Abstract
Forest pathology, the science of forest health and tree diseases, is operating in a rapidly developing environment. Most importantly, global trade and climate change are increasing the threat to forest ecosystems posed by new diseases. Various studies relevant to forest pathology in a changing world are accumulating, thus making it necessary to provide an update of recent literature. In this contribution, we summarize research at the interface between forest pathology and landscape ecology, biogeography, global change science and research on tree endophytes. Regional outbreaks of tree diseases are requiring interdisciplinary collaboration, e.g. between forest pathologists and landscape ecologists. When tree pathogens are widely distributed, the factors determining their broad-scale distribution can be studied using a biogeographic approach. Global change, the combination of climate and land use change, increased pollution, trade and urbanization, as well as invasive species, will influence the effects of forest disturbances such as wildfires, droughts, storms, diseases and insect outbreaks, thus affecting the health and resilience of forest ecosystems worldwide. Tree endophytes can contribute to biological control of infectious diseases, enhance tolerance to environmental stress or behave as opportunistic weak pathogens potentially competing with more harmful ones. New molecular techniques are available for studying the complete tree endobiome under the influence of global change stressors from the landscape to the intercontinental level. Given that exotic tree diseases have both ecologic and economic consequences, we call for increased interdisciplinary collaboration in the coming decades between forest pathologists and researchers studying endophytes with tree geneticists, evolutionary and landscape ecologists, biogeographers, conservation biologists and global change scientists and outline interdisciplinary research gaps.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditionally, forest pathologists have investigated the symptoms and causes of tree diseases, as well as the methods to prevent them or reduce their damage. In the last decades, there has been a shift in the perspective of forest pathologists because of the recognition that tree diseases play an important ecological role in the overall functioning of forest ecosystems and their health [1, 2]. For example, it is now recognized that native fungal diseases of trees contribute in maintaining the tree species diversity of forests, thereby making them more resilient to other disturbances [3, 4]. Moreover, both native and exotic tree diseases can be regarded as biological control tools which diversify uniform plantations of exotic trees, thereby reducing their commercial value but increasing their biodiversity and aesthetics [5, 6]. However, some invasive exotic pathogens can drive tree species close to extinction [7, 8] and threaten whole ecosystems [9, 10].
Forest pathology is operating in a changing context [11, 12]. Forests are changing due to ecological succession, shifts in species distributions, habitat fragmentation, overexploitation, degradation and, in some cases, lack of management. Stakeholder views on forests are also developing, from a traditional focus on the sustainability of timber production to a recognition of the multi-purpose role of many forests, including recreation and the maintenance of clean air and water [13, 14]. At the same time, forest health is challenged worldwide by increased long-distance trade in plant commodities and a rapidly shifting climate [15, 16]. Together, these two global change drivers are likely to increase the opportunities for the establishment, spread and impact of new pests and pathogens.
Researchers interested in forest health are also changing. Taxonomic and morphological expertise is being lost because of the retirement, often without replacement, of many teachers and practitioners [17, 18]. At the same time, modelling is becoming more and more fashionable, also regarding disturbances in forest ecosystems [19, 20]. New molecular methods are being introduced at an accelerating pace, thus making it possible, e.g. to distinguish cryptic species that could previously not be differentiated. Such rapid methodological developments are also a challenge for keeping updated and teaching [21, 22]. In addition, interdisciplinary opportunities are shaping forest pathology in a changing world. For example, the application of landscape ecology tools and perspectives to forest pathology is improving our understanding of regional outbreaks of exotic tree fungal pathogens [23, 24]. New insights on the health of trees have been obtained by investigating the diversity of endophytes of tree species [25, 26]. Interdisciplinary research has also been achieved on the conservation biology implications of exotic tree diseases [27–30].
Various literature reviews are available on these topics, but the subject is developing rapidly so that there is a need for an update focusing on recent studies involving infectious diseases. The main aim of this contribution is, thus, to selectively survey the literature relevant to forest health in a changing world from the last 10 years (but citing previous papers when appropriate). For summaries of previous relevant literature, the reader is referred to other literature reviews (Table 1). A secondary aim of this brief overview is to map some bridges between forest pathology and neighbouring disciplines, from landscape ecology to disease biogeography, global change ecology and research on endophytes. However, these are not the only disciplines at the borders of forest pathology. Due to space constraints, we have for example not covered the literature on (i) environmental pollution and forest health, (ii) tools and indicators for monitoring forest health, (iii) resistance breeding and (iv) defining forest health.
Landscape Pathology
Tree pathogens propagate in heterogeneous landscapes resulting in non-random spatial patterns of disease expression [23]. Using landscape ecology tools and approaches, it is possible to gain a better grasp of the factors associated with variation in tree disease incidence at various sites, e.g. altitude, soil type, slope exposure, stand age and management factors [31–34]. For example, even at the extreme climatic conditions of the treeline environment, topography and moisture-related variables were shown to influence the landscape pattern of white pine (Pinus albicaulis) blister rust incidence, due to Cronartium ribicola [35]. The disease was reported to affect trees in tree islands more than isolated trees.
For generalist plant pathogens, it is important to study their epidemiology not only in the major host of interest, but also in supposedly minor hosts, because these secondary hosts might have a minor economic role, but their co-occurrence can affect the connectivity patterns from the point of view of the pathogen [36, 37]. For example, until the outbreaks in Japanese larch (Larix kaempferi) plantations in 2009, the epidemic of Phytophthora ramorum in Great Britain was largely driven not by the presence of susceptible, yet dead-end host trees such as Castanea sativa, Fagus sylvatica and Quercus ilex, but by the distribution of Rhododendron ponticum, an exotic yet widespread shrub in the UK, which enables sporulation of the pathogen [38].
Landscape features are important determinants of tree disease epidemics, e.g. when dispersal preferentially occurs along streams [39–41] or in combination with the trade in plants [42–44]. Even in soil, tree fungal pathogens can disperse by mycelial growth over considerable distances [45]. For example, genetic analysis of Armillaria gallica, a root rot pathogen, in Massachusetts showed that the average size of the fungal individuals (genets) was 0.13 ha and that basidiospores were able to establish new genets at distances up to 2 km [46]. Even larger genets of Armillaria species have been reported, for example in the case of Armillaria borealis, Armillaria cepistipes and Armillaria ostoyae in Swiss subalpine forests, with a range between 0.2 and ~7 ha [47]. However, the time since establishment is also important: in the Golden Gate Park in San Francisco, which was established in 1871 on sandy dunes unlikely to support mycelium before the planting of trees, the largest genotypes of Armillaria mellea are now about 300 m in length [48]. A host-free barrier can halt mycelial spread, but long distance dispersal limits the efficacy of such control measures [49–51].
Variation in tree disease expression across landscapes can also be influenced by the distribution of host genetic variation [52, 53]. Within European ash (Fraxinus excelsior) tree populations, individual differences in susceptibility to ash dieback, due to Hymenoscyphus fraxineus [54–56], have been reported from Denmark, Germany, Lithuania, Poland and Sweden, thus providing a sign of hope for the future of ash trees and their associated biodiversity [57–64]. Whilst differences in disease resistance or tolerance among tree provenances have long been recognized [65–67], their implications for the outbreaks of tree pests and diseases across landscapes of seminatural forests (rather than tree plantations) have only recently started to be explored [68–71]. A variety of new genomic tools is available to tackle this and other related issues (Table 2).
Often, site biophysical features mask the effect of landscape variation in tree genetic diversity on disease incidence and severity, which can be clarified by excluding such confounding factors, e.g. comparing the evidence obtained from common garden experiments and from the field [72–74]. Confounding factors are nevertheless widespread in nature, where they can reinforce each other. A study of the influences of site, forest type, and tree host species on the presence of Armillaria species in forests of Massachusetts found that stands of Tsuga canadensis are relatively resistant to Armillaria species, but become susceptible when also affected by insect defoliation and drought [75]. Interactions between forest disturbances were also documented by a study of the effects of wildfire on P. ramorum survival in Californian forests, where the pathogen was more likely to persist when wildfires left unburnt patches of bay laurel (Umbellularia californica) [76]. The creation of deadwood by P. ramorum in redwood (Sequoia sempervirens) forests makes wildfires more severe, thus reducing the usual resilience of this tree species to fires [77].
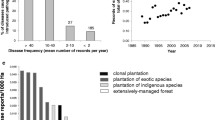
In some tree pathosystems, the landscape patterns of disease incidence and/or severity are affected by interactions with biotic factors. For example, beech bark disease in North America is associated with the invasive beech scale insect Cryptococcus fagisuga which predisposes the trees to attack by Neonectria fungi. A large-scale study across eastern North America showed that the dispersal behaviour of the insect makes it unlikely that any trees or stands will be spared by beech bark disease [78]. Most of the regions where American beech (Fagus grandifolia) is a dominant stand component are affected by the disease, but these areas cover only 30 % of the overall beech distribution [79]. The explanation of this pattern remains a challenge: it is possible that the percentage of American beech unaffected by beech bark disease is higher in northern compared to southern areas of the maritime provinces of New Brunswick, Nova Scotia and Prince Edward Island (Eastern Canada) because the disease arrived later in the north. Yet, more intensive silviculture (which favoured pioneer tree species and reduced the abundance of beech [80]) and colder winters (which are likely to be lethal to the insect [81]) could also play a role. In Europe, where the insect is endemic, beech bark disease has indeed been shown to be more severe in warmer regions [82].
A biotic factor that can reduce tree disease pressure is parasitism on pathogens. The presence in Europe of hypovirulence in the ascomycete Cryphonectria parasitica, the causal agent of chestnut blight, explains the reduced virulence of this introduced tree pathogen in Europe compared to North America [83, 84]. The transmission of the virus depends on the population structure of its host, among other factors [85–87]. Recently, this was also shown for the North American chestnut blight pathosystem, which is characterized by a much higher genetic diversity of C. parasitica compared to Europe [88]. An additional biotic factor affecting tree disease pressure is human management of woodlands [89]. It is well known that thinning creates an unnatural supply of freshly cut stumps, thus favouring root rot pathogens such as Heterobasidion or Armillaria [90]. A study of Armillaria species in 150 km2 of ancient unmanaged forests in the Ukrainian Carpathians documented a relative lack of pathogenic compared to saprotrophic Armillaria species, thus supporting the view that the disturbances accompanying forest management can increase the incidence of tree fungal pathogens [91].
Tree Disease Biogeography
Given the increased ease of travel, human beings are now moving themselves, plants and associated organisms over the planet, without much afterthought about the potential long-term consequences of this unprecedented long-distance mobility. Also forest pathologists now have the opportunity to widen their analyses beyond the local and landscape levels, to regions, countries and continents [92–95]. Broad-scale research on tree pathogens was pioneered in the 1970s by forest pathologists investigating Dutch elm disease, both in North America and in Europe [96–98]. A large-scale approach was also inherent in research on the decline affecting forests in Europe (Waldsterben) and North America during the 1980s [99–101]. Nowadays, broad-scale forest pathology is made necessary by the realization of the common health problems shared by exotic tree plantations in several continents [102, 103].
When tree health is investigated over biogeographic scales, forest pathology and biogeography merge into tree disease biogeography, the study of the factors determining the distribution of tree diseases over large geographic scales. For example, an investigation of records of seven Armillaria species on conifers in Japan showed their association with the host distribution and, thus, with climate [104]. A reconstruction using nucleotide markers of the invasion history in Europe of the fungal virus Cryphonectria hypovirus 1 suggested a role of trade patterns for the spread of hypovirulence (e.g. restrictions in trade between Greece and Turkey; Italy as an important European hub for chestnut cultivation and trade [105]). The host of this hypovirus, C. parasitica, has been shown to have been introduced repeatedly to both North America and Europe from two genetic lineages present in the native Asiatic range, thus highlighting the importance of restricting trade in potentially infected commodities also after a pathogen has been introduced, so as to avoid the enhancement of genetic diversity of the fungus. Higher genetic diversity of the pathogen not only reduces the spread of hypovirulence but also increases the adaptive potential of the pathogen [106].
One important factor now shaping the distribution and severity of tree fungal diseases is indeed the long-distance trade of plant commodities [107–109]. For instance, genetic analyses have shown the role of tree nurseries in the dispersal across South Africa of Fusarium circinatum, which causes pitch canker, a major disease of exotic pine plantations in many countries [110, 111]. In many cases, there is evidence that tree pathogens are likely to have been introduced to a certain region, because of their low levels of genetic diversity and absence of population structure in the invaded area, e.g. for various Phytophthora species [112] and the ash dieback pathogen H. fraxineus [113, 114]. Also, the high levels of virulence and spatial expansion of a disease which was previously unrecorded in a region is an indicator of the presence of an exotic invasive pathogen, as documented, e.g. for H. fraxineus, which is likely to have been introduced to Europe from East Asia [113, 114]. Often, we still do not know the region of origin of such exotic tree diseases, so that surveys in regions with related hosts and a climate similar to the one of the region of the introduction are needed [115]. Also surveys in tree nurseries, together with data about previous long-distance artificial movement of host trees, are useful for reconstructing the invasion history of exotic tree pathogens.
Tree species migrations have happened also in the past and without human help, for example in response to changes in climate, e.g. through the Beringian Strait at times when Asia and North America were connected due to lower sea levels. The fungal pathogen assemblage of Populus angustifolia, a cottonwood species found in western North America, was shown to be similar to the one of Populus species in Asia and dissimilar to the one of Populus trichocarpa, another western North American species, thus confirming the hypothesis that P. angustifolia migrated from Asia to North America [116]. Tree fungal pathogens are interesting not only in their own right: they can also provide evidence to understand the migration history of their host tree species.
Nonetheless, genetic studies of tree fungal pathogens tend not to be carried out together with an analysis of the genetic diversity of their hosts. For example, a genetic study of the root rot pathogen A. mellea in the Western and Eastern USA found genetic divergence between the two regions, with Eastern populations likely to have resulted from multiple introductions [117]. Also Ophiognomonia clavigignenti-juglandacearum, which has caused range-wide mortality of butternut trees (Juglans cinerea) in North America, was shown using genetic analyses to be likely to have been introduced several times, given the geographic clustering of the pathogen genotypes [118]. A single source site in North America and introduction site in central Italy (Castelporziano) was instead inferred for Heterobasidion irregulare, a root rot pathogen whose genetic diversity in the Italian invasive range decreases with the distance from its putative introduction site [119]. A country-wide Swiss study of the genetic diversity of A. cepistipes, a wood-decaying, native fungus that can also be pathogenic when trees have been stressed by other causes, found no isolation by distance despite a long history of forest fragmentation in the Swiss plateau, with fungal gene flow limited by the Alps only [120].
It is important to study the genetic diversity levels of exotic tree pathogen populations because more genetically diverse pathogens are more likely to overcome resistance [121–123]. Resistance (or tolerance) can be present in some tree individuals despite lack of co-evolution with the pathogen (as for European ash, F. excelsior, against ash dieback [64, 113]) or can be obtained after long screening and breeding programmes [124–126]. It is important to preserve the genetic diversity of tree species in such breeding efforts, because this is an insurance against other environmental stresses, pests and diseases.
Global Change and Tree Health
Global change is a process involving the interaction between climate and land use change, increased pollution, trade and urbanization, as well as the invasion of exotic species. All these factors, by modifying the effects of disturbances such as wildfires, droughts, storms, diseases and herbivore outbreaks, are likely to affect the health of forests throughout the planet, although to varying degrees depending on the resilience of each ecosystem [127–129]. Climate shifts over the next decades are expected to lead to novel ecosystems, because of the likely phenological changes and migration of species to cope with the new climatic conditions, together with the artificial long-distance movement of both hosts and pathogens [130–132]. For trees (and their associated organisms), this is likely to lead to selective pressure (in different directions) at the rear, centre and expanding edge of the distribution range [133]. In some cases, tree species are not expected to be able to cope with the rapidity of the climate shifts, so that assisted migration has been suggested to be necessary. This might lead to additional forest health problems in case of unintentional transfer of tree pathogens [134].
Predictions of likely changes in tree disease occurrence and severity under climate change are complicated by model uncertainties in the expected shifts in precipitation, an important factor for the life cycle of many plant pathogens. For example, models of the risk of occurrence of Phytophthora cinnamomi in the southwestern USA under likely future climate change scenarios suggest that even if temperature rises are likely to greatly expand the distribution range of the pathogen, reductions in spring precipitation might still constrain that expansion [135]. A further source of uncertainty is the lack of knowledge of the potential effects of climate change and other global change drivers on competitors, mutualists and enemies of tree diseases and insect herbivores [136]. In some cases, for instance when tree disease severity is already high, climate shifts might not result in additional facilitation of fungal infection, as shown by experiments on the effects of high and low precipitation, increased air temperature and Cytospora chrysosperma canker infection on Salix monticola biomass in Colorado [137]. Many tree host-pathogen interactions currently resulting in disease are dependent on suitable climatic conditions during critical life cycle phases of the pathogen [138]. Climate change might well disrupt such synchronicity, as shown e.g. for the predicted reduction in summer moisture in the 2080s in British Columbia, which would reduce the climate suitability for spore discharge and germination of cedar leaf blight (Didymascella thujina) [139].
Climate change will not operate alone, but together with increased quantities of plant commodities traded over long distances, e.g. bonsai and other ornamental plants, nursery stock, seed, wood and wood packing materials [140–142]. For example, the sudden oak death pathogen P. ramorum and other Phytophthora tree pathogens were widely distributed by the nursery trade in the USA [143–146] and other countries [147–150]. There is also evidence for long-distance dispersal of the ash dieback pathogen by latently infected plants [151] (Fig. 1). This makes it clear that networks spreading information about a certain disease (i.e. the communication channels among researchers, practitioners and other stakeholders) need to be more efficient than networks spreading the disease. However, we have still little knowledge about the structure of plant trade networks compared to animal trade networks and human social networks [109].
Number of ash saplings imported between 2003 and 2011 by the UK from EU countries registered on the Forest Reproductive Material database (Belgium, France, Germany, Hungary, Ireland, the Netherlands). Data were obtained from [151]. The orange colour indicates ash saplings imported from countries which had already reported the presence of ash dieback. The pathogen was described as a new species by Kowalski in 2006 [54]
Much of the plant trade is directed towards urbanized areas, where most of the retailers and customers are located. Given the heat island effect of urbanization, towns provide a repeated experiment combining climate warming with the introduction of exotic plants and pathogens. It would thus make sense to focus some of the monitoring of new tree health problems in and around towns, because this would often be likely to enable early recognition of new outbreaks [152–154]. To some extent, this is already the case given that urbanized areas tend to have more observers than rural regions [155]. Trees in urban alleys, squares and parks are subjected to many sources of stress other than disease, including (i) high levels of air, water and soil pollution, (ii) wounds due to repeated pruning and (iii) soil compaction and sealing. Chronic stress can debilitate urban trees and facilitate the action of secondary pathogens. In the urban forest of Perth, in southwestern Australia, a diversity of Phytophthora species was detected [156], thus confirming the suitability of the urban environment for many tree pathogens. In addition, trees planted in urban parks, gardens and streets often originate from tree nurseries, a hub for the dissemination of the many organisms associated with trees.
Forest pathologists are thus confronted with a changing world, not just because there are now data and tools to study regional outbreaks of tree pathogens over landscape to continental scales, but also because tree health is increasingly challenged by global change drivers and their interactions [157–159]. The increasing number of newly reported tree pathogens over the last decades is a sobering reminder of the tree health problems to come. Indeed, the likely causes of new exotic tree diseases (increased trade, higher temperatures, shifts in host distribution) are supposed to intensify in the next future [160–162]. First reports of crop pests and pathogens have already been reported to have shifted towards the poles, possibly in relation to a warming climate and a stronger increase in economic activities in extratropical countries [163, 164].
Tree Endophytology
In addition to variation in (i) the genetic make-up of hosts, (ii) virulence among pathogen strains and (iii) environmental (including global change) factors, tree health over local to landscape and geographic scales is affected by endophytic assemblages within trees [25, 26, 165, 166]. A beneficial effect of endophytes on tree health has been demonstrated experimentally (reviewed by [26]). Recently, this effect was confirmed for example by studies showing that (i) various leaf endophytes contribute to Melampsora rust resistance in poplar [167] and (ii) tree root endophytes can control soil pathogens [168, 169]. Moreover, enhancement of tolerance to abiotic stresses by endophytes has been reported [170–175]. Endophytes can also enhance pathogen virulence—a potentially useful effect for the control of invasive plant species [176]. It is possible that, by systemic induction of defence responses, some leaf endophytes might enable trees to withstand pathogen attacks to other plant organs [177, 178]. Nevertheless, the importance of root endophytes should not be overlooked just because they have tended to be less studied than leaf endophytes [179].
It has been suggested that tree endophytes could be used as indicators of the health and vitality of trees [180, 181]. Tree (fungal) endophytes would be a suitable bio-indicator because they have been shown to be ubiquitous [25, 182]. Moreover, some endophytes can turn from mutualistic or neutral to pathogenic depending on the environmental and host conditions [25, 183, 184]. Tree endophytes could thus be used to track variations in forest health conditions, by taking into account that the factors shaping tree endophytic assemblages vary in space and time, for example the season of the year [185, 186].
However, using endophytes as health indicators is still problematic because endophytic assemblages are shaped by many further factors, including leaf age [187], host physiological status and genetic variation [188–190]. Host genotype is an important determinant of tree endophytes, as shown for example in Populus balsamifera growing in a common garden in Fairbanks, Alaska [191]. The right host genotype might be required for successful infection by a particular endophyte genotype, as shown by a study of Venturia ditricha, a common foliar endophyte of birch trees [192]. Interestingly, lower frequency and diversity of endophytes have been reported for clones of elms resistant to Dutch elm disease compared to resistant ones [193]. When studied across several dozens of tree species in sub-tropical, cool temperate and sub-boreal forests in Japan, the presence of xylariaceous endophytes was dependent on plant family and leaf traits, thus leading to a certain degree of host recurrence [194].
In addition to host-related traits, variation in tree endophyte assemblages has been shown to be associated with environmental gradients [195, 196]. Relevant factors include the following:
-
altitude, e.g. for F. sylvatica leaves in the Pyrenees [197];
-
latitude, e.g. for Pinus sylvestris needles in Finland [198];
-
temperature, as found in Japan for Fagus crenata [199]
-
as well as precipitation, as documented for Metrosideros polymorpha in the Hawaii [200].
A further issue is the pervasive (but to varying degrees) presence of human influences on forests, e.g. due to silviculture and gradients in land use intensity [201–204]. Given the many confounding factors, studies of endophyte assemblages in single stands and across landscapes are often not conclusive regarding the causal influence of environmental features on endophytic assemblages, because of the co-variation among explanatory factors (e.g. host distribution and climate [205]) and the lack of experimental controls. It can indeed be difficult to clarify the relative contributions of such factors in shaping tree endophyte diversity, as shown by a study of the influence of host identity and location on endophytes of trees of the Cupressaceae family [206]. A study of leaf endophytic fungi of three Nothofagus species growing in four mixed stands in New Zealand found that the diversity of endophytes was more affected by host species than by site [207]. As with tree pathogens, multi-scale studies can help disentangle the factors governing endophyte assemblages at different spatial resolutions [208]. A study of F. sylvatica endophytes in a forest stand in southeastern France found that the differences between assemblages of phyllosphere fungi increased with distance between sampled leaves within a single tree canopy and with genetic distance (rather than spatial distance) between sampled trees within the stand [209].
Although the endophytes of only about 10 % of the ~1000 temperate tree species have been investigated so far [189], tree endophytes have tended to be studied in extratropical regions, particularly in North America, Europe and Japan [195, 210]. Given the high diversity of tree species in the tropics and given that endophytic assemblages appear to be specialized to their hosts, it is reasonable to expect that tropical forests harbour a great diversity of endophytic species, which still need to be studied to better understand their role in ecosystem functioning [211]. This goal is a challenge, because of the difficulties inherent in cultivating tree endophytes and their sheer diversity [212]. Determining the fungal endophyte species hosted by trees, particularly in the tropics, is also hampered by the lack of taxonomic knowledge for many fungal genera [213]. But also outside of the tropics, new fungal endophyte species are routinely encountered, as shown by a study of the phyllosphere of Cephalotaxus harringtonia in Japan and France [214].
Studying diversity data of fungal endophytes that rely only on cultures in the lab can overlook species that are difficult to culture, that grow slowly or those that are rare [215, 216]. Over the last few years, advances in molecular methods have made it easier to obtain more exhaustive data about the diversity of tree endophytes ([189, 217] and literature listed in Table 2). This trend is expected to continue. There is thus an opportunity to consider tree endophytes in local, landscape and regional studies of tree diseases [218]. It is important to realize that there is often a continuum ranging from pathogenic to neutral and mutualistic status and that we still have little knowledge of the asymptomatic hosts for many pathogens with a cryptic biology [219].
Conclusions
Forest pathologists have to act in a world that is rapidly changing in many respects, from the emergence of new, aggressive exotic tree pathogens to the development of just as new molecular techniques. These developments lead to the increased need for interdisciplinary collaboration, e.g. involving (i) forest pathologists in research on assisted migration of tree species, (ii) the collaboration of geneticists of trees and of tree diseases and (iii) surveys of the network connectivity patterns of tree nurseries and their customers, thus leading to data suitable for analysis by network epidemiologists [220–222] (Table 3). This overview of recent literature provides evidence that forest pathology is a subject that has established links with various other disciplines.
Remarkably, the proportion of publications in forest sciences mentioning ‘forest health’ and ‘tree disease(s)’ has remained stable (at about one out of 200, or ~0.5 % and one out of 2000, or ~0.05 %, respectively) over the 1990s and 2000s (Fig. 2), despite, e.g. methodological developments, the rise of electronic publishing and the emergence of various new exotic tree diseases. However, the absolute numbers of research publications on both forests and forest health/tree diseases have increased steadily over the last two decades. Whether the proportion of interdisciplinary studies related to forest health and tree diseases has remained stable or has increased is a knowledge gap that needs collaboration between forest pathology and scientometrics [223–225]. In this concluding section, we point out some research gaps and opportunities for further research at the interface between forest pathology and neighbouring fields, with particular attention to endophytes (Table 3).
Temporal trend in the proportion of publications on forests mentioning a forest health and b tree disease(s) (obtained by dividing the number of papers retrieved each year searching for the keyword ‘forest health’ (or ‘tree disease’) by the number of papers retrieved that year with the keyword ‘forest’), in Google Scholar and Web of Science (1991–2010, as abstracts are searched in Web of Science starting from 1991 only; some papers published after 2010 may still need to be indexed). Data were retrieved in March 2014. Whilst these proportions have remained fairly stable, the absolute number of new yearly publications (both on forests and on forest health/tree diseases) has progressively increased in both databases
Research on landscape features facilitating the establishment and spread of exotic tree diseases appears to have developed largely independently of research on the factors shaping tree endophyte assemblages, but landscape ecology tools and approaches can be beneficial also in the study of tree endophytes. More diverse landscapes are likely to be less conducive to the spread of exotic tree diseases under changing environmental conditions [226]. This insurance effect of landscape diversity applies in some cases also to insect defoliators, despite their ability to jump from patch to patch of suitable hosts [227]. There is evidence from an archipelago in southwestern Finland that birch leaf endophytes are affected to some extent by landscape fragmentation [228], but further studies from other systems are needed to assess whether lack of landscape connectivity generally reduces the protective role of tree endophytes against diseases.
Despite the many landscape (and network) metrics that can be calculated in geographic information systems, field data are important also in landscape studies of tree pathogens, as shown by the better performance of models using direct measurements of the density of P. ramorum hosts compared to models using remotely sensed estimates of host habitat in California [229]. Predicting tree pathogen and endophyte assemblages from satellite measurements might still look like an outlandish research proposal, but could well take place over the next years. Comparative studies of tree microbial assemblages using remotely sensed data vs. field measurements of habitat variables would be needed to test the viability of this idea.
Regional tree mortality due to more frequent and severe forest disturbances can have ecosystem impacts through changes in plant species composition [230]. Widespread tree mortality can lead to the loss of many associated organisms, as feared for ash dieback over the coming years [29, 231–233]. Our understanding of the biodiversity consequences of exotic tree diseases is still limited to a few pathosystems and groups of organisms [234–237]. Relatively, little information is available on the potential consequences of outbreaks of exotic tree pathogens for their associated microbiota, as most research has focused on the effects on endophytes of endemic tree pathogens [238–245]. A similar lack of knowledge applies to the likely impacts of global change drivers on fungal endophytes.
An important requirement for successfully managing exotic tree diseases such as ash dieback, Sudden Oak Death and Dutch elm disease is collaboration with social scientists and engagement with stakeholders [246–248]. Tree health is just one of the many aims of land management, so that multi-criteria risk analyses are needed to assess the impact of various forest management scenarios on forest ecosystem services [249]. Often, national forest inventories deliver only coarse information for the study of specific tree health problems, thus making tailored surveys necessary for particular diseases [250]. For example, many forest inventories clump together all broadleaved tree species in one category, whereas standardized data on e.g. F. excelsior would be needed to assess the potential impacts of ash dieback in various regions.
Exotic tree pathogens have not just environmental and evolutionary consequences [251], but can also be costly economically [252]. Studies of the landscape features associated with tree disease incidence can help prioritize monitoring efforts [253]. A study of limber pine (Pinus flexilis) stands at risk of infestation by C. ribicola across Wyoming (where pine blister rust has long been present) and Colorado (where it is now becoming established) found that about half of the variation among plots in disease incidence could be explained using environmental variables (e.g. climate data at 1-km resolution) available to land managers [254]. There is the need to adopt similar approaches in the study of regional variations of tree endophyte assemblages.
References
Franklin JF, Shugart HH, Harmon ME (1987) Tree death as an ecological process. Bioscience 37:550–556. doi:10.2307/1310665
Teale SA, Castello JD (2011) Regulators and terminators: the importance of biotic factors to a healthy forest. In: Castello JD, Teale SA (eds) Forest health. An integrated perspective. Cambridge University Press, Cambridge, pp 81–114
Hansen EM (1999) Disease and diversity in forest ecosystems. Australas Plant Pathol 28:313–319
Hansen EM, Goheen EM (2000) Phellinus weirii and other native root pathogens as determinants of forest structure and process in western North America. Ann Rev Phytopathol 38:515–539. doi:10.1146/annurev.phyto.38.1.515
Carnus JM, Parrotta J, Brockerhoff E, Arbez M, Jactel H, Kremer A, Lamb D, O’Hara K, Walters B (2006) Planted forests and biodiversity. J For 104:65–77
Lombardero MJ, Alonso-Rodríguez M, Roca-Posada EP (2012) Tree insects and pathogens display opposite tendencies to attack native vs. non-native pines. For Ecol Manag 281:121–129. doi:10.1016/j.foreco.2012.06.036
Roberge JM, Bengtsson SBK, Wulff S, Snäll T (2011) Edge creation and tree dieback influence the patch-tracking metapopulation dynamics of a red-listed epiphytic bryophyte. J Appl Ecol 48:650–658. doi:10.1111/j.1365-2664.2011.01963.x
Cobb RC, Rizzo DM, Hayden KJ, Garbelotto M, Filipe JAN, Gilligan CA, Dillon WW, Meentemeyer RK, Valachovic YS, Goheen E, Swiecki TJ, Hansen EM, Frankel SJ (2013) Biodiversity conservation in the face of dramatic forest disease: an integrated conservation strategy for tanoak (Notholithocarpus densiflorus) threatened by Sudden Oak Death. Madrono 60:151–164. doi:10.3120/0024-9637-60.2.151
Cahill DM, Rookes JE, Wilson BA, Gibson L, McDougall KL (2008) Phytophthora cinnamomi and Australia’s biodiversity: impacts, predictions and progress towards control. Aust J Bot 56:279–310. doi:10.1071/BT07159
Davis RA, Valentine LE, Craig MD, Wilson B, Bancroft WJ, Mallie M (2014) Impact of Phytophthora-dieback on birds in Banksia woodlands in southwest Western Australia. Biol Conserv 171:136–144. doi:10.1016/j.biocon.2014.01.027
Holdenrieder O (1991) Der Forstschutz – Objekte, Probleme, Strategien. Schweiz Z Forstwes 142:795–807
MacDonald WL (2003) Dominating North American forest pathology issues of the 20th century. Phytopathology 93:1039–1040. doi:10.1094/PHYTO.2003.93.8.1039
Hepting GH, Cowling EB (1977) Forest pathology: unique features and prospects. Ann Rev Phytopathol 15:431–450. doi:10.1146/annurev.py.15.090177.002243
Petrokofsky G, Brown ND, Hemery GE, Woodward S, Wilson E, Weatherall A, Stokes V, Smithers RJ, Sangster M, Russell K, Pullin AS, Price C, Morecroft M, Malins M, Lawrence A, Kirby KJ, Godbold D, Charman E, Boshier D, Bosbeer S, Arnold JEM (2010) A participatory process for identifying and prioritizing policy-relevant research questions in natural resource management: a case study from the UK forestry sector. Forestry 83:357–367. doi:10.1093/forestry/cpq018
Wingfield MJ (1990) Current status and future prospects of forest pathology in South Africa. South Afr J Sci 86:60–62
Holdenrieder O (2000) Zur Situation der Forstpathologie in Europa. Nachr Deut Pflanzensch 52:135–139
Gadoury DM, Andrews J, Baumgartner K, Burr TJ, Kennelly MM, Lichens-Park A, MacDonald J, Savary S, Scherm H, Tally A, Wang GL (2009) Disciplinary, institutional, funding, and demographic trends in plant pathology: what does the future hold for the profession? Plant Dis 93:1228–1237. doi:10.1094/PDIS-93-12-1228
MacDonald J, Allen C, Gadoury D, Jacobi W, Kelemu S, Moyer J, Murray T, Ong K, Pearson C, Sherwood J, Vidaver A (2009) Education in plant pathology: present status and future challenges. Plant Dis 93:1238–1251. doi:10.1094/PDIS-93-12-1238
Seidl R, Fernandes PM, Fonseca TF, Gillet F, Jönsson AM, Merganičová K, Netherer S, Arpaci A, Bontemps JD, Bugmann H, González-Olabarria JR, Lasch P, Meredieu C, Moreira F, Schelhaas MJ, Mohren F (2011) Modelling natural disturbances in forest ecosystems: a review. Ecol Model 222:903–924. doi:10.1016/j.ecolmodel.2010.09.040
Mazziotta A, Mönkkönen M, Strandman H, Routa J, Tikkanen OP, Kellomäki S (2014) Modeling the effects of climate change and management on the dead wood dynamics in boreal forest plantations. Eur J For Res 133:405–421. doi:10.1007/s10342-013-0773-3
Hamelin RC (2012) Contributions of genomics to forest pathology. Can J Plant Pathol 34:20–28. doi:10.1080/07060661.2012.665389
Wood L, Gebhardt P (2013) Bioinformatics goes to school—new avenues for teaching contemporary biology. PLoS Comp Biol 9:e1003089. doi:10.1371/journal.pcbi.1003089
Holdenrieder O, Pautasso M, Weisberg P, Lonsdale D (2004) Tree diseases and landscape processes: the challenge of landscape pathology. Trends Ecol Evol 19:446–452. doi:10.1016/j.tree.2004.06.003
Hatala JA, Dietze MC, Crabtree RL, Kendall K, Six D, Moorcroft PR (2011) An ecosystem-scale model for the spread of a host-specific forest pathogen in the Greater Yellowstone Ecosystem. Ecol Appl 21:1138–1153. doi:10.1890/09-2118.1
Sieber TN (2007) Endophytic fungi in forest trees: are they mutualists? Fungal Biol Rev 21:75–89. doi:10.1016/j.fbr.2007.05.004
Witzell J, Martín JA, Blumenstein K (2014) Ecological aspects of endophyte-based biocontrol of forest diseases. In: Verma VC, Gange AC (eds) Advances in endophytic research. Springer, Berlin, pp 321–333. doi:10.1007/978-81-322-1575-2_17
Orwig DA (2002) Ecosystem to regional impacts of introduced pests and pathogens: historical context, questions and issues. J Biogeogr 29:1471–1474. doi:10.1046/j.1365-2699.2002.00787.x
Holzmueller EJ, Jose S, Jenkins MA (2010) Ecological consequences of an exotic fungal disease in eastern U.S. hardwood forests. For Ecol Manag 259:1347–1353. doi:10.1016/j.foreco.2010.01.014
Pautasso M, Aas G, Queloz V, Holdenrieder O (2013) European ash (Fraxinus excelsior) dieback—a conservation biology challenge. Biol Conserv 158:37–49. doi:10.1016/j.biocon.2012.08.026
Shearer BL, Crane CE, Cochrane JA, Dunne CP (2013) Variation in susceptibility of threatened flora to Phytophthora cinnamomi. Australas Plant Pathol 42:491–502. doi:10.1007/s13313-013-0215-1
Bragança H, Simões S, Onofre N, Santos N (2009) Factors influencing the incidence and spread of chestnut blight in northeastern Portugal. J Plant Pathol 91:53–59
Nagle AM, Long RP, Madden LV, Bonello P (2010) Association of Phytophthora cinnamomi with white oak decline in southern Ohio. Phytopathology 94:1026–1034. doi:10.1094/PDIS-94-8-1026
Meentemeyer RK, Haas SE, Václavík T (2012) Landscape epidemiology of emerging infectious diseases in natural and human-altered ecosystems. Ann Rev Phytopathol 50:379–402. doi:10.1146/annurev-phyto-081211-172938
Shearer BL, Crane CE (2014) Phytophthora cinnamomi disease expression and habitat suitability of soils on a topographic gradient across a coastal plain from dunes to forested peneplain. Australas Plant Pathol 43:131–142. doi:10.1007/s13313-013-0255-6
Smith-Mckenna EK, Resler LM, Tomback DF, Zhang H, Malanson GP (2013) Topographic influences on the distribution of white pine blister rust in Pinus albicaulis treeline communities. Ecoscience 20:215–229. doi:10.2980/20-3-3599
Geils BW, Hummer KE, Hunt RS (2010) White pines, Ribes, and blister rust: a review and synthesis. For Pathol 140:147–185. doi:10.1111/j.1439-0329.2010.00654.x
Cox CM, Bockus WW, Holt RD, Fang L, Garrett KA (2013) Spatial connectedness of plant species: potential links for apparent competition via plant diseases. Plant Pathol 62:1195–1204. doi:10.1111/ppa.12045
Purse BV, Graeser P, Searle K, Edwards C, Harris C (2014) Challenges in predicting invasive reservoir hosts of emerging pathogens: mapping Rhododendron ponticum as a foliar host for Phytophthora ramorum and Phytophthora kernoviae in the UK. Biol Invasions 15:529–545. doi:10.1007/s10530-012-0305-y
Reeser PW, Sutton W, Hansen EM, Remigi P, Adams GC (2011) Phytophthora species in forest streams in Oregon and Alaska. Mycologia 103:22–35. doi:10.3852/10-013
Hohl A, Václavík T, Meentemeyer RK (2014) Go with the flow: geospatial analytics to quantify hydrologic landscape connectivity for passively dispersed microorganisms. Int J Geogr Inf Sci 28:1626–1641. doi:10.1080/13658816.2013.854900
Peterson E, Hansen E, Kanaskie A (2014) Spatial relationship between Phytophthora ramorum and roads or streams in Oregon tanoak forests. For Ecol Manag 312:216–224. doi:10.1016/j.foreco.2013.10.002
Harwood TD, Xu XM, Pautasso M, Jeger MJ, Shaw MW (2009) Epidemiological risk assessment using linked network and grid based modelling: Phytophthora ramorum and Phytophthora kernoviae in the UK. Ecol Model 220:3353–3361. doi:10.1016/j.ecolmodel.2009.08.014
Xu XM, Harwood TD, Pautasso M, Jeger MJ (2009) Spatio-temporal analysis of an invasive plant pathogen (Phytophthora ramorum) in England and Wales. Ecography 32:504–516. doi:10.1111/j.1600-0587.2008.05597.x
Chadfield V, Pautasso M (2012) Phytophthora ramorum in England and Wales: which environmental variables predict county disease incidence? For Pathol 42:150–159. doi:10.1111/j.1439-0329.2011.00735.x
Smith ML, Bruhn JN, Anderson JB (1992) The fungus Armillaria bulbosa is among the largest and oldest living organisms. Nature 356:428–431. doi:10.1038/356428a0
Brazee NJ, Marra RE, Wick RL (2012) Genotypic diversity of Armillaria gallica from mixed oak forests in Massachusetts. Mycologia 104:53–61. doi:10.3852/11-113
Bendel M, Kienast F, Rigling D (2006) Genetic population structure of three Armillaria species at the landscape scale: a case study from Swiss Pinus mugo forests. Mycol Res 110:705–712. doi:10.1016/j.mycres.2006.02.002
Travadon R, Smith ME, Fujiyoshi P, Douhan GW, Rizzo DM, Baumgartner K (2012) Inferring dispersal patterns of the generalist root fungus Armillaria mellea. New Phytol 193:959–969. doi:10.1111/j.1469-8137.2011.04015.x
Prospero S, Lung-Escarmant B, Dutech C (2008) Genetic structure of an expanding Armillaria root rot fungus (Armillaria ostoyae) population in a managed pine forest in southwestern France. Mol Ecol 17:3366–3378. doi:10.1111/j.1365-294X.2007.03829.x
Filipe JAN, Cobb RC, Meentemeyer RK, Lee CA, Valachovic YS, Cook AR, Rizzo DM, Gilligan CA (2012) Landscape epidemiology and control of pathogens with cryptic and long-distance dispersal: Sudden Oak Death in northern Californian forests. PLoS Comp Biol 8:e1002328. doi:10.1371/journal.pcbi.1002328
Shearer BL, Crane CE, Fairman RG, Dillon MJ, Buehrig RM (2014) Spatio-temporal variation in invasion of woodlands and forest by Phytophthora cinnamomi. Australas Plant Pathol 43:327–337. doi:10.1007/s13313-014-0274-y
Díaz R, Zas R, Fernández-López J (2007) Genetic variation of Prunus avium in susceptibility to cherry leaf spot (Blumeriella jaapii) in spatially heterogeneous infected seed orchards. Ann For Sci 64:21–30. doi:10.1051/forest:2006084
Hayden KJ, Nettel A, Dodd RS, Garbelotto M (2011) Will all the trees fall? Variable resistance to an introduced forest disease in a highly susceptible host. For Ecol Manag 261:1781–1791. doi:10.1016/j.foreco.2011.01.042
Kowalski T (2006) Chalara fraxinea sp nov associated with dieback of ash (Fraxinus excelsior) in Poland. For Pathol 36:264–270. doi:10.1111/j.1439-0329.2006.00453.x
Queloz V, Grünig CR, Berndt R, Kowalski T, Sieber TN, Holdenrieder O (2011) Cryptic speciation in Hymenoscyphus albidus. For Pathol 41:133–142. doi:10.1111/j.1439-0329.2010.00645.x
Baral H-O, Queloz VK, Hosoya TS (2014) Hymenoscyphus fraxineus, the correct scientific name for the fungus causing ash dieback in Europe. IMA Fungus 5(1):79–80. doi:10.5598/imafungus.2014.05.01.09
McKinney LV, Nielsen LR, Hansen JK, Kjær ED (2011) Presence of natural genetic resistance in Fraxinus excelsior (Oleraceae) to Chalara fraxinea (Ascomycota): an emerging infectious disease. Heredity 106:788–797. doi:10.1038/hdy.2010.119
Pliura A, Lygis V, Suchockas V, Bartkevicius E (2011) Performance of twenty four European Fraxinus excelsior populations in three Lithuanian progeny trials with a special emphasis on resistance to Chalara fraxinea. Balt For 17(1):17–34
Kowalski T, Kraj W, Szeszycki T (2012) Badania nad zamieraniem jesionu w drzewostanach Nadleśnictwa Rokita [The studies on ash decline in Rokita forest district stands]. Acta Agric Silv Ser Silv 50:3–22
Enderle R, Peters F, Nakou A, Metzler B (2013) Temporal development of ash dieback symptoms and spatial distribution of collar rots in a provenance trial of Fraxinus excelsior. Eur J For Res 132:865–876. doi:10.1007/s10342-013-0717-y
Stener LG (2013) Clonal differences in susceptibility to the dieback of Fraxinus excelsior in southern Sweden. Scand J For Res 28:205–216. doi:10.1080/02827581.2012.735699
Enderle R, Nakou A, Thomas K, Metzler B (2014) Susceptibility of autochthonous German Fraxinus excelsior clones to Hymenoscyphus pseudoalbidus is genetically determined. Ann For Sci. doi:10.1007/s13595-014-0413-1
Lobo A, Hansen JK, McKinney LV, Nielsen LR, Kjær ED (2014) Genetic variation in dieback resistance: growth and survival of Fraxinus excelsior under the influence of Hymenoscyphus pseudoalbidus. Scand J For Res. doi:10.1080/02827581.2014.950603
McKinney LV, Nielsen LR, Collinge DB, Thomsen IM, Hansen JK, Kjær ED (2014) The ash dieback crisis: genetic variation in resistance can prove a long-term solution. Plant Pathol. doi:10.1111/ppa.12196
Bingham RT, Hoff RJ, McDonald GI (1971) Disease resistance in forest trees. Ann Rev Phytopathol 9:433–452. doi:10.1146/annurev.py.09.090171.002245
Roll-Hansen F (1972) Scleroderris lagerbergii: resistance and differences in attack between pine species and provenances. A literature review. Eur J For Pathol 2:26–39. doi:10.1111/j.1439-0329.1972.tb00340.x
Illingworth K (1973) Variation in the susceptibility of lodgepole pine provenances to Sirococcus shoot blight. Can J For Res 3:585–589. doi:10.1139/x73-087
Barbour RC, O'Reilly-Wapstra JM, De Little DW, Jordan GJ, Steane DA, Humphreys JR, Bailey JK, Whitham TG, Potts BM (2009) A geographic mosaic of genetic variation within a foundation tree species and its community-level consequences. Ecology 90:1762–1772. doi:10.1890/08-0951.1
Ingwell LL, Preisser EL (2011) Using citizen science programs to identify host resistance in pest-invaded forests. Conserv Biol 25:182–188. doi:10.1111/j.1523-1739.2010.01567.x
Bernhardsson C, Robinson KM, Abreu IN, Jansson S, Albrectsen BR, Ingvarsson PK (2013) Geographic structure in metabolome and herbivore community co-occurs with genetic structure in plant defence genes. Ecol Lett 16:791–798. doi:10.1111/ele.12114
Hamilton MG, Williams DR, Tilyard PA, Pinkard EA, Wardlaw TJ, Glen M, Vaillancourt RE, Potts BM (2013) A latitudinal cline in disease resistance of a host tree. Heredity 110:372–379. doi:10.1038/hdy.2012.106
Hayden KJ, Garbelotto M, Dodd R, Wright JW (2013) Scaling up from greenhouse resistance to fitness in the field for a host of an emerging forest disease. Evol Appl 6:970–982. doi:10.1111/eva.12080
Busby PE, Newcombe G, Dirzo R, Whitham TG (2014) Genetic basis of pathogen community structure for foundation tree species in a common garden and in the wild. J Ecol 101:867–877. doi:10.1111/1365-2745.12112
Shearer BL, Michaelsen BJ, Somerford PJ, Williams M (2014) Forest environment mediated intraspecific resistance of Eucalyptus marginata to Phytophthora cinnamomi. Australas Plant Pathol 43:245–255. doi:10.1007/s13313-013-0263-6
Brazee NJ, Wick RL (2011) Armillaria species distribution and site relationships in Pinus- and Tsuga-dominated forests in Massachusetts. Can J For Res 41:1477–1490. doi:10.1139/X11-076
Beh MM, Metz MR, Frangioso KM, Rizzo DM (2012) The key host for an invasive forest pathogen also facilitates the pathogen’s survival of wildfire in California forests. New Phytol 196:1145–1154. doi:10.1111/j.1469-8137.2012.04352.x
Metz MR, Varner JM, Frangioso KM, Meentemeyer RK, Rizzo DM (2013) Unexpected redwood mortality from synergies between wildfire and an emerging infectious disease. Ecology 94:2152–2159. doi:10.1890/13-0915.1
Garnas JR, Houston DR, Twery MJ, Ayres MP, Evans C (2013) Inferring controls on the epidemiology of beech bark disease from spatial patterning of disease organisms. Agric For Entomol 15:146–156. doi:10.1111/j.1461-9563.2012.00595.x
Morin RS, Liebhold AM, Tobin PC, Gottschalk KW, Luzader E (2007) Spread of beech bark disease in the eastern United States and its relationship to regional forest composition. Can J For Res 37:726–736. doi:10.1139/X06-281
Taylor AR, McPhee DA, Loo JA (2013) Incidence of beech bark disease resistance in the eastern Acadian forest of North America. For Chron 89:690–695
Kasson MT, Livingston WH (2012) Relationships among beech bark disease, climate, radial growth response and mortality of American beech in northern Maine, USA. For Pathol 42:199–212. doi:10.1111/j.1439-0329.2011.00742.x
Jarčuška B, Mihál I, Cicák A, Tsakov H (2013) Beech bark necrosis: partitioning the environmental and spatial variation of the damage severity in Central and South-Eastern Europe. Ann For Res 56:317–338
Prospero S, Rigling D (2012) Invasion genetics of the chestnut blight fungus Cryphonectria parasitica in Switzerland. Phytopathology 102:73–82. doi:10.1094/PHYTO-02-11-0055
Peters FS, Bußkamp J, Prospero S, Rigling D, Metzler B (2014) Genetic diversification of the chestnut blight fungus Cryphonectria parasitica and its associated hypovirus in Germany. Fung Biol 118:193–210. doi:10.1016/j.funbio.2013.11.009
Bryner SF, Rigling D (2011) Temperature‐dependent genotype‐by‐genotype interaction between a pathogenic fungus and its hyperparasitic virus. Am Nat 177:65–74. doi:10.1086/657620
Bryner SF, Rigling D (2012) Hypovirus virulence and vegetative incompatibility in populations of the chestnut blight fungus. Phytopathology 102:1161–1167. doi:10.1094/PHYTO-01-12-0013-R
Brusini J, Robin C (2013) Mycovirus transmission revisited by in situ pairings of vegetatively incompatible isolates of Cryphonectria parasitica. J Virol Meth 187:435–442. doi:10.1016/j.jviromet.2012.11.025
Springer JC, Davelos Baines AL, Fulbright DW, Chansler MT, Jarosz AM (2013) Hyperparasites influence population structure of the chestnut blight pathogen, Cryphonectria parasitica. Phytopathology 103:1280–1286. doi:10.1094/PHYTO-10-12-0273-R
Waring KM, O’Hara KL (2005) Silvicultural strategies in forest ecosystems affected by introduced pests. For Ecol Manag 209:27–41. doi:10.1016/j.foreco.2005.01.008
Garbelotto M, Gonthier P (2013) Biology, epidemiology, and control of Heterobasidion species worldwide. Ann Rev Phytopathol 51:39–59. doi:10.1146/annurev-phyto-082712-102225
Tsykun T, Rigling D, Nikolaychuk V, Prospero S (2012) Diversity and ecology of Armillaria species in virgin forests in the Ukrainian Carpathians. Mycol Prog 11:403–414. doi:10.1007/s11557-011-0755-0
Pinkard EA, Kriticos DJ, Wardlaw TJ, Carnegie AJ, Leriche A (2010) Estimating the spatio-temporal risk of disease epidemics using a bioclimatic niche model. Ecol Model 221:2828–2838. doi:10.1016/j.ecolmodel.2010.08.017
Queloz V, Sieber TN, Holdenrieder O, McDonald BA, Grünig CR (2011) No biogeographical pattern for a root-associated fungal species complex. Glob Ecol Biogeogr 20:160–169. doi:10.1111/j.1466-8238.2010.00589.x
Eschen R, Holmes T, Smith D, Roques A, Santini A, Kenis M (2014) Likelihood of establishment of tree pests and diseases based on their worldwide occurrence as determined by hierarchical cluster analysis. For Ecol Manag 315:103–111. doi:10.1016/j.foreco.2013.12.021
Potter KM, Koch FH (2014) Patterns of forest phylogenetic community structure across the United States and their possible forest health implications. For Sci. doi:10.5849/forsci.13-115
Gibbs JN (1978) Intercontinental epidemiology of Dutch elm disease. Ann Rev Phytopathol 16:287–307. doi:10.1146/annurev.py.16.090178.001443
Houston DR, Parker EJ, Perrin R, Lang KJ (1979) Beech bark disease: a comparison of the disease in North America, Great Britain, France, and Germany. Eur J For Pathol 9:199–211. doi:10.1111/j.1439-0329.1979.tb00679.x
Karnosky DF (1979) Dutch elm disease: a review of the history, environmental implications, control, and research needs. Environ Conserv 6:311–322. doi:10.1017/S037689290000357X
Klein RM, Perkins TD (1988) Primary and secondary causes and consequences of contemporary forest decline. Bot Rev 54:1–43. doi:10.1007/BF02858517
Innes JL, Landmann G, Mettendorf B (1993) Consistency of observations of forest tree defoliation in three European countries. Environ Monit Assess 25:29–40. doi:10.1007/BF00549790
Ferretti M (1997) Forest health assessment and monitoring—issues for consideration. Environ Monit Assess 48:45–72. doi:10.1023/A:1005748702893
Wingfield MJ, Slippers B, Roux J, Wingfield BD (2001) Worldwide movement of exotic forest fungi, especially in the tropics and the southern hemisphere. Bioscience 51:134–140. doi:10.1641/0006-3568(2001)051[013
Wingfield MJ, Roux J, Wingfield BD (2011) Insect pests and pathogens of Australian acacias grown as non-natives—an experiment in biogeography with far-reaching consequences. Divers Distrib 17:968–977. doi:10.1111/j.1472-4642.2011.00786.x
Hasegawa E, Ota Y, Hattori T, Sahashi N, Kikuchi T (2011) Ecology of Armillaria species on conifers in Japan. For Pathol 41:429–437. doi:10.1111/j.1439-0329.2010.00696.x
Bryner SF, Rigling D, Brunner PC (2012) Invasion history and demographic pattern of Cryphonectria hypovirus 1 across European populations of the chestnut blight fungus. Ecol Evol 2:3227–3241. doi:10.1002/ece3.429
Dutech C, Barrès B, Bridier J, Robin C, Milgroom MG, Ravigné V (2012) The chestnut blight fungus world tour: successive introduction events from diverse origins in an invasive plant fungal pathogen. Mol Ecol 21:3931–3946. doi:10.1111/j.1365-294X.2012.05575.x
Brasier CM (2008) The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathol 57:792–808. doi:10.1111/j.1365-3059.2008.01886.x
Santini A et al (2013) Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol 197:238–250. doi:10.1111/j.1469-8137.2012.04364.x
Pautasso M, Jeger MJ (2014) Network epidemiology and plant trade networks. AoB Plants 6:plu007. doi:10.1093/aobpla/plu007
Steenkamp ET, Makhari OM, Coutinho TA, Wingfield BD, Wingfield MJ (2014) Evidence for a new introduction of the pitch canker fungus Fusarium circinatum in South Africa. Plant Pathol 63:530–538. doi:10.1111/ppa.12136
Möykkynen T, Capretti P, Pukkala T (2014) Modelling the potential spread of Fusarium circinatum, the causal agent of pitch canker in Europe. Ann For Sci. doi:10.1007/s13595-014-0412-2
Szabó I, Lakatos F, Sipos G (2013) Occurrence of soilborne Phytophthora species in declining broadleaf forests in Hungary. Eur J Plant Pathol 137:159–168. doi:10.1007/s10658-013-0228-1
Gross A, Holdenrieder O, Pautasso M, Queloz V, Sieber TN (2014) Hymenoscyphus pseudoalbidus, the causal agent of European ash dieback. Mol Plant Pathol 15:5–21. doi:10.1111/mpp.12073
Gross A, Hosoya T, Queloz V (2014) Population structure of the invasive forest pathogen Hymenoscyphus pseudoalbidus. Mol Ecol 23:2943–2960. doi:10.1111/mec.12792
Vélez ML, Coetzee MPA, Wingfield MJ, Rajchenberg M, Greslebin AG (2014) Evidence of low levels of genetic diversity for the Phytophthora austrocedrae population in Patagonia, Argentina. Plant Pathol 63:212–220. doi:10.1111/ppa.12067
Busby PE, Aimé MC, Newcombe G (2012) Foliar pathogens of Populus angustifolia are consistent with a hypothesis of Beringian migration into North America. Fung Biol 116:792–801. doi:10.1016/j.funbio.2012.04.012
Baumgartner K, Travadon R, Bruhn J, Bergemann SE (2010) Contrasting patterns of genetic diversity and population structure of Armillaria mellea sensu stricto in the eastern and western United States. Phytopathology 100:708–718
Broders KD, Boraks A, Sanchez AM, Boland GJ (2012) Population structure of the butternut canker fungus, Ophiognomonia clavigignenti-juglandacearum, in North American forests. Ecol Evol 2:2114–2127. doi:10.1002/ece3.332
Garbelotto M, Guglielmo F, Mascheretti S, Croucher PJP, Gonthier P (2013) Population genetic analyses provide insights on the introduction pathway and spread patterns of the North American forest pathogen Heterobasidion irregulare in Italy. Mol Ecol 22:4855–4869. doi:10.1111/mec.12452
Heinzelmann R, Rigling D, Prospero S (2012) Population genetics of the wood-rotting basidiomycete Armillaria cepistipes in a fragmented forest landscape. Fung Biol 116:985–994. doi:10.1016/j.funbio.2012.07.002
Ježić M, Krstin L, Rigling D, Ćurković-Perica M (2012) High diversity in populations of the introduced plant pathogen, Cryphonectria parasitica, due to encounters between genetically divergent genotypes. Mol Ecol 21:87–99. doi:10.1111/j.1365-294X.2011.05369.x
Prospero S, Lutz A, Tavadze B, Supatashvili A, Rigling D (2013) Discovery of a new gene pool and a high genetic diversity of the chestnut blight fungus Cryphonectria parasitica in Caucasian Georgia. Infect Genet Evol 20:131–139. doi:10.1016/j.meegid.2013.08.009
Vermeulen M, Gryzenhout M, Wingfield MJ, Roux J (2013) Population structure of Chrysoporthe austroafricana in southern Africa determined using Vegetative Compatibility Groups (VCGs). For Pathol 43:124–131. doi:10.1111/efp.12006
Smalley EB, Guries RP (1993) Breeding elms for resistance to Dutch elm disease. Ann Rev Phytopathol 31:325–354. doi:10.1146/annurev.py.31.090193.001545
Santini A, La Porta N, Ghelardini L, Mittempergher L (2008) Breeding against Dutch elm disease adapted to the Mediterranean climate. Euphytica 163:45–56. doi:10.1007/s10681-007-9573-5
Jacobs DF, Dalgleish HJ, Nelson CD (2014) A conceptual framework for restoration of threatened plants: the effective model of American chestnut (Castanea dentata) reintroduction. New Phytol 197:378–393. doi:10.1111/nph.12020
Hicke JA, Allen CD, Desai AR, Dietze MC, Hall RJ, Hogg EHT, Kashian DM, Moore D, Raffa KF, Sturrock RN, Vogelmann J (2012) Effects of biotic disturbances on forest carbon cycling in the United States and Canada. Glob Chang Biol 18:7–34. doi:10.1111/j.1365-2486.2011.02543.x
Matyssek R, Wieser G, Calfapietra C, de Vries W, Dizengremel P, Ernst D, Jolivet Y, Mikkelsen TN, Mohren GMJ, Le Thiec D, Tuovinen JP, Weatherall A, Paoletti E (2012) Forests under climate change and air pollution: gaps in understanding and future directions for research. Environ Pollut 160:57–65. doi:10.1016/j.envpol.2011.07.007
Ayres MP, Hicke JA, Kerns BK, McKenzie D, Littell JS, Band LE, Luce CH, Weed AS, Raymond CL (2014) Disturbance regimes and stressors. In: Peterson DL et al (eds) Climate change and United States forests. Springer, Berlin, pp 55–92. doi:10.1007/978-94-007-7515-2__4
Wingfield MJ, Slippers B, Wingfield BD (2010) Novel associations between pathogens, insects and tree species threaten world forests. N Z J For Sci 40:95–103
Pautasso M, Döring TF, Garbelotto M, Pellis L, Jeger MJ (2012) Impacts of climate change on plant diseases—opinions and trends. Eur J Plant Pathol 133:295–313. doi:10.1007/s10658-012-9936-1
Jeschke JM, Keesing F, Ostfeld RS (2013) Novel organisms: comparing invasive species, GMOs, and emerging pathogens. Ambio 42:541–548. doi:10.1007/s13280-013-0387-5
Kremer A, Potts BM, Delzon S (2014) Genetic divergence in forest trees: understanding the consequences of climate change. Funct Ecol 28:22–36. doi:10.1111/1365-2435.12169
Garbelotto M, Pautasso M (2012) Impacts of exotic forest pathogens on Mediterranean ecosystems: four case studies. Eur J Plant Pathol 133:101–116. doi:10.1007/s10658-011-9928-6
Thompson SE, Levin S, Rodriguez-Iturbe I (2014) Rainfall and temperatures changes have confounding impacts on Phytophthora cinnamomi occurrence risk in the southwestern USA under climate change scenarios. Glob Chang Biol 20:1299–1312. doi:10.1111/gcb.12463
Weed AS, Ayres MP, Hicke JA (2013) Consequences of climate change for biotic disturbances in North American forests. Ecol Monogr 83:441–470. doi:10.1890/13-0160.1
Kaczynski KM, Cooper DJ (2013) Susceptibility of Salix monticola to Cytospora canker under increased temperatures and decreased water levels. For Ecol Manag 305:223–228. doi:10.1016/j.foreco.2013.06.002
Dodd RS, Hüberli D, Mayer W, Harnik TY, Afzal-Rafii Z, Garbelotto M (2008) Evidence for the role of synchronicity between host phenology and pathogen activity in the distribution of sudden oak death canker disease. New Phytol 179:505–514. doi:10.1111/j.1469-8137.2008.02450.x
Gray LK, Russell JH, Yanchuk AD, Hawkins BJ (2013) Predicting the risk of cedar leaf blight (Didymascella thujina) in British Columbia under future climate change. Agric For Meteorol 180:152–163. doi:10.1016/j.agrformet.2013.04.023
Aukema JE, McCullough DG, Von Holle B, Liebhold AM, Britton K, Frankel SJ (2010) Historical accumulation of nonindigenous forest pests in the continental United States. Bioscience 60:886–897. doi:10.1525/bio.2010.60.11.5
Dehnen-Schmutz K, Holdenrieder O, Jeger MJ, Pautasso M (2010) Structural change in the international horticultural industry: some implications for plant health. Sci Hortic 125:1–15. doi:10.1016/j.scienta.2010.02.017
Moslonka-Lefebvre M, Finley A, Dorigatti I, Dehnen-Schmutz K, Harwood T, Jeger MJ, Xu XM, Holdenrieder O, Pautasso M (2011) Networks in plant epidemiology: from genes to landscapes, countries and continents. Phytopathology 101:392–403. doi:10.1094/PHYTO-07-10-0192
Grünwald NJ, Garbelotto M, Goss EM, Heungens K, Prospero S (2012) Emergence of the sudden oak death pathogen Phytophthora ramorum. Trends Microbiol 20:131–138. doi:10.1016/j.tim.2011.12.006
Liebhold AM, Brockerhoff EG, Garrett LJ, Parke JL, Britton KO (2012) Live plant imports: the major pathway for forest insect and pathogen invasions of the US. Front Ecol Environ 10:135–143. doi:10.1890/110198
Bienapfl JC, Balci Y (2014) Movement of Phytophthora spp. in Maryland’s nursery trade. Plant Dis 98:134–144. doi:10.1094/PDIS-06-13-0662-RE
Schoebel CN, Stewart J, Gruenwald NJ, Rigling D, Prospero S (2014) Population history and pathways of spread of the plant pathogen Phytophthora plurivora. PLoS One 9:e85368. doi:10.1371/journal.pone.0085368
Thoirain B, Husson C, Marçais B (2007) Risk factors for the Phytophthora-induced decline of alder in northeastern France. Phytopathology 97:99–105. doi:10.1094/PHYTO-97-0099
Pautasso M (2013) Phytophthora ramorum—a pathogen linking network epidemiology, landscape pathology and conservation biogeography. CAB Rev 8:24. doi:10.1079/PAVSNNR20138024
Prospero S, Vercauteren A, Heungens K, Belbahri L, Rigling D (2013) Phytophthora diversity and the population structure of Phytophthora ramorum in Swiss ornamental nurseries. Plant Pathol 62:1063–1071. doi:10.1111/ppa.12027
Ginetti B, Moricca S, Squires JN, Cooke DEL, Ragazzi A, Jung T (2014) Phytophthora acerina sp. nov., a new species causing bleeding cankers and dieback of Acer pseudoplatanus trees in planted forests in northern Italy. Plant Pathol 63:858–876. doi:10.1111/ppa.12153
Sansford CE (2013) Pest risk analysis for Hymenoscyphus pseudoalbidus for the UK and the Republic of Ireland. Forestry Commission, UK. Accessed May 2014 at http://www.fera.defra.gov.uk/plants/plantHealth/pestsDiseases/documents/hymenoscyphusPseudoalbidusPRA.pdf
Mcpherson EG (1993) Monitoring urban forest health. Environ Monit Assess 26:165–174. doi:10.1007/BF00547494
Tubby KV, Webber JF (2010) Pests and diseases threatening urban trees under a changing climate. Forestry 83:451–459. doi:10.1093/forestry/cpq027
Tomoshevich M, Kirichenko N, Holmes K, Kenis M (2013) Foliar fungal pathogens of European woody plants in Siberia: an early warning of potential threats? For Pathol 43:345–359. doi:10.1111/efp.12036
Liebhold AM, McCullough DG, Blackburn LM, Frankel SJ, Von Holle B, Aukema JE (2013) A highly aggregated geographical distribution of forest pest invasions in the USA. Divers Distrib 19:1208–1216. doi:10.1111/ddi.12112
Barber PA, Paap T, Burgess TI, Dunstan W, Hardy GE (2013) A diverse range of Phytophthora species are associated with dying urban trees. Urban For Urban Green 12:569–575. doi:10.1016/j.ufug.2013.07.009
Jactel H, Petit J, Desprez-Loustau ML, Delzon S, Piou D, Battisti A, Koricheva J (2012) Drought effects on damage by forest insects and pathogens: a meta-analysis. Glob Chang Biol 18:267–276. doi:10.1111/j.1365-2486.2011.02512.x
Barbeito I, Brücker RL, Rixen C, Bebi P (2013) Snow fungi-induced mortality of Pinus cembra at the alpine treeline: evidence from plantations. Arct Antarct Alp Res 45:455–470. doi:10.1657/1938-4246-45.4.455
Gori Y, Cherubini P, Camin F, La Porta N (2013) Fungal root pathogen (Heterobasidion parviporum) increases drought stress in Norway spruce stand at low elevation in the Alps. Eur J For Res 132:607–619. doi:10.1007/s10342-013-0698-x
Pautasso M (2013) Responding to diseases caused by exotic tree pathogens. In: Gonthier P, Nicolotti G (eds) Infectious forest diseases. CABI, Wallingford, pp 592–612
Pautasso M (2013) Fungal under-representation is (slowly) diminishing in the life sciences. Fungal Ecol 6:129–135. doi:10.1016/j.funeco.2012.04.004
Hantula J, Müller MM, Uusivuori J (2014) International plant trade associated risks: laissez-faire or novel solutions. Environ Sci Pollut 37:158–160. doi:10.1016/j.envsci.2013.09.011
Bebber DP, Holmes T, Smith D, Gurr SJ (2014) Economic and physical determinants of the global distributions of crop pests and pathogens. New Phytol 202:901–910. doi:10.1111/nph.12722
Bebber DP, Ramotowski MAT, Gurr SJ (2013) Crop pests and pathogens move polewards in a warming world. Nat Clim Chang 3:985–988. doi:10.1038/nclimate1990
Busby PE, Zimmerman N, Weston DJ, Jawdy SS, Houbraken J, Newcombe G (2013) Leaf endophytes and Populus genotype affect severity of damage from the necrotrophic leaf pathogen, Drepanopeziza populi. Ecosphere 4:125. doi:10.1890/ES13-00127.1
Mayerhofer MS, Kernaghan G, Harper KA (2013) The effects of fungal root endophytes on plant growth: a meta-analysis. Mycorrhiza 23:119–128. doi:10.1007/s00572-012-0456-9
Raghavendra AKH, Newcombe G (2013) The contribution of foliar endophytes to quantitative resistance to Melampsora rust. New Phytol 197:909–918. doi:10.1111/nph.12066
Tellenbach C, Sieber TN (2012) Do colonization by dark septate endophytes and elevated temperature affect pathogenicity of oomycetes? FEMS Microbiol Ecol 82:157–168
Tellenbach C, Sumarah MW, Grünig CR, Miller JD (2013) Inhibition of Phytophthora species by secondary metabolites produced by the dark septate endophyte Phialocephala europaea. Fung Ecol 6:12–18. doi:10.1016/j.funeco.2012.10.003
Rodriguez R, Redman R (2008) More than 400 million years of evolution and some plants still can’t make it on their own: plant stress tolerance via fungal symbiosis. J Exp Bot 59:1109–1114. doi:10.1093/jxb/erm342
Redman RS, Kim YO, Woodward CJDA, Greer C, Espino L, Doty SL, Rodriguez R (2011) Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: a strategy for mitigating impacts of climate change. PLoS One 6:e14823. doi:10.1371/journal.pone.0014823
Hubbard M, Germida J, Vujanovic V (2012) Fungal endophytes improve wheat seed germination under heat and drought stress. Botany 90:137–149. doi:10.1139/B11-091
Giauque H, Hawkes CV (2013) Climate affects symbiotic fungal endophyte diversity and performance. Am J Bot 100:1435–1444. doi:10.3732/ajb.1200568
Goh CH, Veliz Vallejos DF, Nicotra AB, Mathesius U (2013) The impact of beneficial plant-associated microbes on plant phenotypic plasticity. J Chem Ecol 39:826–839. doi:10.1007/s10886-013-0326-8
Zhang Y, Li T, Zhao ZW (2013) Colonization characteristics and composition of dark septate endophytes (DSE) in a lead and zinc slag heap in southwest China. Soil Sedim Contam 22:532–545. doi:10.1080/15320383.2013.750267
Kurose D, Furuya N, Tsuchiya K, Tsushima S, Evans HC (2012) Endophytic fungi associated with Fallopia japonica (Polygonaceae) in Japan and their interactions with Puccinia polygoni-amphibii var. tovariae, a candidate for classical biological control. Fung Biol 116:785–791. doi:10.1016/j.funbio.2012.04.011
Eyles A, Bonello P, Ganley R, Mohammed C (2010) Induced resistance to pests and pathogens in trees. New Phytol 185:893–908. doi:10.1111/j.1469-8137.2009.03127.x
Shoresh M, Harman GE, Mastouri F (2010) Induced systemic resistance and plant responses to fungal biocontrol agents. Ann Rev Phytopathol 48:21–43. doi:10.1146/annurev-phyto-073009-114450
Gómez-Lama Cabanás C, Schiliro E, Valverde-Corredor A, Mercado-Blanco J (2014) The biocontrol endophytic bacterium Pseudomonas fluorescens PICF7 induces systemic defense responses in aerial tissues upon colonization of olive roots. Front Microbiol. doi:10.3389/fmicb.2014.00427
Rajala T, Velmala SM, Tuomivirta T, Haapanen M, Müller M, Pennanen T (2013) Endophyte communities vary in the needles of Norway spruce clones. Fung Biol 117:182–190. doi:10.1016/j.funbio.2013.01.006
Rajala T, Velmala SM, Vesala R, Smolander A, Pennanen T (2014) The community of needle endophytes reflects the current physiological state of Norway spruce. Fung Biol 118:309–315. doi:10.1016/j.funbio.2014.01.002
Izhaki I, Fridman S, Gerchman Y, Halpern M (2013) Variability of bacterial community composition on leaves between and within plant species. Curr Microbiol 66:227–235. doi:10.1007/s00284-012-0261-x
Saikkonen K, Faeth SH, Helander M, Sullivan TJ (1998) Fungal endophytes: a continuum of interactions with host plants. Ann Rev Ecol Syst 29:319–343. doi:10.1146/annurev.ecolsys.29.1.319
Delaye L, García-Guzmán G, Heil M (2013) Endophytes versus biotrophic and necrotrophic pathogens—are fungal lifestyles evolutionarily stable traits? Fung Divers 60:125–135. doi:10.1007/s13225-013-0240-y
Osono T, Masuya H (2012) Endophytic fungi associated with leaves of Betulaceae in Japan. Can J Microbiol 58:507–515. doi:10.1139/w2012-018
Peršoh D (2013) Factors shaping community structure of endophytic fungi—evidence from the Pinus-Viscum-system. Fung Divers 60:55–69. doi:10.1007/s13225-013-0225-x
Scholtysik A, Unterseher M, Otto P, Wirth C (2013) Spatio-temporal dynamics of endophyte diversity in the canopy of European ash (Fraxinus excelsior). Mycol Prog 12:291–304. doi:10.1007/s11557-012-0835-9
Saikkonen K (2007) Forest structure and fungal endophytes. Fung Biol Rev 21:67–74. doi:10.1016/j.fbr.2007.05.001
Unterseher M (2011) Diversity of fungal endophytes in temperate forest trees. In: Pirttilä AM, Frank AC (eds) Endophytes of forest trees: biology and applications. Springer, Berlin, pp 31–46. doi:10.1007/978-94-007-1599-8_2
Unterseher M, Peršoh D, Schnittler M (2013) Leaf-inhabiting endophytic fungi of European beech (Fagus sylvatica L.) co-occur in leaf litter but are rare on decaying wood of the same host. Fung Divers 60:43–54. doi:10.1007/s13225-013-0222-0
Bálint M, Tiffin P, Hallström B, O’Hara RB, Olson MS et al (2013) Host genotype shapes the foliar fungal microbiome of balsam poplar (Populus balsamifera). PLoS One 8:e53987. doi:10.1371/journal.pone.0053987
Ahlholm JU, Helander M, Henriksson J, Metzler M, Saikkonen K (2002) Environmental conditions and host genotype direct genetic diversity of Venturia ditricha, a fungal endophyte of birch trees. Evolution 56:1566–1573. doi:10.1111/j.0014-3820.2002.tb01468.x
Martín JA, Witzell J, Blumenstein K, Rozpedowska E, Helander M, Sieber TN, Gil L (2013) Resistance to Dutch elm disease reduces presence of xylem endophytic fungi in elms (Ulmus spp.). PLoS One 8:e56987. doi:10.1371/journal.pone.0056987
Ikeda A, Matsuoka S, Masuya H, Mori AS, Hirose D, Osono T (2014) Comparison of the diversity, composition, and host recurrence of xylariaceous endophytes in subtropical, cool temperate, and subboreal regions in Japan. Popul Ecol 56:289–300. doi:10.1007/s10144-013-0412-3
Petrini O (1991) Fungal endophytes of tree leaves. In: Andrews JH, Hirano SS (eds) Microbial ecology of leaves. Springer, New York, pp 179–197
Osono T (2014) Diversity and ecology of endophytic and epiphytic fungi of tree leaves in Japan: a review. In: Verma VC, Gange AC (eds) Advances in endophytic research. Springer, Berlin, pp 3–26. doi:10.1007/978-81-322-1575-2_1
Cordier T, Robin C, Capdevielle X, Fabreguettes O, Desprez-Loustau M-L, Vacher C (2012) The composition of phyllosphere fungal assemblages of European beech (Fagus sylvatica) varies significantly along an elevation gradient. New Phytol 196:510–519. doi:10.1111/j.1469-8137.2012.04284.x
Terhonen E, Marco T, Sun H, Jalkanen R, Kasanen R, Vuorinen M, Asiegbu F (2011) The effect of latitude, season and needle-age on the mycota of Scots pine (Pinus sylvestris) in Finland. Silva Fenn 45:301–317
Hashizume Y, Fukuda K, Sahashi N (2010) Effects of summer temperature on fungal endophyte assemblages in Japanese beech (Fagus crenata) leaves in pure beech stands. Botany 88:266–274. doi:10.1139/B09-114
Zimmerman NB, Vitousek PM (2012) Fungal endophyte communities reflect environmental structuring across a Hawaiian landscape. Proc Natl Acad Sci U S A 109:13022–13027. doi:10.1073/pnas.1209872109
Helander M, Wäli P, Kuuluvainen T, Saikkonen K (2006) Birch leaf endophytes in managed and natural boreal forests. Can J For Res 36:3239–3245. doi:10.1139/x06-176
Jumpponen A, Jones KL (2009) Massively parallel 454 sequencing indicates hyperdiverse fungal communities in temperate Quercus macrocarpa phyllosphere. New Phytol 184:438–448. doi:10.1111/j.1469-8137.2009.02990.x
Jumpponen A, Jones KL (2010) Seasonally dynamic fungal communities in the Quercus macrocarpa phyllosphere differ between urban and nonurban environments. New Phytol 186:496–513. doi:10.1111/j.1469-8137.2010.03197.x
Matsumura E, Fukuda K (2013) A comparison of fungal endophytic community diversity in tree leaves of rural and urban temperate forests of Kanto district, eastern Japan. Fung Biol 117:191–201. doi:10.1016/j.funbio.2013.01.007
Lau MK, Arnold AE, Johnson NC (2013) Factors influencing communities of foliar fungal endophytes in riparian woody plants. Fung Ecol 6:365–378. doi:10.1016/j.funeco.2013.06.003
Hoffman MT, Arnold AE (2008) Geographic locality and host identity shape fungal endophyte communities in cupressaceous trees. Mycol Res 112:331–344. doi:10.1016/j.mycres.2007.10.014
Johnston PR, Johansen RB, Williams AFR, Wikie JP, Park D (2012) Patterns of fungal diversity in New Zealand Nothofagus forests. Fung Biol 116:401–412. doi:10.1016/j.funbio.2011.12.010
Vaz ABM, Fontenla S, Rocha FS, Brandao LR, Vieira MLA, De Garcia V, Goes-Neto A, Rosa CA (2014) Fungal endophyte β-diversity associated with Myrtaceae species in an Andean Patagonian forest (Argentina) and an Atlantic forest (Brazil). Fung Ecol 8:28–36. doi:10.1016/j.funeco.2013.12.008
Cordier T, Robin C, Capdevielle X, Desprez-Loustau ML, Vacher C (2012) Spatial variability of phyllosphere fungal assemblages: genetic distance predominates over geographic distance in a European beech stand (Fagus sylvatica). Fung Ecol 5:509–520. doi:10.1016/j.funeco.2011.12.004
U’Ren JM, Lutzoni F, Miadlikowska J, Laetsch AD, Arnold AE (2012) Host and geographic structure of endophytic and endolichenic fungi at a continental scale. Am J Bot 99:898–914. doi:10.3732/ajb.1100459
Kembel SW, Mueller RC (2014) Plant traits and taxonomy drive host associations in tropical phyllosphere fungal communities. Botany 92:303–311. doi:10.1139/cjb-2013-0194
Kemler M, Garnas J, Wingfield MJ, Gryzenhout M, Pillay K-A et al (2013) Ion torrent PGM as tool for fungal community analysis: a case study of endophytes in Eucalyptus grandis reveals high taxonomic diversity. PLoS One 8:e81718. doi:10.1371/journal.pone.0081718
Gazis R, Rehner S, Chaverri P (2011) Species delimitation in fungal endophyte diversity studies and its implications in ecological and biogeographic inferences. Mol Ecol 20:3001–3013. doi:10.1111/j.1365-294X.2011.05110.x
Langenfeld A, Prado S, Nay B, Cruaud C, Lacoste S, Bury E, Hachette F, Hosoya T, Dupont J (2013) Geographic locality greatly influences fungal endophyte communities in Cephalotaxus harringtonia. Fung Biol 117:124–136. doi:10.1016/j.funbio.2012.12.005
Newcombe G (2011) Endophytes in forest management: four challenges. In: Pirttilä AM, Frank AC (eds) Endophytes of forest trees. Springer, Berlin, pp 251–262. doi:10.1007/978-94-007-1599-8_16
Unterseher M, Gazis R, Chaverri P, García Guarniz CF, Zavaleta Tenorio DH (2013) Endophytic fungi from Peruvian highland and lowland habitats form distinctive and host plant-specific assemblages. Biodiv Conserv 22:999–1016. doi:10.1007/s10531-013-0464-x
Prior R, Görges K, Yurkov A, Begerow D (2014) New isolation method for endophytes based on enzyme digestion. Mycol Prog 13:849–856. doi:10.1007/s11557-014-0968-0
García-Guzmán G, Heil M (2014) Life histories of hosts and pathogens predict patterns in tropical fungal plant diseases. New Phytol 201:1106–1120. doi:10.1111/nph.12562
Malcolm GM, Kuldau GA, Gugino BK, Jiménez-Gasco MM (2013) Hidden host plant associations of soilborne fungal pathogens: an ecological perspective. Phytopathology 103:538–544. doi:10.1094/PHYTO-08-12-0192-LE
Koch FH, Yemshanov D, Haack RA, Magarey RD (2014) Using a network model to assess risk of forest pest spread via recreational travel. PLoS One 9:e102105. doi:10.1371/journal.pone.0102105
Lenda M, Skórka P, Knops JMH, Moron D, Sutherland WJ, Kuszewska K, Woyciechowski M (2014) Effect of the internet commerce on dispersal modes of invasive alien species. PLoS One 9:e99786. doi:10.1371/journal.pone.0099786
Shaw MW, Pautasso M (2014) Networks and plant disease management: concepts and applications. Ann Rev Phytopathol 52:477–493. doi:10.1146/annurev-phyto-102313-050229
Hickey GM, Nitschke CR (2005) Crossing disciplinary boundaries in forest research: an international challenge. For Chron 81:321–323. doi:10.5558/tfc81321-3
Dobbertin MK, Nobis MP (2010) Exploring research issues in selected forest journals 1979–2008. Ann For Sci 67:800. doi:10.1051/forest/2010052
Bojović S, Matić R, Popović Z, Smiljanić M, Stefanović M, Vidaković V (2014) An overview of forestry journals in the period 2006–2010 as basis for ascertaining research trends. Scientometrics 98:1331–1346. doi:10.1007/s11192-013-1171-9
Pautasso M, Dehnen-Schmutz K, Holdenrieder O, Pietravalle S, Salama N, Jeger MJ, Lange E, Hehl-Lange S (2010) Plant health and global change—some implications for landscape management. Biol Rev 85:729–755. doi:10.1111/j.1469-185X.2010.00123.x
Rigot T, van Halder I, Jactel H (2014) Landscape diversity slows the spread of an invasive forest pest species. Ecography 37:648–658. doi:10.1111/j.1600-0587.2013.00447.x
Helander M, Ahlholm J, Sieber TN, Hinneri S, Saikkonen K (2007) Fragmented environment affects birch leaf endophytes. New Phytol 175:547–553. doi:10.1111/j.1469-8137.2007.02110.x
Dillon WW, Haas SE, Rizzo DM, Meentemeyer RK (2014) Perspectives of spatial scale in a wildland forest epidemic. Eur J Plant Pathol 138:449–465. doi:10.1007/s10658-013-0376-3
Cobb RC, Eviner VT, Rizzo DM (2013) Mortality and community changes drive sudden oak death impacts on litterfall and soil nitrogen cycling. New Phytol 200:422–431. doi:10.1111/nph.12370
Jönsson MT, Thor G (2012) Estimating coextinction risks from epidemic tree death: affiliate lichen communities among diseased host tree populations of Fraxinus excelsior. PLoS One 7:e45701. doi:10.1371/journal.pone.0045701
Lõhmus A, Runnel K (2014) Ash dieback can rapidly eradicate isolated epiphyte populations in production forests: a case study. Biol Conserve 169:185–188. doi:10.1016/j.biocon.2013.11.031
Mitchell RJ, Beaton JK, Bellamy PE, Broome A, Chetcuti J, Eaton S, Ellis CJ, Gimona A, Harmer R, Hester AJ, Hewison RL, Hodgetts NG, Iason GR, Kerr G, Littlewood NA, Newey S, Potts JM, Pozsgai G, Ray D, Sim DA, Stockan JA, Taylor AFS, Woodward S (2014) Ash dieback in the UK: a review of the ecological and conservation implications and potential management options. Biol Conserv 175:95–109. doi:10.1016/j.biocon.2014.04.019
Tomback DF, Achuff P (2010) Blister rust and western forest biodiversity: ecology, values and outlook for white pines. For Pathol 40:186–225. doi:10.1111/j.1439-0329.2010.00655.x
Garneau DE, Lawler ME, Rumpf AS, Weyburne ES, Cuppernull TM, Boe AG (2012) Potential effects of beech bark disease on small mammals and invertebrates in northeastern US forests. Northeast Nat 19:391–410. doi:10.1656/045.019.0303
Lovett GM, Arthur MA, Weathers KC, Griffin JM (2013) Effects of introduced insects and diseases on forest ecosystems in the Catskill Mountains of New York. Ann NY Acad Sci 1298:66–77. doi:10.1111/nyas.12215
Brunet J, Bukina Y, Hedwall PO, Holmström E, von Oheimb G (2014) Pathogen induced disturbance and succession in temperate forests: evidence from a 100-year data set in southern Sweden. Basic Appl Ecol 15:114–121. doi:10.1016/j.baae.2014.02.002
Sieber TN (1989) Endophytic fungi in twigs of healthy and diseased Norway spruce and white fir. Mycol Res 92:322–326
Ragazzi A, Moricca S, Capretti P, Dellavalle I, Mancini F, Turco E (2001) Endophytic fungi in Quercus cerris: isolation frequency in relation to phenological phase, tree health and the organ affected. Phytopathol Medit 40:165–171
Ragazzi A, Moricca S, Capretti P, Dellavalle I, Turco E (2003) Differences in composition of endophytic mycobiota in twigs and leaves of healthy and declining Quercus species in Italy. For Pathol 33:31–38. doi:10.1046/j.1439-0329.2003.3062003.x
Baird RE, Watson CE, Woolfolk S (2007) Microfungi from bark of healthy and damaged American beech, fraser fir, and eastern hemlock trees during an all taxa biodiversity inventory in forests of the Great Smoky Mountains National Park. Southeast Nat 6:67–82. doi:10.1656/1528-7092(2007)6[67:MFBOHA]2.0.CO;2
Giordano L, Gonthier P, Varese GC, Miserere L, Nicolotti G (2009) Mycobiota inhabiting sapwood of healthy and declining Scots pine (Pinus sylvestris L.) trees in the Alps. Fung Divers 38:69–83
Kowalski T, Andruch K (2012) Mycobiota in needles of Abies alba with and without symptoms of Herpotrichia needle browning. For Pathol 42:183–190. doi:10.1111/j.1439-0329.2011.00738.x
Moricca S, Ginetti B, Ragazzi A (2012) Species- and organ-specificity in endophytes colonizing healthy and declining Mediterranean oaks. Phytopathol Medit 51:587–598
Takemoto S, Masuya H, Tabata M (2014) Endophytic fungal communities in the bark of canker-diseased Toxicodendron vernicifluum. Fung Ecol 7:1–8. doi:10.1016/j.funeco.2013.10.004
Mills P, Dehnen-Schmutz K, Ilbery B, Jeger M, Jones G, Little R, MacLeod A, Parker S, Pautasso M, Pietravalle S, Maye D (2011) Integrating natural and social science perspectives on plant disease risk, management and policy formulation. Phil Trans Roy Soc B 366:2035–2044. doi:10.1098/rstb.2010.0411
Lee CA, Alexander JM, Frankel SJ, Valachovic Y (2012) Evolution of an invasive species research program and implications for large-scale management of a non-native, invasive plant pathogen. Environ Nat Resour Res 2:99–111
Pautasso M, Dehnen-Schmutz K, Ilbery B, Jeger MJ, Jones G, Little R, MacLeod A, Maye D, Parker S, Pietravalle S, Mills P (2012) Plant health challenges for a sustainable land use and rural economy. CAB Rev 7:63. doi:10.1079/PAVSNNR20127063
Jactel H, Branco M, Duncker P, Gardiner B, Grodzki W, Langström B, Moreira F, Netherer S, Nicoll B, Orazio C, Piou D, Schelhaas MJ, Tojic K (2012) A multi-criteria risk analysis to evaluate impacts of forest management alternatives on forest health in Europe. Ecol Soc 17:52
Wulff S, Lindelöw A, Lundin L, Hansson P, Axelsson AL, Barklund P, Wijk S, Ståhl G (2012) Adapting forest health assessments to changing perspectives on threats—a case example from Sweden. Environ Monit Assess 184:2453–2464. doi:10.1007/s10661-011-2130-7
Burdon JJ, Thrall PH, Ericson L (2013) Genes, communities & invasive species: understanding the ecological and evolutionary dynamics of host–pathogen interactions. Curr Opin Plant Biol 16:400–405. doi:10.1016/j.pbi.2013.05.003
Hansen EM (2008) Alien forest pathogens: Phytophthora species are changing world forests. Boreal Environ Res 13:33–41
Horie T, Haight RG, Homans FR, Venette RC (2013) Optimal strategies for the surveillance and control of forest pathogens: a case study with oak wilt. Ecol Econ 86:78–85. doi:10.1016/j.ecolecon.2012.09.017
Kearns HSJ, Jacobi WR, Reich RM, Flynn RL, Burns KS, Geils BW (2014) Risk of white pine blister rust to limber pine in Colorado and Wyoming, USA. For Pathol 44:21–38. doi:10.1111/efp.12065
Manion PD (2003) Evolution of concepts in forest pathology. Phytopathology 93:1052–1055. doi:10.1094/PHYTO.2003.93.8.1052
Rizzo DM, Garbelotto M, Hansen EM (2005) Phytophthora ramorum: integrative research and management of an emerging pathogen in California and Oregon forests. Ann Rev Phytopathol 43:309–335. doi:10.1146/annurev.phyto.42.040803.140418
Hamelin RC (2006) Molecular epidemiology of forest pathogens: from genes to landscape. Can J Plant Pathol 28:167–181. doi:10.1080/07060660609507285
Desprez-Loustau ML, Marçais B, Nageleisen LM, Piou D, Vannini A (2006) Interactive effects of drought and pathogens in forest trees. Ann For Sci 63:597–612. doi:10.1051/forest:2006040
Desprez-Loustau ML, Robin C, Buée M, Courtecuisse R, Garbaye J, Suffert F, Sache I, Rizzo DM (2007) The fungal dimension of biological invasions. Trends Ecol Evol 22:472–480. doi:10.1016/j.tree.2007.04.005
Jeger MJ, Pautasso M, Holdenrieder O, Shaw MW (2007) Modelling disease spread and control in networks: implications for plant sciences. New Phytol 174:279–297. doi:10.1111/j.1469-8137.2007.02028.x
La Porta N, Capretti P, Thomsen IM, Kasanen R, Hietala AM, Von Weissenberg K (2008) Forest pathogens with higher damage potential due to climate change in Europe. Can J Plant Pathol 30:177–195. doi:10.1080/07060661.2008.10540534
Lonsdale D, Pautasso M, Holdenrieder O (2008) Wood-decaying fungi in the forest: conservation needs and management options. Eur J For Res 127:1–22. doi:10.1007/s10342-007-0182-6
Rackham O (2008) Ancient woodlands: modern threats. New Phytol 180:571–586. doi:10.1111/j.1469-8137.2008.02579.x
Loo JA (2009) Ecological impacts of non-indigenous invasive fungi as forest pathogens. Biol Invasion 11:81–96. doi:10.1007/s10530-008-9321-3
Ostry ME, Laflamme G (2009) Fungi and diseases—natural components of healthy forests. Botany 87:22–25. doi:10.1139/B08-090
MacLeod A, Pautasso M, Jeger MJ, Haines-Young R (2010) Evolution of the international regulation of plant pests and challenges for future plant health. Food Sec 2:49–70. doi:10.1007/s12571-010-0054-7
Grünig CR, Queloz V, Sieber TN (2011) Structure of diversity in dark septate endophytes: from species to genes. In: Pirttilä AM, Frank AC (eds) Endophytes of forest trees. Springer, Berlin, pp 3–30. doi:10.1007/978-94-007-1599-8_1
Stenlid J, Oliva J, Boberg JB, Hopkins AJM (2011) Emerging diseases in European forest ecosystems and responses in society. Forests 2:486–504. doi:10.3390/f2020486
Sturrock RN, Frankel SJ, Brown AV, Hennon PE, Kliejunas JT, Lewis KJ, Worrall JJ, Woods AJ (2011) Climate change and forest diseases. Plant Pathol 60:133–149. doi:10.1111/j.1365-3059.2010.02406.x
Döring TF, Pautasso M, Finckh MR, Wolfe MS (2012) Concepts of plant health—reviewing and challenging the foundations of plant protection. Plant Pathol 61:1–15. doi:10.1111/j.1365-3059.2011.02501.x
Hansen EM, Reeser PW, Sutton W (2012) Phytophthora beyond agriculture. Ann Rev Phytopathol 50:359–378. doi:10.1146/annurev-phyto-081211-172946
Waller M (2013) Drought, disease, defoliation and death: forest pathogens as agents of past vegetation change. J Quatern Sci 28:336–342. doi:10.1002/jqs.2631
Oliva J, Stenlid J, Martínez-Vilalta J (2014) The effect of fungal pathogens on the water and carbon economy of trees: implications for drought-induced mortality. New Phytol 203:1028–1035. doi:10.1111/nph.12857
Grünwald NJ, Goss E (2011) Evolution and population genetics of exotic and re-emerging pathogens: novel tools and approaches. Ann Rev Phytopathol 49:249–267. doi:10.1146/annurev-phyto-072910-095246
Lindahl BD, Kuske CR (2013) Metagenomics for study of fungal ecology. In: Martin F (ed) The ecological genomics of fungi. Wiley, Chichester, pp 279–303. doi:10.1002/9781118735893.ch13
Taylor DL, Hollingsworth TN, McFarland JW, Lennon NJ, Nusbaum C, Ruess RW (2014) A first comprehensive census of fungi in soil reveals both hyperdiversity and fine-scale niche partitioning. Ecol Monogr 84:3–20. doi:10.1890/12-1693.1
Peay KG, Baraloto C, Fine PVA (2013) Strong coupling of plant and fungal community structure across western Amazonian rainforests. ISME J 7:1852–1861. doi:10.1038/ismej.2013.66
Vannini A, Bruni N, Tomassini A, Franceschini S, Vettraino AM (2013) Pyrosequencing of environmental soil samples reveals biodiversity of the Phytophthora resident community in chestnut forests. FEMS Microbiol Ecol 85:433–442. doi:10.1111/1574-6941.12132
Mueller RC, Paula FS, Mirza BS, Rodrigues JLM, Nüsslein K, Bohannan BJM (2014) Links between plant and fungal communities across a deforestation chronosequence in the Amazon rainforest. ISME J 8:1548–1550. doi:10.1038/ismej.2013.253
Lee-Cruz L, Edwards DP, Tripathi BM, Adams JM (2013) Impact of logging and forest conversion to oil palm plantations on soil bacterial communities in Borneo. Appl Environ Microbiol 79:7290–7297. doi:10.1128/AEM. 02541-13
Coince A, Cordier T, Lengellé J, Defossez E, Vacher C, Robin C, Buée M, Marçais B (2014) Leaf and root-associated fungal assemblages do not follow similar elevational diversity patterns. PLoS One 9:e100668. doi:10.1371/journal.pone.0100668
Fierer N, Leff JW, Adams BJ, Nielsen UN, Bates ST, Lauber CL, Owens S, Gilbert JA, Wall DH, Caporaso JG (2012) Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc Natl Acad Sci U S A 109:21390–21395. doi:10.1073/pnas.1215210110
Damon C, Lehembre F, Oger-Desfeux C, Luis P, Ranger J, Fraissinet-Tachet L, Marmeisse R (2012) Metatranscriptomics reveals the diversity of genes expressed by eukaryotes in forest soils. PLoS One 7:e28967. doi:10.1371/journal.pone.0028967
Eyre CA, Kozanitas M, Garbelotto M (2013) Population dynamics of aerial and terrestrial populations of Phytophthora ramorum in a California forest under different climatic conditions. Phytopathology 103:1141–1152. doi:10.1094/PHYTO-11-12-0290-R
Quinn L, O'Neill PA, Harrison J, Paskiewicz KH, McCracken AR, Cooke LR, Grant MR, Studholme DJ (2013) Genome-wide sequencing of Phytophthora lateralis reveals genetic variation among isolates from Lawson cypress (Chamaecyparis lawsoniana) in Northern Ireland. FEMS Microbiol Lett 344:179–185. doi:10.1111/1574-6968.12179
Ross-Davis AL, Stewart JE, Hanna JW, Kim MS, Knaus BJ, Cronn R, Rai H, Richardson BA, McDonald GI, Klopfenstein NB (2013) Transcriptome of an Armillaria root disease pathogen reveals candidate genes involved in host substrate utilization at the host–pathogen interface. For Pathol 43:468–477. doi:10.1111/efp.12056
Acknowledgments
Many thanks to J. Talbot and E. Sayer for organizing the symposium on the forest microbiome at the British Ecological Society Centenary Meeting and this special issue and also to A. Gross, C. Grünig, M. Jeger, J. Landolt, I. Mohammed, K. Noetzli, V. Queloz, L. Paul, M. Schmid, T. Sieber and S. Stroheker for their time and discussions as well as to V. Queloz and the anonymous reviewers for their helpful comments on the previous draft.
Author information
Authors and Affiliations
Corresponding author
Additional information
The positions and opinions presented in this article are those of the authors alone and are not intended to represent the views or scientific works of EFSA.
Rights and permissions
About this article
Cite this article
Pautasso, M., Schlegel, M. & Holdenrieder, O. Forest Health in a Changing World. Microb Ecol 69, 826–842 (2015). https://doi.org/10.1007/s00248-014-0545-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-014-0545-8