Abstract
We evaluate the validity of cardiac index (CI) measurements utilizing the Ultrasonic Cardiac Output Monitor (USCOM), a non-invasive Doppler ultrasound device, by comparing measurements to cardiac catheterization-derived CI measurements in patients with single-ventricle physiology. USCOM measurements were repeated three times for each patient at the beginning of a cardiac catheterization procedure for twenty-six patients undergoing elective pre-Glenn or pre-Fontan catheterization. CI was measured by USCOM and was calculated from cardiac catheterization data using Fick’s method. Bland–Altman analysis for CI showed bias of 0.95 L/min/m2 with the 95% limits of agreement of − 1.85 and 3.75. Pearson’s correlation coefficient was 0.89 (p < 0.001) indicating a strong positive relationship between USCOM and cardiac catheterization CI measurements. When excluding two patients with significant dilation of the neo-aortic valve (z-score > + 5), the bias improved to 0.66 L/min/m2 with the 95% limits of agreement of − 1.38 and 2.70. Percent error of limits of agreement was 34%. There was excellent intra-operator reproducibility of USCOM CI measurements with an intra-class coefficient of 0.96. We demonstrate the use of USCOM to measure CI in patients with single-ventricle physiology for the first time, showing acceptable agreement of the CI measurements between USCOM and cardiac catheterization with a high intra-operator reproducibility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Assessment of cardiac output (CO) or index (CI) is important in managing critically ill patients [1,2,3,4,5,6]. Since the introduction of the Swan Ganz pulmonary artery catheter (PAC) to measure CO via thermodilution, modalities for monitoring CO have evolved over the years [5,6,7,8]. Despite widely used in the adult population, PAC is invasive and often not applicable in pediatric patients due to the size. A pulse power analysis method such as the PiCCO (Pulse index Contour Continuous Cardiac Output) system allows less invasive CO monitoring by thermodilution but still requires both central venous and arterial catheters of specific sizes, limiting its use based on the patient’s size [3, 5, 9, 10]. Patients with congenital heart disease, especially with single-ventricle physiology, pose more challenges as cardiac output monitoring has been mostly created for those with normal cardiac anatomy. Currently, we rely on routine hemodynamic monitoring parameters such as arterial line blood pressure, mixed venous saturation, central venous pressure, or lactate level to guide our management of patients with single-ventricle physiology in the cardiac critical care unit [1, 11, 12]. Although information amassed from these parameters is useful, it does not provide real-time CO. Cardiac catheterization is generally considered as the gold standard method to obtain CO or CI in patients with congenital heart disease, but it is an invasive procedure with its own risks and a limited applicability to critically ill patients. Cardiac output can be also derived from an echocardiogram, but the estimate may be inaccurate, and it requires the presence of sub-specialized trained personnel.
The ultrasonic cardiac output monitor (USCOM) 1A (USCOM Ltd, Sydney, Australia) is a non-invasive Doppler ultrasound monitor that provides CO or CI by measuring the velocity of blood flow through either the aortic or pulmonary valve [13]. It is a portable and relatively simple-to-use Doppler machine that automatically calculates CO or CI using its own built-in algorithm. Since its introduction for clinical use in 2001, adult and animal studies have shown that CO measured by USCOM is comparable to the value measured by PAC or echocardiogram [14,15,16,17,18,19]. However, reports of USCOM validity in the pediatric population have been limited [20,21,22,23,24,25]. Moreover, the use of USCOM has been demonstrated only in patients with two-ventricle physiology and without significant congenital heart disease. The use and validity of USCOM in patients with single-ventricle physiology have never been shown. Given their complexity and fragility, a direct and reliable assessment of CI can serve as an invaluable clinical tool in managing patients with single-ventricle physiology. In this study, we aim to investigate the agreement of CI measurements between USCOM and cardiac catheterization using Fick’s method in patients with single-ventricle physiology.
Materials and Methods
This was a single-center prospective study approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center. Patients with single-ventricle physiology scheduled for elective pre-Glenn or pre-Fontan cardiac catheterization were approached between February 2020 and March 2021, and consent was obtained from their parents or legal guardians. Patients undergoing non-elective pre-Glenn or pre-Fontan catheterization were excluded from the study.
Anesthesia Care
All patients had general anesthesia with endotracheal intubation provided by a cardiac anesthesia team. Twenty-two (85%) out of twenty-six patients were anesthetized with a combination of sevoflurane, fentanyl, propofol, and rocuronium. One patient received additional midazolam, while dexmedetomidine was used instead of propofol for another patient. Two remaining patients received sevoflurane and rocuronium along with dexmedetomidine and ketamine, or etomidate.
USCOM Cardiac Output Measurement
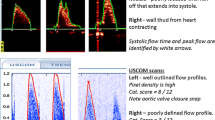
To avoid inter-operator variability, one operator trained by the USCOM company representative made all USCOM measurements. This operator was blinded to the hemodynamic data from the cardiac catheterization until all USCOM measurements were calculated and recorded. Patient’s data including the height, weight, gender, and aortic or neo-aortic valve diameter were entered into the USCOM machine. Following anesthesia induction and intubation, the USCOM Doppler measurement was obtained in a suprasternal notch position to capture blood flow through the native aortic or neo-aortic valve using the 2.2 MHz continuous-wave Doppler probe. USCOM measurements were obtained while cardiac catheterization staff was performing routine draping and preparation for the procedure, thereby not increasing total procedural or anesthetic time. The operator captured three satisfactory flow profile measurements, which are characterized by well-defined waveforms with minimal background noise with a clear audible sound within a 5-min period. CO is the product of heart rate (HR) and stroke volume (SV). The USCOM flow profile is automatically traced and integrated to derive the velocity time integral (VTI) and HR. The SV is the product of VTI and the cross-sectional area of the chosen valve, which is either neo-aortic or aortic in our patient population. [23,24,25,26] The USCOM built-in program estimates the aortic or pulmonary valve based on the patient’s height and gender. However, this is based on normal two ventricle anatomy, thereby does not apply to our study population [13, 27]. Therefore, the diameter of the neo-aortic or aortic valve was manually measured from the echocardiogram by the same USCOM operator prior to the USCOM measurements. An apical 5-chamber view or parasternal long axis view was used for the aortic or neo-aortic valve annulus measurement in mid-systole from the echocardiogram obtained within 7 days of the cardiac catheterization. When looking at the function of aortic/neo-aortic valve, all 26 patients had no stenosis of aortic/neo-aortic valve. Twenty-four patients (92%) had no or trivial aortic/neo-aortic regurgitation while two remaining patients had mild aortic/neo-aortic regurgitation. The cardiac output was indexed automatically using the body surface area based on the patient’s height and weight. Three CI measurements were captured and averaged.
Cardiac Catheterization Cardiac Output Measurement
CO or CI was calculated using Fick’s method, considered the gold standard method in patients with congenital heart disease and single-ventricle physiology [28, 29]. Arterial access was obtained through the femoral artery, and venous access was obtained through the femoral vein (for pre-Glenn evaluation) with additional right internal jugular vein access for those with Glenn physiology. All hemodynamic measurements were performed on FiO2 21%. Fick’s method uses arterial and venous saturation obtained from baseline hemodynamics along with oxygen consumption (VO2); our catheterization laboratory directly measures real-time VO2 utilizing the GE Carescape Monitor B850 and E-CAiOV module (GE Healthcare, Chicago, IL, USA) with the appropriate flow sensor (i.e., Pedi-lite or D-lite; Datex-Ohmeda Division, Instrumentarium Corp., Helsinki, Finland) to directly measure VO2 [30]. We used arterial saturation obtained from the descending aorta and venous saturation from the superior venous cava (SVC). The cardiac output was indexed by the body surface area based on patient’s weight and height.
CI Comparison
We focused on comparing CI measurements between USCOM and cardiac catheterization since it is a more meaningful hemodynamic parameter in children as compared to an absolute CO. It was noted for patients following stage I palliation with either Blalock–Taussig (BT) shunt or patent ductus arteriosus (PDA) stent, the flow across the neo-aortic valve includes both the systemic (Qs) and pulmonary (Qp) flows. Therefore, the USCOM CI value represents both Qs plus Qp in this subgroup, and we combined the Qs and Qp flows derived from cardiac catheterization when comparing to the USCOM CI value. For patients following stage I palliation with right ventricle to pulmonary artery conduit (Sano shunt) or Glenn operation, the USCOM CI value represents systemic CI (Qs) from cardiac catheterization.
Statistics
Cardiac index and heart rate, continuous variables, are described as medians with interquartile ranges (IQRs). Bland–Altman analysis was used to evaluate the agreement between the USCOM and cardiac catheterization measurements [31]. The agreement between two methods is reported with the bias (mean difference between the two methods), limits of agreement, and percent error of limits of agreement (2 standard deviations/mean). Percent error of ± 30% within limits of agreement has generally been considered as the threshold for acceptability, but recent data suggest that up to ± 45% within limits of agreement is more clinically realistic and applicable for a variety of non-invasive hemodynamic monitoring tools [31,32,33,34,35,36,37,38,39,40]. Agreement between the USCOM and cardiac catheterization measurements was also evaluated with Pearson’s correlation coefficient. The Bland–Altman plots and scattered diagrams were generated using the R software (3.6.1, R Core Team 2019, Vienna, Austria). Intra-class correlation (ICC) was computed to evaluate for intra-observer agreement using the “icc” function of the “irr” package in R (version 0.84.1; Gamer, Lemon, Fellows, and Singh, 2019; https://cran.r-project.org/web/packages/irr).
Results
Patient Characteristics
Twenty-six patients were approached and consented (Table 1). Nineteen patients were male with a median age of 24 months. The median weight and height were 8.6 kg and 74.5 cm, respectively. The most common diagnosis was hypoplastic left heart syndrome (HLHS) or its variants. Thirteen patients presented for pre-Glenn cardiac catheterization following stage 1 palliation. Of those, six patients had a Norwood with Sano shunt, six patients had a Norwood with BT shunt, and one patient had a hybrid procedure with PDA stent placement. The remaining thirteen patients had undergone Glenn operation and presented for pre-Fontan cardiac catheterization.
Overall CI and HR Comparison
The median CI measured by USCOM and cardiac catheterization were 5.95 L/min/m2 and 5.01 L/min/m2, respectively. Bland–Altman analysis demonstrates a bias of 0.95 L/min/m2 for CI with the 95% limits of agreement of − 1.85 and 3.75 (Fig. 1A). Percent error was 45% with four outliers falling beyond ± 30% of limits of agreement; three out of these four outliers were patients undergoing pre-Glenn catheterization (Fig. 1B). We observed two significant outliers in the Bland–Altman plot for cardiac index that fell beyond ± 45% of limits of agreement. These two subjects (one pre-Glenn patient and the other pre-Fontan patient) both had HLHS and were found to have significant neo-aortic valve dilation with annulus diameter z-scores of + 5 and + 7. When excluding these two outliers, the bias lowered to 0.66 L/min/m2 for CI with the 95% limits of agreement of − 1.38 and 2.70, and the percent error improved to 34% (Fig. 2).
Pearson’s correlation coefficient was 0.89 for CI (p < 0.001) indicating a strong positive relationship between USCOM and cardiac catheterization CI measurements (Fig. 3A).
Since the USCOM and cardiac catheterization measurements were not done simultaneously, we also compared the HR between the two methods. The median HR measured by USCOM and cardiac catheterization were 122 and 115, respectively. The bias for HR was 4.82 with the 95% limits of agreement of − 24.26 and 33.90, and percent error was 25% (Fig. 4). There was again a strong positive relationship in the HR measurement between the two methods with Pearson’s correlation coefficient of 0.75 (p < 0.001) (Fig. 3B).
We also observed an excellent intra-operator reproducibility with an intra-class correlation (ICC) coefficient of 0.96 for CI and 0.95 for HR, where ICC > 0.9 indicates an excellent reliability.
Comparison of pre-Glenn Versus pre-Fontan Patients
We further investigated whether there is a potential difference in agreement between pre-Glenn and pre-Fontan physiology. For the pre-Glenn group, the median CIs measured by USCOM and cardiac catheterization were 8.20 L/min/m2 and 6.73 L/min/m2, respectively (Table 2). The bias was 1.33 L/min/m2 with the 95% limits of agreement of − 1.70 and 4.36. The Pearson correlation coefficient was 0.89 (p < 0.001). For the pre-Fontan group, the median CI was lower compared to the pre-Glenn group as expected with 5.03 L/min/m2 by USCOM and 4.12 L/min/m2 by cardiac catheterization (Table 2). The bias for CI was 0.56 L/min/m2 with the 95% limits of agreement of − 1.87 and 3.00. The Pearson correlation coefficient was 0.70 for CI (p = 0.008). When excluding two outliers (one each in pre-Glenn and pre-Fontan group) with significant neo-aortic valve dilation, all pre-Fontan patients’ measurements fell within ± 30% of the limits of agreement while all Glenn group fell within ± 45% of the limits of agreement (Fig. 2).
Discussion
Our study is the first to investigate the validity of USCOM to estimate CI in pediatric patients with single-ventricle physiology and demonstrated good agreement of CI and HR measurements between USCOM and cardiac catheterization-derived cardiac output.
Determining the agreement between two different clinical modalities is not simple and has evolved over the years [31, 34, 35, 37,38,39,40,41]. Traditionally, the correlation coefficient has been widely used to evaluate the relationship between two methods’ measurements [31, 37]. Bland–Altman analysis is a newer statistical approach to compare the two methods’ measurements by focusing on the accuracy (bias), precision (limits of agreement), and repeatability/reproducibility of the new method’s measurement compared to the reference method’s measurement [31,32,33, 35, 36, 40, 42].
We noted overall positive bias in both CI and HR by USCOM (i.e., over-estimation) compared to cardiac catheterization. Since the USCOM measurements were done 5–10 min prior to cardiac catheterization hemodynamic assessment to maximize sterility of the procedure, earlier stage of anesthesia with higher HR but still within the agreement between two modalities might have led to higher CI during the USCOM measurements as CI is dependent on HR. There also was a substantial over-estimation by USCOM in the presence of valve dilation as USCOM CI measurement is calculated based on the valve diameter as well in addition to HR. The accuracy of USCOM measurements in a setting of substantial valve dilation or stenosis is not known and could be one of the limitations of USCOM.
When further comparing the degree of measurement agreement between the pre-Glenn and pre-Fontan groups, we detected a smaller bias between the USCOM and Fick’s CI measurements in the pre-Fontan group compared to those in the pre-Glenn group. This is likely due to a much wider range of CIs in the pre-Glenn group depending on the type of stage I palliation operation. USCOM’s CI measurement for those with BT shunt or PDA stent represents a combination of two cardiac outputs, systemic output, and pulmonary output, whereas that for those with right ventricle to pulmonary artery conduit represents just systemic output (one cardiac output). This results in a wide range of CIs in the pre-Glenn group, while CI measured by USCOM for pre-Fontan group represents a true systemic output.
Our data suggest that a wide spectrum of single-ventricle physiology likely affects USCOM measurements. The USCOM technology was developed based on normal two-ventricle circulations which are in series. For patients with single-ventricle physiology, their circulation at birth as well as following stage I palliation (pre-Glenn) is in parallel, with the single functioning ventricle pumping to provide both systemic and pulmonary circulation. Therefore, USCOM measurements in patients following stage 1 palliation with BT shunts or PDA stents represent both the systemic and pulmonary circulations across the aortic/neo-aortic valve. Studies have shown increased discrepancy/variability in USCOM measurements among patients in a high cardiac output state [18, 22, 32]. Although we summate Qp and Qs from catheterization to account for USCOM measurements in these patients, the residual discrepancy may be related to the limitation of USCOM at these higher flows. For those with Sano shunt, an intricate balance between systemic and pulmonary resistance determines how the output from the single ventricle gets divided between the systemic and pulmonary flows. Any minimal change in patient’s condition can deter the systemic and pulmonary output, thereby potentially accentuating discrepancy between USCOM and cardiac catheterization measurements. Thus, USCOM may be better applied in patients with Glenn and Fontan physiology, in whom the circulations are in series. For those with BT shunt or PDA stent, other traditional clinical parameters such as mixed venous saturation, lactate level, blood pressure, or evaluation of end organ function may be combined to further determine true systemic output based on total combined cardiac outputs provided by USCOM.
Given its non-invasive nature and simplicity, USCOM use has evolved in the pediatric population. Much effort has been made to establish normal cardiovascular parameters for USCOM in healthy children, and it has been shown to serve as a valuable non-invasive hemodynamic monitoring tool for resuscitation of septic shock patients in the pediatric intensive care unit [23,24,25,26, 43,44,45,46]. The use of USCOM in the postoperative pediatric patients in the cardiac intensive care unit to trend CI and its correlation with resuscitation is yet to be explored. Several studies demonstrate that USCOM measurements agreed well with other modalities including echocardiogram, PAC, or PiCCO in pediatric patients including neonates with normal cardiac anatomy, as well as adult patients with critical illness, pregnancy, and sepsis with good intra- and inter-operator reliability [15,16,17,18,19,20, 22, 26, 43, 44, 47, 48]. Further studies also have attempted to identify issues related to USCOM measurement discrepancies [20, 21, 43, 44, 47, 49, 50]. As alluded earlier, USCOM calculates SV based on VTI derived from the Doppler flow profile and valve diameter (aortic or pulmonary). However, USCOM’s built-in program that provides the valve diameter is based on data derived from children with normal cardiac anatomy. Therefore, this may lead to inaccurate USCOM CO or CI measurements in patients with congenital heart disease, especially involving valvar disease. To mitigate this source of error, the diameter measured from the echocardiogram can be manually entered into USCOM as was done in our study. Also, when comparing hemodynamic measurements between USCOM and an echocardiogram, it is to be noted that USCOM uses continuous-wave Doppler while echocardiogram uses pulse wave Doppler, which can pose as another source of error in USCOM measurement [21, 50, 51]. Pulse wave Doppler from an echocardiogram measures the flow velocity at the valve annulus, which can be optimized with direct visualization by the operator. With continuous-wave Doppler, velocity is measured along the entire length of the ultrasound beam, with no way to visualize the angle of insonation [21, 50, 51]. This raises the importance of the proficiency and reliability of the operator to capture the highest quality flow profile from only the aortic or pulmonary outflow and not additional flow from additional vessels to generate an accurate VTI [21, 50, 51]. These limitations must be considered when evaluating the use of USCOM.
In this pilot study, we demonstrate USCOM use in patients with single ventricle, who are known for their heterogeneous and intricate physiology and anatomy, for the very first time. Although our study faced heteroscedasticity of data and a small sample size due to the anatomy and physiology of our patients [41], our data demonstrate good agreement between USCOM and cardiac catheterization measurements. Additional studies focusing on patients with Glenn or Fontan physiology might be helpful when comparing the USCOM measurements with the systemic cardiac output from cardiac catheterization, which could be more applicable in daily clinical settings. The clinical use of USCOM in patients with BT shunt or PDA stent may be limited as it provides a total cardiac output or index, rather than separate systemic versus pulmonary output. A future study with a larger sample size will also help further investigate inter-operator reproducibility, which was not evaluated in our study, as well as accuracy of USCOM in the presence of significant valve dilation.
Conclusion
Direct and accurate measurements of CO or CI are crucial in managing critically ill patients. Currently available bedside modalities to estimate CI are not well validated for pediatric patients with single-ventricle physiology. USCOM is a non-invasive tool with minimal risk, and its use with proficiency and reproducibility can be mastered in a relatively short period of time when compared to training in echocardiography. Our data present preliminary evidence that the USCOM monitor may provide valuable, non-invasive, and real-time hemodynamic data to monitor patients with single-ventricle physiology, especially those following the Glenn and Fontan operations. Findings from our pilot study also facilitate further exploration to study how USCOM may be utilized to trend CI in patients in the intensive care unit to help manage and direct care.
References
Bronicki RA (2016) Hemodynamic monitoring. Pediatr Crit Care Med 17(8 Suppl 1):S207–S214
Chang AC (2012) Determination of cardiac output in critically ill children: are we any closer to the ideal methodology? Pediatr Crit Care Med 13(1):99
Huygh J, Peeters Y, Bernards J, Malbrain ML (2016) Hemodynamic monitoring in the critically ill: an overview of current cardiac output monitoring methods. F1000Res 5:2855
Lemson J, Nusmeier A, van der Hoeven JG (2011) Advanced hemodynamic monitoring in critically ill children. Pediatrics 128(3):560–571
Mehta Y, Arora D (2014) Newer methods of cardiac output monitoring. World J Cardiol 6(9):1022–1029
Vincent JL, Rhodes A, Perel A, Martin GS, Della Rocca G, Vallet B et al (2011) Clinical review: update on hemodynamic monitoring—a consensus of 16. Crit Care 15(4):229
Saugel B, Thiele RH, Hapfelmeier A, Cannesson M (2020) Technological assessment and objective evaluation of minimally invasive and noninvasive cardiac output monitoring systems. Anesthesiology 133(4):921–928
Swan HJ, Ganz W (1983) Hemodynamic measurements in clinical practice: a decade in review. J Am Coll Cardiol 1(1):103–113
Alhashemi JA, Cecconi M, Hofer CK (2011) Cardiac output monitoring: an integrative perspective. Crit Care 15(2):214
Kobe J, Mishra N, Arya VK, Al-Moustadi W, Nates W, Kumar B (2019) Cardiac output monitoring: technology and choice. Ann Card Anaesth 22(1):6–17
Chandler HK, Kirsch R (2016) Management of the low cardiac output syndrome following surgery for congenital heart disease. Curr Cardiol Rev 12(2):107–111
Charpie JR, Dekeon MK, Goldberg CS, Mosca RS, Bove EL, Kulik TJ (2000) Serial blood lactate measurements predict early outcome after neonatal repair or palliation for complex congenital heart disease. J Thorac Cardiovasc Surg 120(1):73–80
USCOM Ltd (2006) USCOM 1A user manual. USCOM Ltd, Sydney
Critchley LA, Peng ZY, Fok BS, Lee A, Phillips RA (2005) Testing the reliability of a new ultrasonic cardiac output monitor, the USCOM, by using aortic flowprobes in anesthetized dogs. Anesth Analg 100(3):748–53 (table of contents)
Horster S, Stemmler HJ, Strecker N, Brettner F, Hausmann A, Cnossen J et al (2012) Cardiac output measurements in septic patients: comparing the accuracy of USCOM to PiCCO. Crit Care Res Pract 2012:270631
McNamara H, Barclay P, Sharma V (2014) Accuracy and precision of the ultrasound cardiac output monitor (USCOM 1A) in pregnancy: comparison with three-dimensional transthoracic echocardiography. Br J Anaesth 113(4):669–676
Su BC, Lin CC, Su CW, Hui YL, Tsai YF, Yang MW et al (2008) Ultrasonic cardiac output monitor provides accurate measurement of cardiac output in recipients after liver transplantation. Acta Anaesthesiol Taiwan 46(4):171–177
Tan HL, Pinder M, Parsons R, Roberts B, van Heerden PV (2005) Clinical evaluation of USCOM ultrasonic cardiac output monitor in cardiac surgical patients in intensive care unit. Br J Anaesth 94(3):287–291
Wong LS, Yong BH, Young KK, Lau LS, Cheng KL, Man JS et al (2008) Comparison of the USCOM ultrasound cardiac output monitor with pulmonary artery catheter thermodilution in patients undergoing liver transplantation. Liver Transpl 14(7):1038–1043
Aslan N, Yildizdas D, Horoz OO, Coban Y, Demir F, Erdem S et al (2020) Comparison of cardiac output and cardiac index values measured by critical care echocardiography with the values measured by pulse index continuous cardiac output (PiCCO) in the pediatric intensive care unit: a preliminary study. Ital J Pediatr 46(1):47
Beltramo F, Menteer J, Razavi A, Khemani RG, Szmuszkovicz J, Newth CJ et al (2016) Validation of an ultrasound cardiac output monitor as a bedside tool for pediatric patients. Pediatr Cardiol 37(1):177–183
van Lelyveld-Haas LE, van Zanten AR, Borm GF, Tjan DH (2008) Clinical validation of the non-invasive cardiac output monitor USCOM-1A in critically ill patients. Eur J Anaesthesiol 25(11):917–924
Cattermole GN, Leung PY, Mak PS, Chan SS, Graham CA, Rainer TH (2010) The normal ranges of cardiovascular parameters in children measured using the Ultrasonic Cardiac Output Monitor. Crit Care Med 38(9):1875–1881
He SR, Sun X, Zhang C, Jian Z, Sun YX, Zheng ML et al (2013) Measurement of systemic oxygen delivery and inotropy in healthy term neonates with the Ultrasonic Cardiac Output Monitor (USCOM). Early Hum Dev 89(5):289–294
He SR, Zhang C, Liu YM, Sun YX, Zhuang J, Chen JM et al (2011) Accuracy of the ultrasonic cardiac output monitor in healthy term neonates during postnatal circulatory adaptation. Chin Med J (Engl) 124(15):2284–2289
Doni D, Nucera S, Rigotti C, Arosio E, Cavalleri V, Ronconi M et al (2020) Evaluation of hemodynamics in healthy term neonates using ultrasonic cardiac output monitor. Ital J Pediatr 46(1):112
Nidorf SM, Picard MH, Triulzi MO, Thomas JD, Newell J, King ME et al (1992) New perspectives in the assessment of cardiac chamber dimensions during development and adulthood. J Am Coll Cardiol 19(5):983–988
Karpman VL (1975) The theoretical analysis of Fick’s equation. On the centennial of the use of Fick’s principle in physiology. Z Kardiol 64(9):801–8
Bergersen L (2009) The congenital cardiac catheterization manual. Springer, New York, pp x, 169
Seckeler MD, Hirsch R, Beekman RH 3rd, Goldstein BH (2014) Validation of cardiac output using real-time measurement of oxygen consumption during cardiac catheterization in children under 3 years of age. Congenit Heart Dis 9(4):307–315
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307–310
Chong SW, Peyton PJ (2012) A meta-analysis of the accuracy and precision of the ultrasonic cardiac output monitor (USCOM). Anaesthesia 67(11):1266–1271
Critchley LA, Critchley JA (1999) A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput 15(2):85–91
LaMantia KR, O’Connor T, Barash PG (1990) Comparing methods of measurement: an alternative approach. Anesthesiology 72(5):781–783
Mantha S, Roizen MF, Fleisher LA, Thisted R, Foss J (2000) Comparing methods of clinical measurement: reporting standards for Bland and Altman analysis. Anesth Analg 90(3):593–602
Peyton PJ, Chong SW (2010) Minimally invasive measurement of cardiac output during surgery and critical care: a meta-analysis of accuracy and precision. Anesthesiology 113(5):1220–1235
Bland JM, Altman DG (1994) Correlation, regression, and repeated data. BMJ 308(6933):896
Bland JM, Altman DG (1995) Comparing two methods of clinical measurement: a personal history. Int J Epidemiol 24(Suppl 1):S7-14
Bland JM, Altman DG (1996) Measurement error. BMJ 312(7047):1654
Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8(2):135–160
Lu MJ, Zhong WH, Liu YX, Miao HZ, Li YC, Ji MH (2016) Sample size for assessing agreement between two methods of measurement by Bland–Altman method. Int J Biostat. https://doi.org/10.1515/ijb-2015-0039
Cecconi M, Rhodes A, Poloniecki J, Della Rocca G, Grounds RM (2009) Bench-to-bedside review: the importance of the precision of the reference technique in method comparison studies—with specific reference to the measurement of cardiac output. Crit Care 13(1):201
Fraga MV, Dysart KC, Rintoul N, Chaudhary AS, Ratcliffe SJ, Fedec A et al (2019) Cardiac output measurement using the ultrasonic cardiac output monitor: a validation study in newborn infants. Neonatology 116(3):260–268
Stewart GM, Nguyen HB, Kim TY, Jauregui J, Hayes SR, Corbett S (2008) Inter-rater reliability for noninvasive measurement of cardiac function in children. Pediatr Emerg Care 24(7):433–437
Brierley J, Peters MJ (2008) Distinct hemodynamic patterns of septic shock at presentation to pediatric intensive care. Pediatrics 122(4):752–759
Deep A, Goonasekera CD, Wang Y, Brierley J (2013) Evolution of haemodynamics and outcome of fluid-refractory septic shock in children. Intensive Care Med 39(9):1602–1609
Chaiyakulsil C, Chantra M, Katanyuwong P, Khositseth A, Anantasit N (2018) Comparison of three non-invasive hemodynamic monitoring methods in critically ill children. PLoS ONE 13(6):e0199203
Dey I, Sprivulis P (2005) Emergency physicians can reliably assess emergency department patient cardiac output using the USCOM continuous wave Doppler cardiac output monitor. Emerg Med Australas 17(3):193–199
Knirsch W, Kretschmar O, Tomaske M, Stutz K, Nagdyman N, Balmer C et al (2008) Cardiac output measurement in children: comparison of the ultrasound cardiac output monitor with thermodilution cardiac output measurement. Intensive Care Med 34(6):1060–1064
Nguyen HB, Banta DP, Stewart G, Kim T, Bansal R, Anholm J et al (2010) Cardiac index measurements by transcutaneous Doppler ultrasound and transthoracic echocardiography in adult and pediatric emergency patients. J Clin Monit Comput 24(3):237–247
Hatle L (1985) Assessment of aortic blood flow velocities with continuous wave Doppler ultrasound in the neonate and young child. J Am Coll Cardiol 5(1 Suppl):113S-S119
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koh, W., Schneider, K.A., Zang, H. et al. Measurement of Cardiac Output Using an Ultrasonic Cardiac Output Monitor (USCOM) in Patients with Single-Ventricle Physiology. Pediatr Cardiol 43, 1205–1213 (2022). https://doi.org/10.1007/s00246-022-02840-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-022-02840-0