Abstract
Hemoptysis in the pediatric population, while infrequent, poses significant challenges for both the family and healthcare practitioners. The severity of hemoptysis dictates management decisions. Most cases being mild and self-limiting are treated conservatively. However, “life-threatening hemoptysis” may occur, and is defined as any degree of blood loss that endangers the airway and is arbitrarily considered to be > 8 ml/kg in 24 h in children. It requires prompt airway management and resuscitation followed by a tailored approach consisting of bronchoscopy, computed tomography (CT), interventional radiology, and/or surgery depending on the patient ‘s clinical status and cardiopulmonary comorbidities. Bronchial arteries are hypertrophied in myriad conditions and account for 90–95% cases of hemoptysis due to their systemic pressure levels; the rest being contributed by pulmonary artery pathologies. Despite similar pathogenic mechanisms, the etiologies of pediatric hemoptysis differ from those in adults, with acute lower respiratory tract infections being the predominant cause. Imaging plays a crucial role in identifying the source and cause of hemorrhage. Multidetector computed tomography (MDCT) has emerged as a prime modality in the diagnostic evaluation of hemoptysis and provides a roadmap for potential interventional procedures. This article discusses the etiopathogenesis of hemoptysis along with a brief mention of the diagnostic modalities. It provides a structured reporting format and uses it to illustrate the imaging features in hemoptysis, with emphasis on CT angiography. The key findings in the lung parenchyma, airways, bronchial and non-bronchial systemic collaterals, and pulmonary arteries are elaborated upon. It further addresses the nuances of interventional management, particularly emphasizing the applications of bronchial artery embolization and pulmonary artery embolization in the pediatric population. The article also underscores the potential complications and factors influencing recurrence rates.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The term “hemoptysis” refers to expectoration of blood from the lower respiratory tract or alveoli. While infrequent in the pediatric population, it gives rise to substantial apprehension for the family and health care practitioners. Hemoptysis must be differentiated from bleeding from the upper respiratory tract and gastrointestinal sources, which are relatively more frequent. This, however, is not easy as history is not always forthcoming, and children ingest their own sputum. Hence, a high index of suspicion and meticulous clinical examination are crucial.
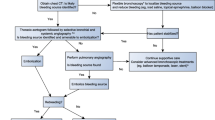
The severity of hemoptysis dictates subsequent management decisions. In most cases, bleeding is mild and self-limiting, hence treated conservatively. “Life-threatening hemoptysis” is defined as any degree of blood loss that endangers the airway or leads to signs of systemic hypotension and is arbitrarily considered to be > 8 ml/kg in 24 h in children [1, 2]. As death in hemoptysis results from asphyxiation rather than exsanguination, securing the airway and resuscitation are the initial steps in critical care. The further clinical pathway may include a combination of bronchoscopy, computed tomography (CT), interventional radiology, and/or surgery and is guided by the availability, the patient ‘s clinical status, and cardiopulmonary comorbidities (Fig. 1).
The primary purpose of imaging in hemoptysis is to confirm the presence and identify the site and source of hemorrhage, and suggest the underlying cause as well as furnish a roadmap for potential interventional procedures. Multidetector computed tomography (MDCT) forms the workhorse of imaging as it facilitates comprehensive evaluation of the airways, pulmonary parenchyma, and vasculature. Together, multidetector CT and catheter angiography have taken center stage in the diagnosis and treatment of childhood hemoptysis [3]. This is true especially in cases with life-threatening and recurrent non-massive amounts of blood loss despite conservative therapy. Consequently, the scope of diagnostic and therapeutic bronchoscopy and its inherent complications have diminished. This article presents a comprehensive review of the etiopathogenesis, imaging manifestations, and the role of interventional radiology in the management of pediatric hemoptysis.
Etiopathogenesis
The lungs have a dual blood supply—99% of which is contributed to by the low-pressure pulmonary circulation and only 1% by the bronchial arteries, with six times the pulmonary perfusion pressure [4, 5]. The pulmonary blood flow steeps through the alveoli and respiratory bronchioles participating in respiration while bronchial arteries nourish the airways and vasa vasorum of the pulmonary arteries. Paradoxically, despite the significant differences in volume, bronchial arteries are the most common “culprit arteries” in hemoptysis, accounting for as much as 90–95% of cases [6]. Their elevated pressure levels predispose them to dilatation and rupture. The bleeding almost always occurs at the level of the walls of distal bronchoalveolar spaces and vasa vasorum of pulmonary arteries where the thin-walled systemic and pulmonary capillaries anastomose [7].
In the setting of congenital or acquired pulmonary artery narrowing (congenital heart diseases, chronic embolic disease and extrinsic compression like fibrosing mediastinitis), there is reflex increase in the bronchial blood flow from 1 to 30% of the cardiac output and recruitment of the systemic–pulmonary anastomotic networks [1, 8]. Eventually, these fragile vessels may rupture leading to pulmonary hemorrhage.
Chronic infection, inflammation, and bronchiectasis also produce hypoxia via “obliterative vasculitis” of the small pulmonary arterioles and trigger a local cytokine cascade [9]. The result is proliferation of leaky channels in the lung interstitium that are vulnerable to rupture. Neoplasms release similar angiogenic growth factors. Furthermore, inflammatory and neoplastic processes that have a necrotic component are the “culprit lesions” that can destroy the lung directly and erode into vessels [10] (Fig. 2). This leads to complications like pseudoaneurysms, which are catastrophic but potentially treatable causes of pulmonary hemorrhage. Certain vascular disorders like infarcts and vasculitis demonstrate similar pathophysiology. It should be emphasized here that most of the lung pathologies operate via multiple mechanisms to cause hemoptysis, which may necessitate distinct therapeutic approaches.
Split-bolus computed tomography angiography in a 16-year-old boy with acute lymphoblastic leukemia presenting with hemoptysis. a Coronal image (mediastinal window) reveals hypodense filling defects in both descending pulmonary arteries (arrows) and enlarged mediastinal nodes (asterisk). The right bronchial artery is not dilated (curved arrow). b Axial image (lung window) shows peripheral wedge-shaped infarcts with ground glass opacities in bilateral lower lobes (arrows) indicative of angioinvasive fungal disease. The patient was treated with antifungals
The etiology of hemoptysis in children differs significantly from the adult population. In children, acute lower respiratory tract infections are the most common underlying pathology, accounting for as much as 40% of cases [11,12,13,14] (Table 1). Other common causes are post-infectious sequelae, bronchiectasis especially, in association with cystic fibrosis, foreign body aspiration, and congenital heart disease. This contrasts with adults in whom post-infectious bronchiectasis and neoplasms are predominant. Among children, the causative factors are further influenced by both age of the child and geographical distribution. In a study by Sim et al., infection, milk protein allergy, and congenital heart disease exhibited greater frequency in early childhood, whereas vasculitis, endobronchial neoplasms, and bronchiectasis affected primarily the older children [15, 16].
In the low-income/lower middle income countries, as anticipated, infections, particularly tuberculosis in both acute stage and chronic sequelae, constitute the majority of cases. Acute infections are also predominant in western nations, but are mostly related to acute necrotizing bacterial pneumonia and lung abscess instead. This is followed by cystic fibrosis and foreign body aspiration in terms of incidence [1].
Imaging modalities
The prime role of imaging in hemoptysis is to identify the proximate source of hemorrhage and discern the possible etiology. A range of imaging modalities may prove valuable, depending upon the urgency and gravity of the clinical presentation.
Chest radiography
Chest radiography serves as a rapid screening tool to guide subsequent management strategies. It can lateralize the bleeding and detect various parenchymal and pleural pathologies. Some common findings are alveolar opacities in active infections and pulmonary hemorrhage, bronchiectasis indicating post-infectious sequelae, and abnormalities of cardiac size and contour in congenital heart diseases. Radiopaque foreign bodies can be directly visualized with ancillary findings of air trapping and atelectasis. Nevertheless, in 30% of cases, radiographs remain normal necessitating further workup [14, 17].
Multidetector computed tomography/computed tomography angiography
CT angiography acts as a one-stop shop for imaging evaluation of hemoptysis. This is due to its ability to rapidly and non-invasively depict the anatomy and pathology of the lung parenchyma and vasculature in exquisite detail. CT is considered “usually appropriate” both for life-threatening and non-life-threatening presentations as per the American College of Radiology Appropriateness Criteria [18]. It demonstrates the site, source, and cause of bleeding and assists in forming a three-dimensional anatomical model of the arteries to guide the interventionalist. CT has the potential to image even the distal airways, beyond the reach of a bronchoscope and enables safer bronchoscopic procedures by revealing any vascular variants. For these reasons, multidetector CT is considered the first-line investigation, ahead of bronchoscopy in the setting of hemoptysis except in life-threatening situations [19,20,21,22].
Magnetic resonance imaging and ultrasonography
Although magnetic resonance imaging (MRI) and ultrasonography (US) have no role in the diagnostic evaluation of acute hemoptysis, they are sometimes preferred for diagnosing and monitoring the underlying pathologies due to the lack of harmful radiation exposure. US with or without contrast enhancement can be used to characterize empyema, consolidation, peripheral abscesses, and masses with additional capability of performing certain guided interventions. MRI can also depict various infectious and neoplastic lung pathologies and is now routinely utilized for the follow-up of cystic fibrosis-related and non-cystic fibrosis-related bronchiectasis in children. MR angiography provides non-contrast evaluation of vascular anomalies with flow quantification which has important surgical implications. Future advances such as ferumoxytol (a non-gadolinium-based contrast agent), ultra-short echo time (UTE) sequences (which capture signal before T2* signal decay of lung parenchyma), and inhalational agents like hyperpolarized xenon (for serial functional assessment) may further expand the scope of MRI [23,24,25,26,27].
Systematic reporting of computed tomography angiography
CT angiography technique: CT angiography protocol should be tailored to the underlying cause. Typically, a systemic trigger (such as the left ventricle or descending aorta) is used to assess hypertrophied bronchial arteries. However, if a pulmonary artery cause is suspected, a right ventricle or pulmonary trunk trigger is necessary. A combined split-bolus protocol is increasingly favored, particularly when the cause of hemoptysis is unknown. This approach allows simultaneous opacification and assessment of both pulmonary and systemic circulations in a single acquisition, reducing radiation exposure and may be preferred in pediatric cases. Additionally, it reveals dilated pulmonary arterial or venous branches corresponding to areas of shunting and potential sites of active hemorrhage. In a similar context, dual energy CT angiography adds value by reducing contrast dose, scan time, and the need for sedation.
At our institution, the protocol involves injecting three-fourths of non-ionic contrast (containing 400 mg/ml iodine) at a high rate of 3–5 ml/s to enable aortic opacification, followed by a second slower bolus (consisting of one-fourth of contrast) at a rate of 1.5–3 ml/s for pulmonary enhancement. A saline chase of equal volume and rate as the second bolus is administered. In children < 5 years, the rate is adjusted according to the cannula size. The scan is started at the peak of systemic arterial enhancement (threshold of 100 Hounsfield units in the ascending aorta) with bolus tracking [1, 28, 29].
A systematic approach should be adopted in examining the various structures on CT and angiographic reconstructions to assess the bleeding site, culprit vessel, culprit lesion (etiology), and angioarchitecture of the abnormal vessels. A structured reporting format such as the one presented in Table 2 can be utilized for this purpose.
Lung parenchyma
The lung parenchyma should be assessed for consolidation, nodules, and ground glass opacities. These may be the manifestations of an infection causing hemoptysis or may represent parenchymal hemorrhage itself. However, when these findings are confined to a specific lung region, they help to localize the site of hemorrhage (Table 3).
In a patient with fibroparenchymal opacities and other sequelae of previous infection, new infiltrates in a lung segment point towards the epicenter of active bleeding so that embolization procedures can be prioritized accordingly. Diffuse infiltrates may be associated with a widespread infectious process or diffuse alveolar hemorrhage and have no localizing value; bronchoscopy is indicated for this purpose [19, 30].
Low density areas or cavitation within a consolidation or nodule should be specifically looked for as it is the necrotizing nature of infections (bacterial, mycobacterial, and fungal) that makes them capable of significant lung and vascular damage. A contrast-filled outpouching abutting the wall of a cavity should alert the radiologist to the presence of a pseudoaneurysm, which can be directly implicated in the current episode of hemoptysis and requires urgent embolization. A soft tissue nodule within the cavity can indicate aspergilloma colonization, blood clot, or neoplasm (Fig. 3).
Computed tomography angiography of a 14-year-old girl with a past history of tuberculosis who presented with hemoptysis. a Axial image (lung window) shows fibrobronchiectatic changes with cavitations in both upper lobes (arrows). b Axial image (mediastinal window) shows hypodense soft tissue in one of the cavities in the left upper lobe (aspergilloma) (arrow) with pleural thickening (broken arrow). An enlarged left intercostal artery (arrowheads) supplies the lesion. c Coronal maximum intensity projection image (mediastinal window) reveals an enlarged right intercostobronchial trunk (arrow). The diagnosis was chronic cavitatory pulmonary aspergillosis. Bronchial artery embolization with antifungal treatment was given
It is prudent to realize, however, that aspirated blood from a proximal source can look similar to localized parenchymal hemorrhage. A subtle distinguishing feature is that aspiration causes smaller, clustered tree-in-bud centrilobular nodules in the distal airways, thus initiating scrutiny along the tracheobronchial tree for a more proximal pathology. In contrast, sentinel parenchymal hemorrhage shows a more regional distribution of ground glass opacities and hazy consolidation and is generally associated with the culprit lesion [31].
Airways
The airways are examined for the presence of any narrowing due to intraluminal (foreign body, neoplasm, or blood clot) or extraluminal (lymphadenopathy, broncholith, or fibrosing mediastinitis) causes and airway dilatation (bronchiectasis). Minimum intensity projection images can illustrate the pathologies of airways and areas of air trapping to a greater advantage. The exact site of focal stenosis, its length, distance from carina or bronchial branching, visualization of the distal airway, and post-obstructive parenchymal changes are important elements of the radiology report [32].
The presence of blood clots and/or fluid density within the bronchi is exceedingly rare and acts as soft pointers for the site of hemorrhage. Foreign body aspiration in the acute setting is promptly diagnosed and treated with bronchoscopy. However, a small organic foreign body trapped within the distal airways commonly has a delayed presentation with complications like chronic recurrent pneumonia and focal bronchiectasis. CT can adequately depict these findings as well as the bronchial artery hypertrophy caused by recurrent bouts of inflammation. Endoluminal virtual bronchoscopic images can locate and characterize the foreign body in terms of size, shape, and density for bronchoscopic retrieval. Rare carcinoids of the tracheobronchial tree will demonstrate stippled calcifications and intense enhancement, sometimes with neovascularity. A peribronchial calcified or necrotic lymph node can directly erode the bronchi and hilar vessels with catastrophic hemorrhage resulting from pseudoaneurysm formation [15].
Bronchiectasis should be characterized in terms of distribution and morphology which gives a clue to its etiology. For instance, upper lobe distribution frequently results from post-tubercular sequelae and cystic fibrosis. In tuberculosis, the exuberant fibroparenchymal changes produce traction and irregular bronchial dilatation. In contrast, cystic fibrosis demonstrates less fibrosis; predominant findings being cylindrical and cystic bronchiectasis with frequent mucus plugging, centrilobular nodules, air trapping, and parenchymal infiltrates (Fig. 4).
Images of a 17-year-old boy with acute exacerbation of cystic fibrosis. a Posteroanterior chest radiograph reveals tubular and cystic bronchiectatic changes in right upper and left lower zones (arrows). b Coronal maximum intensity projection image of computed tomography angiography (mediastinal window) depicts a hypertrophied right intercostobronchial trunk (white arrow) and right and left branches of common bronchial artery (black arrows). c Digital subtraction angiography check run shows abnormally dilated common bronchial artery (arrow) with parenchymal blush, which was subsequently embolized. Sputum culture showed pseudomonas superinfection which was managed with antibiotics
Lower lobe distribution of bronchial dilatation is seen in recurrent aspiration and immunodeficiency disorders (Fig. 5). Primary ciliary dyskinesia is a rare inherited disorder of the ciliary epithelium presenting as sinusitis, infertility, and bronchiectasis in lower and middle lobe distribution [33]. Nevertheless, all pathologies and morphologies of bronchiectasis cause hemoptysis by shrinkage of the normal pulmonary microcirculation and enlargement of abnormal leaky bronchial arteries which may rupture during the bouts of infection.
A 16-year-old girl with recurrent chest infections and hemoptysis. a Axial computed tomography angiography image (lung window) reveals left lower lobe collapse with bronchiectasis (thin arrow). Similar bronchiectatic changes are noted in bilateral lung bases with centrilobular nodules (broken arrows). Bronchocele with air-fluid levels is seen in the right middle lobe (thick black arrow). b Coronal maximum intensity projection image of computed tomography angiography (mediastinal window) depicts hypertrophied right intercostobronchial trunk (arrow) and left bronchial artery (broken arrow). c, d Digital subtraction angiography images show right intercostobronchial trunk (arrow) and left bronchial artery (broken arrow) which were embolized
Bronchial and non-bronchial systemic collaterals
Bronchial and non-bronchial systemic collaterals are the major vascular sources of hemoptysis, blamed for up to 90–95% of cases [6]. However, it is prudent to consider that they are only a manifestation of pulmonary hypoperfusion. Hence, the underlying etiology should be explored and addressed along with occlusion of the bleeding vessel for effective management.
The bronchial arteries are direct branches of the descending aorta arising at thoracic 5–6 vertebral levels in up to 70% of cases [1, 34]. They course through the hilum paralleling the bronchi. Ectopic bronchial arteries originate elsewhere, including the inferior aspect of the aortic arch, subclavian, vertebral, internal mammary, and coronary arteries, but they still traverse through the hilum. Normal bronchial arteries may be visualized as enhancing nodules and lines in the posterior mediastinum just below the aortic arch. Multiplanar and maximum intensity projection (MIP) reformats are used to determine their number, origin, diameter, and course. Cauldwell et al. described four major types of bronchial artery branching patterns, out of which type one—consisting of one right intercostobronchial trunk and two left bronchial arteries—is the most common, seen in 40% of the population [35]. A diameter exceeding 2 mm at origin, increased tortuosity, or, more specifically, lack of distal tapering with visualization of arterial lumen up to the hilum are considered signs of abnormality [9, 34] (Table 4).
Non-bronchial systemic collaterals are branches from brachiocephalic, subclavian, internal mammary, inferior phrenic, and coeliac axis that communicate with peripheral branches of pulmonary arteries via pleural adhesions and inferior pulmonary ligament and do not accompany the bronchi. Any non-bronchial collateral apparently supplying the lung abnormality, irrespective of its size, is always abnormal. Asymmetrically dilated intercostal arteries in the extrapleural space that are associated with pleural thickening > 3 mm should be deemed pathological [15, 19]. The location of the lung abnormality provides an important clue to the possibly hypertrophied collateral; upper lobe involvement suggests subclavian artery collaterals, while lower lung diseases are mainly supplied by hypertrophied inferior phrenic vessels. It is imperative for the radiologist to be aware of the non-bronchial vascular supply before contemplating interventional procedures as omission of these vessels during catheterization stands as one of the most common reasons for recurrence [28] (Fig. 6).
Axial (a–c) and coronal (d–f) computed tomography angiography (mediastinal windows) in a 6-year-old boy who was diagnosed with cystic fibrosis (a), a 17-year-old boy who was diagnosed with pulmonary tuberculosis (b–d), a 16-year-old girl who presented with recurrent chest infections and hemoptysis (e), and a 15-year-old girl, a known case of Takayasu’s arteritis, who presented with mild hemoptysis (f). The images depict the frequently encountered hypertrophied bronchial and non-bronchial systemic arteries. a Common bronchial (arrow), b Left inferior phrenic (arrow), c Left intercostal (arrow), d Left internal mammary (arrow), e Right intercostobronchial (arrow), and f Left bronchial (arrow) arteries
Every attempt should be made to identify systemic–pulmonary shunts which are arterioarterial or arteriovenous connections between the bronchial or non-bronchial collaterals and the pulmonary circulation (Fig. 7). On CT, they appear as a cluster of tortuous vessels in an area of lung abnormality associated with a prominent non-tapering branch of the pulmonary artery. This branch shows differential hyperattenuation due to more densely opacified blood from the bronchial circulation. Systemic–pulmonary shunts are markers of sites of active hemorrhage, thus helping to prioritize embolization. Furthermore, these shunts warrant the use of larger sized embolic particles or coils due to the risk of non-target dissemination and pulmonary infarction [29].
A 15-year-old boy with past history of foreign body aspiration, complained of recurrent chest infections and hemoptysis. a Axial post-contrast computed tomography image (mediastinal window) reveals left lower lobe collapse with bronchiectasis and fluid bronchograms (arrow) consequent to upstream airway stricture (not shown). b Oblique coronal computed tomography angiography zoomed image (mediastinal window) shows a dilated left bronchial artery (arrow) supplying the left lower lobe lesion. c Oblique sagittal computed tomography angiography zoomed image (mediastinal window) shows distal pulmonary artery segment with clustering of vessels surrounding it (arrow) suggesting systemic to pulmonary shunting. Tr trachea
Rarely, bronchial artery aneurysms are seen in the context of infections like tuberculosis and vasculitis particularly polyarteritis nodosa [36, 37].
Pulmonary arteries
Bronchial artery hypertrophy in the setting of congenital or acquired pulmonary stenosis can result in hemoptysis. Such conditions include recurrent pulmonary infections, vasculitis, mediastinal fibrosis, and congenital cardiovascular disorders. Pulmonary vasculitis like Takayasu’s arteritis and Behcet’s disease are associated with mural thickening and enhancement along the pulmonary arteries (Fig. 8). Ancillary features include pulmonary artery aneurysms, diffuse alveolar hemorrhage, and cavitating lung nodules with ground glass halo. Mediastinal fibrosis can also cause unilateral or bilateral uniform narrowing of the pulmonary arteries. It presents as a focal calcified mass or an infiltrative multicompartmental soft tissue encasing and compressing the mediastinal structures [15].
Computed tomography angiography of a 15-year-old girl, a known case of Takayasu’s arteritis who presented with mild hemoptysis. a Axial image (mediastinal window) shows mural thickening along the ascending and descending aorta (arrows) and pulmonary trunk (broken black arrow). Main and right pulmonary arteries are dilated suggesting pulmonary artery hypertension. b On coronal image (mediastinal window), there are eccentric filling defects in lower lobe branch of the right pulmonary artery (arrows) and a large filling defect in the left pulmonary artery (broken arrow) suggestive of chronic thrombotic sequelae. c Coronal image (mediastinal window) shows prominent left bronchial artery (arrow). The patient was managed with medical therapy
Irregular narrowing of the pulmonary artery with acute angulations and intraluminal web-like filling defects is indicative of chronic pulmonary embolism. In the acute form of this condition, peripheral wedge-shaped pulmonary infarcts may be observed. They produce only minor hemoptysis and pink frothy sputum.
Certain congenital heart diseases are associated with pulmonary artery atresia or hypoplasia and branch vessel stenosis including Tetralogy of Fallot, ventricular septal defect with pulmonary atresia, and other conotruncal anomalies (Fig. 9). The lung perfusion, particularly in cases with closed ductus, depends on major aortopulmonary collateral arteries which are persistent embryologic connections between aorta or its branches and the pulmonary circulation. Major aortopulmonary collateral arteries provide blood flow to the lungs at systemic pressure and may lead to torrential hemorrhage upon rupture. They supply a pulmonary segment either as a standalone vessel (essential major aortopulmonary collateral artery) or in combination with pulmonary artery (non-essential major aortopulmonary collateral artery). Non-essential major aortopulmonary collateral arteries need to be ligated or embolized. In this context, the radiologist should report the origin, size, and course of each collateral, detailed vascular supply of each pulmonary segment, and adequacy of the native pulmonary circulation [38]. Congenital left to right shunts like ventricular septal defects that initially cause pulmonary hyperperfusion eventually result in endothelial injury and obliterative changes.
A 15-year-old boy with D-transposition of great arteries underwent computed tomography angiography. a Axial image (mediastinal window) shows ascending aorta (asterisk) anterior and slightly to the right of pulmonary trunk (oval). Aorta was arising from the right ventricle and pulmonary artery from the left ventricle (not shown). Pulmonary artery origin is stenotic (arrow) resulting in the formation of multiple major aortopulmonary collaterals (as shown in b–d). b Coronal maximum intensity projection image (mediastinal window) shows dilated and tortuous right bronchial artery (arrow). c, d Coronal volume rendered technique images show hypertrophied left internal mammary artery (arrow in c) and dilated inferior phrenic artery (broken arrow in d)
Focal smooth stenosis/absence of the proximal pulmonary artery within 1 cm of its origin is referred to as proximal interruption of pulmonary artery. It occurs opposite the side of aortic arch. Ipsilateral pulmonary vessels are diminished; the lung is small and opaque and supplied by systemic collaterals [4].
Focal pulmonary artery dilatations (aneurysms) are one of the few causes of hemoptysis originating directly from the pulmonary artery, typically associated with infection and trauma. Although implicated in 5–10% of cases of hemoptysis, their mortality rates can reach up to 50% [39]. Necrotic infections, such as tuberculosis, invasive fungal pneumonia, and septic emboli, have the propensity to erode pulmonary artery walls. In the context of tuberculosis, the pseudoaneurysm is typically termed “Rasmussen aneurysm” (Fig. 10). Notably, the parent artery is often normal or reduced in caliber and may not be traceable on CT scan. The size, location, and relationship of the pseudoaneurysm to the bronchial tree should be documented. It is imperative to meticulously screen for this finding along the walls of necrotic cavities using MIP reconstructions as it necessitates additional venous catheterization for pulmonary artery embolization. In cases involving multiple pseudoaneurysms associated with septic embolism and vasculitis, it is vital to identify the actively bleeding culprit lesion. It is distinguishable by the presence of a perianeurysmal ground glass halo and requires immediate embolization. Conservative management often suffices for the remaining lesions.
Computed tomography angiography of a 15-year-old girl who is a known case of pulmonary tuberculosis. a Axial image (mediastinal window) shows a right upper lobe cavity with an enhancing focus in the posterior wall suggesting pseudoaneurysm (arrow). There is significant adjoining pleural thickening (asterisk) with dilated intercostal collateral (thick black arrow) also leading to it. Also note the dilated right bronchial artery (thin black arrow). b Axial image (lung window) depicts the cavity with bronchiectatic changes in the right upper lobe (arrow). Combined bronchial and pulmonary artery embolization was required in this case
Other causes of pulmonary artery-related hemorrhage include pulmonary arteriovenous malformations and traumatic catheterization. Pulmonary arteriovenous malformation appears as a tangle of vessels in the periphery of the lung, representing an aneurysm or nidus connecting one or more pulmonary arteries and veins. It is assessed in terms of the number, segmental localization, and number and caliber of the feeding pulmonary arteries [40].
Other findings
Findings in other compartments of the thoracic cavity, such as the mediastinum, pleura, and chest wall should be documented following standard reporting protocols. Cardiac findings should be delineated to the extent feasible on non-gated scans. Exhaustive reporting of the cardiac details, however, is not essential. Likewise, observations on the visualized abdomen should be included. These findings can potentially contribute as collaborative evidence for the underlying etiology.
Interventional management
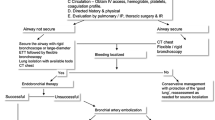
Management of pediatric hemoptysis hinges on several factors including the severity of presentation, patient’s general condition, and available clinical expertise. The treatment modalities ranging from conservative approach, bronchial artery embolization, bronchoscopy, and surgery are selected and tailored to each specific case scenario. Acute infections are the predominant cause of hemoptysis in children. As hemoptysis is mild and self-limiting, medical management generally suffices.
While the role of bronchoscopy has diminished in the current medical landscape, it remains a valuable tool, particularly in cases of bilateral extensive disease and for unstable patients. It has a high accuracy in localizing the bleeding site and facilitates prompt hemostatic measures. It is also useful for foreign body retrieval and obtaining tissue for histopathology [22].
Bronchial artery embolization
Bronchial artery embolization is a well-established, robust modality for therapy of adult hemoptysis; however, in the pediatric population, it is relatively nascent. It is indicated for both massive and recurrent non-massive hemoptysis if conservative treatment has failed. It is the preferred procedure in bilateral extensive disease, unresolving hemoptysis due to cryptogenic factors, and for patients who are not suitable candidates for surgery. In contrast, if a pulmonary lesion is localized, such as a neoplasm or aspergilloma or bleeding is secondary to pulmonary vascular injury, surgical resection of the lesion is the definitive management [22, 28]. Nevertheless, even in preoperative patients, embolization can be performed for emergency palliation and reducing blood loss during surgery. Bronchial artery embolization involves the occlusion of all the hypertrophied bronchial and non-bronchial systemic collaterals, effectively reducing perfusion in the distal fragile vascular bed and controlling hemoptysis in 66–90% of patients [3]. Before undertaking the procedure, it is crucial to review the CT scans to detect (i) the hypertrophied vessels (bronchial artery and other systemic collaterals), (ii) any pulmonary or bronchial artery pseudoaneurysm and pulmonary arteriovenous malformations which may alter the approach, and (iii) the putative site of active hemorrhage in multifocal diseases.
The procedure is performed under general anesthesia in young children to reduce motion artifacts. The preferred access route is the femoral artery. 4F or 5F coaxial angiographic catheter with end hole is used to selectively hook the bronchial arteries. A region encompassing 1 cm on either side of the left bronchial crossing point over the aorta is the angiographic landmark for orthotopic bronchial artery origin. Abnormal bronchial vessels are dilated and tortuous and demonstrate parenchymal blush and shunting. Any non-bronchial collateral supplying the diseased lung is deemed abnormal. A word of caution in cases with central pulmonary artery narrowing is that the systemic collaterals may be the sole supply to a lung segment and embolization may cause pulmonary infarction. It is thus crucial to detect and embolize only the culprit vessel or lesion in such cases [28].
Polyvinyl alcohol particles of size 350–500 u is the most employed embolization material with or without supplemental gel foam (Fig. 11). Polyvinyl alcohol embolizes the distal vascular bed. Isolated use of gel foam is discouraged as it causes more proximal occlusion and has a high propensity to recanalize. Larger sized polyvinyl alcohol particles (> 700 u) or coils are required for treating systemic to pulmonary shunts. Smaller particles may be hazardous as they can cross bronchopulmonary anastomosis and result in pulmonary and systemic infarcts. Polyvinyl alcohol-contrast mixture is injected in small aliquots, carefully observing for any spinal artery opacification or reflux. The procedure is discontinued when the target vessel exhibits stasis. All the abnormal arteries, in descending order of their severity, are embolized until the maximum contrast dose limit is reached [28].
A 12-year-old girl with Kartagener’s syndrome and massive hemoptysis. a Axial computed tomography angiography image (lung window) shows right sided aortic arch and dextrocardia. Fibrobronchiectatic changes with peribronchial thickening are seen in lingula and both lower lobes (arrows). Ground glass density centrilobular nodules in left lower lobe (asterisk) suggest post aspiration changes. b Coronal maximum intensity projection computed tomography angiography image (mediastinal window) shows the right and left limbs of common bronchial artery (arrows). Digital subtraction angiography pre (c) and post (d) embolization images show a hypertrophied common bronchial artery (arrows in c) which was subsequently embolized using polyvinyl alcohol particles
Alternatively, glue can be used for embolization, particularly in recurrent cases. It offers better visibility and control than polyvinyl alcohol and has lesser rates of recanalization. However, glue requires considerable expertise and a stable catheter position, as the risk of non-target dissemination is higher. Coils lodge in the more proximal vessel; hence, distal revascularization and recurrence frequently occur. Future access to the vessel is also obliterated. However, they can be deployed in large shunts and act as tools to prevent non-target embolization.
Recurrence rates following bronchial artery embolization can be as high as 10–60% [2, 41]. Early recurrences occur within 3 months of the procedure and are usually due to incomplete embolization. It is thus prudent to scrutinize the pre-procedure CT images to identify all abnormal vessels and non-bronchial collaterals in particular. Late recurrence is associated with recanalization of the target vessels and progression of the underlying pathology. It cannot be overemphasized that bronchial artery embolization is only a palliative procedure; definitive treatment of the underlying etiology is imperative. Various factors associated with recurrences are inadequate technique, extensive revascularization, presence of non-bronchial systemic collaterals, bronchopulmonary shunting, aspergilloma, active cavitatory tuberculosis, and localization of lung opacities to more than two segments on the radiograph. Bronchial artery embolization is also indicated in recurrent hemoptysis [28, 42].
The overall complication rate of bronchial artery embolization in children is reportedly low, the most frequent being chest pain [41]. The most dreaded complication, spinal cord ischemia, occurs in 0.2–6.5% of patient population and is usually transient [28, 42]. There is inadvertent embolization of the radiculomedullary artery that shares its origin with the intercostal arteries. By careful monitoring and ensuring that the tip of the catheter is well beyond the origin of spinal artery, this can be prevented. Other forms of non-target embolization can cause phrenic nerve palsy, bronchial necrosis, and myocardial ischemia.
Concerning children, particularly those < 15 kg, there is an increased risk of in situ vascular thrombosis up to 8–10% [43]. Doppler examination can promptly distinguish thrombosis from vasospasm, also a common condition in this age group. In this context, the smallest possible needles and catheters should be employed whenever possible. Furthermore, these patients are susceptible to volume overload from multiple contrast injections. Other complications like hypothermia and hypoglycemia are common in neonates.
Pulmonary artery embolization
Hemoptysis related to the pulmonary artery occurs in 5–10% of cases, the predominant cause being Rasmussen aneurysm [19, 39]. It is suspected on imaging or in cases of recurrent hemoptysis despite technically adequate bronchial artery embolization. Pulmonary artery embolization necessitates femoral or jugular vein cannulation with subsequent catheter placement via the right ventricle into the pulmonary artery. The embolic substance should be placed as close to the aneurysm as possible to decrease the amount of lung infarction and collateral reperfusion. Ideally, the pseudoaneurysm should be occluded by sandwich technique with both proximal and distal embolization to prevent recurrence. This is especially true for mycotic aneurysms due to their propensity for collateralization. However, in cases of massive hemorrhage or neoplastic processes, proximal closure may suffice [44].
Embolic materials for pulmonary artery embolization include coils, glue, graft stent, and vascular plugs. Glue is the preferred agent in pulmonary pseudoaneurysms due to their higher propensity to rupture. In situations where endovascular treatment fails, typically when pulmonary and systemic feeders are occluded, alternative interventions such as ultrasound-guided thrombin injection and surgical resection may be considered. It is important to note that due to the possibility of systemic vessel recruitment by the pulmonary pseudoaneurysms, pulmonary artery embolization is always attempted in conjunction with embolization of the bronchial artery [44]. Pulmonary arteriovenous malformations in children have a propensity to reperfusion after embolization. Hence, only large lesions (feeding artery size > 3 mm) and those associated with hypoxemia and paradoxical emboli are treated. Nevertheless, all children with pulmonary arteriovenous malformations, whether treated or not, should undergo regular surveillance to detect interval growth and recanalization [40, 41].
Conclusion
The management of pediatric hemoptysis is complex and depends mainly on the patient’s presentation. While medical therapy may be sufficient for mild cases, massive hemoptysis represents a life-threatening condition that demands immediate attention. Swift resuscitation and precise localization of the bleeding site and underlying cause are paramount to salvage the patient. The collaborative use of bronchoscopy and CT helps to guide appropriate treatment strategies. Meticulous reporting of CT findings is imperative to facilitate subsequent surgical or interventional planning.
Advances in both hardware and techniques have made interventional radiology increasingly prominent in pediatric healthcare. However, it is crucial to prioritize patient safety by minimizing radiation exposure, and adhering to the principles of “ALARA” (as low as reasonably achievable). Moreover, it is imperative to recognize that “kids are not small adults,” and, as such, hardware and scanning protocols must be tailored to the specific needs of the pediatric population to ensure the highest standard of patient care.
Data availability
Raw data used for generating the figures for this article is not publicly available to preserve individuals’ privacy, but can be accessed on reasonable request from the corresponding author.
References
Shera TA, Bhalla AS, Naranje P et al (2022) Role of computed tomography angiography in the evaluation of haemoptysis in children: decoding the abnormal vessels. Indian J Med Res 155:356–363
Roebuck DJ, Barnacle AM (2008) Haemoptysis and bronchial artery embolization in children. Pediatr Respir Rev 9:95–104
Larici AR, Franchi P, Occhipinti M et al (2014) Diagnosis and management of hemoptysis. Diagn Interv Radiol 20:299–309
Castañer E, Gallardo X, Rimola J et al (2006) Congenital and acquired pulmonary artery anomalies in the adult: radiologic overview. Radiographics 26:349–371
Deffebach ME, Charan NB, Lakshminarayan S, Butler J (1987) The bronchial circulation: small, but a vital attribute of the lung. Am Rev Respir Dis 135:463–481
Gupta A, Sands M, Chauhan NR (2018) Massive hemoptysis in pulmonary infections: bronchial artery embolization. J Thorac Dis 10:S3458–S3464
Miyano Y, Kanzaki M, Onuki T (2017) Bronchial artery embolization: first-line option for managing massive hemoptysis. Asian Cardiovasc Thorac Ann 25:618–622
Almeida J, Leal C, Figueiredo L (2020) Evaluation of the bronchial arteries: normal findings, hypertrophy and embolization in patients with hemoptysis. Insights Imaging 11:1–5
Walker CM, Rosado-de-Christenson M, Martínez-Ji S et al (2015) Bronchial Arteries: anatomy, function, hypertrophy, and anomalies. Radiographics 35:32–49
Osiro S, Wear C, Hudson R et al (2012) A friend to the airways: a review of the emerging clinical importance of the bronchial arterial circulation. Surg Radiol Anat 34:791–798
Bannister M (2016) Pediatric haemoptysis and the otorhinolaryngologist: systematic review. Int J Pediatr Otorhinolaryngol 92:99–102
Turcios NL, Vega M (1987) The child with hemoptysis. Hosp Pract 22:214–218
Batra PS, Holinger LD (2001) Etiology and management of pediatric hemoptysis. Arch Otolaryngol-Head Neck Surg 127:377–382
Tom LW, Weisman RA, Handler SD (1980) Hemoptysis in children Ann Otol 89:419–424
Singh D, Bhalla AS, Veedu PT, Arora A (2013) Imaging evaluation of hemoptysis in children. World J Clin Pediatr 2:54–64
Sim J, Kim H, Lee H et al (2009) Etiology of hemoptysis in children: a single institutional series of 40 cases. Allergy Asthma Immunol Res 1:41–44
Ittrich H, Bockhorn M, Klose H, Simon M (2017) The diagnosis and treatment of hemoptysis. Dtsch Arztebl Int 114:371–381
Olsen KM, Shwadi MP, Donnelly EF et al (2020) American College of Radiology ACR appropriateness criteria® hemoptysis. J Am Coll Radiol 17:S148–S159
Khalil A, Fedida B, Parrot A et al (2015) Severe hemoptysis: from diagnosis to embolization. Diagn Interv Imaging 96:775–788
McGuinness G, Beacher JR, Harkin TJ et al (1994) Hemoptysis: prospective high-resolution CT/bronchoscopic correlation. Chest 105:1155–1162
Vohra S, Chowdhury V (2017) Multi-detector CT in evaluation of hemoptysis. Curr Radiol Rep 5:1–11
Kettenbach J, Ittrich H, Gaubert JY et al (2022) CIRSE standards of practice on bronchial artery embolisation. Cardiovasc Intervent Radiol 45:721–732
Wielpütz MO, Puderbach M, Kopp-Schneider A et al (2014) Magnetic resonance imaging detects changes in structure and perfusion, and response to therapy in early cystic fibrosis lung disease. Am J Respir Crit Care Med 189:956–965
Fain S, Schiebler ML, McCormack DG, Parraga D (2010) Imaging of lung function using hyperpolarized helium-3 magnetic resonance imaging: review of current and emerging translational methods and applications. J Magn Reson 32:1398–1408
Alexopoulou E, Prountzos S, Raissaki M et al (2024) Imaging of acute complications of community-acquired pneumonia in the pediatric population—from chest radiography to MRI. Children 11:122
Adams LC, Jayapal P, Ramasamy SK et al (2023) Ferumoxytol-enhanced MRI in children and young adults: state of the art. AJR Am J Roentgenol 220:590–603
Gräfe D, Prenzel F, Hirsch FW (2023) Chest magnetic resonance imaging in cystic fibrosis: technique and clinical benefits. Pediatr Radiol 53:640–648
Singhal R, Santhosh Babu KB, Naranje P et al (2023) Society of Chest Imaging and Interventions consensus guidelines for the interventional radiology management of hemoptysis. Indian J Radiol Imaging 33:361–372
Meena P, Bhalla AS, Goyal A et al (2022) Imaging findings as predictors of the site of bleeding in patients with hemoptysis: comparison between split-bolus dual-energy CT angiography and digital subtraction angiography. Diagn Interv Radiol 28:344–351
Park MS (2013) Diffuse alveolar hemorrhage Tuberc Respir Dis 74:151–162
Marquis KM, Raptis CA, Rajput MZ et al (2021) CT for evaluation of hemoptysis. Radiographics 41:742–761
Sundarakumar DK, Bhalla AS, Sharma R et al (2011) Multidetector CT evaluation of central airways stenoses: comparison of virtual bronchoscopy, minimal-intensity projection, and multiplanar reformatted images. Indian J Radiol Imaging 21:191–194
Milliron B, Henry TS, Veeraraghavan S, Little BP (2015) Bronchiectasis: mechanisms and imaging clues of associated common and uncommon diseases. Radiographics 35:1011–1030
Marshall TJ, Jackson JE (1997) Vascular intervention in the thorax: bronchial artery embolization for haemoptysis. Eur Radiol 7:1221–1227
Cauldwell EW, Siekert RG (1948) The bronchial arteries: an anatomic study of 150 human cadavers. Surg Gynecol Obstet 86:395–412
Kabilan K, Gulati M, Banday IA et al (2022) Myriad faces of active tuberculosis: intrapulmonary bronchial artery pseudoaneurysm. Vasc Endovascular Surg 56:212–215
Lee YJ, Park SS, Kim SY et al (2010) A case of systemic polyarteritis nodosa involving bronchial artery. Sarcoidosis Vasc Diffuse Lung Dis 27:164–168
Alex A, Ayyappan A, Valakkada J et al (2022) Major aortopulmonary collateral arteries. Radiol Cardiothorac Imaging 4:e210157
Shin S, Shin TB, Choi H, Cho JS (2010) Peripheral pulmonary arterial pseudoaneurysms: therapeutic implications of endovascular treatment and angiographic classifications. Radiol 256:656–664
Kaufman CS, McDonald J, Balch H, Whitehead K (2022) Pulmonary arteriovenous malformations: what the interventional radiologist should know. Semin Intervent Radiol 39:261–270
Marra P, Di Fazio B, Dulcetta L et al (2022) Embolization in pediatric patients: a comprehensive review of indications, procedures, and clinical outcomes. J Clin Med 11:6626
Panda A, Bhalla AS, Goyal A (2017) Bronchial artery embolization in hemoptysis: a systematic review. Diagn Interv Radiol 23:307–317
Heran MK, Marshalleck F, Temple M et al (2010) Joint quality improvement guidelines for pediatric arterial access and arteriography: from the Societies of Interventional Radiology and Pediatric Radiology. Pediatr Radiol 40:237–250
Guillaume B, Vendrell A, Stefanovic X et al (2017) Acquired pulmonary artery pseudoaneurysms: a pictorial review. Br J Radiol 90:20160783
Author information
Authors and Affiliations
Contributions
Study conception, supervision, and design: A.B. Literature search and manuscript preparation: A.G., P.N., and D.K. Final manuscript editing was done by A.B. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garg, A., Bhalla, A., Naranje, P. et al. Pediatric hemoptysis: diagnostic and interventional challenges. Pediatr Radiol (2024). https://doi.org/10.1007/s00247-024-06002-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00247-024-06002-7