Abstract
Background
Studies have demonstrated that 70-kilovolt (kV) imaging enhances the contrast of iodine, potentially affording a reduction in radiation dose while maintaining the contrast-to-noise ratio (CNR). There is a maximum amount of image noise beyond which increased contrast does not improve structure visualization. Thus, noise should be constrained during protocol optimization.
Objective
This phantom study investigated the effect of 70-kV imaging for pediatric thoracic CT angiography on image quality and radiation dose in a pediatric population when a noise constraint was considered.
Materials and methods
We measured contrast and noise using anthropomorphic thoracic phantoms ranging in size from newborn age equivalent to 10-year-old age equivalent. We inserted contrast rods into the phantoms to simulate injected contrast material used in a CT angiography study. The image-quality metric “iodine CNR with a noise constraint” was used to determine the relative dose factor for each phantom size, kV setting (70–140 kV) and noise constraint (1.00–1.20). A noise constraint of 1.20 indicates that noise should not increase by more than 20% of the noise level in images performed at the reference kV, selected to be 80 kV in this study. The relative dose factor can be applied to the original dose obtained at 80 kV in order to maintain iodine CNR with the noise constraint. A relative dose factor <1.0 indicates potential for dose reduction while a relative dose factor >1.0 indicates a dose penalty.
Results
Iodine contrast was highest for 70 kV and decreased with higher kV settings for all phantom sizes. The relative dose factor at 70 kV was <1.0 for all noise constraint >1.0, indicating potential for dose reduction, for the newborn, 1-year-old and 5-year-old age-equivalent phantom sizes. For the 10-year-old age-equivalent phantom, relative dose factor at 70 kV=1.22, 1.11, 1.01, 0.92 and 0.83 for noise constraint=1.00, 1.05, 1.10, 1.15, 1.20, respectively, indicating a dose penalty for noise constraint ≤1.10 and potential for dose reduction for noise constraint >1.10.
Conclusion
Using 70 kV does allow for radiation dose reduction if the radiologist is willing to accept a higher level of image noise as a trade-off for increased vessel contrast. This increase in noise is small (<5%) for the nominal newborn, 1- and 5-year-old but is >10% for the 10-year-old. Therefore, we recommend limiting 70 kV thoracic CT angiography to newborn through 5-year-old patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
CT imaging performed at 70 kV has shown potential to achieve improved image quality and reduction in radiation dose for chest and cardiac applications [1–4]. Based on the physics of CT imaging, the greatest potential advantage exists for examinations that use intravenous iodinated contrast agents [5]. The high atomic number of iodine results in greater attenuation of low-energy photons and increased subject contrast in vessels. This translates to a measurable increase in image contrast. If image contrast is increased, image noise can also be increased while maintaining the contrast-to-noise ratio (CNR). Although CNR is a commonly measured image-quality metric, it is not a comprehensive measure of diagnostic quality, especially at high noise levels where increased contrast does not improve visualization of small structures [6].
Several studies have attempted to quantify the advantages of 70-kV imaging in both patient-based and combined patient-phantom studies [1, 2, 7, 8]. Previous studies have had deficiencies related to patient demographics in terms of age or presence of contrast material. Zhang et al. [1] demonstrated that adult coronary CT angiography using a high pitch at 70 kV resulted in less radiation dose and decreased contrast medium volume without detriment to diagnostic image quality. However children were not included in this study. Niemann et al. [7] showed no appreciable difference in image quality between patients scanned at 70 kV and 80 kV and similar radiation dose levels. However the exams analyzed were a mix of contrast-enhanced (I+) and non-contrast (I-) exams, which failed to quantify the theoretical advantage of 70-kV imaging (improved contrast of injected iodine). Durand et al. [2] measured the CNR of various iodine dilutions in 10 ovoid phantoms as part of a phantom study and in the left ventricle of the heart as part of a patient study. Improved CNR at 70 kV was observed and recommended for contrast-enhanced scanning of small children, while 80 kV was recommended for non-contrast scanning. The authors noted a significant increase in noise at 70 kV compared with 80 kV and commented that “there may be a maximal level of acceptable noise regardless of contrast attenuation” [2].
An approach of maintaining a fixed CNR does not account for the potential negative impact of increased noise [6]. To account for this limitation, Yu et al. [6] proposed a new optimization metric, called “iodine CNR with a noise constraint,” that limits the amount of dose reduction achievable solely by gains in CNR. For example, if a low kV acquisition resulted in a doubling of image contrast in a vessel containing iodine relative to a higher kV, noise could be allowed to double while maintaining CNR. Using an approximation from basic X-ray statistics, dose could then be reduced to 1/22=25% of the reference dose, although the exact noise/dose relationship should be determined experimentally. This is termed the relative dose factor. However, a drastic increase in the absolute noise level could render an image non-diagnostic, particularly when small anatomy is concerned. Therefore noise must be constrained and the relative dose factor must be limited. In the prior example, if the noise increase were limited to 20% (i.e. noise constraint=1.20), the relative dose factor would be limited to the higher of 1/22=25% (determined by the gains in CNR) and 1/(1.2)2=69% (determined by the noise constraint) of the dose at the reference kV.
The goals are to maximize CNR until image noise reaches a noise limit defined by the diagnostic task. The noise constraint is task-dependent: higher noise constraints are appropriate for studies of relatively large structures with high attenuation, while lower noise constraints are required to visualize small structures with low subject contrast. Noise constraints are also size-dependent [9] because less noise is tolerated for smaller patients with less adipose tissue and smaller anatomy.
The purpose of this phantom study was to investigate the effect of 70-kV imaging for pediatric thoracic CT angiography on image quality and radiation dose in a pediatric population when a noise constraint was considered.
Materials and methods
Phantoms
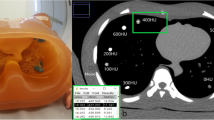
Four anthropomorphic pediatric thoracic phantoms (CIRS Inc., Norfolk, VA) were scanned to simulate pediatric thoracic CT angiography images (Fig. 1). For convenience, phantom age is used to denote nominal size for the remainder of the article. The phantoms were constructed from water-equivalent epoxy (Hounsfield Unit, HU=0), with lung regions (HU=-810) and a bone-equivalent insert to simulate the spine (HU=742). Phantoms contained holes of 1-cm diameter in the center and periphery for insertion of CT dose chambers or material inserts (rods). Contrast rod inserts were manufactured with iodine concentrations of 3 mg/ml, 6 mg/ml, 8 mg/ml, 10 mg/ml and 15 mg/ml (HU=85, 170, 230, 290, 450) and placed into the phantom center between the lung regions (Fig. 1).
Multi-detector CT parameters
All scans were performed on a 40-slice multi-detector CT scanner (Biograph mCT-S; Siemens, Erlangen, Germany). Each phantom was scanned with our clinical pediatric CT angiography kV setting (80 kV) and additionally scanned at 70 kV, 100 kV, 120 kV and 140 kV. The milliamperes (mA) was fixed and adjusted for each kilovolt (kV) to match the CT dose index (CTDI) of 2.02 milligrays (mGy) (32 cm) obtained for the 5-year-old age-equivalent phantom scanned using the clinical protocol with CARE Dose4D enabled. Images were reconstructed using iterative reconstruction (SAFIRE™ [sinogram-affirmed iterative reconstruction] I30, strength=2). All images were acquired in helical mode (rotation time, 0.3 s; pitch, 0.6; collimation, 40x0.6 mm; image thickness, 2.0 mm; CTDI, 2.02 mGy). A pitch of 0.6 was used to remove the limitation of mA limits at 70 kV from the experimental design. This should be a consideration in a clinical setting but given the wide range of mA limits between single- and dual-source scanners, we ignored this issue in the current study. It was not necessary to match CTDI for the purpose of calculations because only relative dose, i.e. relative dose factor, was considered and the absolute CTDI is irrelevant for the purpose of calculating relative dose factor. To simplify data collection and protocol design, CTDI was matched across kV and phantom size and is not intended to reflect a clinically appropriate dose level for all patient sizes.
Phantom analysis
We measured contrast (C), noise (σ) and contrast-to-noise ratio (CNR) on each of five contiguous images at the center of each phantom on each image series. One circular region of interest (ROI) was placed in the center of the contrast insert (ROIInside) and a second ROI (ROIOutside) was placed 1.5 cm above the contrast insert in an area of uniform tissue-equivalent epoxy. Each ROI was equal to 50% of the contrast insert area (ROI, 50 mm2; contrast insert, 1 cm2). Contrast was calculated as the difference in mean HU between ROIInside and ROIOutside. We measured noise as the standard deviation (σ) of HU for ROIOutside. Measurements from each image series were averaged to reduce measurement variability.
Using equation 12 in Yu et al. [6], we calculated the relative dose factor for each kV with five noise constraint parameters (noise constraint=1.00, 1.05, 1.10, 1.15 and 1.20). We used 80 kV as the reference kV (kVRef) in the relative dose factor calculation because it reflected the kV setting of our clinical CT angiography protocol prior to this study. A noise constraint equal to 1.00 denoted zero tolerance for increased noise. A noise constraint of 1.05 indicated a 5% increase in noise tolerance and values of noise constraint=1.10, 1.15, and 1.20 indicated an increase in noise of 10%, 15% and 20%, respectively. The maximum value of noise constraint=1.20 was selected because the relative dose factor did not change when noise constraint was greater than 1.20. In other words, the relative dose factor was determined solely by gains in CNR for noise constraint >1.20. Because only relative contrast as compared to the reference kV (i.e. CkV/CkV-ref) affects the relative dose factor calculation [6], and CkV/CkV-ref is unaffected by changes in iodine concentration, the contrast ratio was averaged across all iodine concentrations for each kV and phantom size. This allowed us to reduce statistical fluctuations and calculate a single relative dose factor for each kV, noise constraint and phantom size.
Results
Contrast and noise
Values for contrast (C), noise (σ) and CNR for all kV settings are shown in Table 1 for reference. Noise increased for all kV with larger phantom sizes as expected because CTDI was matched across kV settings and patient sizes. The relative noise between 70 kV and 80 kV was very similar for the newborn, 1- and 5-year-old phantom sizes (σ70/ σ80=1.01, 1.00 and 1.02, respectively). The noise was noticeably higher at 70 kV for the 10-year-old age-equivalent phantom (σ70/ σ80=1.11). The relative contrast was higher for 70 kV compared with 80 kV for all phantom sizes (C70/C80=1.18 for newborn, 1.18 for 1-year-old, 1.19 for 5-year-old, and 1.20 for 10-year-old). Absolute contrast decreased with increased kV and larger phantom sizes as expected because of decreased overlap of the spectrum with the k-edge of iodine and beam hardening with larger phantoms, respectively (Table 1).
Relative dose factor
The relative dose factor at 70 kV, 80 kV and 100 kV for all phantom sizes and noise constraints is shown in Fig. 2. Only 70 kV, 80 kV and 100 kV are shown in Fig. 2 for readability, but the relative dose factor values for all kV settings are included in Table 2 for reference. Because kVRef=80, the relative dose factor equals 1.00 at 80 kV for all noise contraints settings. Above 80 kV, the relative dose factor was >1.0 for all noise constraints and increased with increasing kilovoltage.
Relative dose factor at 70 kV, 80 kV and 100 kV for the four phantom sizes and noise constraints of (a) 1.00, (b) 1.05, (c) 1.10, (d) 1.15 and (e) 1.20. Note that the relative dose factor is 1.00 at 80 kV for all patient sizes and noise constraints because 80 kV was selected as the reference kilovoltage. NB newborn, yo year old
At noise constraint=1.00, relative dose factor at 70 kV was 1.04, 1.00 and 1.06 for the newborn, 1-year-old and 5-year-old age-equivalent phantoms, respectively. The relative dose factor at 70 kV was 1.22 for the 10-year-old phantom, indicating a 22% dose penalty at 70 kV for this phantom size and noise constraint setting. For noise constraint >1.00, relative dose factor at 70 kV was <1.0 for the newborn, 1-year-old and 5-year-old age-equivalent phantoms and decreased with higher noise constraint values. Relative dose factor at 70 kV was 1.01 at NC=1.10 for the 10-year-old phantom and decreased for noise constraint >1.10. All phantom sizes reached a minimum relative dose factor at 70 kV at noise constraint 1.20. The effect of noise constraint and patient size on relative dose factor at 70 kV compared to 80 kV is shown in Fig. 3.
Discussion
Clinical considerations
The decision to perform pediatric thoracic CT angiography at 70 kV should be made in the context of patient size and tolerance of increased noise for the clinical indication. For all phantom sizes used in this study, there is a dose penalty (relative dose factor >1.0) above 80 kV caused by poor subject contrast at higher kV. As such, we consider kV settings >80 kV to be inappropriate for pediatric CT angiography and therefore focus on the differences between 70 kV and 80 kV for the remainder of the discussion. The term “dose penalty” is intended to reflect the necessity for increased dose while maintaining the diagnostic value of the study as quantified by the “iodine CNR with a noise constraint.” There would be no such dose penalty if only CNR were used as the image-quality metric; however this poses the risk of non-diagnostic exams resulting from objectionable noise. It is noteworthy that in situations where there is either no dose advantage or even a dose penalty at 80 kV, contrast is always higher at 70 kV. Thus the dose/image quality balance is not a zero-sum endeavor when both contrast and a noise constraint are considered. In other words, when the relative dose factor at 70 kV is close to 1.00, it is possible to increase contrast at 70 kV even though there is no potential for dose reduction.
The range of noise constraint (1.00–1.20) was much smaller than that proposed by Yu et al. [6] (e.g., 1.5–2.0). Because we chose 80 kV as the reference kV, the gains in contrast between 70 kV and 80 kV are much smaller compared with gains in contrast between 80 kV and 120 kV (the reference kV used in [6]). In addition, the phantom sizes used in the current study were much different from the phantom sizes used by Yu et al. [6]. For the relatively small phantom sizes used here, the effect of kV on noise for a matched CTDI is much less than the effect in larger phantoms. As a result, for noise constraint >1.20, relative dose factor at 70 kV is not limited by noise constraint and depends solely on the difference in contrast. Hence, the smaller range (1.00–1.20) was valid for our study.
Patient dose reduction
First consider the case when the noise level is kept constant (noise constraint=1.00). For all phantom sizes, there is no dose advantage at 70 kV. For the newborn, 1- and 5-year phantom sizes, 70 kV and 80 kV are approximately dose-neutral (i.e. relative dose factor ~1.0). For phantom sizes of 10 years and larger, there is a dose penalty at 70 kV (i.e. relative dose factor > 1.0 ) and the magnitude of this dose penalty would be expected to increase for larger phantom sizes.
For the increased noise scenario (noise constraint >1.0), 70 kV might present a dose advantage (relative dose factor < 1.0) while maintaining diagnostic yield in terms of the “iodine CNR with a noise constraint.” However the noise constraint at which this dose advantage exists is size–dependent. There is potential for dose reduction at 70 kV for the newborn, 1- and 5-year phantom sizes when noise constraint is >1.0. However a noise increase of more than 10% (noise constraint >1.10) is required to realize a dose advantage for the 10-year-old phantom size.
Limitations
There are several limitations to our study. First, we used phantoms instead of actual patients because it is unethical to double-scan children at two different kV settings. This limitation was largely overcome because our phantoms accurately mimicked the attenuation properties of real patients. Second, we did not include the presence of artifacts in our assessment. Beam-hardening artifacts from high-attenuation material (bone and metal implants) would be more severe at 70 kV, and in these cases higher kV techniques should be used. A third limitation was the single pitch factor of 0.6 for our CT scanning protocol. This low pitch value was selected to avoid potential tube peaking limitations at low kV imaging; however motion should also be considered when imaging people rather than patients Because different scanner models have different power ratings, we thought it best to eliminate potential tube peaking limits in the experimental design, although these limits should be considered before building low-kV pediatric protocols. A fourth limitation was the dose metric used to calculate the relative dose factor. Although CTDI and size-specific dose estimate are equivalent for the purpose of calculating relative dose factor, both are metrics of average absorbed dose to a phantom (either a CTDI phantom or water-equivalent phantom, respectively) and do not account for variations in organ dose. Lower kV would result in increased superficial organ dose (e.g., anteriorly to the breast and posteriorly to red bone marrow in the spine). This increased superficial organ dose was not accounted for in the current relative dose factor calculations because the measurements of organ dose are difficult and require anatomically equivalent phantoms (e.g., with breast tissue) and locations to insert special dosimeters such as metal-oxide-semiconductor field-effect transistor (MOSFET) or optically stimulated luminescence (OSL) dosimeters. This information was beyond the scope of the current study. In situations that are dose-neutral (i.e. relative dose factor ~1.0), it might be more appropriate to use the higher kV setting (i.e. 80 kV) because superficial organ dose (e.g., breasts, red bone marrow) and effective dose would be increased at low kV settings because of higher absorption in shallow tissue. The issue of increased superficial organ dose would be magnified in larger patients. Finally, we did not consider limitations on scanner hardware that could prohibit 70-kV scanning in large patients. So-called tube loading limits are different for each scanner model and might limit the maximum mA to a value below what is required to produce adequate image quality in larger patients.
Recommendations
The relative dose factor at 70 kV is ~1.0 for the newborn, 1- and 5-year phantom sizes at a noise constraint=1.0 and falls below 1.0 for all other noise constraint values. Thus, image quality can be improved by gains in CNR with minimal or zero increase in radiation dose. By contrast, maintaining image quality for the 10-year phantom would require a higher dose for noise constraint <1.10. Another consideration is the developing breast issue in older patients that could result in increased superficial organ dose for patients >10 years old scanned at 70 kV. Finally, scanning speed introduces a limitation for larger patients. The maximum radiation output of 70 kV is limited for a high helical pitch and a short rotation time. In the case of larger pediatric patients, 70 kV may not be able deliver sufficient dose in a fast scanning speed, depending on the scanner power limit. In light of these factors, we suggest an upper limit of 5 years (nominal anteroposterior=14.5 cm, lateral=18.0 cm) be set for 70-kV thoracic CT angiography. It is possible to extend this upper limit to include the 10-year-old patient size in order to realize more contrast if a marginal increase in image noise (~1.5 HU in Table 1) can be tolerated and the mA limit of the scanner is not reached. This increased noise tolerance and higher noise constraint deserves further clinical validation.
Conclusion
Using 70 kV does allow for radiation dose reduction; however the radiologist must be willing to accept a higher level of image noise as a trade-off to increased vessel contrast. This increase in noise is small (<5%) for the nominal newborn, 1- and 5-year-old patients but is >10% for the 10-year-old patient. Thus, we recommend limiting 70-kV thoracic CT angiography to the newborn through 5-year-old patient body sizes. Future studies are needed to assess the effect of 70-kV scanning on organ dose because this could place further limitations on the appropriateness of 70-kV imaging.
References
Zhang LJ, Qi L, De Cecco CN et al (2014) High-pitch coronary CT angiography at 70 kVp with low contrast medium volume: comparison of 80 and 100 kVp high-pitch protocols. Medicine 93, e92
Durand S, Paul JF (2014) Comparison of image quality between 70 kVp and 80 kVp: application to paediatric cardiac CT. Eur Radiol 24:3003–3009
Nakagawa M, Ozawa Y, Sakurai K et al (2015) Image quality at low tube voltage (70 kV) and sinogram-affirmed iterative reconstruction for computed tomography in infants with congenital heart disease. Pediatr Radiol 45:1472–1479
Siegel MJ, Ramirez-Giraldo JC, Hildebolt CD et al (2013) Automated low-kilovoltage selection in pediatric computed tomography angiography: phantom study evaluating effects on radiation dose and image quality. Invest Radiol 48:584–589
MacDougall RD, Strauss KJ, Lee EY (2013) Managing radiation dose from thoracic multidetector computed tomography in pediatric patients. Radiol Clin North Am 51:743–760
Yu L, Li H, Fletcher JG et al (2009) Automatic selection of tube potential for radiation dose reduction in CT: a general strategy. Med Phys 37:234–243
Niemann T, Henry S, Duhamel A et al (2014) Pediatric chest CT at 70 kVp: a feasibility study in 129 children. Pediatr Radiol 44:1347–1357
Gnannt R, Winklehner A, Goetti R et al (2012) Low kilovoltage CT of the neck with 70 kVp: comparison with a standard protocol. AJNR Am J Neuroradiol 33:1014–1019
Brisse HJ, Brenot J, Pierrat N et al (2009) The relevance of image quality indices for dose optimization in abdominal multi-detector row CT in children: experimental assessment with pediatric phantoms. Phys Med Biol 54:1871
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
MacDougall, R.D., Kleinman, P.L., Yu, L. et al. Pediatric thoracic CT angiography at 70 kV: a phantom study to investigate the effects on image quality and radiation dose. Pediatr Radiol 46, 1114–1119 (2016). https://doi.org/10.1007/s00247-016-3588-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-016-3588-9