodiversity of intertidal estuarine fungi on Phragmites at Mai Po marshes, Hong Kong. Bot Mar 41:141–156
Poon MOK, Hyde KD (1998) Evidence for the vertical distribution of saprophytic fungi on senescent Phragmites australis culms at Mai Po marshes, Hong Kong. Bot Mar 41:285–292
Prasannarai K, Sridhar KR (2001) Diversity and abundance of higher marine fungi on woody substrates along the west coast of India. Curr Sci 81:304–311
Rodrigues M, Oliveira A, Queiroga H, Brotas V (2012) Seasonal and diurnal water quality and ecological dynamics along a salinity gradient (Mira channel, Aveiro lagoon, Portugal). Procedia Environ Sci 13:899–918
Sadaba RB, Vrijmoed LLP, Jones EBG, Hodgkiss IJ (1995) Observations on vertical distribution of fungi associated with standing senescent Acanthus ilicifolius stems at Mai Po mangrove, Hong Kong. Hydrobiologia 295:119–126
Samiaji J, Barlocher F (1996) Geratology and decomposition of Spartina alterniflora Loisel in a New Brunswick saltmarsh. J Exp Mar Bio Ecol 201:233–252
Sánchez JM, Otero XL, Izco J, Macías F (1997) Growth form and population density of Spartina maritima (Curtis) Fernald in northwest Spain. Wetlands 17:368–374
Sánchez JM, SanLeon DG, Izco J (2001) Primary colonisation of mudflat estuaries by Spartina maritima (Curtis) Fernald in Northwest Spain: vegetation structure and sediment accretion. Aquat Bot 69:15–25
Sarma VV, Hyde KD (2001) A review on frequently occurring fungi in mangroves. Fungal Divers 8:1–34
Serôdio J, Catarino F (2000) Modelling the primary productivity of intertidal microphytobenthos: time scales of variability and effects of migratory rhythms. Mar Ecol Prog Ser 192:13–30
Sguros PL, Simms J (1964) Role of marine fungi in the biochemistry of the oceans—IV Growth responses to seawater inorganic macroconstituents. J Bacteriol 88:346–355
Shoemaker RA, Babcock CE (1989) Phaeosphaeria. Can J Bot 67:1500–1599
Sousa AI, Lillebø AI, Caçador I, Pardal MA (2008) Contribution of Spartina maritima to the reduction of eutrophication in estuarine systems. Environ Pollut 156:628–635
Tan TK, Leong WF, Jones EBG (1989) Succession of fungi on wood of Avicennia alba and A. lanata in Singapore. Can J Bot 2686–2691
Torzilli AP, Andrykovitch G (1986) Degradation of Spartina lignocellulose by individual and mixed cultures of salt-marsh fungi. Can J Bot 64:2211–2215
Torzilli AP, Sikaroodi M, Chalkley D, Gillevet PM (2006) A comparison of fungal communities from four salt marsh plants using automated ribosomal intergenic spacer analysis (ARISA). Mycologia 98:690–698
Van Ryckegem GV, Verbeken A (2005) Fungal ecology and succession on Phragmites australis in a brackish tidal marsh. I. Leaf sheaths. Fungal Divers 19:157–187
Van Ryckegem GV, Verbeken A (2005) Fungal ecology and succession on Phragmites australis in a brackish tidal marsh. II. Stems. Fungal Divers 20:209–233
Van Ryckegem GV, Gessner MO, Verbeken A (2007) Fungi on leaf blades of Phragmites australis in a brackish tidal marsh: diversity, succession, and leaf decomposition. Microb Ecol 53:600–611
Vrijmoed LLP (2000) Isolation and culture of higher filamentous fungi. In: Hyde KD, Pointing SB (eds) Marine Mycology—a practical approach. Fungal Diversity Press, Hong Kong, pp 1–20
Walker AK, Campbell J (2010) Marine fungal diversity: a comparison of natural and created salt marshes of the north-central Gulf of Mexico. Mycologia 102:513–521
Zhou D, Hyde KD (2001) Host-specificity, host- exclusivity, and host-recurrence in saprobic fungi. Mycol Res 105:1449–1457
Acknowledgments
The authors would like to thank to Cristina Oliveira and Rita Severino for their precious help in the culture experiment. This study was supported financially by Foundation for Science and Technology (FCT) through a PhD grant (SFRH/BD/48525/2008).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
(PDF 1534 kb)
Rights and permissions
About this article
Cite this article
Calado, M.d., Carvalho, L., Pang, KL. et al. Diversity and Ecological Characterization of Sporulating Higher Filamentous Marine Fungi Associated with Spartina maritima (Curtis) Fernald in Two Portuguese Salt Marshes. Microb Ecol 70, 612–633 (2015). https://doi.org/10.1007/s00248-015-0600-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-015-0600-0