Abstract
Background
Image-guided percutaneous microwave ablation has been used to treat adult osteoid osteomas but has not been thoroughly evaluated in the pediatric population.
Objective
To evaluate the technical feasibility and clinical efficacy of microwave ablation to treat osteoid osteomas in pediatric patients.
Materials and methods
The electronic medical record and imaging archive were reviewed for 24 consecutive patients who had undergone microwave ablation of osteoid osteomas between January 1, 2015, and May 31, 2018, at a single tertiary care pediatric hospital. All patients were diagnosed by clinical and imaging criteria, and referred by a pediatric orthopedic surgeon after failing conservative management with pain medication. The average age of the patients was 13.3 years (range: 3–18 years), and the average size of the osteoid osteoma nidus was 8.8 mm (range: 5–22 mm). Technical success was defined as placement of the microwave antenna at the distal margin of the lesion nidus and achievement of the target ablation temperature. Clinical findings were assessed pre- and post-ablation and clinical success was defined as complete relief of pain without pain medication at 1-month follow-up. The number and severity of complications were also documented.
Results
Clinical success was achieved in 100% of patients (24/24), with all reporting complete cessation of pain medication use 1 week after treatment and 0/10 pain at 1 month. There were 4 minor complications (17%) including access site numbness and a minor soft-tissue infection. There were no major complications.
Conclusion
Microwave ablation is a technically feasible and clinically effective treatment for pediatric osteoid osteomas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoid osteomas are benign but often painful neoplasms. They are most commonly found in the long bones of the lower extremities but can occur throughout the appendicular and axial skeleton [1]. As the third most-common benign skeletal tumor, these often small (<1.5 cm) solitary tumors have a characteristic history of severe nocturnal pain relieved by nonsteroidal anti-inflammatory drugs (NSAIDS) [1,2,3,4]. Pain from the lesion can sometimes be debilitating and result in functional limitations. Other reported complications include limb-length discrepancies, scoliosis and contractures particularly for juxta-articular lesions [5, 6].
Osteoid osteomas can be treated with operative excision or curettage. Percutaneous ablation is now the standard of care and validated as a safe, highly effective and less invasive treatment option [7]. Radiofrequency ablation (RFA) is the most widely used and studied modality for percutaneous treatment of osteoid osteomas [4, 8], but microwave ablation is increasingly being utilized. Treatment of osteoid osteomas with microwave ablation provides several theoretic and practical advantages over RFA, and has been shown to be effective in adults [9,10,11,12]. However, there are few reports evaluating this technique in children. The purpose of this study was to retrospectively evaluate the technical feasibility and clinical efficacy of microwave ablation in the treatment of pediatric osteoid osteomas. This study hypothesizes that microwave ablation is a safe and effective modality for the treatment of osteoid osteomas in the pediatric population.
Materials and methods
Institutional review board approval was obtained for this retrospective study with waiver of informed consent. The electronic medical records and archived images were reviewed for all osteoid osteomas treated with microwave ablation between January 1, 2015, and May 31, 2018, at our single-center tertiary care children’s hospital. All patients were referred for treatment by the pediatric orthopedic surgery service after having failed conservative management with pain medication and were separately evaluated in a pediatric interventional radiology clinic. Inclusion criteria included all patients with osteoid osteomas diagnosed by clinical presentation and imaging characteristics [13]. Patients with lesions abutting articular surfaces and lesions not amenable to safe percutaneous access were excluded from ablative treatment. Imaging and demographic data were collected and are summarized (Table 1).
Procedure protocol
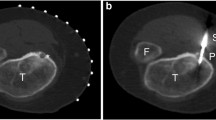
Informed consent was obtained from the parents/guardians before the induction of general anesthesia under the supervision of a pediatric anesthesiologist. All microwave ablations were performed under fluoroscopic guidance with cone-beam computed tomography (Philips Healthcare, Andover, MD). A cone-beam CT was performed to determine persistence of the lesion as well as for planning the needle trajectory (Fig. 1). Navigational overlay with Xper Guide (Philips Healthcare, Andover, MD) was then used for real-time needle guidance and probe localization (Fig. 2) with subsequent cone-beam CT for final confirmation of probe position before ablation.
Real-time needle guidance (same patient as in Fig. 1). Entry (a) and progress (b) views with navigation overlay demonstrate the access needle positioned at the superficial margin of the nidus
Procedures were performed by three board-certified interventional radiologists (E.J.M., 3 years of post-fellowship experience; K.S.H.K., 3 years of post-fellowship experience, and G.M.S., with 4 years of post-fellowship experience) using the Neuwave Certus system (Ethicon US, LLC, Somerville, NJ). In all cases, an 11-gauge Arrow OnControl bone access cortical drill (Teleflex, Inc., Morrisville, NC) was used to traverse the bone cortex, followed by coaxial advancement of a 13-gauge coring needle into the lesion nidus using intermittent imaging guidance. The coring needle was then removed and the 17-gauge Neuwave PR antenna (Ethicon US, LLC, Somerville, NJ) was advanced just beyond the distal edge of the lesion to cover the entire nidus within the antenna’s ablation zone (Fig. 3). A minimum of three ablation cycles were performed at a power setting of 30 watts, with a target temperature of 90 degrees Celsius and a 30-s cooling period between cycles. Each ablation cycle lasted 30 s. If the target temperature was not reached after three ablation cycles, additional ablation cycles were performed to reach the target temperature. Ultrasound-guided hydrodissection was performed at the discretion of the operator when the ablation zone or microwave probe was in close proximity to neurovascular structures.
Microwave ablation probe is placed through the nidus (bracket) with the tip (arrowhead) just past the deep margin to optimize the geometry of the ablation zone (same patient as in Fig. 1)
After the procedure, patients recovered in a dedicated post-procedural unit where cardiovascular status was monitored for 3–6 h. All patients were discharged home on the same day with activity restrictions to include no heavy lifting and, in the case of lower extremity lesions, no running for a week.
Outcome measures
Follow-up data were obtained for all patients using a standardized telephone questionnaire, as well as interventional radiology and orthopedic clinical follow-up notes. Procedural information collected included total and fluoroscopic procedural time, lesion location, lesion size and peak temperature of ablation cycles. Clinical data included pain at 1 week and 1 month post ablation, use of pain medication after ablation and post-procedure complications. Pain was assessed via an ordinal 11-point pain rating scale where 0 represents no pain and 10 represents the worst possible level of pain.
Technical success was defined as localizing the microwave antenna at the distal margin of the lesion nidus and reaching the target ablation temperature of 90 degrees Celsius. Clinical success was defined as complete relief of pain without pain medication at 1-month follow-up. The number and severity of complications were recorded as defined by the Society of Interventional Radiology (SIR) guidelines [14].
Results
A total of 24 patients were treated, with outcomes summarized in Table 2. The average age was 13.3 years (range: 3–18 years). The average size of the lesions was 8.8 mm (range: 5–22 mm). The majority of patients (21/24) were treated with a single ablation antenna. Due to the ellipsoid morphology and angle of approach required to pass the bone cortex, three patients required placement of two ablation antennas to create an ablation zone that covered the entire lesion nidus. Two patients had previously failed RFA treatment of their osteoid osteoma and one had previously failed surgical curettage. Complete relief of pain was achieved in 21/24 (88%) of patients at 1 week, and 24/24 (100%) at 1 month. Before ablation, 23/24 (96%) of patients used an NSAID for pain control, while 1/24 (4%) used acetaminophen. The average duration of symptoms before ablation was 11 months (range: 1–37 months, standard deviation [SD]: 7.9). Post-ablation average duration of pain medication use was 1.1 days (range: 0–7 days, SD: 1.8 days), and by 1 week post-procedure all patients had ceased medical therapy. Mean follow-up time was 5 months (range: 1–14 months, SD: 4.9 days). Five patients (21%) received clinical follow-up beyond 12 months. Hydrodissection to protect lower extremity neurovascular structures was performed in 7/24 (29%) patients. As defined by SIR criteria, minor complications were seen in 4/24 (17%) patients [14]. These included three patients with local skin numbness in the region of the access site (Class A complication, two of which had spontaneously resolved at their 3-month follow-up), and one patient with a soft-tissue infection at the access site (Class B complication, resolved with antibiotic treatment). There were no major complications.
The average number of ablation cycles per antenna placed was 3.4 (range: 3–6, SD: 0.7), for a total average ablation time of 1.7 min (range: 1.5–3, SD: 0.4). Average procedure and fluoroscopy time was 94 min (range: 26–248 min, SD: 13.1), and 7.8 min (range: 1.6–21.5, SD: 4.6), respectively.
Discussion
Percutaneous thermal ablation is a well-established technique to treat pediatric osteoid osteomas [4, 8, 15, 16]. While RFA is the most widely used method of pediatric osteoid osteoma ablation, there is limited literature specifically evaluating the use of microwave ablation in this population [4, 8, 17]. The objective of this study was to confirm the technical feasibility and clinical efficacy of microwave ablation to treat osteoid osteomas in an exclusively pediatric population. Although this study was not designed to establish superiority of microwave ablation compared with other ablation techniques, the larger sample size and longer follow-up builds on established data demonstrating that microwave ablation is a safe and effective technique, and supports microwave ablation as an option to treat pediatric osteoid osteomas [10,11,12, 18].
RFA energy is delivered by resistive heating, which then spreads through the surrounding tissue via conduction. In contrast, microwave ablation creates heat by rapidly agitating water molecules within its field. The higher frequency of energy delivered in microwave ablation allows for superior tissue propagation and is less susceptible to the heat-sink effect [9]. In addition, the thermal properties of microwave ablation result in decreased tissue carbonization, which can limit power application at the periphery of the ablation zone [19]. These differences in energy dispersion are relevant when treating lesions in bone and lung, which have intrinsically high baseline impedance and have been associated with suboptimal outcomes with RFA [20, 21]. Compared with RFA, microwave ablation allows for faster tissue heating and can decrease the time necessary to reach target temperature. In the pediatric population, a reduced procedure time is particularly relevant due to concerns regarding cumulative anesthetic use [22, 23]. The radiative dispersion of microwave energy does not require grounding pads to establish conductive pathways used in RFA [9, 24,25,26]. This eliminates the potential for skin burns related to pad placement and suboptimal pad positioning encountered with smaller patients. At our institution, the use of RFA to treat osteoid osteoma was sometimes delayed by automatic generator shutoff during ablations, presumably related to high tissue impedance from bone charring. Due to these concerns, our institution has moved to perform bone ablations exclusively via microwave. A systematic review of the literature on RFA found an average ablation time of 6.8 min for treatment of osteoid osteoma [27]. Although the literature describing ablation time in microwave ablation for osteoid osteomas is more limited, our average ablation time of 1.7 min falls in the reported range of 1–3 min [10,11,12].
Due to the angle of approach and lesion morphology, three patients required placement of two ablation probes to achieve full coverage of their lesion. A single lesion in our series measured >1.5 cm, qualifying as an osteoblastoma. These larger lesions were otherwise treated in similar fashion, and outcomes were indistinguishable from the majority of lesions, which were treated with a single probe. Although not necessary for our procedures, microwave ablation probes can be positioned and phased to overlap fields and achieve more efficient, faster heating to treat larger lesions [28].
Microwave ablation has already been shown to be a safe and clinically efficacious treatment of osteoid osteomas in mixed age populations [10,11,12] but have been limited by a small sample size of pediatric patients. The outcomes of this study support that microwave ablation can be successfully used to treat osteoid osteomas in an exclusively pediatric population. The early clinical success rate of 100% in this series is comparable to similarly powered pediatric RFA and cryoablation series [8, 16, 17]. Based on symptoms, there were no instances of osteoid osteoma recurrence over an average follow-up interval of 5 months, which is consistent with findings in studies evaluating microwave ablation treatment of osteoid osteomas in adults [10,11,12]. There were no major complications in this study. The minor complication rate was 17%, within the range of 9.5–22% demonstrated by other studies describing ablative techniques for treatment of osteoid osteomas [8, 17, 18]. Our complications included a single superficial infection at the probe access site and three instances of subjective numbness around the access site, all of which had either resolved or reached subclinical severity at subsequent follow-up.
The primary limitation of this study is the retrospective nature and lack of a control group. A larger sample size would add additional power to the results. Although the majority of recurrences of osteoid osteomas following thermal ablation occur in the first 12 months, a recent series demonstrated recurrence of osteoid osteomas up to 1.4 years after initially successful RFA [27, 29]. Our mean follow-up time of 5 months is adequate to assess for initial treatment response but is inadequate to capture late recurrences.
Additionally, the majority of interventions were based on clinical and imaging diagnosis, and were not biopsy-proven osteoid osteomas. Intraprocedural biopsy was not performed due to the increased time and resources required. Although using clinical and imaging features alone is an accepted method of diagnosing osteoid osteoma, it is unclear whether intraoperative consultation with Pathology would have yielded a diagnosis of osteoid osteoma, as some studies have shown that other benign bone diseases may mimic osteoid osteoma [1, 12, 30]. Lastly, bone access in this series was achieved with a relatively large-gauge coring needle as opposed to the smaller Kirschner wires reported in many series. While all clinical benefits have been attributed to microwave ablation, the effects of ablation may have been additive with mechanical disruption and excision, similar to techniques shown to be effective as stand-alone treatment [31].
Conclusion
This study demonstrates that microwave ablation is a safe and efficacious technique for treating pediatric osteoid osteomas. The theoretical advantages of this technique require further study to determine non-inferiority to other established techniques and to evaluate long-term clinical response. Specific areas of future research could include determining the specific benefit of faster ablative techniques on procedural and anesthetic duration.
References
Boscainos PJ, Cousins GR, Kulshreshtha R et al (2013) Osteoid osteoma. Orthopedics 36:792–800
Zhang Y, Rosenberg AE (2017) Bone-forming tumors. Surg Pathol Clin 10:513–535
Hakim DN, Pelly T, Kulendran M, Caris JA (2015) Benign tumours of the bone: A review. J Bone Oncol 4:37–41
Natali GL, Paolantonio G, Fruhwirth R et al (2016) Paediatric musculoskeletal interventional radiology. Br J Radiol 89:20150369
Laurence N, Epelman M, Markowitz RI et al (2012) Osteoid osteomas: a pain in the night diagnosis. Pediatr Radiol 42:1490–1501
Ghanem I (2006) The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr 18:36–41
Miyazaki M, Arai Y, Myoui A et al (2016) Phase I/II multi-institutional study of percutaneous radiofrequency ablation for painful osteoid osteoma (JIVROSG-0704). Cardiovasc Intervent Radiol 39:1464–1470
Donkol RH, Al-Nammi A, Moghazi K (2008) Efficacy of percutaneous radiofrequency ablation of osteoid osteoma in children. Pediatr Radiol 38:180–185
Lubner MG, Brace CL, Hinshaw JL, Lee FT Jr (2010) Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 21:S192–S203
Prud'homme C, Nueffer J-P, Runge M et al (2017) Prospective pilot study of CT-guided microwave ablation in the treatment of osteoid osteomas. Skeletal Radiol 46:315–323
Kostrzewa M, Diezler P, Michaely H et al (2014) Microwave ablation of osteoid osteomas using dynamic MR imaging for early treatment assessment: preliminary experience. J Vasc Interv Radiol 25:106–111
Basile A, Failla G, Reforgiato A et al (2014) The use of microwaves ablation in the treatment of epiphyseal osteoid osteomas. Cardiovasc Intervent Radiol 37:737–742
Lee EH, Shafi M, Hui JH (2006) Osteoid osteoma: a current review. J Pediatr Orthop 26:695–700
Sacks D, McClenny TE, Cardella JF, Lewis CA (2003) Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 14(9 Pt 2):S199–S202
Brown SD, vanSonnenberg E (2007) Issues in imaging-guided tumor ablation in children versus adults. AJR Am J Roentgenol 189:626–632
Whitmore MJ, Hawkins CM, Prologo JD et al (2016) Cryoablation of osteoid osteoma in the pediatric and adolescent population. J Vasc Interv Radiol 27:232–237
Earhart J, Wellman D, Donaldson J et al (2013) Radiofrequency ablation in the treatment of osteoid osteoma: results and complications. Pediatr Radiol 43:814–819
Perry BC, Monroe EJ, McKay T et al (2017) Pediatric percutaneous osteoid osteoma ablation: cone-beam CT with fluoroscopic overlay versus conventional CT guidance. Cardiovasc Intervent Radiol 40:1593–1599
de Baere T, Deschamps F (2014) New tumor ablation techniques for cancer treatment (microwave, electroporation). Diagn Interv Imaging 95:677–682
Abbas G, Schuchert MJ, Pennathur A et al (2007) Ablative treatments for lung tumors: radiofrequency ablation, stereotactic radiosurgery, and microwave ablation. Thorac Surg Clin 17:261–271
Moser T, Buy X, Goyault G et al (2008) Image-guided ablation of bone tumors: thermal ablation. J Radiol 89:461–471
Montana MC, Evers AS (2017) Anesthetic neurotoxicity: new findings and future directions. J Pediatr 181:279–285
Ing C, DiMaggio C, Whitehouse A et al (2012) Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics 130:e476–e485
Schramm W, Yang D, Haemmerich D (2006) Contribution of direct heating, thermal conduction and perfusion during radiofrequency and microwave ablation. Conf Proc IEEE Eng Med Biol Soc 1:5013–5016
Wright AS, Sampson LA, Warner TF et al (2005) Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 236:132–139
Krokidis M, Ahmed I (2013) Overview of thermal ablation devices: Radiofrequency Ablation. In: Clark T, Sabharwal T (eds) Interventional radiology techniques in ablation, 1st edn. Springer-Verlag London, London, pp 5–11
Lanza E, Thouvenin Y, Viala P et al (2014) Osteoid osteoma treated by percutaneous thermal ablation: when do we fail? A systematic review and guidelines for future reporting. Cardiovasc Intervent Radiol 37:1530–1539
Trembly BS, Douple EB, Ryan TP, Hoopes PJ (1994) Effect of phase modulation on the temperature distribution of a microwave hyperthermia antenna array, in vivo. Int J Hyperth 10:691–705
Shields DW, Sohrabi S, Crane EO et al (2017) Radiofrequency ablation for osteoid osteoma - Recurrence rates and predictive factors. Surgeon 16:156–162
Iyer RS, Chapman T, Chew FS (2012) Pediatric bone imaging: diagnostic imaging of osteoid osteoma. AJR Am J Roentgenol 198:1039–1052
Towbin R, Kaye R, Meza MP et al (1995) Osteoid osteoma: percutaneous excision using a CT-guided coaxial technique. AJR Am J Roentgenol 164:945–949
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rinzler, E.S., Shivaram, G.M., Shaw, D.W. et al. Microwave ablation of osteoid osteoma: initial experience and efficacy. Pediatr Radiol 49, 566–570 (2019). https://doi.org/10.1007/s00247-018-4327-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-018-4327-1