Abstract
Mid-aortic syndrome (MAS) is an uncommon condition characterized by severe narrowing of the abdominal aorta, usually involving visceral and renal arteries. Most patients are asymptomatic and typically present with incidental hypertension which might evolve into end-organ damage if untreated. Our aim was to review 8 new pediatric MAS cases. A retrospective observational study of all pediatric patients with MAS diagnosis (April 1992–November 2021) was conducted. Patients underwent systematic evaluation (medical and family history; 12-lead electrocardiogram; echocardiogram; angiography and/or computed tomography or magnetic resonance angiography). 8 pediatric patients with MAS were included. Median age at diagnosis was 2.6 [0.2–4.7] years; median follow-up time was 8.6 [6.6–10.0] years. 6/8 patients presented with incidental hypertension, 1/8 with heart murmur, and 1/8 with heart failure symptoms. All patients were on antihypertensive treatment. 1/8 patients underwent surgery and 7/8 an endovascular treatment. At the end of the study period, among the 6 patients that underwent a successful endovascular procedure, 2 achieved good blood pressure (BP) control, 2 acceptable BP control, 1 stage 1 hypertension and, another, stage 2 hypertension. There was 1 death during follow-up. BP monitoring in pediatric patients is crucial for early recognition of MAS. Treatment should be based on the individual clinical characteristics of patients with careful planning of surgical revascularisation, if possible, after adult growth is completed. Our study demonstrates that endovascular treatment might be a good alternative to surgery. Nevertheless, further trials with larger sample size and longer-term follow-up are required to determine the best treatment approach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mid-aortic syndrome (MAS) was first described by Senet al. in 1963 [1]. It is characterized by severe narrowing of the distal thoracic and abdominal aorta, frequently involving visceral and renal arteries [2]. It is an uncommon condition accounting for the 0.5–2% of the thoraco-abdominal aortic coarctation [3, 4]. It results in severe renovascular hypertension (HT) mainly affecting children and young adults [5, 6].
MAS etiology is unknown, being the majority of cases idiopathic [2, 7]. Nevertheless, MAS has been reported associated with congenital disorders (neurofibromatosis) or acquired disorders (Takayasu arteritis) [1, 5, 8,9,10].
Most patients are asymptomatic and typically present with incidental HT, often unresponsive to antihypertensive agents. However, symptoms like headache, dyspnea or even claudication, abdominal angina, and heart failure have been reported [2].
Angiography is the gold standard diagnostic for MAS [6]. Magnetic resonance angiography (MRA) or computed tomography angiography (CTA) are good non-invasive alternatives, not only for diagnosis but also for treatment planning [11,12,13].
Treatment options include antihypertensive medication, percutaneous transluminal angioplasty (PTA) (± stent implantation), surgical revascularisation, or even unilateral nephrectomy [2, 11].
This study aimed to report the presentation, diagnosis, management and long-term follow-up of 8 pediatric patients with MAS.
Methods
Data Collection
A retrospective observational study was conducted including all consecutive pediatric individuals (aged ≤ 18 years) with a diagnosis of MAS seen at Hospital Sant Joan de Déu between April 1992 and November 2021. Medical records were systematically reviewed. The study was approved by our institutional Research Board and consent waived in view of the retrospective data collection.
Clinical Evaluation
Patients underwent systematic evaluation, including personal and family medical history; physical examination; resting 12-lead ECGs; two-dimensional, color and Doppler echocardiography; abdominal-vessel Doppler ultrasound; and angiography, CTA, or MRA. Genetic testing was performed in those patients with other associated anomalies and suspected genetic syndromes. Clinical data were collected at baseline and during follow-up until patients were transitioned to adult services at 18 years of age, until the end of the study period or until patient’s death.
Blood pressure (BP) response after treatment was classified as follows: good BP control (normotensive) when BP was < 90th centile for gender and height centile according to current guidelines [14]; acceptable BP control when BP was between 90 and 95th centile; stage 1 HT when BP was > 95th centile but < 5 mmHg above 99th centile; and stage 2 HT when BP was ≥ 5 mmHg above 99th centile. Renal dysfunction was defined as estimated glomerular filtration rate (eGFR) < 90 ml/min/1.73 m2, using the Schwartz formula [15].
Nonpharmacological and Pharmacological Treatment
At present, there are a paucity of long-term follow-up studies to guide choice of antihypertensive agents in pediatric patients with HT [16, 17]. Nonetheless, current pediatric guidelines recommend initial lifestyle and nonpharmacological interventions to diminish BP [16, 17]. In pediatric population with HT, the optimal BP level with nonpharmacological and medical therapy to be achieved should be < 90th percentile or < 130/80 mmHg if ≥ 13 years old, whichever is lower (grade C, moderate recommendation) [17]. If nonpharmacological therapy fail to lower BP, especially in the context of left ventricular hypertrophy (LVH) on echocardiography, symptomatic HT, stage 2 HT with no modifiable causes (namely obesity), current international pediatric guidelines propose to initiate treatment with a single drug at the lowest recommended dose and up-titrate it until the maximum tolerated or recommended dose is achieved, with close monitoring of side effects and until target BP value is reached (Fig. 1) [16,17,18]. The American Academy of Pediatrics (AAP) proposes, as initial treatment, angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), long-acting calcium channel blockers, or thiazide diuretics (grade B, moderate recommendation) [17]. Beta-blockers are not recommended as first-line pediatric treatment [18]. A second or third drug should be initiated if monotherapy or bitherapy (respectively) does not reach cut-off BP [18]. In adults, resistant HT is described as persistently high BP regardless of treatment with ≥ 3 antihypertensive drugs of different classes. However, there are currently no data to confirm that treatment-resistant HT in childhood population exists [17]. In pediatric patients with suspected treatment resistance, AAP proposes to consider the evaluation and management strategies recommended in adults with resistant HT [17, 19].
Statistics
Non-normally distributed data are presented as a median [interquartile range (IQR)]. Categorical variables are presented as number (n) and percentages (%).
Results
8 pediatric patients with MAS diagnosis were included in this study. Table 1 shows patients’ demographic and clinical characteristics. The median age at diagnosis was 2.6 [0.2–4.7] years and the median follow-up time was 8.6 [6.6–10.0] years. No patients had relevant family history.
Presenting Features
Six patients (75.0%) were referred for cardiac screening because incidental finding of HT [median age 2.9 (0.1–4.9) years], one patient (patient 2, 12.5%) because of the presence of a heart murmur in routine medical examination, and the last (patient 8, 12.5%) due to heart failure symptoms. All patients but one (patient 4, 12.5%) had LVH on echocardiogram. Patient 4 was additionally found to have isolated pericardial effusion without hemodynamic compromise which self-resolved over the following months.
Patient 8 presented at the age of 8 months with fever and shortness of breath and cardiomegaly on her chest-XR. She was subsequently admitted to the pediatric intensive care unit (PICU) for suspected myocarditis vs dilated cardiomyopathy as her echocardiogram demonstrated dilated left ventricle (LV) with impaired ejection fraction (EF). During her admission, she was found to be hypertensive and her angiography confirmed MAS diagnosis. Her LVEF gradually improved after diuretics and ACEi were started. Her LV end-diastolic diameter and LVEF progressively normalized over the next months.
Associated Findings
Genetic analysis was performed in 5 patients (62.5%). Patient 2 and 4 were heterozygous for a pathogenic variant in SMAD4 gene [p.Ile500Val (NM_005359.5:c.1498A > G)] consistent with Myhre syndrome. Patient 3 was found to carry variants of unknown significance (VUS) in LZTR1 [p.Asp185fs (NM_006767.3:c.552_553dupTG], FLNC [p.Thr788Met (NM_001458.4:c.2363C > T)] and TSC1 genes [p.Thr798Met (c.2393C > T)]. Patient 5 was not found to present any mutations included in a genetic panel for vascular diseases. Patient 6 was found to carry a pathogenic variant in HNF1B gene with no available data for the exact variant identified. Patient 7 was clinically diagnosed with Neurofibromatosis type 1 (NF1) although genetic analysis was negative for this condition.
Extent of the Disease
Table 2 shows the extent of the disease for each patient. All patients presented narrowing of the abdominal aorta at different levels with involvement of the renal arteries in 7 (87.5%). Precisely, six patients (75.0%) had bilateral renal artery stenosis (RAS) and one (12.5%, patient 6) unilateral RAS (left artery stenosis).
Seven patients (87.5%) had intestinal arterial involvement. However, none of them presented with mesenteric ischemia. Five (62.5%) patients had iliac artery narrowing and one (12.5%, patient 3) occluded iliac arteries. Patient 6 (12.5%) developed claudication during follow-up which improved after performing percutaneous transluminal angioplasty (PTA) of the abdominal artery together with the implantation of a stent at the same level and in the left renal artery (see treatment section for further details).
Five patients underwent a cerebral CTA or MRA: three were normal (60.0%), one (patient 5, 20.0%) had a hypoplastic left internal carotid artery, and another (patient 3, 20.0%) showed severe obstruction of the origin of the left carotid artery which was also hypoplastic. Moreover, this later patient had right aortic arch and hypoplastic pulmonary arteries.
Treatment, Follow-Up and Final Outcome
Medical Therapy
Medical treatment was the first-line treatment for all patients, requiring a combination of up to a median of 2.5 [1.3–3.0] antihypertensive agents (Table 3).
While on ACEi treatment, patient 5 presented transient deterioration in renal function (eGFR 69 ml/min/1.73 m2). ACEi dose was reduced with subsequent improvement of her renal function. Patient 4 received antihypertensive treatment only. However, the remaining seven patients (87.5%) required additional treatment with PTA (± stent implantation) and/or surgical treatment.
Endovascular Treatment
Seven patients (87.5%) underwent cardiac catheterization at a median age of 8 [0.5–63.0] months. PTA was performed more than once in 6 patients (85.7%) with a median of 2.0 [1.0–4.0] interventions. PTA was unsuccessful in patient 7 (28.6%). Three patients (42.9%) underwent PTA for unilateral or bilateral RAS with stenting. Four children (57.1%) had balloon dilatation and stenting of the aorta (for patient 2 at the level of the thoracic aorta, for the remaining patients at the abdominal aorta).
Surgical Treatment
Two patients (25.0%) underwent surgery for aortic coarctation: patient 2 underwent extended coartectomy of the aortic arch with term-terminal anastomosis and patient 8 an aortorenal bypass and a splenorenal bypass (see below for further details). Patient six’s parents rejected surgical revascularization for potential complications.
Outcome
At the end of the study, all patients were on antihypertensive treatment with a median of 3 [1–3] agents. Among the 6 patients that underwent a successful PTA procedure, two (25.0%) had good BP control, two (25.0%) acceptable, but one (12.5%) remained in stage 1 HT, and another (12.5%) in stage 2 despite antihypertensive treatment. One patient (12.5%, Patient 3) died during follow-up due to congestive heart failure. All patients had normal renal function and ophthalmological examination at the end of the follow-up period.
Patients’ Treatment Details and Follow-Up
Patient 1
A 3-day-old boy presented with hypertension. His echocardiogram demonstrated distal thoracic aorta coarctation. Angiography and CTA confirmed MAS diagnosis (Fig. 2A–C). Owing to his young age, it was decided to start medical treatment only. Nevertheless, due to persistent HT despite optimized medical treatment, at the age of 6.9 years, he underwent a PTA of the descending thoracic aorta where 3 stents were implanted in a telescopic fashion (Fig. 3A, B, videos 1–4). His subsequent CTA revealed a reduction of the previously demonstrated collateral formation. He is currently on decreasing doses of his three antihypertensive agents with acceptable BP measurements 8 months after his last procedure.
Computed tomography angiography (CTA) of patient 1. A, B Three-dimensional CTA showing a hypoplastic thoracic aorta and another segment of the distal descending aorta with collateral circulation. Additionally, it demonstrates hypoplastic abdominal aorta, renal, and iliac arteries. C CTA images showing the hypoplastic thoracic aorta with calcification at this level
Catheter angiography of patient 1. A Angiography taken before the procedure showing hypoplastic thoracic aorta with prominent collateral circulation before percutaneous transluminal angioplasty. B Angiography after performing a percutaneous transluminal angioplasty with implantation of 3 stents at the level of the descending thoracic aorta
Patient 2
An 11-day-old male new-born was referred for evaluation of a heart murmur. His echocardiogram revealed a thoracic aortic coarctation. A PTA of this region was subsequently performed with good results. At 2 months of age, he presented at the emergency department with shortness of breath and feeding difficulties. His repeated echocardiography demonstrated recoarctation of the aorta at the isthmus level, undergoing a second PTA, without significant improvement. Hence, surgical intervention with extended coartectomy with term-terminal anastomosis was performed 10 days later. Despite initial success, he required three further PTAs of the thoracic aorta due to recoarctation suspected by echocardiography (Fig. 4A–C). Additionally, a stent was placed at the level of the proximal descending aorta during the latter procedure. At the age of 1.7 years, an echocardiogram revealed stent stenosis. Therefore, he underwent PTA of the implanted stent and a stent within a stent implantation. He was diagnosed with MAS after a CTA was performed at 2.3 years (Table 2). Owing to his young age and the high complexity of his arterial disease, surgical intervention was excluded at that time. At the age of 7.0 and 8.5 years, he underwent two further PTAs for in-stent restenosis. He is currently on 3 antihypertensive agents with stage 1 HT, 1.3 years after his last procedure.
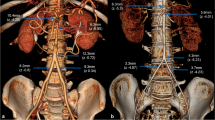
Computed tomography angiography (CTA) of patients 2, 3, and 7. A, B CTA of Patient 2 showing the stents placed at the descending thoracic aorta. C Hypoplastic descending thoracic aorta starting from the last stent, with a diameter of 3 × 3 mm and a length of 30 mm (red arrow). D CTA of patient 3 showing hypoplastic abdominal aorta. E, F Three-dimensional CTA images of patient 7 showing aortic coarctation of the abdominal aorta, narrowing of the visceral branches (coeliac trunk and superior and inferior mesenteric artery), mild narrowing of the ostium of the right renal artery and narrowing of the left renal artery
Patient 3
She was prenatally diagnosed with tricuspid valve dysplasia with severe tricuspid valve regurgitation. Furthermore, her fetal cerebral magnetic resonance imaging showed subependymal cysts at the caudothalamic groove, right ventriculomegaly, and suspected bilateral germinal matrix hemorrhage. At 10 days of age, while admitted at the neonatal intensive care unit, she presented with significant BP discrepancy between upper and lower extremities. Thus, a CTA was performed confirming MAS diagnosis (Fig. 4D). As aforementioned, this patient additionally had right aortic arch, diffuse hypoplastic pulmonary arteries, and severe obstruction of the origin of the left carotid artery with a hypoplastic vessel. She underwent a catheter angiography at 20 days of age with aortoiliac balloon dilatation. Following this, she achieved acceptable BP control. Surgical intervention was excluded for the high complexity and widespread of the arterial and venous disease. She presented clinical deterioration with heart failure despite optimisation of medical therapy and she died at 36 days of age.
Patient 4
A 2.9-year-old boy was referred for HT. CTA confirmed MAS diagnosis. He has not required any interventions. However, his BP recently rose to stage 2 HT and he is currently on increasing doses of his antihypertensive treatment.
Patient 5
A 4.9-year-old girl was referred for incidental HT. Her CTA was in keeping with MAS diagnosis. For uncontrolled HT and severe bilateral RAS, she underwent a PTA of the abdominal aorta and a bioresorbable stent was placed in each renal artery at the age of 5.3 years. One month later, due to refractory HT, she underwent an unsuccessful PTA attempt of the left renal artery. As no hypoperfusion renal areas were observed during the procedure, it was decided not to perform further attempts. At the age of 8.5 years, she continued to be hypertensive despite optimized medical treatment. Furthermore, she was symptomatic with headache and dyspnea on exertion. She subsequently underwent another PTA of the right renal artery with insertion of a bioabsorbable stent. Although her BP improved, she remained hypertensive. At 10.9 years, her CTA showed a re-stenosis of the right renal artery. Hence, she underwent a PTA of this vessel and a stent was placed at the coeliac artery, at the abdominal aorta and at the right renal artery. This latter procedure ameliorated her symptoms but had minimal impact on her BP. At the age of 12.4 years, she continued to be hypertensive and a slight increase of her LVH was observed. A renal ultrasound was therefore performed and revealed diminished arterial perfusion of the right kidney. Thus, she underwent another PTA of the right renal artery. She is currently asymptomatic with good BP control with three antihypertensive medications after 6.5-year follow-up.
Patient 6
A 14.0-year-old boy presented with incidental HT. His MRA confirmed MAS diagnosis. At that time, his parents refused aortic surgery due to potential complications. After three years of medical treatment, he had uncontrolled BP and developed claudication. For these reasons, at the age of 17.8 years, he underwent a PTA of the left renal artery with stent implantation with significant improvement of his BP. Two months later, he underwent stent dilatation and a PTA of the abdominal aorta, where another stent was inserted. His BP and symptoms initially improved although he is currently hypertensive (stage 2 HT) in the context of poor medication adherence.
Patient 7
A 4.1-year-old boy was referred for incidental HT. The CTA performed was in keeping with MAS diagnosis and he was subsequently started on medical treatment with initial acceptable BP control. At the age of 6.6 years, for persistent HT and in view of progressive left renal artery stenosis and reduced left renal volume on his CTA (Fig. 4E, F), he underwent an unsuccessful attempt at the left renal artery dilatation. At the age of 6.8 years, he had another unsuccessful attempt. It was then decided to continue on medical therapy and not to perform further attempts. He is currently asymptomatic with stage 1 HT on three antihypertensive agents after 6.7-year follow-up.
Patient 8
An 8-month-old girl was admitted to PICU as she presented with heart failure symptoms. During her admission, she was found to be hypertensive and an angiography confirmed MAS diagnosis. To resolve the right renal artery stenosis, she underwent right aortorenal bypass using femoral artery homograft with initial good result. At the age of 1.0 year, due to partial occlusion of the right renal artery observed on her follow-up CTA, she required a PTA of this artery with a stent placement. A repeated CTA at 1.5 years showed severe left renal artery stenosis for which she underwent a left splenorenal bypass. Nevertheless, there was a left renal function loss right after the latter surgery. Her follow-up CTAs demonstrated progressive in-stent stenosis, requiring a PTA of the right renal artery stent at 3.3 years of age. She is currently with good BP control on monotherapy after 10.1 years of follow-up.
Discussion
MAS is a rare disease mainly affecting children and young adults [8, 20, 21]. Nevertheless, recently, Tummolo et al. reported a lower median age (2.7 years) at presentation in patients with MAS [6]. A similar pattern was found in our study were a median age at diagnosis [2.6 (0.2–4.7) years] was witnessed, with the youngest patient being diagnosed at 3 days of age. This trend toward a younger age at diagnosis could potentially be the result of an earlier detection of the disease reflecting a higher awareness of MAS diagnosis along with improved diagnostic techniques [6, 7]. As shown previously, incidental HT was the most common presenting feature among our patients [2].
In line with previous reports, all the patients from our study had RAS [8]. Nonetheless, none of them had renal dysfunction at the end of the study period, conversely to other studies reporting an incidence of up to 24% of a chronic kidney disease [6]. Besides, all our patients but one had involvement of splanchnic arteries. However, none of them presented mesenteric ischemia symptoms, potentially due to extensive collateral circulation [7]. This is consistent with previous reports where no patients presented mesenteric ischemia [6]. Moreover, our findings and those from prior studies concordantly show similar mortality rate, which was 12.5% in our study [6].
A genetic condition is found in less than 10% of patients with MAS [7]. This concurs with our results, where one patient (12.5%) was genetically and clinically diagnosed with Myhre syndrome, disease that has been associated with aortic coarctation, and mild narrowing of the descending aorta [22, 23]. Furthermore, another patient was clinically diagnosed with NF1 which has been previously reported to be associated with MAS [6]. In addition, one patient was found to carry a VUS in LZTR1 and TSC1 genes. A pathogenic variant of these genes has already been described in patients with Noonan syndrome and tuberous sclerosis, respectively. Despite aortic coarctation having been rarely reported in Noonan syndrome and coarctation of the abdominal aorta and renal artery stenosis in tuberous sclerosis, it is difficult to determine the contribution of these two variants in our patient’s aortic phenotype [24, 25]. Another patient was found to carry a pathogenic mutation in HNF1B gene which has been described associated with congenital anomalies of the kidney and urinary tract; nevertheless, to our knowledge, MAS has not been described associated with this mutation [26].
In our study, medical therapy represented the first-line treatment for all patients. Panayiotopoulos et al. reported that medical management was able to control BP and to reduce the risk for complications [7, 27]. In the present study, the only patient that was treated with antihypertensive agents alone had stage 2 HT at the end of the follow-up period. Nevertheless, this increase in BP was recently found and he is currently on increasing doses of his monotherapy.
Long-term outcomes of endovascular treatment in MAS are not consistent in all studies [28]. Some suggest that it has a transient benefit in patients with MAS [10, 29], failing to produce a lasting clinical and angiographic improvement [7, 8, 20, 21]. On the other hand, more recent reports describe PTA to be efficacious in patients with MAS providing good long-term clinical outcomes [28, 30]. In our study, PTA was performed more than once in 6 patients (85.7%) for persistent HT secondary to a narrowing of the abdominal aorta and/or renal arteries, suggesting a transient benefit of angioplasty in these patients as previous studies reported; nevertheless, lower rates of reintervention compared to our results had been described (16.7%) [6]. This could be explained by the fact that, in our center, a primary angioplasty strategy was preferred in order to enable a full adult growth to consider surgical approach, when better outcomes have been reported [6, 31]. Additionally, PTA allowed good/acceptable BP control in four out of the 6 patients that underwent a successful PTA procedure. In two of these patients, these procedures were done 16 days before the death (patient 3) and 1 month before the end of the study period (patient 8). Hence, longer-term follow-up with a larger sample size is warranted to determine long-term benefit of this procedure in these patients.
The fact that only 1 patient from our series underwent surgery for MAS could be due to the median age at last follow-up was 10.7 [7.6–11.4] years. Previous studies have described a preference to postpone surgical reconstruction until adolescence, if clinical situation allows it, as prosthetic vascular conduits lack growth potential. Therefore, its use in young pediatric patients may require a further surgical intervention during or after puberty, when idiopathic MAS appears to stabilize and best long-term results have been achieved [5,6,7]. In this regard, prior studies proposed medical treatment as the first step, angioplasty (including stent implantation) as a second choice and to reserve surgery to older patients, when surgical approach tends to be the definitive treatment [6, 32]. Nevertheless, the timing of surgical intervention is controversial and difficult to decide as surgical therapy has also been reported successful in younger children [8, 32].
Additionally, 2 patients (25.0%) had hypoplastic carotid artery. Concomitant cerebral disease in patients with MAS has been previously described [7, 32]. Hence, cerebral imaging may be considered in these patients in order to rule out cerebrovascular disease.
Limitations
This study is limited by the small sample size and its retrospective design. Furthermore, owing to our institutional preference, angioplasty was performed as an attempt to delay surgical intervention. Therefore, it was not feasible to statistically compare the different treatment options not only due to the small sample size but also to the number of patients undergoing surgery.
Conclusion
In conclusion, this study stresses the paramount importance that regular and comprehensive BP monitoring in all pediatric patients plays in early recognition of MAS, which clinicians should contemplate in the differential diagnosis of HT. For the above-mentioned reasons and for the low incidence of this disease and the small sample size of the published studies, it is difficult to establish the best treatment approach in pediatric patients with MAS. Hence, in our institution, we preferred a more conservative strategy, with endovascular treatment as the primary strategy as an attempt to delay surgical intervention, which we demonstrated that might be a good alternative approach to surgery. Overall, a stepwise treatment approach considering medical treatment, angioplasty, and/or surgery should be individualized to the clinical characteristics of each patient, with a multidisciplinary management strategy. Nevertheless, further studies with larger sample size are warranted to better define the best treatment strategy.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Sen PK, Kinare SG, Engineer SD, Parulakar GB (1963) The middle aortic syndrome. Br Heart J 25:610–618
Lin YJ, Hwang B, Lee PC, Yang LY, Laura Meng CC (2008) Mid-aortic syndrome: a case report and review of the literature. Int J Cardiol 123(3):348–352
Goldstein SA, Evangelista A, Abbara S, Arai A, Asch FM, Badano LP et al (2015) Multimodality imaging of diseases of the thoracic aorta in adults: from the american society of echocardiography and the european association of cardiovascular imaging: endorsed by the society of cardiovascular computed tomography and society for cardiova. J Am Soc Echocardiogr 28(2):119–182
Rajakaruna R, Porter KR, Ok MS (2021) Mid-aortic syndrome. Anesthesiology 6:938
Sandmann W, Dueppers P, Pourhassan S, Voiculescu A, Klee D, Balzer KM (2014) Early and long-term results after reconstructive surgery in 42 children and two young adults with renovascular hypertension due to fibromuscular dysplasia and middle aortic syndrome. Eur J Vasc Endovasc Surg. 47(5):509–16. https://doi.org/10.1016/j.ejvs.2013.12.012
Tummolo A, Marks SD, Stadermann M, Roebuck DJ, McLaren CA, Hamilton G et al (2009) Mid-aortic syndrome: long-term outcome of 36 children. Pediatr Nephrol 24(11):2225–2232
Sethna CB, Kaplan BS, Cahill AM, Velazquez OC, Meyers KEC (2008) Idiopathic mid-aortic syndrome in children. Pediatr Nephrol 23(7):1135–1142
Panayiotopoulos YP, Tyrrell MR, Koffman G, Reidy JF, Haycock GB, Taylor PR (1996) Mid-aortic syndrome presenting in childhood. Br J Surg 83(2):235–240
Pelliccia A, Fagard R, Bjørnstad HH, Anastassakis A, Arbustini E, Assanelli D et al (2005) Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of My. Eur Heart J 26(14):1422–1445
Carretero JM, Mortera C, Prada F, Vila Cots J (2010) Hipoplasia de aorta abdominal. Tratamiento con stent. An Pediatría 73(5):295–7
Ten Dam K, Van Der Palen RLF, Tanke RB, Schreuder MF, De Jong H (2013) Clinical recognition of mid-aortic syndrome in children. Eur J Pediatr 172(3):413–416
Prakken FJ, Kitslaar PJEHM, van de Kar N, Robben SF, Leiner T (2006) Diagnosis of abdominal aortic hypoplasia by state-of-the-art MR angiography. Pediatr Radiol. 36(1):57–60
Kusel K, Zubrowski H, Weerakkody Y (2021) Sonographic findings in mid-aortic syndrome. BJR Case Rep 7(1):20200123
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 114(2 Suppl):555–76
Schwartz GJ, Feld LG, Langford DJ (1985) A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Urol 133(1):154–154. https://doi.org/10.1016/S0022-5347(17)48857-4
Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A et al (2016) European Society ofHypertension guidelines for themanagement of high blood pressure in children and adolescents. J Hypertens 34:1887–1920
Flynn JT, Falkner BE (2017) New clinical practice guideline for the management of high blood pressure in children and adolescents. Hypertension 70(4):683–686
Göknar N, Çalışkan S (2020) New guidelines for the diagnosis, evaluation, and treatment of pediatric hypertension. Turkish Arch Pediatr 55(1):11–22
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M et al (2018) 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 39(33):3021–104
Messina LM, Reilly LM, Goldstein J (1986) Middle aortic syndrome: effectiveness and durability of complex arterial revascularization techniques. Ann Surg 204(3):331–340
Connolly JE, Wilson SE, Lawrence PL, Fujitani RM (2002) Middle aortic syndrome: distal thoracic and abdominal coarctation, a disorder with multiple etiologies. J Am Coll Surg 194(6):774–781
Starr LJ, Grange DK, Delaney JW, Yetman AT, Hammel JM, Sanmann JN et al (2015) Myhre syndrome: clinical features and restrictive cardiopulmonary complications. Am J Med Genet Part A 167(12):2893–2901
Lin AE, Michot C, Cormier-Daire V, L’Ecuyer TJ, Matherne GP, Barnes BH et al (2016) Gain-of-function mutations in SMAD4 cause a distinctive repertoire of cardiovascular phenotypes in patients with Myhre syndrome. Am J Med Genet Part A 170(10):2617–2631
Zmolikova M, Puchmajerova A, Hecht P, Lebl J, Trkova M, Krepelova A (2014) Coarctation of the aorta in Noonan-like syndrome with loose anagen hair. Am J Med Genet Part A 164(5):1218–1221
Flynn PM, Robinson MB, Stapleton FB, Roy S, Koh G, Tonkin IL (1984) Coarctation of the aorta and renal artery stenosis in tuberous sclerosis. Pediatr Radiol 14(5):337–9
Bockenhauer D, Jaureguiberry G (2016) HNF1B-associated clinical phenotypes: the kidney and beyond. Pediatr Nephrol 31(5):707–714
Keith DS, Markey B, Schiedler M (2002) Successful long-term stenting of an atypical descending aortic coarctation. J Vasc Surg 35(1):166–167
Patel PA, Cahill AM (2021) Renovascular hypertension in children. CVIR Endovasc 4(1):10. https://doi.org/10.1186/s42155-020-00176-5
DiSouza SJA, Tsai WS, Silver MM, Chait P, Benson LN, Silverman E et al (1998) Diagnosis and management of stenotic aorto-arteriopathy in childhood. J Pediatr 132(6):1016–1022
Che W, Xiong H, Jiang X, Dong H, Zou Y, Yang Y et al (2018) Stenting for middle aortic syndrome caused by Takayasu arteritis-immediate and long-term outcomes. Catheter Cardiovasc Interv 91:623–631
Lobeck IN, Alhajjat AM, Dupree P, Racadio JM, Mitsnefes MM, Karns R et al (2018) The management of pediatric renovascular hypertension: a single center experience and review of the literature. J Pediatr Surg 53(9):1825–31. https://doi.org/10.1016/j.jpedsurg.2017.12.008
Stadermann MB, Montini G, Hamilton G, Roebuck DJ, McLaren CA, Dillon MJ et al (2010) Results of surgical treatment for renovascular hypertension in children: 30 year single centre experience. Nephrol Dial Transplant 25(3):807–813
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
L-BG is the primary author, involved in participants’ recruitment, drafted the manuscript, image acquisition, and image preparation. F-HP involved in participants’ recruitment, image acquisition, and critical revision of the manuscript. A-LS involved in participants’ recruitment and critical revision of the manuscript. J-SdT involved in critical revision of the manuscript. All authors have read and approved the final manuscript. JM-CB is responsible for the overall content and involved in participants’ recruitment, image acquisition and image preparation, and critical revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical Approval
This study was approved by the institutional Research Board of Hospital Sant Joan de Déu and consent waived in view of the retrospective data collection.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brunet-Garcia, L., Prada Martínez, F.H., Lopez Sainz, A. et al. Mid-aortic Syndrome in a Pediatric Cohort. Pediatr Cardiol 44, 168–178 (2023). https://doi.org/10.1007/s00246-022-03036-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-022-03036-2