Abstract
Background
18F-2-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET) shows tumor activity in most neuroblastomas, but the role of 18F-FDG PET/CT in neuroblastoma remains to be defined.
Objective
This study explored the prognostic significance of 18F-FDG PET in newly diagnosed neuroblastic tumors.
Materials and methods
This retrospective study reviewed all 18F-FDG PET/CT examinations performed for a new diagnosis of suspected neuroblastoma. MYCN amplification status, tumor recurrence and survival were abstracted from the medical record. Primary tumors were manually segmented to measure maximum standardized uptake value (SUVmax), mean standardized uptake value (SUVmean), tumor volume and total lesion glycolysis. Univariate and multivariable analyses using Cox proportional hazards regression testing assessed the predictive performance of PET indices for event-free survival and overall survival with thresholds determined using receiver operating characteristic curve analysis.
Results
Fifty-five children were included, with a median age of 2.9 years (interquartile range [IQR] 1.8–3.0 years). SUVmax, tumor volume and total lesion glycolysis were higher in MYCN-amplified tumors (P=0.012, P<0.0001, P<0.0001, respectively) and in higher International Neuroblastoma Risk Group (INRG) stages (P=0.0008, P=0.0017, P=0.0017, respectively). After adjusting for age, tumor SUVmax (P=0.028) and SUVmean (P=0.045) were associated with overall survival. An SUVmax threshold of 4.77 (P=0.028) best predicted overall survival, with median overall survival of 2,604 days (SUVmax>4.77) vs. >2,957 days (SUVmax≤4.77). No PET parameters were independently significantly associated with overall survival or event-free survival after controlling for MYCN status, stage or treatment risk stratification.
Conclusion
Tumor metabolic activity is higher in higher-stage MYCN-amplified neuroblastic tumors. Higher SUVmax and SUVmean were associated with worse overall survival but were not independent of other prognostic markers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neuroblastoma is the most common extracranial solid tumor of childhood, accounting for 8–10% of all childhood malignancies and 15% of pediatric cancer-related deaths [1]. Despite advances in multi-modal therapy including dose-intensive and myeloablative therapy with hematopoietic stem cell support, radiation therapy and immunotherapy, the survival for children presenting with metastatic neuroblastoma (International Neuroblastoma Risk Group Staging System [INRGSS] Stage M) remains poor, with a 3-year event-free survival of 60% [2].
While 123I-metaiodobenzylguanidine (MIBG) scintigraphy remains the dominant disease-specific imaging modality for neuroblastoma, 18F-2-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET) shows tumor activity in the majority of cases [3]. Despite showing tumor activity, the contribution of 18F-FDG to the management of children with neuroblastoma remains incompletely defined. Currently, 18F-FDG PET/CT is selectively used at some sites because of MIBG availability [4] and is used for non-MIBG-avid tumors but is otherwise not widely applied in children with neuroblastoma.
18F-FDG has the potential to contribute to the management of children with neuroblastoma. Studies have shown that 18F-FDG might have higher sensitivity than MIBG for detecting soft-tissue lesions [5] and stage 1 or stage 2 disease [6]. Other studies have suggested that pretreatment 18F-FDG PET/CT might have prognostic significance in newly diagnosed neuroblastoma, with a few studies showing that maximum standardized uptake value (SUVmax) is correlated with longer event-free survival and overall survival [5, 7, 8]. However, findings are not consistent across studies, and the role of 18F-FDG PET/CT remains to be elucidated. The purpose of this study was to explore the prognostic significance of 18F-FDG PET in children with newly diagnosed neuroblastic tumors by reviewing our clinical experience.
Materials and methods
Patients
The institutional review board at Cincinnati Children’s Hospital Medical Center approved this retrospective study and waived the requirement for written informed consent. We reviewed the electronic medical record (Epic; Epic Systems Corp., Verona, WI) and imaging record for all children who had undergone clinically indicated 18F-FDG PET/CT imaging for a new diagnosis of suspected neuroblastoma at our institution between July 2006 and July 2019. Children were excluded if they had undergone prior treatment for neuroblastoma, including surgery or chemotherapy, or if a primary tumor other than a neuroblastic tumor was subsequently identified. When available, additional data were collected from the electronic medical record including age, gender, height, weight, tumor histology characteristics, MYCN amplification status, lactate dehydrogenase (LDH) levels and treatment risk stratification.
Imaging
18F-FDG PET/CT examinations were performed according to our standard clinical protocol. After fasting for at least 4 h, 3.7–5.2 MBq/kg of 18F-FDG was administered intravenously and imaging was performed approximately 1 h after radiopharmaceutical administration. A low-dose non-contrast CT scan was obtained during shallow breathing from the skull vertex to toes for the purposes of localization and attenuation correction. Attenuation-corrected PET images were reconstructed with a 144×144 matrix.
Data analysis
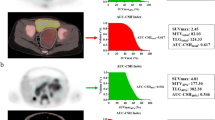
For the purpose of this study, we reviewed attenuation-corrected PET images and reconstructed localization CT images using a commercially available viewing platform (MIM Software, Cleveland, OH). Using the PET-edge tool, a single observer (A.J.S., pediatric radiology fellow) drew regions of interest around the primary tumor to measure SUVmax, mean standardized uptake value (SUVmean), tumor volume and total lesion glycolysis (defined as: SUVmean × tumor volume) (Fig. 1). The primary tumor was defined as the dominant soft-tissue mass and all immediately adjacent, inseparable tumor-involved lymph nodes. Mean liver standardized uptake value (SUVliver) was measured from a spherical volume of interest placed in the right hepatic lobe. This volume of interest was positioned to avoid large vessels and tumor-involved liver parenchyma. All measurements were reviewed and adjusted as needed by a board-certified nuclear radiologist with 6 years of post-fellowship experience (A.T.T.).
Sample tumor segmentation on axial 18F-FDG positron emission tomography (PET) (a), axial localization CT (b) and fused axial PET/CT (c) images in a 3.5-year-old boy with left suprarenal neuroblastoma. Maximum standardized uptake value was 6.5, mean standardized uptake value was 2.6, tumor volume was 65 mL and total lesion glycolysis was 170
Statistical analysis
Continuous variables were summarized with means ± standard deviations and medians with interquartile ranges (IQR) as appropriate, and categorical variables were summarized with counts and percentages. Event-free survival was defined as the time from diagnosis to tumor progression, recurrence or death. Overall survival was defined as the time from diagnosis to death or last follow-up. We employed univariate and multivariable (including age, MYCN amplification, stage and treatment risk stratification) analyses using Cox proportional hazards regression models to assess the predictive performance of PET indices for event-free survival and overall survival. Optimal thresholds for the PET indices were determined using receiver operating characteristic (ROC) curve analysis, and the survival significance of these thresholds was determined using a log-rank test. We used Kaplan–Meier estimator to illustrate the difference in overall survival between two groups based on the threshold. Student’s t-test was used for comparison of means. A P-value of less than 0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Study population
During the study period, 130 children had undergone FDG PET/CT for suspected neuroblastoma. Of these, 55 children were ultimately confirmed to have a neuroblastic tumor: ganglioneuroma (n=2), ganglioneuroblastoma (n=6), neuroblastoma (n=47). The remaining 75 children were excluded for the following reasons: 59 had prior treatment at the time of the PET/CT scan, 5 had no primary tumor or mass identified and 11 had processes other than neuroblastoma (1 renal cell carcinoma, 1 Ewing sarcoma, 1 low-grade fibromyxosarcoma, 1 infantile fibrosarcoma, 1 rhabdomyosarcoma, 1 pheochromocytoma, 1 sclerosing mediastinitis, 1 fibroid, 1 lymphatic malformation, 1 inflammatory tissue, 1 periportal fibrosis).
The demographics and clinical characteristics of the 55 included children are summarized in Table 1. The median age at the time of PET/CT was 2.9 years (IQR 1.8–3.0 years) and 29 (53%) patients were boys.
18F-FDG positron emission tomography findings
The metabolic indices for the primary tumor were as follows (mean ± standard deviation [range]): SUVmax 4.4±2.2 (1.1–11.2); SUVmax/SUVliver 5.2±2.6 (1.5–13.0); SUVmean 2.0±0.8 (0.8–4.7); tumor volume 178±232 mL (0.80–1,039 mL); total lesion glycolysis 418±601 (0.8–2,681).
Outcomes
Median follow-up for the included population was 1,508 days (range 27–4,433 days). During the study period, 14 children (25% of 55) experienced an event and 11 children (20% of 55) died. In comparing children with events and those without events, there were statistically significant differences in the primary tumor site, bone marrow involvement, SUVmax/SUVliver, SUVmean and total lesion glycolysis (Table 1). All PET/CT measures were significantly higher in higher INRG stages (Table 2).
Prognostic performance of 18F-FDG positron emission tomography
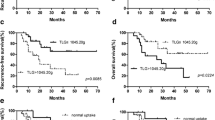
Univariate analysis revealed tumor SUVmax (P=0.025) to be significantly associated with overall survival, with a hazard ratio of 1.29 (95% confidence interval [CI] 1.03–1.62). After adjusting for age, SUVmax (P=0.028) and SUVmean (P=0.045) were statistically significantly associated with overall survival, with age-adjusted hazard ratios of 1.30 (95% CI 1.03–1.63) and 2.04 (95% CI 1.02–4.08), respectively. An SUVmax threshold of 4.77 (P=0.028) best predicted overall survival with a median overall survival of 2,604 days for children with SUVmax above this threshold versus >2,957 days (median not reached during the study period) for children with SUVmax below this threshold (Fig. 2). Tumor volume and total lesion glycolysis were not significantly associated with overall survival, and no parameters were significantly associated with event-free survival. No parameters were significantly associated with overall survival or event-free survival after adjustment for MYCN amplification, tumor stage and risk classification. Among children with stage M disease, no parameters were significantly associated with either overall survival or event-free survival.
Imaging parameters and MYCN status
Forty-nine children (89% of 55) had MYCN fluorescence in situ hybridization (FISH) analysis performed; 14 of these 49 (29%) had MYCN-amplified tumors. Children with MYCN-amplified tumors were older than those without (3.6±2.2 years vs. 2.3±1.6 years, P=0.04). Additionally, mean SUVmax (5.9±2.3 vs. 4.1±2.1, P=0.012), SUVmax/SUVliver (7.2±2.6 vs. 4.8±2.3, P=0.002), tumor volume (409±339 mL vs. 106±104 mL, P<0.0001) and total lesion glycolysis (987±852 vs. 243±335, P<0.0001) were higher in MYCN-amplified tumors versus non amplified tumors (Fig. 3).
Discussion
In this study, we report the metabolic indices from pretreatment 18F-FDG PET/CT in children with newly diagnosed neuroblastic tumors, and their associations with other indicators of disease severity and survival status. Tumor metabolic activity measured on 18F-FDG PET was significantly greater in children with MYCN amplification and children with higher-stage disease. Further, in our population of 55 children, SUVmax and SUVmean were significantly negatively associated with overall survival after adjustment for age. Specifically, tumors with greater metabolic activity had lower overall survival.
Based on receiver operating characteristic (ROC) curve analysis, we identified a threshold SUVmax of 4.77 to optimally stratify our patient population based on overall survival. In children with a primary tumor SUVmax of 4.77 or less, median overall survival was not reached for the study population during the study period. However, for children with a primary tumor with SUVmax of greater than 4.77, median overall survival was 2,604 days. Prior studies have similarly shown 18F-FDG PET metabolic indices to predict survival of children with neuroblastoma. In a cohort of 50 children, Lee et al. [7] showed primary tumor SUVmax to predict event-free survival but not overall survival. In that study, a threshold value of 4.0 showed a 2-year event-free survival of more than 80% with low-FDG-uptake tumors and less than 30% with high-FDG-uptake tumors. Papathanasiou et al. [9], in a cohort of 28 children, all with International Neuroblastoma Staging System (INSS) stage 4 neuroblastoma, showed SUVmax to be significantly associated with both progression-free survival and overall survival at an SUVmax threshold of 5.3. Finally, Kang et al. [8], in a series of 20 children, reported SUVmax to be significantly associated with both progression-free survival and overall survival, but at a higher threshold of 7.78. Taken together, the results of our study and these prior studies clearly demonstrate a relationship between metabolic activity of primary neuroblastic tumors and patient survival. The specific SUVmax threshold identified in each of these studies to optimally stratify survival varies, likely based on population and technical differences.
Our study also identified differences between MYCN-amplified and non-amplified primary tumors. Specifically, MYCN-amplified tumors were, on average, larger in volume with higher SUVmax and total lesion glycolysis than non-amplified tumors. These findings are concordant with those from Liu et al. [10], who demonstrated higher SUVmax, metabolic tumor volume and total lesion glycolysis in MYCN-amplified tumors. These authors also showed SUVmax, metabolic tumor volume and total lesion glycolysis to be significant predictors of overall survival. While these findings are similar to those in our study, there is an important methodological difference: Liu et al. [10] used a 40% SUVmax cut-off threshold to calculate metabolic tumor volume and total lesion glycolysis, which might have enriched their study data with higher-grade (more metabolically active) tumor components.
Interestingly, when multivariate Cox proportional hazard regression models controlled for MYCN status, stage and treatment risk stratification, none of the PET parameters was independently significantly associated with overall survival or event-free survival in our cohort. This result is similar to findings by Lee et al. [7], although a single parameter, Tmax/Lmean (total tumor SUVmax/mean liver SUV), did remain associated with overall survival in their analysis (hazard ratio 3.2, 95% CI 1.2–8.9, P=0.02). In conjunction with our findings of higher SUV in higher-stage or MYCN-amplified tumors, this suggests an interplay among tumor metabolic activity, tumor stage and MYCN amplification status.
There are several limitations to our study. First, as a retrospective study, our results are inherently subject to selection bias. Although 18F-FDG PET/CT is now performed in most newly diagnosed neuroblastoma cases in our practice, historically it was only performed for MIBG-negative tumors and when MIBG scans were not initially readily available. Second, we did not account for the various treatment regimens to which our study population might have been exposed. While treatment paradigms for low- and intermediate-risk tumors — accounting for 45% of our cohort — did not change substantially over the period of the study, the treatment paradigms for high-risk tumors have changed significantly over the last 15 years (e.g., tandem stem cell transplants and dinutuximab immunotherapy have become standard of care). These changes might have impacted outcomes, but cohorts treated under each paradigm are too small to make statistically meaningful conclusions. Third, because this study was focused on 18F-FDG PET/CT, we did not explore or incorporate findings from 123I-MIBG imaging performed in the included patients. Last, though ours is the largest cohort described to date, our sample size of 55 is still relatively small. With several studies now published on pretreatment FDG PET/CT in neuroblastoma, a meta-analysis would provide greater statistical power and might provide further insight into the relevance of metabolic features.
Conclusion
Tumor metabolic activity is higher in newly diagnosed higher-stage, MYCN-amplified neuroblastic tumors. Higher SUVmax and SUVmean were associated with worse overall survival but were not independent of other prognostic markers.
References
Maris JM, Hogarty MD, Bagatell R et al (2007) Neuroblastoma. Lancet 369:2106–2120
Park JR, Kreissman SG, London WB et al (2019) Effect of tandem autologous stem cell transplant vs. single transplant on event-free survival in patients with high-risk neuroblastoma: a randomized clinical trial. JAMA 322:746–755
Monclair T, Brodeur GM, Ambros PF et al (2009) The international neuroblastoma risk group (INRG) staging system: an INRG task force report. J Clin Oncol 27:298–303
Liu Y-L, Lu M-Y, Chang H-H et al (2016) Diagnostic FDG and FDOPA position emission tomography scans distinguish the genomic type and treatment outcome of neuroblastoma. Oncotarget 7:18774–18786
Kushner BH, Yeung HW, Larson SM et al (2001) Extending positron emission tomography scan utility to high-risk neuroblastoma: fluorine-18 fluorodeoxyglucose positron emission tomography as sole imaging modality in follow-up of patients. J Clin Oncol 19:3397–3405
Sharp SE, Shulkin BL, Gelfand MJ et al (2009) 123I-MIBG scintigraphy and 18F-FDG PET in neuroblastoma. J Nucl Med 50:1237–1243
Lee JW, Cho A, Yun M et al (2015) Prognostic value of pretreatment FDG PET in pediatric neuroblastoma. Eur J Radiol 84:2633–2639
Kang SY, Rahim MK, Kim Y-I et al (2016) Clinical significance of pretreatment FDG PET/CT in MIBG-avid pediatric neuroblastoma. Nucl Med Mol Imaging 51:154–160
Papathanasiou ND, Gaze MN, Sullivan K et al (2011) 18F-FDG PET/CT and 123I-metaiodobenzylguanidine imaging in high-risk neuroblastoma: diagnostic comparison and survival analysis. J Nucl Med 52:519–525
Liu C-J, Lu M-Y, Liu Y-L et al (2017) Risk stratitfication of pediatric patients with neuroblastoma using volumetric parameters of 18F-FDG and 18F-DOPA PET/CT. Clin Nucl Med 42:e142–e148
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sung, A.J., Weiss, B.D., Sharp, S.E. et al. Prognostic significance of pretreatment 18F-FDG positron emission tomography/computed tomography in pediatric neuroblastoma. Pediatr Radiol 51, 1400–1405 (2021). https://doi.org/10.1007/s00247-021-05005-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-021-05005-y