Abstract
Fetal ventriculomegaly is the most common central nervous system abnormality detected by prenatal imaging. It has a high association with other anomalies. Etiologies and prognoses for fetal ventriculomegaly range from normal outcomes to significant neurodevelopmental sequelae. In this paper, we review the development, terminology, pathogenesis, imaging and prognosis of fetal ventriculomegaly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fetal ventriculomegaly is defined as in utero enlargement of the lateral ventricles. It is the most common central nervous system (CNS) abnormality detected by prenatal US, with an incidence of approximately 1% [1, 2]. Considered a “tip of the iceberg” finding, fetal ventriculomegaly has a high association with other CNS and non-CNS anomalies and should prompt a thorough investigation of the entire brain and body [2,3,4,5]. Etiologies and prognoses for fetal ventriculomegaly range from normal outcomes to significant neurodevelopmental sequelae. In this article, we review the development, terminology, pathogenesis, imaging and prognosis of fetal ventriculomegaly.
Ventricular development
The lateral ventricles undergo a dramatic transformation throughout gestational life. These changes are largely related to the development of the surrounding structures. The inward folding and maturation of the cerebral surfaces result in a decrease in the volume of the lateral ventricles. Early on during the vesicular period, at 10–13 weeks of gestation, the lateral ventricles occupy much of the fetal brain. At that time, the frontal horns, body and atrium are rudimentary in appearance. As the brain develops, the ventricles take shape with the appearance of four distinct horns. This morphology becomes recognizable on imaging at approximately 16 weeks [6,7,8,9,10]. With time, the lateral ventricles narrow and appear less prominent. By term, they appear small with respect to the developed brain. Despite the apparent evolution, the atrial diameter remains fairly constant (Fig. 1).
Terminology
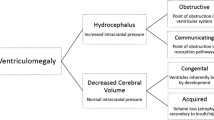
Ventriculomegaly is the preferred terminology for enlarged ventricles identified in utero. The term hydrocephalus describes a subset of ventriculomegaly patients that also have increased cerebrospinal fluid (CSF) pressure within the ventricular system, obstruction being a common etiology. Given the challenge of making this distinction prenatally, the term “hydrocephalus” is typically reserved for fetuses with a more severe degree of ventriculomegaly, obvious site of obstruction, or enlarged head size [2,3,4,5].
Pathogenesis
While the causes of ventriculomegaly are complex and variable, the mechanisms can be broadly divided into obstructive and nonobstructive etiologies as in postnatal assessment. Obstructive ventriculomegaly is the result of either a defect in CSF circulation or resorption, also known as noncommunicating and communicating obstructive ventriculomegaly, respectively. Noncommunicating obstruction can be further subdivided into intrinsic and extrinsic etiologies, depending upon whether the obstruction is intrinsic to the ventricular system or extrinsic and thereby causing mass effect upon ventricular drainage. Communicating obstructive ventriculomegaly, while rare in utero, represents a lack of resorption of CSF from the subarachnoid space. Nonobstructive ventriculomegaly may be the result of CSF overproduction (very rare), a malformative process or brain destruction. Table 1 provides an overview of the various causes of fetal ventriculomegaly [11].
In practice, there is often an overlap in mechanisms for ventriculomegaly. For example, congenital infection can cause ventriculomegaly from parenchymal destruction, ventricular obstruction, as well as maldevelopment (Fig. 2). Similarly, a vein of Galen malformation can result in ventriculomegaly from any combination of decreased CSF resorption secondary to increased venous pressure, brain injury and extrinsic obstruction upon the cerebral aqueduct (Fig. 3).
Cytomegalovirus infection in a 28-week fetus. a Axial single-shot turbo spin-echo (SSTSE) fetal MR image reveals bilateral ventriculomegaly (arrowheads). b Coronal SSTSE fetal MR image of the same fetus demonstrates abnormally small gyri of the superior convexities with a sawtooth appearance concerning for polymicrogyria (arrows)
Vein of Galen malformation. a Sagittal single-shot turbo spin-echo (SSTSE) fetal MR image of a 35-week fetus illustrates a dilated median prosencephalic vein (MPV) of Markowski (colloquially vein of Galen malformation; arrowheads). b Axial SSTSE fetal MR image of the same fetus shows mild ventriculomegaly (asterisk). c Sagittal SSTSE fetal MR image of a different fetus, at 28 weeks of gestation, also demonstrates a dilated MPV (arrowheads). d Axial SSTSE fetal MR image of the fetus in (c) reveals diffuse cystic encephalomalacia of the bilateral cerebral hemispheres (arrows) resulting in ex-vacuo enlargement of the lateral ventricles
Diagnosis
Evaluation of the ventricles is a standard component of the obstetric US exam. Ventriculomegaly is typically first detected during the second-trimester fetal survey at 18–20 weeks of gestation, though it might not be identified till late second or third trimesters. Because of the complex and wide-ranging pathogenesis of ventriculomegaly, a prenatal diagnosis of ventriculomegaly on screening US often results in a detailed anatomical US scan, fetal echocardiography, as well as fetal MRI. In addition to imaging evaluation, genetic testing, TORCH (toxoplasmosis, other [syphilis], rubella, cytomegalovirus and herpes simplex virus) screening, and alloimmune thrombocytopenia screening might also be performed.
As mentioned, ventricular morphology changes over the gestational period, largely because of the growth and evolution of the surrounding brain structures [6,7,8,9,10]. Despite this transformation, the atrial diameter remains relatively consistent from 14 weeks to 40 weeks, with the mean atrial diameter ranging 5.4–7.6 mm. Furthermore, the atria are often the first regions to dilate in ventriculomegaly. It is for both those reasons that we characterize ventriculomegaly by the atrial diameter. Fetal ventriculomegaly is defined as an atrial diameter greater than or equal to 10 mm, which is 2.5 to 4.0 standard deviations above the mean. It is classified as mild, moderate or severe based on the degree of enlargement: mild: 10–12 mm; moderate: 12–15 mm, severe: >15 mm [1, 2, 12, 13]. Approximately 50–60% of ventriculomegaly cases are unilateral. That said, asymmetry of normal-size lateral ventricles is not uncommon. When both ventricles are <10 mm, isolated lateral ventricular asymmetry does not appear to be associated with adverse neurodevelopmental outcomes [14,15,16].
Sonographic evaluation of lateral ventricular size, specifically the atria, is performed in the axial plane. The atrium is where the body, occipital horn and temporal horn converge. It is easily identified by the prominent choroid plexus glomus [2, 13]. Measurements are made from the inner walls of the atria, at the level of the glomus of the choroid plexus and the parieto-occipital groove, perpendicular to the long axis of the lateral ventricle (Fig. 4). The frontal horns and cavum septum pellucidum should be within the axial image [2, 13, 17, 18]. Technique is imperative because small variations can result in incorrect interpretation, particularly when the atrial diameter is borderline (close to 10 mm).
Fetal MRI is an important adjunct to US. The strength of MRI lies in its utility for characterizing subtle intracranial findings, such as cortical migration anomalies, hemorrhage and parenchymal destruction [19,20,21,22]. This is of great importance given that presence of additional CNS as well as non-CNS malformations decreases the likelihood of a good outcome. In cases of seemingly isolated ventriculomegaly detected by prenatal US in which no additional anomalies were identified, fetal MRI has been shown to detect additional abnormalities in up to 50% of cases [22]. Note that this is highly dependent on the quality of US [19,20,21,22,23]. A systematic approach to fetal MRI evaluation is thus crucial. The search pattern should include, but not be limited to, evaluation of the ventricular morphology, ependyma, caudothalamic notch, corpus callosum, septum pellucidum, posterior fossa, parenchymal signal and sulcation, head size and extracranial findings.
MRI measurements can be obtained in both the axial and coronal planes (Fig. 5). Axial measurements are performed at the level of the thalami, while coronal measurements are performed at the level of the choroid plexus. Coronal images should be parallel to the brainstem. Generally, most studies suggest excellent agreement between US and MRI measurements [17, 24, 25], although coronal MRI measurements have been shown to be more reliably concordant with US than axial [3].
The evaluation of the remainder of the ventricular system is also crucial in the workup of fetal ventriculomegaly. Normal reference values for gestational age exist and should be referenced in clinical practice [11].
Etiologies
Obstructive noncommunicating ventriculomegaly
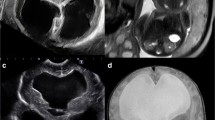
Obstructive noncommunicating ventriculomegaly is the consequence of a blockage of CSF flow, either intrinsic or extrinsic to the ventricular system. Aqueductal stenosis is the classic example of intrinsic obstruction. It might be secondary to prior hemorrhage or infection with resultant webs or gliotic tissue effacing the cerebral aqueduct, and it is often seen in isolation. On imaging, there is a congenital supratentorial pattern of obstructive ventriculomegaly, often moderate to severe in degree (i.e. hydrocephalus) (Fig. 6). Findings that significantly increase the probability of aqueductal stenosis include enlarged third ventricular recesses, aqueduct funneling, and hemorrhage in the cerebral aqueduct [26]. The fourth ventricle is typically normal in size. Although aqueductal stenosis is often sporadic, imaging can provide clues to the diagnosis of several possible genetic and syndromic associations. X-linked hydrocephalus is a notable genetic hydrocephalus syndrome, caused by a mutation of the L1CAM gene on the X chromosome (Xq28). L1CAM codes for a neural cell adhesion molecule transmembrane glycoprotein and, as such, is essential to nervous system development, including neuronal migration and differentiation. L1CAM mutations cause a variety of X-linked neurologic syndromes. Collectively, these syndromes have been referred to as L1 syndrome, and the phenotype might include corpus callosal agenesis, Hirschsprung disease and other intestinal pseudo-obstruction, and limb anomalies (e.g., adducted thumbs) [27]. Congenital aqueductal stenosis is also seen in association with rhombencephalosynapsis, defined as lack of division of the cerebellar hemispheres with complete or partial absence of the vermis [28] (Fig. 7). Up to 65% of patients with rhombencephalosynapsis have coexisting aqueductal stenosis.
Aqueductal stenosis in a 31-week fetus. a Sagittal single-shot turbo spin-echo (SSTSE) fetal MR image reveals severe lateral ventriculomegaly with bowing of the corpus callosum (white arrowheads) as well as enlargement of the third ventricle (black arrowhead) and third ventricular recesses caused by aqueductal stenosis (arrow). Note the normal size of the fourth ventricle (asterisk). b Axial SSTSE fetal MR image of the same fetus shows the severe lateral ventriculomegaly (arrows)
Aqueductal stenosis and rhombencephalosynapsis in a 27-week fetus. a Sagittal single-shot turbo spin-echo (SSTSE) fetal MR image shows findings of aqueductal stenosis (arrow) resulting in third-ventricular enlargement, aqueductal funneling and bowing of the corpus callosum. b Axial SSTSE fetal MR image of the same fetus reveals a small cerebellum with lack of normal division, consistent with rhombencephalosynapsis (arrowheads)
There are many causes of extrinsic obstructive noncommunicating ventriculomegaly. The Chiari II malformation is an important and common indication for prenatal consultation at dedicated fetal care centers. It is the sequelae of chronic in utero CSF leakage through an open spinal dysraphism and is characterized by a constellation of intracranial findings, the hallmark being a small posterior fossa with hindbrain herniation [29] (Fig. 8). The hindbrain herniation can result in obstruction to CSF flow at the level of the foramen magnum, leading to ventriculomegaly. Postnatal CSF diversion/shunting is required in most children. In utero repair of the open spinal dysraphism has been shown to decrease the need for postnatal ventricular shunting and can improve neurologic outcomes in some children [30, 31].
Chiari II malformation in a 23-week fetus. a Sagittal balanced turbo field echo (bTFE) fetal MR image of a fetus with a myelomeningocele (arrows) demonstrates findings of a Chiari II malformation with a small, crowded posterior fossa and hindbrain herniation (arrowhead). b Axial bTFE fetal MR image of the same fetus shows moderate lateral ventriculomegaly (asterisks)
Obstructive communicating ventriculomegaly
Obstructive communicating ventriculomegaly is rare in utero. It represents impaired resorption of CSF from the subarachnoid space. CSF is normally resorbed from the subarachnoid space at several levels: along the cranial nerve sheaths (particularly the olfactory bulbs) into head and neck lymphatics, along the spinal nerve sheaths into perispinal lymphatics, into meningeal lymphatics, and into dural sinuses via arachnoid granulations [32]. Subarachnoid pathology can reduce absorptive capacity. Specifically, subarachnoid hemorrhage or inflammation can cause a communicating ventriculomegaly by subarachnoid scarring. Impaired CSF resorption can also occur when cranial venous sinus pressures are elevated, such as in the setting of vein of Galen malformation (Fig. 3), dural sinus malformation or dural sinus thrombosis. On imaging, there is dilation of the entire ventricular system, including the fourth ventricle, without a site of obstruction.
Nonobstructive ventriculomegaly
Nonobstructive ventriculomegaly might be caused by CSF overproduction, a malformation of development, or brain injury (Table 1). CSF overproduction in utero is extremely rare. Choroid plexus tumors as well as choroid plexus villous hyperplasia have been implicated as causes of CSF overproduction. While congenital brain tumors are uncommon, choroid plexus tumors are frequent among the ones that do occur — most of which represent papillomas. Ventriculomegaly from choroid plexus tumors might be from any combination of CSF overproduction, mechanical obstruction or impaired CSF resorption (from hemorrhage). On MRI, these are cauliflower-like masses most commonly located in the atrium of the lateral ventricles in children. Choroid plexus papillomas are associated with Aicardi syndrome and, as such, should prompt evaluation for other findings of this syndrome when suspected on fetal imaging (i.e. callosal dysgenesis, polymicrogyria, heterotopia, intracranial cysts, ocular coloboma). There is also an association with choroid plexus tumors and Li–Fraumeni syndrome, a cancer-predisposing syndrome caused by mutation of the TP53 gene. The prognosis for children with congenital brain tumors is generally poor; however, it is favorable in the context of choroid plexus tumors [33].
Malformative lesions of the brain are a frequent cause of enlarged or dysplastic-appearing ventricles. Specifically, agenesis/dysgenesis of the corpus callosum is among the most common malformations of cerebral development and results in a very distinctive ventricular appearance. The corpus callosum is a C-shaped structure in the midline brain that serves as the main pathway through which neocortical commissural fibers cross. These connections are important for the functional integration of sensory, motor, visuomotor and cognitive processes. The corpus callosum helps provide shape to the roof of the frontal horns and body, as well as the margins of the occipital horns. In its absence, the leaves of the septum pellucidum will also be absent, as will the cingulate gyrus. On imaging in the midline sagittal plane, a radial array of sulci emanate in a perpendicular fashion from the dilated third ventricle in a sunburst pattern. In the axial plane, the lateral ventricles parallel one another, with the frontal horns appearing narrow and the occipital horns dilated (i.e. colpocephalic). In the coronal plane, the lateral ventricles are widely separated and vertically oriented, likened to the appearance of Texas longhorns (Fig. 9). Associated brain anomalies are common including interhemispheric cysts and lipomas, as well as posterior fossa abnormalities. In addition, non-CNS anomalies have been reported in as many as 60% of cases [34]. The cause of the disrupted callosum can be genetic, infectious (TORCH infections, Zika virus), vascular or toxic (fetal alcohol syndrome). Genetic factors are most common, with more than 200 genetic syndromes that include disorder of the corpus callosum as a feature (e.g., Aicardi syndrome (Fig. 10), MASA syndrome (L1CAM), Acrocallosal syndrome, Vici syndrome) [35].
Agenesis of the corpus callosum in a 32-week fetus. a Sagittal single-shot turbo spin-echo (SSTSE) fetal MR image reveals complete absence of the corpus callosum. b, c Axial (b) and coronal (c) SSTSE fetal MR images of the same fetus illustrate borderline-enlarged lateral ventricles with teardrop-shaped occipital horns and trident-shaped frontal horns, respectively (arrowheads)
Aicardi syndrome in a 29-week fetus. a Axial single-shot turbo spin-echo (SSTSE) fetal MR image demonstrates agenesis of the corpus callosum (asterisk) with enlarged, dysmorphic lateral ventricles (arrowheads). b Axial SSTSE fetal MR image of the same fetus, at the level of the orbits, reveals an ocular coloboma on the right (white arrowhead), a single nodule of periventricular heterotopia on the right (arrow) and mass-like subcortical heterotopia on the left (black arrowheads)
Brain destruction leading to ex-vacuo enlargement of the ventricles might be the result of several different pathogenic mechanisms including ischemia, infection, inborn error of metabolism and others [1,2,3, 36]. Insults can be diffuse or focal, sometimes pattern-specific, offering insight into the underlying etiology (Fig. 11). Perinatal stroke encompasses a group of heterogeneous conditions in which a focal disruption of cerebral blood flow secondary to arterial or cerebral venous thrombosis or embolization often results in a pattern-specific injury. Periventricular venous infarction is part of the spectrum of germinal matrix and intraventricular hemorrhage and occurs primarily in utero. It is thought to be caused by the obstruction of the medullary and terminal veins by the associated intraventricular hemorrhage, resulting in venous congestion, ischemia and hemorrhagic infarction [37]. Acute imaging demonstrates a germinal matrix hemorrhage with parenchymal extension (Fig. 12). Diffusion-weighted imaging reveals a rind of cytotoxic edema. In the chronic phase, there is focal cystic encephalomalacia of the periventricular white matter, termed “caudal triangle,” that is often lined by residual blood products. The underlying ventricle is enlarged as a result thereof (Fig. 12). Interestingly, despite the focal nature of the injury, studies have shown that gray matter volume is diffusely diminished ipsilateral to the side of the infarct — suggesting retrograde neuronal degeneration and disrupted migration [38, 39].
Brain destruction. a Axial single-shot turbo spin-echo (SSTSE) fetal MR image in a 23-week fetus shows a region of cystic encephalomalacia (arrows) from prior right middle cerebral artery infarction resulting in ex-vacuo enlargement of the lateral ventricle. b Axial SSTSE fetal MR image of a different fetus, at 25 weeks of gestation, reveals large bilateral schizencephalic clefts (arrowheads) resulting in severe ventriculomegaly. c Axial SSTSE fetal MR image of a third fetus, at 30 weeks of gestation, demonstrates absence of the cerebral hemispheres with a fluid-filled cranial vault (asterisks), consistent with hydranencephaly
Hemorrhagic periventricular venous infarction in a 23-week fetus. a Coronal single-shot turbo spin-echo (SSTSE) fetal MR image shows bilateral germinal matrix hemorrhages with parenchymal extension, right (arrow) larger than left (arrowhead). b Follow-up imaging of the same fetus at 31 weeks of gestation demonstrates maturation of the injury with focal cystic encephalomalacia of the periventricular white matter (arrowheads) and ex-vacuo enlargement of the underlying lateral ventricle
Associated anomalies
Associated CNS and non-CNS abnormalities in fetal ventriculomegaly are common. In severe ventriculomegaly, additional CNS anomalies are seen in 50–85% of cases. Fetal ventriculomegaly is also associated with an increased risk for a chromosomal abnormality, which is higher when the ventriculomegaly is severe or associated abnormalities are present. Even in cases of apparent isolated mild ventriculomegaly, a meta-analysis found up to 4.7% of fetuses had an abnormal karyotype,most commonly trisomy 21 [40]. When additional fetal anomalies are found, the incidence of chromosomal abnormalities increases to 18% [41]. A diverse array of genetic disorders is associated with ventriculomegaly, including trisomies 13, 18 and 21, many microdeletion syndromes, neurofibromatosis and tuberous sclerosis, and a range of single gene disorders [41, 42].
Prognosis
The goal of prenatal diagnosis and evaluation is to better inform and prepare the family, as well as to determine the ideal birth environment. Fetal ventriculomegaly encompasses a wide range of diagnoses and outcomes. The synthesis of all available data becomes crucial in these discussions. Detection of associated anomalies on imaging, genetic evaluation, and in utero infection screening is of great importance. When the cause of ventriculomegaly can be identified, the counseling and prognostication is based on the diagnosis. Often, the etiology cannot be determined prenatally, making counseling challenging. Ultimately, a range of potential outcomes is discussed with the family.
Two important factors in counseling families about prognosis are whether the ventriculomegaly is isolated, and the severity of the ventriculomegaly. In cases of isolated mild–moderate ventriculomegaly, the incidence of abnormal development is 0–8% [40, 43]. Similarly, the prognosis of unilateral mild ventriculomegaly is also good [44]. Assuming the diagnosis is confirmed on a high-quality fetal MRI, isolated ventriculomegaly portends a good prognosis for neurodevelopmental outcome. However, families need to be counseled that additional findings not seen on prenatal imaging are discovered on postnatal imaging in up to 11% of patients [40]. Isolated severe ventriculomegaly is associated with higher rates of long-term developmental sequelae when compared with isolated mild and moderate ventriculomegaly. The rate of normal outcome in severe ventriculomegaly is 42%, with an additional 18% having mild–moderate disability and 40% having severe disability [45].
The presence of associated intra- or extracranial malformations increases the morbidity/mortality from 6% in isolated ventriculomegaly to 56%, and it increases the likelihood of neurologic delay from 9–36% in isolated ventriculomegaly to 84% [41, 46, 47]. The major difficulty in prenatal counseling is that most studies do not characterize the long-term disability with any granularity, and many only distinguish between “normal” and “abnormal” outcomes. While the presence of additional anomalies increases the risk of neurologic sequelae, the severity is dependent upon the nature of the additional anomalies [48].
Last, progression of ventriculomegaly in utero is important to document [3, 5]. The natural history of fetal ventriculomegaly is resolution in 29%, stability in 57% and progression in 14% [49], with worse neurologic outcomes and postnatal abnormalities in fetuses demonstrating progression [50, 51]. Conversely, stability or improvement in ventricular size favors better outcomes [5, 12]. As such, most fetuses undergo follow-up US examination at 28–34 weeks to assess progression or regression. Furthermore, a second US identifies a previously undetected associated abnormality in up to 13% of cases [52].
Given the complexity in diagnosis and prognosis, family counseling requires a multidisciplinary approach, including experts in maternal–fetal medicine, obstetrics, child neurology, neurosurgery and radiology.
Conclusion
Fetal ventriculomegaly is the most common CNS abnormality found by prenatal US. It has a wide variety of associations and outcomes. Imaging plays an important role in workup, prognostication and counseling.
References
Barzilay E, Bar-Yosef O, Dorembus S et al (2017) Fetal brain anomalies associated with ventriculomegaly or asymmetry: an MRI-based study. AJNR Am J Neuroradiol 38:371–375
D’Addario V, Rossi AC (2012) Neuroimaging of ventriculomegaly in the fetal period. Semin Fetal Neonatal Med 17:310–318
Garel C, Luton D, Oury J-F, Gressens P (2003) Ventricular dilatations. Childs Nerv Syst 19:517–523
Nyberg DA, Mack LA, Hirsch J et al (1987) Fetal hydrocephalus: sonographic detection and clinical significance of associated anomalies. Radiology 163:187–191
Weichert J, Hartge D, Krapp M et al (2010) Prevalence, characteristics and perinatal outcome of fetal ventriculomegaly in 29,000 pregnancies followed at a single institution. Fetal Diagn Ther 27:142–148
Dubois J, Benders M, Cachia A et al (2008) Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex 18:1444–1454
Huang H, Xue R, Zhang J et al (2009) Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J Neurosci 29:4263–4273
Kyriakopoulou V, Vatansever D, Elkommos S et al (2014) Cortical overgrowth in fetuses with isolated ventriculomegaly. Cereb Cortex 24:2141–2150
Li Z, Xu F, Zhang Z et al (2019) Morphologic evolution and coordinated development of the fetal lateral ventricles in the second and third trimesters. AJNR Am J Neuroradiol 40:718–725
Taketani K, Yamada S, Uwabe C et al (2015) Morphological features and length measurements of fetal lateral ventricles at 16-25 weeks of gestation by magnetic resonance imaging. Congenit Anom 55:99–102
Kline-Fath B, Bahado-Singh R, Bulas D (2014) Fundamental and advanced fetal imaging: ultrasound and MRI. Wolters Kluwer Health, Philadelphia
Gaglioti P, Oberto M, Todros T (2009) The significance of fetal ventriculomegaly: etiology, short- and long-term outcomes. Prenat Diagn 29:381–388
Society for Maternal-Fetal Medicine, Fox NS, Monteagudo A et al (2018) Mild fetal ventriculomegaly: diagnosis, evaluation, and management. Am J Obstet Gynecol 219:B2–B9
Achiron R, Yagel S, Rotstein Z et al (1997) Cerebral lateral ventricular asymmetry: is this a normal ultrasonographic finding in the fetal brain? Obstet Gynecol 89:233–237
Sadan S, Malinger G, Schweiger A et al (2007) Neuropsychological outcome of children with asymmetric ventricles or unilateral mild ventriculomegaly identified in utero. BJOG 114:596–602
Meyer R, Bar-Yosef O, Barzilay E et al (2018) Neurodevelopmental outcome of fetal isolated ventricular asymmetry without dilation: a cohort study. Ultrasound Obstet Gynecol 52:467–472
Behrendt N, Zaretsky MV, West NA et al (2017) Ultrasound versus MRI: is there a difference in measurements of the fetal lateral ventricles? J Matern Fetal Neonatal Med 30:298–301
Pisapia JM, Sinha S, Zarnow DM et al (2017) Fetal ventriculomegaly: diagnosis, treatment, and future directions. Childs Nerv Syst 33:1113–1123
Benacerraf BR, Shipp TD, Bromley B, Levine D (2007) What does magnetic resonance imaging add to the prenatal sonographic diagnosis of ventriculomegaly? J Ultrasound Med 26:1513–1522
Griffiths PD, Reeves MJ, Morris JE et al (2010) A prospective study of fetuses with isolated ventriculomegaly investigated by antenatal sonography and in utero MR imaging. AJNR Am J Neuroradiol 31:106–111
Manganaro L, Savelli S, Francioso A et al (2009) Role of fetal MRI in the diagnosis of cerebral ventriculomegaly assessed by ultrasonography. Radiol Med 114:1013–1023
Morris JE, Rickard S, Paley MNJ et al (2007) The value of in-utero magnetic resonance imaging in ultrasound diagnosed foetal isolated cerebral ventriculomegaly. Clin Radiol 62:140–144
Salomon LJ, Ouahba J, Delezoide AL et al (2006) Third-trimester fetal MRI in isolated 10- to 12-mm ventriculomegaly: is it worth it? BJOG 113:942–947
Levine D, Feldman HA, Tannus JFK et al (2008) Frequency and cause of disagreements in diagnoses for fetuses referred for ventriculomegaly. Radiology 247:516–527
Perlman S, Shashar D, Hoffmann C et al (2014) Prenatal diagnosis of fetal ventriculomegaly: agreement between fetal brain ultrasonography and MR imaging. AJNR Am J Neuroradiol 35:1214–1218
Heaphy-Henault KJ, Guimaraes CV, Mehollin-Ray AR et al (2018) Congenital aqueductal stenosis: findings at fetal MRI that accurately predict a postnatal diagnosis. AJNR Am J Neuroradiol 39:942–948
Kenwrick S, Jouet M, Donnai D (1996) X linked hydrocephalus and MASA syndrome. J Med Genet 33:59–65
Ishak GE, Dempsey JC, Shaw DWW et al (2012) Rhombencephalosynapsis: a hindbrain malformation associated with incomplete separation of midbrain and forebrain, hydrocephalus and a broad spectrum of severity. Brain 135:1370–1386
Mirsky DM, Schwartz ES, Zarnow DM (2015) Diagnostic features of myelomeningocele: the role of ultrafast fetal MRI. Fetal Diagn Ther 37:219–225
Adzick NS, Thom EA, Spong CY et al (2011) A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 364:993–1004
Tulipan N, Wellons JC, Thom EA et al (2015) Prenatal surgery for myelomeningocele and the need for cerebrospinal fluid shunt placement. J Neurosurg Pediatr 16:613–620
Jessen NA, Munk AS, Lundgaard I, Nedergaard M (2015) The glymphatic system: a beginner’s guide. Neurochem Res 40:2583–2599
Severino M, Schwartz ES, Thurnher MM et al (2010) Congenital tumors of the central nervous system. Neuroradiology 52:531–548
Bedeschi MF, Bonaglia MC, Grasso R et al (2006) Agenesis of the corpus callosum: clinical and genetic study in 63 young patients. Pediatr Neurol 34:186–193
Edwards TJ, Sherr EH, Barkovich AJ, Richards LJ (2014) Clinical, genetic and imaging findings identify new causes for corpus callosum development syndromes. Brain 137:1579–1613
Neuberger I, Garcia J, Meyers ML et al (2018) Imaging of congenital central nervous system infections. Pediatr Radiol 48:513–523
Lee S, Mirsky DM, Beslow LA et al (2017) Pathways for neuroimaging of neonatal stroke. Pediatr Neurol 69:37–48
Li D, Hodge J, Wei XC, Kirton A (2012) Reduced ipsilesional cortical volumes in fetal periventricular venous infarction. Stroke 43:1404–1407
Kirton A, Deveber G, Pontigon AM et al (2008) Presumed perinatal ischemic stroke: vascular classification predicts outcomes. Ann Neurol 63:436–443
Pagani G, Thilaganathan B, Prefumo F (2014) Neurodevelopmental outcome in isolated mild fetal ventriculomegaly: systematic review and meta-analysis. Ultrasound Obstet Gynecol 44:254–260
Wax JR, Bookman L, Cartin A et al (2003) Mild fetal cerebral ventriculomegaly: diagnosis, clinical associations, and outcomes. Obstet Gynecol Surv 58:407–414
Tully HM, Dobyns WB (2014) Infantile hydrocephalus: a review of epidemiology, classification and causes. Eur J Med Genet 57:359–368
Signorelli M, Tiberti A, Valseriati D et al (2004) Width of the fetal lateral ventricular atrium between 10 and 12 mm: a simple variation of the norm? Ultrasound Obstet Gynecol 23:14–18
Atad-Rapoport M, Schweiger A, Lev D et al (2015) Neuropsychological follow-up at school age of children with asymmetric ventricles or unilateral ventriculomegaly identified in utero. BJOG 122:932–938
Carta S, Agten AK, Belcaro C, Bhide A (2018) Outcome of fetuses with prenatal diagnosis of isolated severe bilateral ventriculomegaly: systematic review and meta-analysis. Ultrasound Obstet Gynecol 52:165–173
D’Addario V, Pinto V, Di Cagno L, Pintucci A (2007) Sonographic diagnosis of fetal cerebral ventriculomegaly: an update. J Matern Fetal Neonatal Med 20:7–14
Lee CS, Hong SH, Wang K-C et al (2006) Fetal ventriculomegaly: prognosis in cases in which prenatal neurosurgical consultation was sought. J Neurosurg 105:265–270
Yamasaki M, Nonaka M, Bamba Y et al (2012) Diagnosis, treatment, and long-term outcomes of fetal hydrocephalus. Semin Fetal Neonatal Med 17:330–335
McKechnie L, Vasudevan C, Levene M (2012) Neonatal outcome of congenital ventriculomegaly. Semin Fetal Neonatal Med 17:301–307
Baffero GM, Crovetto F, Fabietti I et al (2015) Prenatal ultrasound predictors of postnatal major cerebral abnormalities in fetuses with apparently isolated mild ventriculomegaly. Prenat Diagn 35:783–788
Devaseelan P, Cardwell C, Bell B, Ong S (2010) Prognosis of isolated mild to moderate fetal cerebral ventriculomegaly: a systematic review. J Perinat Med 38:401–409
Melchiorre K, Bhide A, Gika AD et al (2009) Counseling in isolated mild fetal ventriculomegaly. Ultrasound Obstet Gynecol 34:212–224
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mirsky, D.M., Stence, N.V., Powers, A.M. et al. Imaging of fetal ventriculomegaly. Pediatr Radiol 50, 1948–1958 (2020). https://doi.org/10.1007/s00247-020-04880-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-020-04880-1