Abstract
Background
In the medicolegal literature, focal concavities or notching of the corpus callosum has been thought to be associated with fetal alcohol spectrum disorders. Recent work suggests corpus callosum notching is a dynamic and normal anatomical feature, although it has not yet been defined in early life or infancy.
Objective
Our purpose was to characterize the dorsal contour of the corpus callosum during the first 2 years of life by defining the prevalence, onset and trajectory of notching on midsagittal T1-weighted images.
Materials and methods
We reviewed retrospectively 1,157 consecutive patients between birth and 2 years of age. Corpus callosum morphology was evaluated and described. A notch was defined as a dorsal concavity of at least 1 mm in depth along the dorsal surface of the corpus callosum. Patient age as well as notch depth, location, number and presence of the pericallosal artery in the notch were noted.
Results
Two hundred thirty-three notches were identified in 549 patients: 36 anterior, 194 posterior and 3 patients with undulations. A statistically significant (R2=0.53, Beta=0.021, P=0.002) positive correlation between posterior notch prevalence and age in months was noted. A positive correlation between age and depth of the posterior notch was also statistically significant (r=0.32, n=179, P≤0.001). A trend for increased anterior notch prevalence with age was identified with significant correlation between visualized pericallosal artery indentation and anterior notching (r=0.20, n=138, P=0.016). Sub-analysis of the first month of life showed corpus callosum notching was not present.
Conclusion
The presence of posterior notching increased significantly with age and was more frequent than that of anterior notching. Corpus callosum notching was absent in the first week of life, building on prior studies suggesting corpus callosum notching is acquired. This study provides baseline data on normative corpus callosum notching trajectories by age group during early life, a helpful correlate when associating corpus callosum morphology with disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The corpus callosum is the largest white matter tract in the brain and the dominant commissure, facilitating transmission and synthesis of information between the two cerebral hemispheres. Approximately 200 million fibers within the corpus callosum interconnect all the major subdivisions of the cerebral cortex, the majority of which are high-level associative areas [1,2,3]. Callosal neuronal networks allow for interhemispheric integration of sensory, motor and high-order cognitive information such as language and abstract reasoning [4]. From anterior to posterior, the corpus callosum is defined by divisions: rostrum, genu, body/trunk, isthmus and splenium. Pericallosal vessels that supply the corpus callosum arise from both the anterior and posterior circulation, with large diameter arteries in contact with the corpus callosum in some locations [5, 6].
Corpus callosum morphology evolves with changes in midsagittal size, thickness, structural integrity and myelination over a life span [1, 3, 7,8,9,10,11,12,13,14,15]. While the number of corpus callosum fibers is determined around the time of birth, corpus callosum volume demonstrates alternating phases of expansion and shrinkage depending on varying degrees of axonal myelination, redirection and pruning [3]. On magnetic resonance imaging (MRI), corpus callosum signal intensity is associated with myelination, while thickness reflects the number of fibers and associated myelin volume [16, 17].
Corpus callosum thickness can serve as an important developmental feature with thinning suggestive of underlying disease [17]. Abnormal development of the corpus callosum has been implicated in adverse motor outcomes [18], impaired numerical processing [19], poor spatial integration [20], attention deficit/hyperactivity disorder [21], autism spectrum disorder [22], schizophrenia [23, 24], fetal alcohol spectrum disorders [25,26,27,28] and the pathogenesis of certain forms of epilepsy [1, 29,30,31]. Focal thinning or concavity of the corpus callosum, what we refer to as notching, has been implicated as a diagnostic feature of fetal alcohol spectrum disorders in the medicolegal literature [32]. A 1995 article by Riley et al. [25] remains highly cited in medicolegal literature for establishing focal abnormalities of the corpus callosum as part of the fetal alcohol spectrum [25, 32]. Subsequent literature suggests focal thinning or concavity of the corpus callosum, which we refer to as notching, to be an indicator of brain damage from fetal alcohol exposure [32, 33]. As a result of this literature and the clarity and ease of corpus callosum imaging on MRI, isolated concavity or notching on a defendant’s corpus callosum is used in the U.S. court system when trying to introduce fetal alcohol spectrum disorders as part of a legal defense strategy [32].
Given the dynamic growth patterns of normal corpus callosum development, we sought to build upon recent work on corpus callosum notching in a normal population [34] by examining the prevalence of these notches in a large cohort within the first 2 years of life. Previous studies by Krause et al. [34] analyzed corpus callosum thickness and notching using the same methodology as employed here and included a broad age distribution, but infants were underrepresented in their sample. The objective of our study was to investigate notching prevalence during the first years of life to gain insight into the developmental trajectory of notching and to determine if notching is a congenital or acquired finding.
Materials and methods
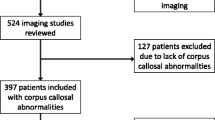
Institutional review board approval was obtained with a waiver of patient consent. Potential subjects for this retrospective cohort study were obtained from a search of the PACS imaging archive for all MRI examinations of the brain performed at our institution, a major tertiary care university medical center. The PACS system was queried for all consecutive brain MRI examinations on patients up to 2 years of age between Jan. 1, 2014, and Dec. 31, 2016, containing the keywords “normal” or “no acute process.” Patients were included if they contained diagnostic-quality midline sagittal T1-weighted images without cerebral pathology. Exclusion criteria included but were not limited to cerebral malformation, developmental abnormalities, hydrocephalus, intracranial hemorrhage, infarction, encephalomalacia, demyelinating disease, trauma, malignancy, surgery, and pre- and perinatal hypoxic ischemic injury.
All MRIs were performed using 1.5-T or 3-T machines. T1-weighted sagittal slices were obtained with 1- to 4-mm section thickness. For each radiographically normal patient, the morphology of the corpus callosum was evaluated using a previously described protocol [34]. The midsagittal dorsal contour of the corpus callosum was inspected by a team of neuroradiologists and neurosurgeons for any focal concavity or notch, and all notches were then reviewed by the senior author (J.M.P. with 17 years of experience in neuroradiology). A notch was defined as a focal depression or concavity measuring greater than 1 mm in depth from a tangential line to the dorsal surface of the corpus callosum (Fig. 1). In addition to depth, each notch was categorized as having either an anterior or posterior location relative to the mid-body of the corpus callosum (Fig. 2). Each notch was reviewed for a pericallosal artery flow void contacting the nadir of the notch (Fig. 3). A corpus callosum was designated normal if the midsagittal dorsal surface was flat or convex and contained no notches. A corpus callosum was considered undulating if there were more than two notches, of which the deepest anteriorly and posteriorly were measured.
Statistical analysis was performed to determine associations between age, notch presence, depth and an associated artery in the notch. MRI examinations were sorted by patient age at the time of imaging and grouped into 2-month increments. Notch prevalence for each age group was obtained by dividing the number of examinations with notching by the total number of imaging exams in the age group. Anterior and posterior notching data were analyzed both cumulatively and independently using the 2-month interval age group as the independent variable in linear regression models. Pearson correlation analysis was used to determine whether the depth of a posterior notch changed with time. Spearman’s Rho was used for anterior notch depth changes with time. Anterior and posterior notch depth were analyzed separately. Pearson correlation was considered significant at the 0.01 level (2-tailed). Spearman’s Rho test was used to determine if the presence of a flow void along the dorsal contour of the corpus callosum significantly correlated with the presence of an anterior notch. Sub-analysis of notch prevalence within the youngest population grouped into 1-week increments over the first month of life was additionally performed. Statistical analysis was performed using SPSS Statistics, Version 24 (IBM, Armonk, NY). P-values <0.05 were considered significant.
Results
MRI examinations from 1,157 patients were reviewed. Five hundred forty-nine patients met the inclusion criteria and were classified. Two hundred thirty-three notches were identified in those 549 patients: 36 anterior, 194 posterior and 3 patients with undulations. The prevalence of anterior, posterior and undulating notches was 6.6%, 35.3% and 0.5%, respectively (Table 1). Overall notch prevalence for the entire population was 42.4%.
Both anterior and posterior notch prevalence increased with age. The linear regression model found a statistically significant association between posterior notch prevalence and age (R2=0.53, Beta=0.021, P=0.002). There was no statistically significant association between anterior notch prevalence and age despite a trend of increased anterior notch prevalence over time (Fig. 4).
Posterior notch depth significantly increased with patient age in our study population (Fig. 5). A statistically significant positive correlation between patient age group and posterior notch depth was identified (r=0.32, n=179, P≤0.001). There was no statistically significant correlation between patient age and anterior notch depth (r=0.008, n=14, P=0.98).
Anterior notches were associated with the presence of the pericallosal artery in the notch.
The pericallosal artery flow void contacted the nadir of the anterior notch in 29 of the 36 cases. Using the Spearman’s Rho correlation test, we found there was a significant correlation between visualization of the artery and the presence of an anterior notch (r=0.20, P=0.016, n=138).
MRI examinations of the 62 patients younger than 1 month were evaluated by age groups for the first, second, third and fourth weeks of life and were comprised of 19, 24, 9 and 10 patients, respectively. No notching was identified within the 1st or 4th week of life. Highest posterior notch prevalence was 11% (n=1) during the third week of life. Anterior and posterior notches were both present in the second week of life, seen in 8% (n=2) and 4% (n=1), respectively. There was no undulating morphology present during the first month of life (Table 2).
Discussion
A thorough understanding of the spectrum of normal anatomical morphology informs neuroimaging diagnosis. Whereas generalized thinning of the corpus callosum may indicate underlying disease [17], alteration in focal callosal thickness or notching may represent normal anatomical variability in the course of dynamic structural development. To our knowledge, this is the first study describing the normal MRI appearance of the dorsal surface of the midline corpus callosum during early life. Corpus callosum notches were a common finding during the first two years of life, appearing in 42% of MRI studies in this age group. However, no notches were present in scans of children performed during the first week of life. The prevalence of posterior notching and posterior notch depth both significantly increased with age during the first 2 years of life (Figs. 3 and 4). The prevalence of anterior notching increased during early life without reaching statistical significance. However, there was a significant positive correlation with the presence of the pericallosal artery in the anterior notch.
Our study complements a study by Krause et al. [34] that described the prevalence of callosal notching throughout the life span but had a relatively small number of subjects in the group ages 0–2 years. In a retrospective review of 1,639 MRI examinations in patients ranging in age from 0 to 89 years, Krause et al. [34] demonstrated a statistically significant correlation in both the prevalence and depth of anterior notching as age increased. Conversely, they found a significant decrease in the prevalence of posterior notching as age increased. When they analyzed their 0- to 1-year-old population, Krause et al. [34] found that anterior and posterior corpus callosum notch prevalence reached 12% and 21%, respectively. However, the significance of this finding was limited by a small sample size [34]. Our data, combined with the Krause et al. [34] study, suggest that posterior notch prevalence rapidly increases over the first 2 years of life, peaks at 10–15 years of age and then gradually decreases in prevalence throughout the rest of life. The significant increases in posterior notch prevalence during the 4- to 6-month age group (Fig. 4) are likely a manifestation of disproportionate increases in the thickness of the splenium also described by morphological studies [17, 35, 36]. Posterior notch prevalence plateauing at 10–15 years of age is also supported by prior morphological studies that showed a differential age-related growth of the corpus callosum during embryological development until a critical period around 9–12 years old [3, 37, 38].
While the prevalence of the posterior notch is dynamic and likely the result of early thickening of the splenium followed by stabilization [36], the anterior notch prevalence appears strongly associated with the pericallosal artery contacting the dorsal surface of the corpus callosum and extending into the notch. In our study, the pericallosal artery was seen in 29 of the 36 (81%) anterior notches. Krause et al. [34] found the pericallosal artery in 490 of 823 (60%) anterior notches. While there was a trend in our data for anterior notch prevalence to increase with age, it did not reach statistical significance. We believe this result is secondary to the relatively short time frame and small number of anterior notches in our study. Our data in combination with the Krause et al. [34] study suggests that anterior notches are slowly accumulated over the human lifetime and many (60–80%) are associated with the pericallosal artery. It may be that progressive increases in arterial tortuosity from the neonatal period through infancy and into adulthood contribute to the increased prevalence of anterior notches with advancing age [39] in a mechanism similar to development of brainstem deformity secondary to dolichoectatic vertebral arteries.
One of the aims of the study was to determine if notches were congenital or acquired. Our study demonstrated mean anterior and posterior notch prevalence across all age groups from birth to 2 years of age of 6% and 35%, respectively. If notches were a congenital manifestation reflecting the in utero environment, then notches should have a constant prevalence in the population and be seen in the earliest age groups. Our study shows that posterior notches are highly dynamic with a significant increase in prevalence with age while anterior notches are slowly accumulated over time. Of note, we could not identify any notches in the first week of life. These data suggest both anterior and posterior notches are acquired and represent manifestations of normal development and growth.
Fetal alcohol spectrum disorders have been associated with anomalies of the corpus callosum for decades [25, 27]. In the medicolegal realm, the diagnosis of fetal alcohol spectrum disorders has been considered on the basis of a solitary morphological feature: focal concavity (notching) of the corpus callosum [32]. In an era where the U.S. judicial system may accept brain imaging as evidence of injury or preexisting conditions [32], the importance of differentiating spectrums of normal morphology from pathology is paramount. Our study does not directly address the prevalence of notching in fetal alcohol spectrum disorders, but the overall prevalence of notches in our population is much higher than the expected prevalence of fetal alcohol spectrum disorders, which is reported to have a prevalence of 2–5% of the U.S. population [40, 41]. This discrepancy between the reported fetal alcohol spectrum disorders prevalence and notch prevalence, along with the dynamic changes of notch prevalence associated with increasing age, makes it unlikely for notching to be secondary to fetal alcohol spectrum disorders.
Intrinsic limitations of our study relate to the narrow focus of examining the dorsal surface of the corpus callosum in the cohort ages 0–2 years. Additionally, an inherent selection bias may be present by the retrospective nature of including studies from a hospital database. We did not collect or analyze data on the gender of the patients in relation to the prevalence of notches. Some previous studies revealed gender-specific differences in callosal morphology, but the influence of gender has been inconsistent [3, 35,36,37]. However, because there was no difference between patient gender and corpus callosum notching (P=0.88) in the Krause et al. [34] study addressing corpus callosum dorsal morphology, we did not collect gender information in the current study population. The study is also limited by the relatively small sample sizes in the first month of life; our youngest patients were often excluded due to motion artifacts, which obscured visualization of the corpus callosum. We did not collect data on interobserver reliability. However, all notches were reviewed by the senior author, a neuroradiologist with 17 years of experience. Another limitation was our reliance on the appearance of flow voids on T1-weighted imaging to infer the presence of the pericallosal artery in the anterior notch rather than using dedicated angiographic imaging; however, the relative lack of angiographic studies in our patient group necessitated this. Furthermore, our study relied on radiographically normal imaging in a group of patients sent for clinical imaging rather than normal healthy volunteers. As a purely radiologic study, associations between notches and developmental, intellectual or behavioral milestones were not correlated. Functional deficits of the corpus callosum may not always be associated with sagittal midline morphology changes and would potentially go unrecognized in our study [42, 43].
Conclusion
This manuscript provides the first comprehensive characterization of the dorsal contour of the corpus callosum in the group ages 0–2 years. We found posterior notching was more frequent than anterior notching and that posterior notch prevalence significantly increased with age during the first two years of life. Progressive tortuosity of physiological pericallosal artery development may play a role in the development of anterior notching while age-related changes in myelination and axon caliber may account for changes observed in posterior notch development. Importantly, corpus callosum notching was absent in the first week of life, building on prior studies suggesting that corpus callosum notching is acquired. These data will be helpful for comparison to corpus callosum morphology associated with abnormal development during early childhood.
References
Meissner TW, Friedrich P, Ocklenburg S et al (2017) Tracking the functional development of the corpus callosum in children using behavioral and evoked potential interhemispheric transfer times. Dev Neuropsychol 42:172–186
Lenroot RK, Giedd JN (2006) Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev 30:718–729
Luders E, Thompson PM, Toga AW (2010) The development of the corpus callosum in the healthy human brain. J Neurosci 30:10985–10990
Edwards TJ, Sherr EH, Barkovich AJ, Richards LJ (2014) Clinical, genetic and imaging findings identify new causes for corpus callosum development syndromes. Brain 137(Pt 6):1579–1613
Ture U, Yasargil MG, Krisht AF (1996) The arteries of the corpus callosum: a microsurgical anatomic study. Neurosurgery 39:1075–1084
Kahilogullari G, Comert A, Ozdemir M et al (2013) Arterial vascularization patterns of the splenium: an anatomical study. Clin Anat 26:675–681
Sakai T, Mikami A, Suzuki J et al (2017) Developmental trajectory of the corpus callosum from infancy to the juvenile stage: comparative MRI between chimpanzees and humans. PLoS One 12:e0179624
Hasan KM, Kamali A, Iftikhar A et al (2009) Diffusion tensor tractography quantification of the human corpus callosum fiber pathways across the lifespan. Brain Res 1249:91–100
Hasan KM, Kamali A, Kramer LA et al (2008) Diffusion tensor quantification of the human midsagittal corpus callosum subdivisions across the lifespan. Brain Res 1227:52–67
Imperati D, Colcombe S, Kelly C et al (2011) Differential development of human brain white matter tracts. PLoS One 6:e23437
Lebel C, Beaulieu C (2011) Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci 31:10937–10947
McLaughlin NC, Paul RH, Grieve SM et al (2007) Diffusion tensor imaging of the corpus callosum: a cross-sectional study across the lifespan. Int J Dev Neurosci 25:215–221
Pujol J, Vendrell P, Junque C et al (1993) When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann Neurol 34:71–75
Ren T, Anderson A, Shen WB et al (2006) Imaging, anatomical, and molecular analysis of callosal formation in the developing human fetal brain. Anat Rec A Discov Mol Cell Evol Biol 288:191–204
Rakic P, Yakovlev PI (1968) Development of the corpus callosum and cavum septi in man. J Comp Neurol 132:45–72
Luders E, Narr KL, Bilder RM et al (2007) Positive correlations between corpus callosum thickness and intelligence. Neuroimage 37:1457–1464
Andronikou S, Pillay T, Gabuza L et al (2015) Corpus callosum thickness in children: an MR pattern-recognition approach on the midsagittal image. Pediatr Radiol 45:258–272
Malavolti AM, Chau V, Brown-Lum M et al (2017) Association between corpus callosum development on magnetic resonance imaging and diffusion tensor imaging, and neurodevelopmental outcome in neonates born very preterm. Dev Med Child Neurol 59:433–440
Cantlon JF, Davis SW, Libertus ME et al (2011) Inter-parietal white matter development predicts numerical performance in young children. Learn Individ Differ 21:672–680
Fornari E, Knyazeva MG, Meuli R, Maeder P (2007) Myelination shapes functional activity in the developing brain. Neuroimage 38:511–518
Hynd GW, Semrud-Clikeman M, Lorys AR et al (1991) Corpus callosum morphology in attention deficit-hyperactivity disorder: morphometric analysis of MRI. J Learn Disabil 24:141–146
Belmonte M, Egaas B, Townsend J, Courchesne E (1995) NMR intensity of corpus callosum differs with age but not with diagnosis of autism. Neuroreport 6:1253–1256
David AS (1993) Callosal transfer in schizophrenia: too much or too little? J Abnorm Psychol 102:573–579
Raine A, Harrison GN, Reynolds GP et al (1990) Structural and functional characteristics of the corpus callosum in schizophrenics, psychiatric controls, and normal controls. A magnetic resonance imaging and neuropsychological evaluation. Arch Gen Psychiatry 47:1060–1064
Riley EP, Mattson SN, Sowell ER et al (1995) Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res 19:1198–1202
Donald KA, Eastman E, Howells FM et al (2015) Neuroimaging effects of prenatal alcohol exposure on the developing human brain: a magnetic resonance imaging review. Acta Neuropsychiatr 27:251–269
Roebuck TM, Mattson SN, Riley EP (1998) A review of the neuroanatomical findings in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res 22:339–344
Hoyme HE, Kalberg WO, Elliott AJ et al (2016) Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics:138. https://doi.org/10.1542/peds.2015-4256
Bocci T, Caleo M, Giorli E et al (2011) Transcallosal inhibition dampens neural responses to high contrast stimuli in human visual cortex. Neuroscience 187:43–51
Bocci T, Caleo M, Restani L et al (2016) Altered recovery from inhibitory repetitive transcranial magnetic stimulation (rTMS) in subjects with photosensitive epilepsy. Clin Neurophysiol 127:3353–3361
Unterberger I, Bauer R, Walser G, Bauer G (2016) Corpus callosum and epilepsies. Seizure 37:55–60
Mardia K, Bookstein F, Kent J (2013) Alcohol, babies and the death penalty: saving lives by analysing the shape of the brain. Significance 10:12–16
Bookstein FL, Connor PD, Huggins JE et al (2007) Many infants prenatally exposed to high levels of alcohol show one particular anomaly of the corpus callosum. Alcohol Clin Exp Res 31:868–879
Krause KL, Howard D, Pettersson DR et al (2019) Defining the normal dorsal contour of the corpus callosum with time. AJNR Am J Neuroradiol 40:86–91
Garel C, Cont I, Alberti C et al (2011) Biometry of the corpus callosum in children: MR imaging reference data. AJNR Am J Neuroradiol 32:1436–1443
Westerhausen R, Fjell AM, Krogsrud SK et al (2016) Selective increase in posterior corpus callosum thickness between the age of 4 and 11 years. Neuroimage 139:17–25
Giedd JN, Blumenthal J, Jeffries NO et al (1999) Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuro-Psychopharmacol Biol Psychiatry 23:571–588
Keshavan MS, Diwadkar VA, DeBellis M et al (2002) Development of the corpus callosum in childhood, adolescence and early adulthood. Life Sci 70:1909–1922
Bullitt E, Zeng D, Mortamet B et al (2010) The effects of healthy aging on intracerebral blood vessels visualized by magnetic resonance angiography. Neurobiol Aging 31:290–300
May PA, Baete A, Russo J et al (2014) Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics 134:855–866
May PA, Gossage JP, Kalberg WO et al (2009) Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev 15:176–192
Fenlon LR, Richards LJ (2015) Contralateral targeting of the corpus callosum in normal and pathological brain function. Trends Neurosci 38:264–272
Tovar-Moll F, Monteiro M, Andrade J et al (2014) Structural and functional brain rewiring clarifies preserved interhemispheric transfer in humans born without the corpus callosum. Proc Natl Acad Sci U S A 111:7843–7848
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Simpson, L.N., Schneble, E.J., Griffin, E.D. et al. Morphological changes of the dorsal contour of the corpus callosum during the first two years of life. Pediatr Radiol 50, 543–549 (2020). https://doi.org/10.1007/s00247-019-04585-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-019-04585-0