Abstract
Background
Dipyridamole and adenosine are traditional pharmacological stressors for myocardial perfusion. Regadenoson, a selective adenosine A2A agonist, has a lower side effect profile with lower incidence of bronchospasm and bradycardia. There is a growing need for myocardial perfusion assessment within pediatrics. There is no report on the utility of regadenoson as a stress agent in children.
Objective
To observe the safety and feasibility of regadenoson as a pharmacologic stressor for perfusion cardiac MR in a pilot cohort of pediatric patients weighing more than 40 kg who have congenital heart disease and pediatric acquired heart disease.
Materials and methods
We reviewed our initial experience with regadenoson stress cardiac MR in 31 pediatric patients 15.8 ± 1.7 years (range 12–22 years) with congenital heart disease and acquired heart disease. Mean patient weight was 60 ± 15 kg (range of 40–93 kg). All patients underwent cardiac MR because of concern for ischemia. The cohort included a heterogeneous group of patients at a pediatric institution with potential risk for ischemia. Subjects’ heart rate and blood pressure were monitored and pharmacologic stress was induced by injection of 400 mcg of regadenoson. We evaluated their hemodynamic response and adverse effects using changes in vital signs and onset of symptoms. A pediatric cardiologist and radiologist qualitatively assessed myocardial perfusion and viability images.
Results
One child was unable to complete the stress perfusion portion of the examination, but did complete the remaining portion of the CMR. Resting heart rate was 72 ± 14 beats per minute (bpm) and rose to peak of 124 ± 17 bpm (95 ± 50% increase, P < 0.005) with regadenoson. Image quality was considered good or diagnostic in all cases. Three patients had irreversible perfusion defects. Four patients had reversible perfusion defects. Nine of the patients underwent cardiac catheterization with angiography and the findings showed excellent agreement.
Conclusion
Regadenoson might be a safe and feasible pharmacologic stress agent for use in cardiac MR in older pediatric patients with congenital heart disease and acquired heart disease. The ease of use as a bolus and the advantage of a prolonged hyperemia make its use appealing in pediatrics. In a limited number of cases, regadenoson stress perfusion showed excellent agreement with cardiac catheterization. Regadenoson might be a viable pharmacologic stress agent in this population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Myocardial ischemia can occur in a number of clinical settings within the pediatric population, although its prevalence is much lower than in the adult population. Coronary artery disease can develop after surgical repair of congenital heart disease that involves the coronary arteries, such as arterial switch for transposition of the great arteries (TGA), anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) and anomalous aortic origin of the coronary artery (AAOCA) [1–3]. Additionally, acquired pediatric heart disease such as Kawasaki disease can result in coronary artery disease [4]. The coronary lesions can cause myocardial ischemia, which is a known risk factor for morbidity and mortality in children [5]. Cardiac stress testing can be performed with different modalities to assess for signs of impaired myocardial perfusion. Nuclear medicine studies have commonly been used within the pediatric population to assess patients with both congenital and acquired pediatric heart disease who are at risk for myocardial ischemia [6]. Although this technique has shown utility in detecting ischemia, nuclear medicine studies are limited by lower spatial resolution for small defects, suboptimal localization of pathology in the setting of complex anatomy, attenuation artifacts related to the body wall and diaphragm, relatively high incidence of false-positive findings [7], and potentially harmful effects of ionizing radiation. Likewise, X-ray coronary angiography might help to identify areas of coronary stenosis or kinking but does not depict the functional significance of the coronary abnormality and is also invasive and associated with ionizing radiation.
Cardiac magnetic resonance imaging is a noninvasive technique that provides high-quality imaging of the cardiac structures with good spatial resolution, and it is not associated with ionizing radiation. Cardiac MR perfusion imaging — using the first-pass perfusion (FPP) kinetics of a gadolinium bolus to detect myocardial ischemia — has shown excellent sensitivity and specificity in the adult population [8, 9]. Likewise cardiac MR perfusion has been performed in pediatrics for a variety of conditions that carry a risk of myocardial ischemia, with good results [10, 11]. To achieve pharmacologic stress by coronary hyperemia, adenosine or dipyridamole has traditionally been used to augment coronary blood flow [12]. These medications induce coronary vasodilation in normal coronary circulations and uncover a myocardial perfusion deficit in territories fed by a stenosed coronary artery. Adenosine’s mechanism of action is to act non-selectively upon the multitude of adenosine receptors, whereas dipyridamole works indirectly by inhibiting the uptake of adenosine [13]. However adenosine has a very short half-life and must be administered as a continuous infusion. While the half-life of dipyridamole is considerably longer than that of adenosine, it must still be given as a continuous infusion. Regadenoson is a newer myocardial stressor that is the first agent approved by the U.S. Food and Drug Administration to selectively target the A2A receptor [14]. By being a selective adenosine A2A agonist, regadenoson does not act upon the A1 receptors associated with negative chronotropic and dromotropic effects or the A3 receptors associated with mast cell degranulation and bronchospasm. Regadenoson is a potent vasodilator that selectively acts upon the coronary vasculature. It has been shown in adults to be a safe pharmacologic stress agent and has proved to be an effective coronary vasodilator [15, 16] with a rapid onset of action and a longer half-life than adenosine. Additionally it can be administered as a single intravenous bolus, which allows the cardiac MR examination to be performed with a single intravenous (IV) access line, unlike other stressors such as adenosine or dobutamine, which require a dedicated IV line for a continuous infusion. All of these features make regadenoson an attractive candidate as a stressor for perfusion cardiac MR in adults and children. However no series has described the use of regadenoson in a pediatric population.
The purpose of our study is to report on our initial experience with the safety and feasibility of regadenoson as a pharmacologic stressor in an older pediatric and young adult population with congenital and acquired cardiovascular disease and weighing more than 40 kg, and to provide comparative results from other modalities, including nuclear stress testing and cardiac catheterization when available.
Materials and methods
The research ethics board and institutional review board of Baylor College of Medicine both approved this retrospective study.
Study population
We conducted a retrospective evaluation of our experience using regadenoson for stress perfusion cardiac MR in 31 consecutive patients (25 male, 6 female) from April 2014 to December 2015. We excluded any patient requiring sedation or weighing less than 40 kg. The mean age of the patient at the time of the study was 15.8 ± 1.7 years (range 12–22 years). Twenty-nine of the patients were 18 years or younger. The mean weight of the patients was 60 ± 15 kg (range 40–93 kg) and the average body surface area was 1.67 ± 0.25 m2 (range of 1.3–2.14 m2). The clinical diagnoses are listed in Table 1. The cohort included 11 patients with D-transposition of great arteries after an arterial switch procedure (D-TGA), 9 with Kawasaki disease and coronary aneurysm, 6 with aortic root replacement, 3 who had repair of AAOCA, 1 with left ventricular hypertrophy and 1 with coarctation. All but two of the patients had a lesion considered to have an elevated risk of myocardial ischemia. The other two had a history of coarctation repair and left ventricular hypertrophy, respectively, and demonstrated nonspecific ST segment changes during an exercise stress test.
We reviewed patient characteristics including age, diagnosis, surgical history, and signs or symptoms concerning for myocardial ischemia. Nine of the 31 patients (29%) underwent cardiac catheterization within 3 months of the cardiac MR, and 4 patients (13%) had fractional flow reserve (FFR) performed with continuous infusion of adenosine by an experienced interventional cardiologist, which is the reference standard for the diagnosis of myocardial perfusion [17]. FFR directly assesses coronary blood flow, both before and during coronary hyperemia, and is a more accurate assessment of functionality than anatomical imaging alone. Sixteen of the 31 patients (52%) had undergone nuclear medicine perfusion scan (Sestamibi) within 1 year of the stress cardiac MR (4.3 ± 3.2 months; range 0.3–11.9 months, weight 63 ± 19 kg).
Cardiac magnetic resonance imaging protocol
All patients were instructed to avoid caffeinated products 24 h prior to the examination. Pre-study counseling was performed by the cardiologist (C.V.N.) as to the procedure and possible side effects of the stress agent including chest pain, flushing, dyspnea, bradycardia, sinus pause and seizure. All patients received the usual adult dose of 400 mcg of regadenoson (Lexiscan; Astellas Pharma; Northbrook, IL). Continuous heart rate and pulse oximetry monitoring was performed as well as periodic blood pressure assessment before and every 3 min after regadenoson administration, and at the conclusion of the study. A 50-mg dose of aminophylline was administered intravenously after stress imaging was completed to reverse the pharmacologic stress agent, regardless of symptoms. The time difference between stress and resting perfusion sequences, as well as the time from regadenoson to heart rate recovery, were recorded. A pediatric nurse, a cardiac MR technologist, a cardiologist and a pediatric radiologist were present during the examination. A cardiac resuscitation cart was placed outside the cardiac MR room. All studies were performed on awake patients without the need for sedation to allow for communication of any adverse effects. Patients were monitored for an hour following the examination for any adverse effects.
The initial 12 studies were performed on a 1.5-tesla (T) clinical MRI scanner (Achieva; Philips Medical Systems, Best, The Netherlands), with the remainder being performed on a 3-T clinical MRI scanner (Philips Achieva). A 16-channel XL torso coil was used with the 1.5-T scanner. A 32-channel cardiac coil was used with the 3-T scanner. Cardiac synchronization and heart rate monitoring were performed with vector electrocardiographic (ECG) gating. The cardiac MR protocol changed slightly over the time period of the study, with the initial three patients undergoing stress perfusion after the resting perfusion had been completed. This was the initial order due to concern that pharmacologic stress could potentially cause the pediatric patient to abort the remainder of the exam, and thus make a complete interpretation difficult. A total of three patients were performed in this manner; however none of the patients had any adverse events, and all tolerated the entire examination without issue. Because the initial patients showed no ill effects with pharmacologic stress, and due to concerns about the lingering effect of the prior gadolinium-based contrast injection during the stress perfusion assessment, the stress perfusion sequence was performed initially and was followed by resting perfusion assessment in the remainder of the patients [18]. The most recent and commonly used protocol is described later and listed in Fig. 1.
Initial survey imaging was performed. Respiratory and vector electrocardiograph (ECG)-gated, black-blood T1-W echoplanar (EPI) imaging in the axial plane was performed for general overview of the cardiac and mediastinal anatomy. Breath-hold cine imaging using an ECG-gated, steady-state free precession (SSFP) pulse sequence in two-chamber, four-chamber and short-axis planes was performed for quantification of biventricular size and ejection fraction. Stress-myocardial first-pass perfusion was performed 60 s following administration of regadenoson, with 0.1 mmol/kg of gadolinium (Bayer HealthCare, Whippany, NJ) injected at a rate of 3.5 mL/s. A single-shot (acquisition of the complete image within the same heartbeat), T1-weighted saturation recovery gradient echo sequence with a parallel acceleration (SENSE) factor of 2, no shared pre-pulse, repetition time (TR)/echo time (TE)/flip angle = 5/2.5 ms/17°, voxel size 2–2.5 * 2–2.5 * 7 mm3 was used for FPP imaging at three short-axis slices. A saturation delay of about 120 ms was used. Following first-pass perfusion, while hyperemia was still present, myocardial wall motion was assessed using cine SSFP sequences at three ventricular short-axis levels, with a temporal resolution of approximately 20 ms, as previously described [19]. Following stress-first-pass perfusion imaging, 50 mg of aminophylline was administered intravenously for reversal, regardless of clinical symptoms. Upon the return of heart rate to baseline, rest-first-pass perfusion was performed using the same sequence as described, adjusted for heart rate. Coronary imaging was performed with a vector ECG and respiratory navigator-gated, fat-suppressed 3-D SSFP sequence covering the coronary artery origins and course. Approximately 8 min following the final administration of gadolinium, myocardial viability of the entire left ventricle was assessed with phase-sensitive inversion-recovery sequences in short-axis orientation and four-chamber orientation.
Patient assessment for hemodynamic response and adverse effects
The hemodynamic response and presence of adverse effects was evaluated during the study using changes in vital signs and onset of new symptoms. Patients were monitored for 1 h after the study for signs and symptoms and for abnormalities in vital signs. They were examined by a physician prior to discharge. While they were monitored, they were instructed to fill out a follow-up questionnaire (Table 2) to assess for side effects of the medication. The questionnaire was a short document of yes-or-no questions investigating the presence of side effects during or after the stress cardiac MR.
Image analysis
Myocardial perfusion was assessed from first-pass perfusion images by both a cardiologist (C.V.N.) with 4 years of experience and a pediatric radiologist (R.K.) with 14 years of experience, both in pediatric cardiac MR. Both readers qualitatively assessed the image quality of first-pass perfusion images at stress and rest, and they graded them as good (homogeneity of the myocardium at all levels, with no significant artifacts obscuring the ventricle), diagnostic (near homogeneity of the ventricular myocardium or minimal artifact seen in the ventricle) or non-diagnostic (significant inhomogeneity of the myocardium or significant affect from artifact that obscures a large portion of the ventricular myocardium). They noted the presence of any artifacts on the first-pass perfusion and cine SSFP images. They evaluated the first-pass perfusion sequences for decreased signal intensity within the myocardium in either a subendocardial or transmural pattern. In addition, the high-temporal-resolution cine SSFP sequences performed at three ventricular short-axis levels were assessed in the same manner for normal wall motion, hypokinesia, akinesia and dyskinesia. A perfusion defect seen at stress and not at rest was deemed to be a reversible perfusion defect. A perfusion defect seen at both stress and rest with a corresponding area of hyperenhancement on myocardial viability imaging was classified as an irreversible perfusion defect. Any other pattern of perfusion abnormality in the absence of delayed enhancement was characterized as non-ischemic in origin, or an artifact. Wall motion abnormalities were used as an independent marker of ischemia.
Statistics
Numerical data were reported as mean values and standard deviations or as median values and ranges where appropriate. We compared variables using a paired Student’s t-test. A P-value <0.05 was considered as statistically significant.
Results
Feasibility, hemodynamic response and adverse effects
There were no significant adverse events in any of the stress studies. Hemodynamic response to regadenoson is shown in Table 1. The average resting heart rate was 72 ± 14 beats per minute (bpm) and rose to a peak of 124 ± 17 bpm (95 ± 50% increase, P < 0.005) with regadenoson. At 3 min after administration of regadenoson, the heart rate remained high at an average of 124 ± 19 bpm. The systolic blood pressure at rest was 121 ± 7.3 mmHg and dropped to a peak of 118 ± 8 mmHg (P < 0.005), demonstrating a slight significance. The diastolic blood pressure at rest was 79 ± 6.2 mmHg, and during pharmacologic stress was 77 ± 6.7 mmHg (P = 0.03). The time until return of the resting heart rate from regadenoson administration was 12 ± 4 min (range 8–21 min). No serious adverse events were encountered during or after the examination, including second-degree atrioventricular block, significant dyspnea, or severe chest pain. One patient complained of “not feeling well” within 10 s of administration. This was an autistic patient who was unable to complete the stress perfusion portion of the examination but was able to complete the remainder of the cardiac MR examination including rest perfusion and delayed myocardial enhancement after aminophylline was administered. No abnormal heart rhythm was detected in this patient. Three other patients had minor adverse events: one complained of nausea during the examination, the second had mild dizziness and the third had mild flushing. All patients had complete resolution shortly after finishing the examination and sustained no additional effects. The questionnaire administered at the conclusion of the examination showed that none of the patients experienced chest pain, shortness of breath, palpitations or headache. The patients who did experience minor side effects had those effects at the time of regadenoson administration, and none of the patients experienced side effects after the examination during the hour-long monitoring period. Please refer to Table 2 for patient responses to the questionnaire.
Image analysis
Image quality was good (19/31, 61%) or diagnostic (12/31, 39%) in all studies. Heart rates were elevated during the pharmacologic stress, with the shortening of the R-R interval in 8/31 (26%) to the degree that image acquisition occurred during every other heartbeat. Because of the size of the pediatric patients versus adults, the authors preserved spatial resolution by maintaining slice coverage and compromising temporal resolution. A known feature of stress cardiac MR FPP imaging is the dark rim artifact, which was present in 8/31 (26%) patients. The timing of the hypointensity, the symmetry of the lesion, the comparison to the resting first-pass perfusion and delayed enhancement images, and the location in relation to the area of pre-test concern all aided in the distinguishing a dark rim artifact from a true perfusion defect. The time from stress to resting perfusion sequence averaged 28 ± 4 min (range 22–38 min).
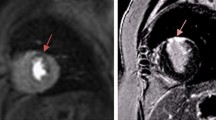
A total of 7/31 (23%) patients had a perfusion deficit on the first-pass perfusion images. None of the patients demonstrated an abnormality on delayed myocardial enhancement imaging without a corresponding perfusion deficit. Five of the seven patients with abnormal perfusion had a concomitant wall motion abnormality, whereas none of the patients with normal perfusion had a wall motion abnormality. Three of the seven patients with abnormal perfusion had an irreversible perfusion defect. The first patient had a diagnosis of D-TGA with a Yacoub type D coronary pattern, was lost to follow-up for more than 5 years, and subsequently presented with ST changes on resting and exercise ECG testing. The stress perfusion images are seen in Fig. 2. The defect was thought to be irreversible because of the extent of the delayed enhancement that corresponded to the perfusion defect (Fig. 2). Coronary angiography at cardiac catheterization demonstrated complete occlusion of the circumflex artery. The second patient with an irreversible perfusion defect had undergone unroofing for anomalous origin of the right coronary artery from the left sinus of Valsalva. The final patient with an irreversible defect had Kawasaki disease with an aneurysm at the bifurcation of the left anterior descending and circumflex coronary arteries. During cardiac catheterization that followed the cardiac MR, he was found to have near-occlusion of the circumflex coronary artery and severe stenosis of the proximal left anterior descending coronary artery. Four additional patients were found to have inducible myocardial ischemia with stress perfusion. Two of these patients had Kawasaki disease, one of whom had undergone right and left internal mammary bypass grafts (Fig. 3). Another patient had previously undergone an arterial switch operation for D-TGA and was found to have a reversible defect of the interventricular septum after complaining of chest pain with exertion. The final patient had undergone an aortic root replacement from a peri-aortic abscess; she was diagnosed after complaining of chest pain while hiking.
Cardiac MR in the left ventricular short-axis plane at the mid-ventricular level in an 18-year-old man diagnosed with D-transposition of the great arteries status post arterial switch operation. a Stress perfusion image demonstrates a pattern of decreased signal at the inferior and inferolateral walls, consistent with abnormal perfusion. b Delayed hyperenhancement image demonstrates increased signal in the inferior and inferolateral walls, corresponding to the area of abnormal perfusion seen in (a)
Cardiac MR imaging in the left ventricular short-axis plane at the mid-ventricular level in a 10 year-old boy with Kawasaki disease who had previously undergone a RIMA (right internal mammary artery bypass graft) and LIMA (left internal mammary artery bypass graft) due to coronary stenosis. a Stress perfusion image demonstrates a reversible subendocardial perfusion defect in the anterior and posterior interventricular septum. b Delayed hyperenhancement image demonstrates no increased signal intensity, indicating a reversible perfusion deficit
The remaining patients (24/31) demonstrated normal myocardial perfusion during first-pass perfusion at rest and stress, without any wall motion abnormalities during hyperemia or evidence of delayed myocardial hyperenhancement. The global left ventricular ejection fraction (LVEF) in the patients with no perfusion defect was 60.5 ± 5.5 vs. 56.8 ± 5.7% in the patients with the perfusion defect (P = 0.1, Student’s two-tailed equal variance t-test).
Cardiac catheterization and Sestamibi
A portion of the patients, 9/31 (29%), underwent cardiac catheterization in close temporal proximity to the cardiac MR study (0.6 ± 0.3 months; range 0.2–3.1 months). See Table 3 for indications and results of the cardiac catheterization findings. In summary, 5/7 patients who were found by stress cardiac MR to have a perfusion deficit underwent cardiac catheterization with coronary angiography. In addition, four patients who were not found to have perfusion deficits by stress cardiac MR underwent cardiac catheterization, with three of them also undergoing FFR testing. Two of these four patients had an abnormal nuclear myocardial perfusion study — one had Kawasaki disease with a medium-size aneurysm of the left anterior descending artery (LAD) and one had coarctation. The coronary angiography in these four patients did not demonstrate stenosis of the coronary arteries in any patient. Additionally, FFR testing was performed in three of these four patients (in all but the one with coarctation) and was normal (0.96 ± 0.03) in all three patients.
Six of the 13 patients who also had a nuclear medicine perfusion scan had a discrepant result from the stress cardiac MR. Four of the six also underwent cardiac catheterization (Table 3 shows a comparison among modalities). Five of the six discrepancies were a result of the nuclear medicine examination being interpreted as positive for ischemic insult, while one discrepancy was a result of the nuclear medicine examination being interpreted as normal in the context of a completely occluded circumflex coronary artery.
Discussion
The primary outcome of this study is the demonstration of the feasibility of regadenoson use in children and young adults with congenital and pediatric acquired coronary artery disease. A secondary outcome is comparing stress cardiac MR outcomes with cardiac catheterization and nuclear medicine results in the studied pediatric cohort. This study is the first to report the feasibility of using regadenoson for pharmacologic myocardial stress perfusion cardiac MR in a series of pediatric patients. Although there is a paucity of studies detailing the use of stress perfusion in pediatrics, our study complements one that demonstrated adenosine stress in a heterogeneous pediatric population [11], as well as a study demonstrating the feasibility of dobutamine stress cardiac MR in a heterogeneous pediatric population [19]. Although the study by Buechel et al. [11] included a higher number of patients, the percentage of patients found to have a perfusion defect by stress cardiac MR was fairly similar, 29% compared to 23%. However in their study there was a much larger percentage of patients who underwent coronary angiography for comparison to the cardiac MR findings. Like the study using adenosine, our study had no significant medical complications with the administration of a pharmacologic stress agent. In comparison to the study by Strigl et al. [19], our study is very similar, with a comparable number of patients included, although that study demonstrated feasibility of dobutamine. However in our study all cardiac MR examinations were performed in non-sedated patients who could communicate their symptoms and adverse events, whereas in the study of dobutamine a portion of the patients was sedated, and thus patient comfort could not be assessed. Additionally, in our study there were no significant adverse events or arrhythmias and patient comfort was very high; however the study by Strigl et al. [19] demonstrated that 3/32 (9%) patients terminated the examination because of symptoms, with one patient demonstrating atrial ectopy during dobutamine infusion. Neither study by Buechel et al. or Strigl et al. was designed to fully assess the accuracy of stress cardiac MR for the detection of a perfusion defect. Our study supplements these previous studies by the use of an alternative pharmacologic stress agent, regadenoson.
There is a growing need for stress perfusion cardiac MR assessment in pediatrics. The multiparametric nature of cardiac MR allows not only the analysis of myocardial perfusion but also morphological and functional analysis, and assessment of myocardial viability in the same setting. Combined with the excellent spatial resolution and lack of ionizing radiation, cardiac MR has been shown to be an excellent choice for assessing myocardial perfusion, with high specificity and sensitivity in large adult trials [8, 9]. Additionally the prognostic value of a negative stress perfusion cardiac MR study is excellent in adults [20, 21]. However it is difficult to extrapolate the results from adults with atherosclerotic coronary artery disease and apply them to pediatrics, where myocardial ischemia has different etiologies [10–12]. The ability of the myocardium to adapt to ischemia and the development of collateral blood flow might be different in a pediatric population. In addition, the safety profile for different pharmacologic stressors might be different in children. Monitoring for ischemic symptoms is limited in children. They also have a higher risk of symptomatic bronchospasm because of the smaller size of the airways [22]. An appropriate choice of pharmacologic stressor in children is critical to ensuring safety as well as high sensitivity and specificity for a given clinical indication.
All of the patients in this study, with two exceptions (coarctation repair, left ventricular hypertrophy), had a cardiac diagnosis that portended an increased risk of myocardial ischemia from discrete, fixed narrowing of the proximal coronary arterial course (D-TGA following arterial switch repair, AAOCA repair, and Kawasaki disease with coronary stenosis), with similarity to the discrete narrowing seen in atherosclerotic coronary artery disease. A coronary hyperemia agent was thought to be the best choice, and regadenoson was selected because of its inherent advantages over adenosine and dipyridamole. Although this study was not designed to assess the accuracy of regadenoson for the diagnosis of perfusion defects, it is certainly worth noting that in the nine patients who underwent coronary angiography by cardiac catheterization, including four who also had FFR testing, the stress cardiac MR results corresponded in all nine cases.
Regadenoson has numerous features that make it an ideal choice to diagnose myocardial ischemia in children. Foremost among these qualities is the selective adenosine A2A receptor targeting of regadenoson, which allows maximal vasodilation effect and rapid termination of action [14]. This has been demonstrated in trials on the safety of regadenoson to result in a lower likelihood of adverse events, particularly cardiac adverse events, compared to adenosine [23]. Adenosine is a non-selective agonist for the adenosine A1, A2A, A2B and A3 receptors. While the A2A receptor activation results in coronary vasodilation, activation of other receptors is responsible for atrioventricular block and bronchospasm. The higher affinity of regadenoson for the adenosine A2A receptor causes a more exaggerated tachycardic response than adenosine or dipyridamole [16].
In our study population, we witnessed a very brisk heart rate response. In the study in adults by Vasu et al. [16], the resting heart rate was similar to that in our population, 63 ± 12 bpm versus 72 ± 14 bpm. However in the Vasu study the heart rate rose to 95 ± 11 bpm while in our study the heart rate response was more pronounced, rising to 124 ± 19 bpm. Although we did see a statistically significant change in the systolic blood pressure, no clinical symptom was identified in association with the slight change, and the diastolic blood pressure did not demonstrate a significant change. Pharmacokinetic information in pediatric patients is needed to further understand the hemodynamic changes seen within this younger population. Although the pharmacokinetic data regarding regadenoson is scarce, studies have shown that the maximum tolerated dose is 20 μg/kg in the supine position [24]. The authors elected to have a maximum dose of 50% of that level, in an attempt to avoid significant side effects that could limit the exams, thus the lower limit of 40 kg was calculated (40 kg × 10 mcg/kg = 400 mcg). There were no serious adverse events in any of the patients, including bradycardia, bronchospasm, myocardial infarction, significant chest pain or arrhythmia. Only four patients demonstrated minor side effects, all of which resolved shortly after the examination. The administration of aminophylline in the majority of our patients might have contributed to the lack of adverse events. Although we did not have invasive FFR measurements to evaluate the effectiveness of hyperemia, the heart rate increase was significant and an indication of vasoactive response; however coronary artery vasodilation could not be confirmed.
Another aspect of regadenoson that makes it attractive in pediatric imaging is the ability to administer it as a single intravenous bolus. This allows the examination to be completed with a single IV. Additionally, the peak hyperemic effect of regadenoson occurs in 60–90 s. Regadenoson has a longer duration of action than adenosine, lasting for 2.4 min after the peak onset [24]. This characteristic is valuable because it allows the ability to assess wall motion abnormalities following first-pass perfusion. This has the potential to improve the sensitivity of the ischemia assessment without significantly prolonging the time of the study [25]. We elected to administer a fixed dose of aminophylline to our patients in an attempt to increase the tolerability of regadenoson and decrease its adverse events [23]. Aminophylline has a very minimal side effect profile and has been shown when given as a fixed dose to decrease the length and likelihood of adverse events during pharmacologic stress. The authors, in discussion with our pharmacy department, elected to administer a fixed dose of 50 mg to our patient population, which is a slightly lower dose than is typically given in adult studies [26].
There are several limitations to our study, including its retrospective design. A primary limitation is the small number of patients studied. Although our study has demonstrated feasibility and safety in a subset of pediatric and young adult patients, diagnostic accuracy requires a larger number of patients to be evaluated with quantification and a true reference standard. Additionally, we did not assess inter-observer variability. There was no gold standard for assessment of ischemia. We did not perform an invasive assessment of FFR as a reference standard for hyperemia and flow-limiting lesions in our patient population to determine the efficacy and effectiveness. Likewise, because this study was not designed to evaluate the effectiveness in diagnosing a perfusion defect, inter- and intraobserver variability was not evaluated in the assessment of a perfusion defect. We did see a brisk heart rate response with regadenoson as a sign of its hemodynamic effects; however this is not a defined marker of coronary hyperemia. Although regadenoson has been shown in adults to be a reliable inducer of ischemia, adults have pathophysiological differences from children so it is a limitation that regadenoson might not be an equal inducer of ischemia in our population. Finally, the current method of fixed dosing of regadenoson is not weight-based. Currently, regadenoson is administered as a bolus dose of 400 mcg. Our study was limited by including only pediatric patients who weighed more than 40 kg for the reasons stated above. However, to be widely adopted across all pediatric age groups, a weight-based dosing regimen needs to be developed. This would allow the use of regadenoson in younger patients, and early after D-TGA and ALCAPA repair when there is concern for ischemia. In addition, the accuracy of regadenoson stress cardiac MR needs to be evaluated against the gold standard of coronary angiography with FFR testing, and we plan to accomplish this in a future study.
Conclusion
We have demonstrated for the first time the feasibility of using regadenoson as a stressor for myocardial perfusion assessment in a series of pediatric patients weighing more than 40 kg. The cardiac MR was successfully completed in all patients, with only four minor adverse events and no serious adverse events.
References
Hauser M, Bengel FM, Kuhn A et al (2001) Myocardial blood flow and flow reserve after coronary reimplantation in patients after arterial switch and Ross operation. Circulation 103:1875–1880
Vogel M, Smallhorn JF, Gilday D et al (1991) Assessment of myocardial perfusion in patients after the arterial switch operation. J Nucl Med 32:237–241
Secinaro A, Ntsinjana H, Tann O et al (2011) Cardiovascular magnetic resonance findings in repaired anomalous left coronary artery to pulmonary artery connection (ALCAPA). J Cardiovasc Magn Reson 13:27
Mavrogeni S, Papadopoulos G, Douskou M et al (2004) Magnetic resonance angiography is equivalent to X-ray coronary angiography for the evaluation of coronary arteries in Kawasaki disease. J Am Coll Cardiol 43:649–652
Bonnet D, Bonhoeffer P, Piechaud JF et al (1996) Long-term fate of the coronary arteries after the arterial switch operation in newborns with transposition of the great arteries. Heart 76:274–279
Hernandez-Pampaloni M, Allada V, Fishbein MC et al (2003) Myocardial perfusion and viability by positron emission tomography in infants and children with coronary abnormalities: correlation with echocardiography, coronary angiography, and histopathology. J Am Coll Cardiol 41:618–626
Tobler D, Motwani M, Wald RM et al (2014) Evaluation of a comprehensive cardiovascular magnetic resonance protocol in young adults late after the arterial switch operation for D-transposition of the great arteries. J Cardiovasc Magn Reson 16:98
Schwitter J, Wacker CM, Wilke N et al (2013) MR-IMPACT II: magnetic resonance imaging for myocardial perfusion assessment in coronary artery disease trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J 34:775–781
Greenwood JP, Maredia N, Younger JF et al (2012) Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 379:453–460
Prakash A, Powell AJ, Krishnamurthy R et al (2004) Magnetic resonance imaging evaluation of myocardial perfusion and viability in congenital and acquired pediatric heart disease. Am J Cardiol 93:657–661
Buechel ER, Balmer C, Bauersfeld U et al (2009) Feasibility of perfusion cardiovascular magnetic resonance in paediatric patients. J Cardiovasc Magn Reson 11:51
Tacke CE, Kuipers IM, Groenink M et al (2011) Cardiac magnetic resonance imaging for noninvasive assessment of cardiovascular disease during the follow-up of patients with Kawasaki disease. Circ Cardiovasc Imaging 4:712–720
Shryock JC, Belardinelli L (1997) Adenosine and adenosine receptors in the cardiovascular system: biochemistry, physiology, and pharmacology. Am J Cardiol 79:2–10
Al Jaroudi W, Iskandrian AE (2009) Regadenoson: a new myocardial stress agent. J Am Coll Cardiol 54:1123–1130
Nguyen KL, Bandettini WP, Shanbhag S et al (2014) Safety and tolerability of regadenoson CMR. Eur Heart J Cardiovasc Imaging 15:753–760
Vasu S, Bandettini WP, Hsu LY et al (2013) Regadenoson and adenosine are equivalent vasodilators and are superior than dipyridamole — a study of first pass quantitative perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson 15:85
Pijls NH, van Nunen LX (2015) Fractional flow reserve, maximum hyperemia, adenosine, and regadenoson. Cardiovasc Revasc Med 16:263–265
Gerber BL, Raman SV, Nayak K et al (2008) Myocardial first-pass perfusion cardiovascular magnetic resonance: history, theory, and current state of the art. J Cardiovasc Magn Reson 10:18
Strigl S, Beroukhim R, Valente AM et al (2009) Feasibility of dobutamine stress cardiovascular magnetic resonance imaging in children. J Magn Reson Imaging 29:313–319
Macwar RR, Williams BA, Shirani J (2013) Prognostic value of adenosine cardiac magnetic resonance imaging in patients presenting with chest pain. Am J Cardiol 112:46–50
Gargiulo P, Dellegrottaglie S, Bruzzese D et al (2013) The prognostic value of normal stress cardiac magnetic resonance in patients with known or suspected coronary artery disease: a meta-analysis. Circ Cardiovasc Imaging 6:574–582
Salgado Garcia C, Jimenez Heffernan A, Sanchez de Mora E et al (2014) Comparative study of the safety of regadenoson between patients with mild/moderate chronic obstructive pulmonary disease and asthma. Eur J Nucl Med Mol Imaging 41:119–125
Cerqueira MD, Nguyen P, Staehr P et al (2008) Effects of age, gender, obesity, and diabetes on the efficacy and safety of the selective A2A agonist regadenoson versus adenosine in myocardial perfusion imaging integrated ADVANCE-MPI trial results. JACC Cardiovasc Imaging 1:307–316
Gordi T, Frohna P, Sun HL et al (2006) A population pharmacokinetic/pharmacodynamic analysis of regadenoson, an adenosine A2A-receptor agonist, in healthy male volunteers. Clin Pharmacokinet 45:1201–1212
Hojjati MR, Muthupillai R, Wilson JM et al (2014) Assessment of perfusion and wall-motion abnormalities and transient ischemic dilation in regadenoson stress cardiac magnetic resonance perfusion imaging. Int J Cardiovasc Imaging 30:949–957
Rangel TB, Assreuy AM, Pires Ade F et al (2011) Crystallization and characterization of an inflammatory lectin purified from the seeds of Dioclea wilsonii. Molecules 16:5087–5103
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Noel, C.V., Krishnamurthy, R., Moffett, B. et al. Myocardial stress perfusion magnetic resonance: initial experience in a pediatric and young adult population using regadenoson. Pediatr Radiol 47, 280–289 (2017). https://doi.org/10.1007/s00247-016-3762-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-016-3762-0