Abstract
Background
Accurate and reproducible means of measuring the portosystemic gradient are essential for risk stratification and treatment of portal hypertension.
Objective
To report the reliability of hepatic venous pressure gradients in children with intrahepatic veno-venous collateralization.
Materials and methods
Between January 2012 and December 2019 (96 months), 39 patients with native livers underwent wedge hepatic venography and hepatic venous pressure gradient measurements at a tertiary pediatric center. All archived images were reviewed for balloon isolation of the hepatic vein and hepatic vein-to-hepatic vein (HV-HV) collaterals. HV-HV collaterals were categorized as present on the basis of non-catheterized segmental venous opacification despite appropriate balloon isolation. Hepatic venous pressure gradient was defined as the difference of wedge and free hepatic venous pressures. Wedge portosystemic gradient was defined as the difference between wedge hepatic venous pressure and right atrial (RA) pressures. For patients subsequently undergoing portal venous catheterization, portosystemic gradient was defined as the difference between main portal vein and RA pressures.
Results
Thirteen of 39 (33.3%) patients demonstrated HV-HV collaterals on wedge hepatic venography. The mean hepatic venous pressure gradient was 5.2±3.8 mmHg (range: 0–15 mmHg). The mean hepatic venous pressure gradient was 3.6±2.6 mmHg (range: 0–9 mmHg) in the presence of HV-HV collaterals and 5.9±4.2 mmHg (range: 1–15 mmHg) in the absence of HV-HV collaterals (P=0.043). Twelve (30.8%) patients were found to have varices: 10 gastroesophageal, 1 rectal and 1 stomal. The mean hepatic venous pressure gradient in patients with varices was 5.4±47 mmHg (range: 0–15 mmHg). For patients with varices, mean hepatic venous pressure gradient was 3.0±2.7 mmHg (range: 0–9 mmHg) in the presence of HV-HV collaterals and 10.3±4.1 mmHg (range: 5–15 mmHg) in the absence of HV-HV collaterals (P=0.004). Four (10.3%) patients had extrahepatic portal vein occlusion: 3 with cavernous transformation and 1 with type Ib Abernethy malformation. All patients with extrahepatic portal vein occlusion demonstrated HV-HV collaterals compared with 8 of 35 (22.9%) patients without extrahepatic portal vein occlusion (P=0.002). Four of 39 (10.3%) patients underwent direct portal pressure measurements: 3 via transhepatic and 1 via trans-splenic portal access. All had demonstrated HV-HV collaterals on wedged imaging. One had extrahepatic portal vein occlusion. The mean time between wedge portosystemic gradient and portosystemic gradient measurement was 3.75 days (range: 0–8 days). The mean wedge portosystemic gradient was 4.5±3.1 mmHg (range: 2–9 mmHg) and the mean portosystemic gradient was 14.5±3.7 mmHg (range: 12–20 mmHg) (P=0.006).
Conclusion
HV-HV collateralization is frequently observed in children undergoing wedged portal venography and leads to misrepresentative hepatic venous pressure gradients. All patients undergoing hepatic venous pressure gradient measurement should have wedged venography to identify HV-HV collaterals and to qualify measured pressures. Additional techniques to obtain representative pressures in the presence of HV-HV collaterals warrant further investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Managing portal hypertension in children may prevent or treat variceal hemorrhage, hypersplenism, exudative enteropathy and ascites [1, 2]. Portal hypertension, whether arising from sinusoidal or extrahepatic obstruction to portal blood flow, can be complicated by a spectrum of conditions ranging from ascites to variceal hemorrhage. Although invasive measurement of the portosystemic pressure gradient is routinely used to assess the risk of variceal hemorrhage in adults, similar practice has not been widely adopted in the management of pediatric portal hypertension. In the past decade, there have been emerging data on the use of catheter-based techniques to predict bleeding risk in children with portal hypertension who have not yet had primary variceal bleeding. Novel interventional radiology approaches may also complement existing medical and surgical therapies for the management of pediatric portal hypertension.
The standard method to measure the portosystemic pressure gradient is by transcatheter free and wedged hepatic venous pressure measurements. Wedging, achieved by either advancing an angiographic catheter into a small hepatic venous tributary or by compliant balloon catheter inflation to isolate a selected hepatic vein, approximates the sinusoidal and transmitted portal pressure. This assumption relies on the absence of collaterals between hepatic veins and the presence of collaterals between venules in the portal tract (Fig. 1). That first assumption may be negated in the setting of hepatic vein-to-hepatic vein (HV-HV) collaterals, which may be observed with or without balloon isolation of hepatic veins (Fig. 2). While hepatic vein collaterals have been previously described and postulated to depress the pressure gradient measurement [3], a comparative analysis of different methods to measure the portosystemic gradient in the presence of intrahepatic vein collaterals has not been done.
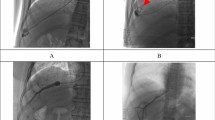
A 17-year-old boy with fulminant liver failure due to hepatitis B. Digital subtraction anteroposterior projection venography during transjugular liver biopsy from the right hepatic vein (rHV) demonstrates collateralization (arrows) to an accessory hepatic vein (aHV). There was neither extrahepatic portal vein occlusion nor gastroesophageal varices. No pressures were measured. The figure is presented for illustrative purposes and this patient is not included in the analysis
Accurate and reproducible means of measuring the portosystemic gradient are essential for risk stratification [4, 5]. Patients with misrepresentative hepatic venous pressure gradients lower than their true portosystemic gradient may have their work-up of portal hypertension prematurely and may not be referred for appropriate endoscopic or endovascular treatment. The purpose of this study was to examine the prevalence of HV-HV collaterals observed during wedged venography in children and to determine their effect on hepatic venous pressure gradients.
Materials and methods
Study design
This study was conducted with institutional review board approval and complied with the Health Insurance Portability and Accountability Act. Informed consent was waived from all participants in the study. This study was assessed using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [6].
Inclusion and exclusion criteria
Children (≤18 years) who underwent both wedged hepatic venography and hepatic venous pressure gradients in native livers at a tertiary pediatric center were included. During the study period of January 2012 and December 2019 (96 months), 39 patients met inclusion criteria for the study. An additional 14 patients identified during our database search did not meet inclusion criteria and were excluded: 1 patient who underwent wedged venography without pressure measurements and 13 patients with liver transplant undergoing wedged hepatic venography and pressure measurements were excluded.
Thirty-five (89.7%) patients underwent concomitant liver biopsy, 31 via a transjugular approach and 4 percutaneously.
Patient demographics
Patient demographics are shown in Table 1. Thirty-nine patients were included in the study. The mean patient age was 9.4±5.4 years (range: 2 months to 18 years). The mean patient weight was 35.7±24.6 kg (range: 3–95 kg).
Techniques
All procedures were performed under general anesthesia. Interventions were performed by six board-certified interventional radiologists (E.J.M., K.S.H.K., G.M.S., and three others who are not authors of this paper) ranging in experience from 1 to 20 years of post-graduate practice. Transjugular hepatic venography and pressure measurements with transjugular liver biopsy were performed through a 9-Fr vascular access sheath. Transjugular liver biopsies were performed using a transjugular liver biopsy set (TLAB; Argon Medical Devices, Frisco, TX). Hepatic venography and pressure measurements without transjugular biopsy were performed through a single 6- or 7-Fr vascular access sheath placed via the jugular vein. Hepatic veins were selectively catheterized. The position was confirmed and the catheters were exchanged for a 5-Fr 13-mm compliant balloon catheter (Python Balloon; Applied Medical, Irvine, CA). Hepatic venography was performed before the balloon inflation to verify uninhibited outflow within the catheterized vein and to obtain free hepatic venous pressure measurement. The balloon catheter was inflated to wall apposition in the right hepatic vein or tributary. Venography, with the intent to confirm wedged position (n=29), was performed until contrast filling of the isolated venous tributaries (Fig. 3). Venography, with the intent to image the portal vein (n=10), was performed with injection of carbon dioxide after balloon isolation. Following the confirmation of appropriate balloon isolation, the balloon was deflated to allow egress of contrast and then reinflated to the same position for measurement of wedge hepatic venous pressure.
An 8-year-old boy with pancytopenia and transaminitis. Anteroposterior projection venography following catheterization and balloon inflation in the right hepatic vein shows appropriate isolation without hepatic vein-to-hepatic vein collaterals. There was neither extrahepatic portal vein occlusion nor gastroesophageal varices
Variables collected and defined
All archived images were reviewed by the principal investigator with verification of appropriate balloon isolation and evaluation for HV-HV collaterals. HV-HV collaterals were categorized as present on the basis of non-catheterized segmental venous opacification despite appropriate balloon isolation (Fig. 4). Hepatic venous pressure gradient was defined as the difference in wedged and free hepatic venous pressures. Wedged portosystemic gradient was defined as the difference between wedge hepatic venous pressure and right atrial (RA) pressures. For patients subsequently undergoing portal venous catheterization and pressure measurements, portosystemic gradient was defined as the difference between the main portal vein and RA pressures.
A 6-year-old girl with a history of congenital hepatic fibrosis and gastroesophageal variceal bleed. Anteroposterior projection carbon dioxide venography following catheterization and balloon occlusion of the anterior right hepatic vein (arrowhead) demonstrates collaterals to the posterior right hepatic vein (prHV) and middle hepatic vein (mHV) without portal vein visualization. The wedged portosystemic gradient and hepatic venous pressure gradient were 9 mmHg. The patient underwent transjugular portosystemic shunt a week later at which time direct pressure measurements yielded a baseline portosystemic gradient of 12 mmHg. There was no extrahepatic portal vein occlusion
The electronic medical record was reviewed for procedural complications, specimen histology, imaging evidence of extrahepatic portal vein occlusion, and sequela of portal hypertension. Complications were recorded according to the Society of Interventional Radiology (SIR) guidelines on adverse events [7].
Statistical analyses
Calculations of means, standard deviations and ranges were performed with statistical spreadsheet software (Excel; Microsoft, Redmond, WA). Statistical comparison of means was performed using a two-sample t-test. A comparison of categorical data was performed using “N-1” chi-squared test (MedCalc, Belgium). A P-value <0.05 was considered significant.
Results
Results are shown in Table 1. Thirteen (33.3%) of 39 patients demonstrated HV-HV collaterals on wedged hepatic venography. The mean hepatic venous pressure gradient was 5.2±3.8 mmHg (range: 0–15 mmHg). The mean hepatic venous pressure gradient was 3.6±2.6 mmHg (range: 0–9 mmHg) in the presence of HV-HV collaterals and 5.9±4.2 mmHg (range: 1–15 mmHg) in the absence of HV-HV collaterals (P=0.043).
Twelve (30.8%) patients had varices: 10 gastroesophageal, 1 rectal and 1 stomal. The mean hepatic venous pressure gradient in patients with varices was 5.4±4.7 mmHg (range: 0–15 mmHg). For patients with varices, the mean hepatic venous pressure gradient was 3.0±2.7 mmHg (range: 0–9 mmHg) in the presence of HV-HV collaterals and 10.3±4.1 mmHg (range: 5–15 mmHg) in the absence of HV-HV collaterals (P=0.004).
Four (10.3%) of 39 patients had extrahepatic portal vein occlusion: 3 with cavernous transformation and 1 with type Ib Abernethy malformation. All patients were suspected to have extrahepatic portal vein occlusion on the basis of Doppler US and underwent transjugular procedures to gather additional information as part of treatment planning. For the patient with suspected Abernethy malformation, venography was requested to categorize the malformation and plan endovascular versus open surgical intervention. For patients with suspected cavernous transformation, venography was requested to identify the intrahepatic portal venous anatomy suitable for meso-Rex bypass versus a dominant channel of cavernous transformation for transjugular intrahepatic portosystemic shunt (TIPS) creation. At the time of venography, all patients with extrahepatic portal vein occlusion demonstrated HV-HV collaterals compared with 8 of 35 (22.9%) patients without extrahepatic portal vein occlusion (P=0.002).
Subsequent analysis was performed for patients without extrahepatic portal vein occlusion (n=35). The mean hepatic venous pressure gradient was 4.4±2.5 mmHg (range: 2–9 mmHg) in the presence of HV-HV collaterals and 5.9±4.2 mmHg (range: 1–15 mmHg) in the absence of HV-HV collaterals (P=0.324). Eight of 35 (22.9%) patients had varices. The mean hepatic venous pressure gradient in patients with varices and no extrahepatic portal vein occlusion was 7.3±4.7 mmHg (range: 3–15 mmHg). For patients with varices and no extrahepatic portal vein occlusion, the mean hepatic venous pressure gradient was 4.3±3.2 mmHg (range: 2–9 mmHg) in the presence of HV-HV collaterals and 10.3±4.1 mmHg (range: 5–15 mmHg) in the absence of HV-HV collaterals (P=0.061).
Four patients with HV-HV collaterals went on to receive direct portal pressure measurements: three during TIPS placement and one via trans-splenic portal access with venography. All four patients had varices. None had extrahepatic portal vein occlusion. The mean time between wedge portosystemic gradient and portosystemic gradient measurement was 3.75 days (range: 0–8 days). The mean wedge portosystemic gradient was 4.5±3.1 mmHg (range: 2–9 mmHg) and the mean portosystemic gradient was 14.5±3.7 mmHg (range: 12–20 mmHg) (P=0.006). A single patient without HV-HV collaterals went on to receive direct portal pressure measurements during TIPS placement 21 days after the initial measurement, revealing a discrepant wedge portosystemic gradient and portosystemic gradient measurements of 9 and 13 mmHg, respectively.
Two patients (5.1%) (patients 28 and 35) has disparities of >3 mmHg between hepatic venous pressure gradient and wedge portosystemic gradient (range: 6–15 mmHg), indicating pressure gradients between the hepatic vein and RA. Neither had varices. Neither had venographic evidence of Budd-Chiari syndrome. One patient had hepatomegaly on imaging and sinusoidal congestion on pathology. The other patient demonstrated hepatomegaly and a cluster of nodules near the hepatic vein confluence on imaging; pathology demonstrated a fibrosing inflammatory process with predominance of plasma cells and eosinophils.
Adverse events
No mild or moderate adverse events occurred. One (2.6%) severe adverse event (SIR Class III, requiring marked escalation of care) occurred: a hepatic arterial injury from transjugular liver biopsy with hemorrhagic shock requiring massive transfusion protocol (Fig. 5). The injury, localized to branches of the middle hepatic artery, was successfully treated with coil embolization and the patient was discharged a week after the procedure. The injury was attributed to anterior angulation of the transjugular liver biopsy needle after anterolateral passes yielded insufficient tissue, with probable capsular transgression.
A 13-year-old girl with splenomegaly and transaminitis. a Anteroposterior (AP) projection fluoroscopic image demonstrates antero-inferior passage of the transjugular biopsy needle from the right hepatic vein. Prior anterolateral passes had yielded insufficient tissue. b Coronal CT angiography following hemodynamic deterioration demonstrates active extravasation anterior to the falciform ligament (arrow) and perihepatic hematoma (asterisks). c AP projection digital subtraction angiography confirms active extravasation (arrows). d AP projection digital subtraction angiography following coil embolization of two middle hepatic arteries demonstrates no further extravasation. Angiography of the replaced right hepatic artery (not shown) was unremarkable
Discussion
In adults, a portosystemic gradient of ≤4 mmHg is considered normal and ≥10 mmHg is associated with portal hypertensive sequela including the formation of varices [8]. Reducing the hepatic venous pressure gradient ≥20% from baseline or ≤12 mmHg decreases complications related to cirrhosis and increases survival [9]. Because of a lack of established pediatric guidelines, management approaches for portal hypertensive complications in children have been extrapolated from those for adults. Portal pressure nomograms in healthy children have yet to be determined. Hepatic venous pressure gradient remains a mainstay in the staging of adult cirrhosis [8]. While feasible and safe in children [10], further investigation is needed to define its clinical use and to determine optimal techniques for acquiring representative measures of the portosystemic gradient.
The results of this study reaffirm the technical feasibility and safety of hepatic venous pressure gradients measurement in children and highlight the importance of concurrent wedged venography. Given the high prevalence of HV-HV collaterals, reliance on pressure measurements without analysis of accompanying wedged venography may result in improper risk stratification and potentially inappropriate management. Wedged venography should be incorporated to document the presence or absence of HV-HV collaterals in addition to confirming appropriate balloon-to-vessel wall apposition. Hepatic venous pressure gradient obtained in the presence of HV-HV collaterals should be qualified as potentially misrepresentative surrogates of a potentially elevated portosystemic gradient.
Zipprich and colleagues [11] compared the reproducibility and reliability of balloon versus straight catheter measurement of hepatic venous pressure gradients in a series of adults, concluding superiority of balloon occlusion. A prior study using a straight catheter to measure wedge hepatic venous pressures at multiple positions demonstrated considerable variability, a phenomenon attributed to heterogeneous sinusoidal involvement by liver disease as well as intrahepatic shunts [12]. Zipprich and colleagues [11] theorized that the more accurate measurements of the balloon occlusion technique result from greater territory coverage and minimization of hemodynamic variations. The result of our study may suggest otherwise. Optimal techniques may be instead determined by the anatomy encountered on venography. Additionally, suspicion for HV-HV collaterals may be influenced by patient population and, specifically, patient age. Pediatric patients with transplanted livers were not included in this study considering the finding of collaterals may be more frequent in pediatric native livers compared to adult donors as well as the surgical disruption of any intersegmental collaterals and accessory hepatic drainage inherent to reduced graft transplantation in the pediatric population.
Additional techniques may also be considered to acquire more representative hepatic venous pressure gradient measurements. Further study may include comparison between balloon and straight catheter measurement of pressures, particularly if a straight catheter position distal to visualized collaterals may be achieved. In the experience of Miraglia and colleagues [3], 7 of 20 pediatric patients undergoing hepatic venous pressure gradient measurement exhibited HV-HV collaterals, all 7 of whom had biliary atresia. They described efforts to catheterize and occlude beyond the point of collateralization, which were successful in 4 of 7 patients, but did not provide details on how catheter position affected pressure measurements. When reliable hepatic venous pressure gradients cannot be acquired, direct portal pressure measurements via transhepatic or trans-splenic catheterization may be considered.
The limitations of this study include a lack of direct portal pressure measurements in all patients for comparison to the observed hepatic venous pressure gradient. Additionally, depressed hepatic venous pressure gradient measurements were confounded by a high prevalence in extrahepatic portal vein occlusion, a condition of pre-hepatic portal hypertension, which itself would be expected to result in discordance of hepatic venous pressure gradient and portosystemic gradient. Indeed, calculations excluding patients with extrahepatic portal vein occlusion showed that hepatic venous pressure gradient measurements were generally lower in the presence of HV-HV collaterals, but the comparison no longer reached statistical significance. While this is acknowledged, it is worth noting that extrahepatic portal vein occlusion was absent in the four patients with HV-HV collaterals who went on to have direct portosystemic gradients significantly elevated above prior wedge portosystemic gradients.
Regardless of HV-HV collaterals, the utility of wedged pressures in the case of extrahepatic portal vein occlusion remains dubious and these are no longer routinely acquired in patients with known extrahepatic portal vein occlusion at the authors’ institution. If not previously documented, however, extrahepatic portal vein occlusion should be considered when HV-HV collaterals are observed in a pediatric patient with portal hypertensive sequela.
Conclusion
HV-HV collateralization is frequently encountered in children undergoing wedged portal venography and leads to misrepresentative hepatic venous pressure gradients. All patients undergoing HPVG measurement should have wedged venography to identify HV-HV collaterals and to qualify measured pressures. Additional techniques to obtain representative pressures in the presence of HV-HV collaterals warrant further investigation.
References
Shneider BL, Bosch J, de Franchis R et al (2012) Portal hypertension in children: expert pediatric opinion on the report of the Baveno v consensus workshop on methodology of diagnosis and therapy in portal hypertension. Pediatr Transplant 16:426–437
Gauthier F (2005) Recent concepts regarding extra-hepatic portal hypertension. Semin Pediatr Surg 14:216–225
Miraglia R, Luca A, Maruzzelli L et al (2010) Measurement of hepatic vein pressure gradient in children with chronic liver diseases. J Hepatol 53:624–629
Groszmann RJ, Wongcharatrawee S (2004) The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology 39:280–282
Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC (2009) The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol 6:573–582
Vandenbroucke JP, von Elm E, Altman DG et al (2007) Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Ann Intern Med 12:1500–1524
Khalilzadeh O, Baerlocher MO, Shyn PB et al (2017) Proposal of a new adverse event classification by the society of interventional radiology standards of practice committee. J Vasc Interv Radiol 28:1432–1437.e3
Merkel C, Montagnese S (2011) Hepatic venous pressure gradient measurement in clinical hepatology. Dig Liver Dis 43:762–767
Thalheimer U, Mela M, Patch D, Burroughs AK (2004) Targeting portal pressure measurements: a critical reappraisal. Hepatology 39:286–290
Woolfson J, John P, Kamath B et al (2013) Measurement of hepatic venous pressure gradient is feasible and safe in children. J Pediatr Gastroenterol Nutr 57:634–637
Zipprich A, Winkler M, Seufferlein T, Dollinger MM (2010) Comparison of balloon vs. straight catheter for the measurement of portal hypertension. Aliment Pharm Ther 32:1351–1356
Keiding S, Vilstrup H (2002) Intrahepatic heterogeneity of hepatic venous pressure gradient in human cirrhosis. Scand J Gastroenterol 37:1344
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Monroe, E.J., Michalsky, W.S., Koo, K.S.H. et al. Intrahepatic veno-venous collateralization and misrepresentative hepatic venous pressure gradients in children. Pediatr Radiol 50, 1579–1586 (2020). https://doi.org/10.1007/s00247-020-04751-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-020-04751-9