Abstract
Background
Considering inherent limitations of transthoracic echocardiography, the diagnostic accuracy of cardiac CT in identifying coronary artery anatomy before arterial switch operation needs to be investigated with recently improved coronary artery visibility using electrocardiogram (ECG)-synchronized dual-source CT.
Objective
To compare diagnostic accuracy between cardiac CT using a dual-source scanner and transthoracic echocardiography in identifying coronary artery anatomy before arterial switch operation in newborns and young infants.
Materials and methods
The study included 101 infants (median age 4 days, range 0 days to 10 months; M:F=78:23) who underwent ECG-synchronized cardiac dual-source CT and transthoracic echocardiography before arterial switch operation between July 2011 and December 2016. We evaluated and classified coronary artery anatomy on cardiac CT and transthoracic echocardiography. With the surgical findings as the reference standard, we compared the diagnostic accuracy for identifying coronary artery anatomy between cardiac CT and transthoracic echocardiography.
Results
The most common coronary artery pattern was the usual pattern (left coronary artery from sinus 1 and right coronary artery from sinus 2; 64.4%, 65/101), followed by a single coronary artery from sinus 2 and a conal branch from sinus 1 (7.9%, 8/101), the inverted pattern (5.9%, 6/101), the right coronary artery and left anterior descending artery from sinus 1 and the left circumflex artery from sinus 2 (5.9%, 6/101), and others. In 96 infants with surgically proven coronary artery anatomy, the diagnostic accuracy of cardiac CT was significantly higher than that of transthoracic echocardiography (91.7%, 88/96 vs. 54.2%, 52/96; P<0.0001).
Conclusion
Diagnostic accuracy of cardiac CT is significantly higher than that of echocardiography in identifying coronary artery anatomy before arterial switch operation in newborns and young infants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary artery anatomy is essential for arterial switch operation because intact transfer of the coronary arteries without kinking, twisting and stretching is a prerequisite for successful surgical outcome [1,2,3]. Therefore, these complex coronary artery patterns have been classified to define high-risk patterns by using the Yacoub and Radley-Smith classification [4] that was subsequently modified by Planché et al. [5] and the Leiden classification [6]. Various surgical techniques of coronary artery transfer during arterial switch operation have been developed, chiefly for high-risk variants to improve early surgical outcome [7].
To facilitate surgical planning of arterial switch operation, transthoracic echocardiography is commonly used to delineate complex coronary artery patterns [8]. However, transthoracic echocardiography is limited by operator-dependence and obscured acoustic window. In fact, the diagnostic performance of transthoracic echocardiography for identifying coronary arteries before arterial switch operation has been reported to be variable in the range of 39.4–98.5%, depending on a patient volume of each center as well as the number of readers [1, 8, 9].
Coronary artery visibility on cardiac CT has been increased up to 97.1% for the origins and proximal segments because of increased scan speed and electrocardiogram (ECG)-synchronized data acquisition, even in small children with congenital heart disease [10,11,12,13,14,15,16]. The diagnostic accuracy of cardiac CT in identifying coronary artery anatomy before arterial switch operation has been rarely reported. By using prospectively ECG-triggered electron beam CT, 82.4% diagnostic accuracy in identifying coronary artery anatomy before arterial switch operation was reported in 34 children with transposition of the great arteries [17]. To the best of my knowledge, a similar study using ECG-synchronized multi-detector row CT has not been reported for this diagnostic purpose. Therefore this study compared diagnostic accuracy between cardiac CT using a dual-source scanner and transthoracic echocardiography in identifying coronary artery anatomy before arterial switch operation in newborns and young infants.
Materials and methods
Study population
The institutional review board approved this study and waived informed consent. We retrospectively reviewed cardiac CT and transthoracic echocardiography examinations performed before arterial switch operation in newborns and young infants between July 2011 and December 2016. Four children with congenitally corrected transposition of the great arteries who underwent double switch operation were excluded because of their older age at the operation. In addition, three infants who underwent aortic translocation leaving coronary arteries in situ, the so-called Nikaidoh operation, were excluded because there was no coronary artery transfer during the operation. Therefore, a total of 101 consecutive infants (median age at CT examination 4 days, range 0 days to 10 months; M:F=78:23) were included in this study. The time intervals were 6.7±8.0 days between cardiac CT and arterial switch operation and 9.8±6.2 days between transthoracic echocardiography and arterial switch operation. The cardiac defects requiring arterial switch operation in the study population were as follows: transposition of the great arteries with intact ventricular septum, 47; transposition of the great arteries with ventricular septal defect, 41; double-outlet right ventricle with subpulmonary ventricular septal defect (Taussig-Bing anomaly), 11; and double-outlet right ventricle with non-committed ventricular septal defect, 2 (Table 1). Associated cardiovascular defects included coarctation of the aorta in 11 patients, interrupted aortic arch in 3, vascular ring in 2 and total anomalous pulmonary venous connection in 1. For palliative procedures prior to arterial switch operation, balloon atrial septostomy was performed in 19 infants, pulmonary artery banding in 3, a modified Blalock-Taussig shunt in 2 and ligation of patent ductus arteriosus in 2.
Cardiac computed tomography
ECG-synchronized cardiac CT was performed by using a 128-slice dual-source scanner (SOMATOM Definition Flash; Siemens Healthcare, Forchheim, Germany) with 2×64×0.6-mm slices with z-flying focal spot technique, a 0.75-mm slice width and a 0.4-mm reconstruction interval during free-breathing in all infants. To sedate the infants, oral choral hydrate (50 mg/kg) was initially used and intravenous midazolam (0.1 mg/kg) or ketamine (1 mg/kg) was additionally administered as required. Prospectively ECG-triggered sequential scan was used in 96 infants, combined prospectively ECG- and respiratory-triggered scan [18] in 3 and prospectively ECG-triggered high-pitch (3.4) spiral scan in 2. Because patient heart rates were high, in the range of 120–160 beats per minute, the end-systolic phase was targeted for ECG-synchronized scans to get the best cardiac rest period in 100 infants. On the contrary, the end-diastolic phase was selected in the 1 remaining infant to evaluate muscular ventricular septal defects. To obtain uniform image noise, a volumetric CT dose index value based on a 32-cm phantom was individually determined based on the cross-sectional area and mean body density measured on an axial CT image obtained approximately 1–2 cm above the dome of the liver for bolus tracking [19]. For tube voltage, 70 kV was used in 84 infants and 80 kV in 17 infants. For image reconstruction, the sinogram-affirmed iterative reconstruction (SAFIRE; Siemens Healthcare, Forchheim, Germany) strength 5 with a medium smooth kernel (I26f) was used. Iodinated contrast agent (Iomeron 400, 400 mg I/mL; Bracco Imaging SpA, Milan, Italy; 1.5–2.0 mL/kg) was intravenously administered at an injection rate of 0.2–0.5 mL/s by using a dual-head power injector and a tri-phasic injection protocol, in which undiluted contrast agent was followed by 50% diluted contrast agent and then by 5% diluted contrast agent, to achieve uniform cardiovascular enhancement and minimal peri-venous streak artifacts from undiluted contrast agent. The scan delay time was determined by a bolus tracking technique with a trigger threshold of 150 Hounsfield units (HU) in the left ventricular cavity. A stack of thin axial cardiac CT data was sent to a commercially available workstation (Advantage Windows 4.6; GE Healthcare, Milwaukee, WI) and multiplanar images of the coronary arteries were evaluated by a pediatric radiologist (H.W.G., 17 years’ experience in pediatric cardiac CT). The volume CT dose index and dose–length product values (mean ± standard deviation) based on a 32-cm phantom of cardiac CT were 1.0±0.1 mGy and 8.3±4.0 mGy·cm, respectively.
Transthoracic echocardiography
Two-dimensional transthoracic echocardiography (iE33; Philips Medical Systems, Andover, MA) was performed by one of our six pediatric cardiologists (2–30 years’ experience in echocardiography) for evaluating congenital heart disease. To sedate the infants, oral chloral hydrate (80 mg/kg) was used. According to standard imaging protocol, parasternal short-axis view at the level of the aortic root with slight clockwise and counterclockwise rotations of the probe, apical four-chamber view, and left oblique subcostal view were used for the echocardiographic evaluation of coronary artery patterns of the children [20].
Evaluation of coronary artery anatomy
To classify coronary artery anatomy, we used the combination of the Leiden classification [6] and abnormal looping of the coronary arteries [5]. Based on the Leiden terminology viewed from an observer standing in the non-coronary sinus and looking toward the pulmonary trunk, sinus 1 is the facing aortic sinus to the right of the observer and sinus 2 is the opposite facing sinus to the left of the observer. To emphasize the importance of spatial relationship between the great arteries and the proximal coronary artery segments, abnormal looping of the coronary arteries needs to be described: anterior looping refers to an abnormal course anterior to the aorta and posterior looping refers to an abnormal course posterior to the pulmonary trunk. Coronary artery anatomy on cardiac CT and transthoracic echocardiography was based solely on the official imaging reports and was not re-evaluated by a specific reviewer to reflect the actual clinical setting in all patients. The initial echocardiographic findings were blinded to the cardiac CT findings and the cardiac CT findings might not be completely blinded to the initial echocardiographic findings. Nonetheless, the cardiac CT findings were not changed according to the discrepant findings at initial echocardiography. On the other hand, echocardiography was frequently repeated before arterial switch operation when there were substantial discrepant findings between initial echocardiography and cardiac CT. Therefore, these repeat echocardiographic examinations were not included in this study. Coronary artery pattern identified during arterial switch operation was regarded as the reference standard. When only a part of the whole coronary arteries could be identified, it was defined as “incomplete evaluation.” When the whole coronary arteries could not be identified, it was defined as “non-assessable.” Specific coronary artery patterns identified on cardiac CT and transthoracic echocardiography were compared with those identified during arterial switch operation. With the surgical findings as the reference standard, we compared the diagnostic accuracy for identifying coronary artery anatomy between cardiac CT and transthoracic echocardiography.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median with range, and categorical variables are expressed as frequency with percentage. McNemar test was used to compare diagnostic accuracy between cardiac CT and transthoracic echocardiography. A P-value of less than 0.05 was considered to indicate statistical significance. Statistical analyses were performed using Excel (Microsoft Corp., Redmond, WA).
Results
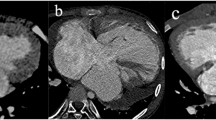
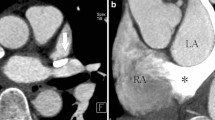
The coronary artery patterns of the study population evaluated with cardiac CT, transthoracic echocardiography, and a surgeon’s eyes are summarized in Table 2. The most common coronary artery pattern of the study population was the usual pattern (64.4%, 65/101), in which the left coronary artery arises from sinus 1 and the right coronary artery originates from sinus 2 (Fig. 1). The second most common finding was a single coronary artery from sinus 2 and a conal branch from sinus 1 (7.9%, 8/101; Figs. 2), followed by the inverted pattern in which the right coronary artery arises from sinus 1 and the left coronary artery originates from sinus 2 (5.9%, 6/101; Fig. 3) and the right coronary artery and left anterior descending artery from sinus 1 and the left circumflex artery from sinus 2 (5.9%, 6/101; Fig. 4). Of note, in a single coronary artery arising from sinus 2 with or without a conal branch from sinus 1 correctly identified on cardiac CT (n=12), cardiac CT could accurately distinguish between a double orifice (n=2; Fig. 5) and a single orifice (n=10; Fig. 6) as well as between an anterior coronary looping (n=2; Fig. 7) and a posterior coronary looping (n=10; Fig. 2).
Imaging in a 3-day-old girl born with transposition of the great arteries and ventricular septal defect. a–c Cardiac CT images show the usual coronary artery pattern in which the left coronary artery arises from sinus 1 and the right coronary artery originates from sinus 2. Axial oblique section (a) demonstrates both coronary artery origins. Coronal oblique section (b) best demonstrates the right coronary artery. Coronal oblique section (c) best demonstrates the left coronary artery. d, e Transthoracic echocardiographic images also demonstrate the usual patterns of the left (d) and right (e) coronary arteries that was subsequently confirmed at arterial switch operation. 1 sinus 1, 2 sinus 2, AA ascending aorta, DA descending aorta, LA left atrium, LAD left anterior descending artery, LCA left coronary artery, LCx left circumflex artery, PT pulmonary trunk, RA right atrium, RCA right coronary artery, RV right ventricle
Imaging in a 5-day-old boy born with transposition of the great arteries, ventricular septal defect and coarctation of the aorta. Axial oblique (a) and coronal oblique (b, c) cardiac CT images of the left (a) and right (b) coronary arteries, and of conal branches (c) show a single coronary artery from sinus 2 and a conal branch from sinus 1 that was proved to be correct at arterial switch operation. Of note, the left coronary artery runs posterior to the pulmonary trunk, constituting posterior looping (a). 1 sinus 1, 2 sinus 2, AA ascending aorta, C conal branch, LA left atrium, LAD left anterior descending artery, LCA left coronary artery, LCx left circumflex artery, LV left ventricle, PT pulmonary trunk, RA right atrium, RCA right coronary artery, RV right ventricle
Imaging in a 2-day-old boy born with transposition of the great arteries and ventricular septal defect and who underwent balloon atrial septostomy 1 day prior. a–c Cardiac CT images show the inverted coronary artery pattern in which the right coronary artery arises from sinus 1 and the left coronary artery originates from sinus 2. Axial oblique section (a) demonstrates both coronary artery origins. Coronal oblique section (b) best demonstrates the right coronary artery. Coronal oblique section (c) best demonstrates the left coronary artery. d Transthoracic echocardiographic image also demonstrates the inverted coronary artery pattern that was subsequently confirmed at arterial switch operation. Side-by-side relationship of the great arteries is noted on both cardiac CT and transthoracic echocardiography. 1 sinus 1, 2 sinus 2, AA ascending aorta, DA descending aorta, LA left atrium, LAD left anterior descending artery, LCA left coronary artery, LCx left circumflex artery, LV left ventricle, PT pulmonary trunk, RA right atrium, RCA right coronary artery, RV right ventricle
Imaging in an 8-day-old boy born with transposition of the great arteries and ventricular septal defect. Axial oblique (a, b) and coronal oblique (c, d) cardiac CT images of the origins (a), right coronary artery (b), left anterior descending (c) and left circumflex artery (d) reveal the right coronary artery and left anterior descending artery arising from sinus 1 with a single ostium and the left circumflex artery originating from sinus 2, which was proved to be correct at arterial switch operation. 1 sinus 1, 2 sinus 2, AA ascending aorta, DA descending aorta, LA left atrium, LAD left anterior descending artery, LCx left circumflex artery, LV left ventricle, PT pulmonary trunk, RA right atrium, RCA right coronary artery
Imaging in a 4-day-old boy born with double-outlet right ventricle, subpulmonary ventricular septal defect and interrupted aortic arch. Axial oblique (a) and sagittal oblique (b) cardiac CT images demonstrate the single but separate coronary artery origins (a) and the proximal right coronary artery (b), which was confirmed at arterial switch operation. 1 sinus 1, 2 sinus 2, DA descending aorta, LA left atrium, LCA left coronary artery, PT pulmonary trunk, RA right atrium, RCA right coronary artery
Imaging in a 4-day-old boy born with transposition of the great arteries and intact ventricular septum. Axial oblique (a) and sagittal oblique (b) cardiac CT images demonstrate the single coronary artery origins (a) and the proximal coronary arteries (b), which was confirmed at arterial switch operation. Almost complete anteroposterior relationship of the great arteries and severe commissural malalignment are noted (a). Patent ductus arteriosus (asterisks) is also seen (b). 1 sinus 1, 2 sinus 2, DA descending aorta, LA left atrium, LCA left coronary artery, PT pulmonary trunk, RA right atrium, RCA right coronary artery, S single coronary artery
Imaging in a 4-day-old girl born with double-outlet right ventricle, non-committed ventricular septal defect (asterisks) and mild pulmonary stenosis. Axial oblique (a, b) and coronal oblique (c) cardiac CT images show a single coronary artery from sinus 2 (a) that was confirmed at arterial switch operation, the left coronary artery (b) running anterior to the pulmonary trunk, constituting anterior looping and the right coronary artery dominance (arrows in c). 1 sinus 1, 2 sinus 2, AA ascending aorta, LAA left atrial appendage, LAD left anterior descending artery, LCA left coronary artery, LCx left circumflex artery, LV left ventricle, PS pulmonary stenosis, PT pulmonary trunk, RCA right coronary artery, RV right ventricle
For evaluating coronary artery anatomy, there was no non-assessable case at cardiac CT and at surgery, while anatomy was non-assessable at transthoracic echocardiography in nine cases (8.9%, 9/101; Table 2). Incomplete evaluation occurred in 19 cases (18.8%, 19/101) for transthoracic echocardiography, in six cases (5.9%, 6/101) for cardiac CT, and in five cases (5.0%, 5/101) for surgery. At transthoracic echocardiography, the left circumflex artery could not be identified in eight cases, the right coronary artery in seven, the left coronary artery in two, the left anterior descending artery in one, and the coronary artery origin in one. At cardiac CT, the left circumflex artery could not be delineated in three cases, the right coronary artery in two, and the left anterior descending artery in one. In the operation field, the left circumflex artery could not be seen in four cases and the left anterior descending artery in one. Consequently, diagnostic accuracy was calculated for the 96 infants with surgically proven coronary artery anatomy (Table 3). The diagnostic accuracy of cardiac CT was significantly higher than that of transthoracic echocardiography (91.7%, 88/96 vs. 54.2%, 52/96; P<0.0001).
For the surgically proven usual coronary artery pattern, the diagnostic accuracy of cardiac CT was also significantly higher than that of transthoracic echocardiography (95.4%, 62/65 vs. 67.7%, 44/65; P=0.0001). In the usual pattern, incorrect echocardiographic cases included no visible left circumflex artery in eight, no visible right coronary artery in seven, single coronary artery arising from sinus 1 in three, non-assessable case in three, no visible left coronary artery in one, and the left circumflex artery arising from the right coronary artery in one. On the other hand, cardiac CT was not useful in making the diagnosis of the usual pattern in two cases with no visible left circumflex artery and one case with no visible right coronary artery. Transthoracic echocardiography showed the worst diagnostic performance (0.0%, 0/8) in identifying a single coronary artery from sinus 2 and a conal branch from sinus 1, while cardiac CT was useful in achieving the correct diagnosis in seven (87.5%) of eight surgically proven cases (Table 2). Of interest, transthoracic echocardiography overcalled two coronary artery variants including the left circumflex artery arising from the right coronary artery and a single coronary artery from sinus 1, but the accurate diagnosis was made in only 25.0% (2/8) and 0.0% (0/4), respectively.
Discussion
Based on the results of this study, cardiac CT can be used to identify coronary artery anatomy before arterial switch operation in newborns and young infants with significantly higher diagnostic accuracy than transthoracic echocardiography, not only for the whole of coronary artery variants, but also for the most common usual pattern. The diagnostic value of cardiac CT for this purpose seems to be even greater for a small surgical volume center where the diagnostic accuracy of transthoracic echocardiography tends to be relatively low, which can be supported by previous echocardiographic studies on the same diagnostic task [1, 8, 9]. Consequently, infants with high-risk coronary artery patterns detected with cardiac CT could be transferred to a high surgical volume center for arterial switch operation in order to improve their long-term surgical outcome. High temporal resolution (75 ms) of the dual-source CT scanner and end-systolic data acquisition were two key elements in assuring high diagnostic accuracy of cardiac CT for identifying coronary artery anatomy in this study by increasing coronary artery visibility in young children with high heart rates.
In a study using prospectively ECG-triggered electron beam CT [17], the diagnostic accuracy was low (67%) in children between 1 month and 2 months and even lower (22%) in children younger than 1 month of age. As mentioned in the article [17], the longitudinal spatial resolution (3 mm) of electron beam CT was clearly lower than that of modern multi-detector row CT (0.5–0.6 mm). In addition, the temporal resolution (75 ms) of the dual-source CT scanner used in this study is higher than that of electron beam CT (100 ms). Of importance, the researchers using electron beam CT used the end-diastolic phase (80% of the RR interval), which is suboptimal for high heart rates [17]. These unfavorable technical factors might be attributed to the lower diagnostic accuracy of electron beam CT. On the other hand, the diagnostic accuracy (91.7%) of a second-generation dual-source CT for evaluating coronary artery anatomy before arterial switch operation in this study is slightly lower than the visibility (97.1%) of the origins and proximal segments of the coronary arteries reported using a first-generation dual-source CT [15]. The equivalent CT acquisition method, i.e. free-breathing prospectively ECG-triggered sequential scan, was used in both studies but patient age was different in the two, i.e. median age 4 days in this study vs. 5 months in the previous study [15].
Compared with transthoracic echocardiography, cardiac CT might have additional benefits in the concomitant evaluation of the spatial relationships between the coronary arteries and the great arteries as well as a degree of commissural malalignment [7, 21, 22], both of which are also important in planning optimal coronary artery transfer during arterial switch operation. Moreover, other cardiovascular, lung and airway abnormalities can be accurately evaluated with cardiac CT [23,24,25]. In contrast, radiation exposure of cardiac CT in this study could be reduced by using prospectively ECG-triggered sequential scan, low tube voltage, and iterative reconstruction algorithm. Contrast agent-induced nephrotoxicity is another shortcoming of cardiac CT but it could be prevented by confirming normal renal function prior to the cardiac CT examination. Therefore the benefits outweigh the potential risks of cardiac CT in delineating coronary artery pattern, which is critical for improved surgical outcome in the study population.
As expected, transthoracic echocardiography evaluated by single readers with different levels of experience in this study led to non-assessable coronary artery anatomy exclusively and the incomplete evaluation of the coronary arteries more frequently than cardiac CT. This is a result of inherent limitations of the imaging modality including poor acoustic window, patient motion, and in some cases less-experienced operators. As demonstrated in a previous study [9], two readers now perform transthoracic echocardiography to evaluate coronary artery anatomy before arterial switch operation at our institution to increase the diagnostic accuracy, a change made in recognition of the results of this study.
MR imaging that does not use ionizing radiation is often recommended for young children. In this regard, three-dimensional ECG-triggered, navigator-gated whole-heart coronary MR angiography can be used in children [16, 26,27,28]. However, in one study diagnostic-quality images could be obtained in only 17% of patients younger than 4 months of age with congenital heart disease even though all the cardiac MR examinations were performed under general anesthesia [27]. MR imaging is generally also limited by low spatial resolution, long examination time, motion artifacts and high cost.
Coronary artery transfer techniques, such as the trapdoor technique, the bay window technique and the upside-down technique, have been created to improve long-term surgical outcomes of arterial switch operation for high-risk coronary artery variants such as single, intramural and looping coronary arteries [7, 21]. The trapdoor technique can minimize the rotation angle of a coronary button by transferring the coronary artery to a medially hinged trapdoor in the neoaorta. In the bay window technique, both coronary arterial orifices are excised as a tall U-shape cuff; the inferior half of the cuff is sewn into a trapdoor incision on the pulmonary stump, and the superior half is folded down inside to form a bay window-shape channel. The upside-down technique consists of turning a single coronary button upside-down and anastomosing the ascending aorta to the front edge of the upside-down button so the coronary arteries open into the neoaorta. An expert center recently reported excellent long-term outcomes, including no late death and 93% freedom from reoperation on the reimplanted coronary arteries at 10 years, after arterial switch operation for intramural coronary arteries [29]. Nevertheless the juxtacommissural coronary origin, single coronary artery, and two ostia from a single sinus in addition to the intramural coronary artery are still critical surgical challenges during arterial switch operation in small surgical volume centers [1]. Therefore cardiac CT with high diagnostic accuracy in evaluating coronary artery anatomy before arterial switch operation would help surgeons in these centers plan an optimal coronary artery transfer technique.
In addition to its high clinical value in preoperative evaluation demonstrated in this study, cardiac CT can be used to evaluate coronary artery stenosis and its mechanisms after arterial switch operation [30, 31]. Such accurate data provided with cardiac CT before and after arterial switch operation help surgeons to minimize postsurgical risks. Although the perioperative mortality of arterial switch operation now reaches an almost negligible level at some centers, the surgical risks vary according to the experience at the centers; therefore surgical risks should be based on a subjective approach, such as the Aristotle comprehensive score or a morbidity score [21]. In this respect, accurate and objective cardiac CT data would be very helpful to establish a complexity stratification of the arterial switch operation.
In a retrospective study from a single institution [32], the optimal time period of arterial switch operation in complete transposition of the great arteries was between 1 day and 3 days. However in the present study the actual median age at operation was 5 days (range, 1–12 days), and infants with surgical delay from an intercurrent pathological process, such as infection or intracranial hemorrhage, were excluded from the study. The inclusion of these patients would further increase the age at operation in clinical practice, as in this study.
This study has several limitations. First, there might be selection bias in performing cardiac CT before arterial switch operation because of the retrospective nature of this study. However almost all patients undergo cardiac CT to evaluate coronary artery anatomy, cardiovascular abnormalities and non-cardiovascular abnormalities before arterial switch operation at our institution. Second, there was no intramural coronary artery, a high-risk coronary artery pattern during arterial switch operation, in this study. However, other high-risk coronary artery patterns were evaluated in this study. In fact, the patient number in this study was large enough to have sufficient statistical power for evaluating the whole and usual coronary artery patterns. Third, there may be an inter-reader variability in interpreting pediatric cardiac CT findings among pediatric radiologists with different expertise. Inter-reader variability of pediatric cardiac CT could not be evaluated because only one pediatric radiologist reads pediatric cardiac CT at our institution.
Conclusion
Diagnostic accuracy of cardiac CT is significantly higher than that of echocardiography in identifying coronary artery anatomy before arterial switch operation in newborns and young infants. Its diagnostic usefulness in surgical planning might be invaluable at institutions whose echocardiography programs are not completely established for this effort-intensive diagnostic task in this population.
References
Baslaim GM (2006) Is preoperative delineation of coronary artery pattern a prerequisite for arterial switch operation? J Card Surg 21:465–470
Angeli E, Formigari R, Pace Napoleone C et al (2010) Long-term coronary artery outcome after arterial switch operation for transposition of the great arteries. Eur J Cardiothorac Surg 38:714–720
Rudra HS, Mavroudis C, Backer CL et al (2011) The arterial switch operation: 25-year experience with 258 patients. Ann Thorac Surg 92:1742–1746
Yacoub MH, Radley-Smith R (1978) Anatomy of the coronary arteries in transposition of the great arteries and methods for their transfer in anatomical correction. Thorax 33:418–424
Planché C, Bruniaux J, Lacour-Gayet F et al (1988) Switch operation for transposition of the great arteries in neonates: a study of 120 patients. J Thorac Cardiovasc Surg 96:354–363
Karl TR, Cochrane A, Brizard CP (1997) Arterial switch operation. Surgical solutions to complex problems. Tex Heart Inst J 24:322–333
Suzuki T (2009) Modification of the arterial switch operation for transposition of the great arteries with complex coronary artery patterns. Gen Thorac Cardiovasc Surg 57:281–292
Pasquini L, Sanders SP, Parness IA et al (1994) Coronary echocardiography in 406 patients with d-loop transposition of the great arteries. J Am Coll Cardiol 24:763–768
Gremmels DB, Tacy TA, Brook MM et al (2004) Accuracy of coronary artery anatomy using two-dimensional echocardiography in d-transposition of great arteries using a two-reviewer method. J Am Soc Echocardiogr 17:454–460
Goo HW, Park IS, Ko JK et al (2005) Visibility of the origin and proximal course of coronary arteries on non-ECG-gated heart CT in patients with congenital heart disease. Pediatr Radiol 35:792–798
Tsai IC, Lee T, Chen MC et al (2007) Visualization of neonatal coronary arteries on multidetector row CT: ECG-gated versus non-ECG-gated technique. Pediatr Radiol 37:818–825
Goo HW, Seo DM, Yun TJ et al (2009) Coronary artery anomalies and clinically important anatomy in patients with congenital heart disease: multislice CT findings. Pediatr Radiol 39:265–273
Ben Saad M, Rohnean A, Sigal-Cinqualbre A et al (2009) Evaluation of image quality and radiation dose of thoracic and coronary dual-source CT in 110 infants with congenital heart disease. Pediatr Radiol 39:668–676
Goo HW (2010) State-of-the-art CT imaging techniques for congenital heart disease. Korean J Radiol 11:4–18
Goo HW, Yang DH (2010) Coronary artery visibility in free-breathing young children with congenital heart disease on cardiac 64-slice CT: dual-source ECG-triggered sequential scan vs. single-source non-ECG-synchronized spiral scan. Pediatr Radiol 40:1670–1680
Goo HW (2015) Coronary artery imaging in children. Korean J Radiol 16:239–250
Chen SJ, Lin MT, Lee WJ et al (2007) Coronary artery anatomy in children with congenital heart disease by computed tomography. Int J Cardiol 120:363–370
Goo HW, Allmendinger T (2017) Combined ECG- and respiratory-triggered CT of the lung to reduce respiratory misregistration artifacts between imaging slabs in free-breathing children: initial experience. Korean J Radiol 18:860–866
Goo HW (2011) Individualized volume CT dose index determined by cross-sectional area and mean density of the body to achieve uniform image noise of contrast-enhanced pediatric chest CT obtained at variable kV levels and with combined tube current modulation. Pediatr Radiol 41:839–847
Freire G, Miller MS (2015) Echocardiographic evaluation of coronary arteries in congenital heart disease. Cardiol Young 25:1504–1511
Lacour-Gayet F (2012) Complexity stratification of the arterial switch operation: a second learning curve. Cardiol Young 22:739–744
Bang JH, Park JJ, Goo HW (2017) Evaluation of commissural malalignment of aortic-pulmonary sinus using cardiac CT for arterial switch operation: comparison with transthoracic echocardiography. Pediatr Radiol 47:556–564
Goo HW (2011) Cardiac MDCT in children: CT technology overview and interpretation. Radiol Clin N Am 49:997–1010
Goo HW (2004) Evaluation of the airways in patients with congenital heart disease using multislice CT. J Korean Pediatr Cardiol Soc 8:37–43
Malik A, Hellinger JC, Servaes S et al (2017) Prevalence of non-cardiovascular findings on CT angiography in children with congenital heart disease. Pediatr Radiol 47:267–279
Kim JW, Goo HW (2013) Coronary artery abnormalities in Kawasaki disease: comparision between CT and MR coronary angiography. Acta Radiol 54:156–163
Tangcharoen T, Bell A, Hegde S et al (2011) Detection of coronary artery anomalies in infants and young children with congenital heart disease by using MR imaging. Radiology 259:240–247
Hussain T, Mathur S, Peel SA et al (2015) Coronary artery size and origin imaging in children: a comparative study of MRI and trans-thoracic echocardiography. BMC Med Imaging 15:48
Fricke TA, Bulstra AE, Naimo PS et al (2016) Excellent long-term outcomes of the arterial switch operation in patients with intramural coronary arteries. Ann Thorac Surg 101:725–729
Ou P, Celermajer DS, Marini D et al (2008) Safety and accuracy of 64-slice computed tomography coronary angiography in children after the arterial switch operation for transposition of the great arteries. JACC Cardiovasc Imaging 1:331–339
Ou P, Khraiche D, Celermajer DS et al (2013) Mechanisms of coronary complications after the arterial switch for transposition of the great arteries. J Thorac Cardiovasc Surg 145:1263–1269
Anderson BR, Ciarleglio AJ, Hayes DA et al (2014) Earlier arterial switch operation improves outcomes and reduces costs for neonates with transposition of the great arteries. J Am Coll Cardiol 63:481–487
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Goo, H.W. Identification of coronary artery anatomy on dual-source cardiac computed tomography before arterial switch operation in newborns and young infants: comparison with transthoracic echocardiography. Pediatr Radiol 48, 176–185 (2018). https://doi.org/10.1007/s00247-017-4004-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-017-4004-9