Abstract
The aim of this study was to test the feasibility of measuring right ventricular (RV) volumes, ejection fraction (EF), and systolic function indices in neonates using three-dimensional speckle-tracking echocardiography (3D-STE). Thirty-eight neonates underwent complete echocardiographic evaluation, including the acquisition of three full-volume 3D datasets or more from each of the apical, parasternal, and subcostal windows while naturally sleeping. Datasets were analyzed using a commercially available software (Tomtec). Global RV 3D volumes and EF were measured. In addition, 2D free wall longitudinal strain (LS), tricuspid valve annulus (TVA), tricuspid annular plane systolic excursion (TAPSE) and its index to RV length (TAPSEi), and fractional area change (FAC) were obtained from a non-shortened apical 4-chamber view of the RV, derived from the 3D dataset. Three or more datasets obtained from the apical window were available for analysis for each subject. At least one dataset was adequate for analysis in all subjects. Mean indexed 3D diastolic, systolic, stroke volumes, and EF were measured at 28.5 ± 3.4 ml/m2, 13 ± 2.0 ml/m2, 15.6 ± 1.9 ml/m2, and 54.6 ± 3.2%, respectively. Free wall 2D LS was calculated at (− 27.9 ± 2.5%). In addition, mean TVA measured 11.1 ± 0.8 mm, TAPSE measured 6.8 ± 0.9 mm, and TAPSEi and FAC were calculated at 24.2 ± 2.1 and 46 ± 3.4%, respectively. 3D-STE is feasible in normal neonates without the need for sedation. Reference values of RV 3D volumes and 2D indices of systolic function were obtained. These data could be helpful in patients where the size or systolic function of the RV is in question. Larger studies are required to establish nomograms for the above indices in this age group.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Assessment of RV dimensions and function is among the challenging tasks in the echocardiography laboratory. Smith et al. eloquently demonstrate the need for reliable quantitative assessment of the RV, especially by pediatric echocardiographers [1]. Historically those measurements have been estimated using M-mode (MM) and 2D echocardiography [2,3,4], with several and serious limitations. Over the last two decades, 3D echocardiography (3DE) has been reported to be a robust tool in the evaluation of the left ventricular (LV) volumes and function in clinical practice, both in adult and child [5,6,7]. However, evaluation of RV function has been hampered by its complex geometrical shape that does not fit any known mathematical model, the presence of an infundibulum, trabecular myocardium, and muscle bundles. In spite of all the work that has been done, the need for normative LV and RV data and validation against gold standards in neonatal population remains.
Speckle-tracking echocardiography permits the tracking of chamber walls irrespective of the angle of incidence of the ultrasound beam. 3D-STE has been found to be very useful in the evaluation of the LV deformation at different age groups and LV strain derived from 3D-STE has been validated against CMRI in children and adults [8]. This modality was found to be useful in the evaluation of the LV mechanics and volumes in neonates as well [9]. Moreover, the RV volumes obtained by 3D-STE were reported to correlate well with volumes obtained from MRI, both in healthy adults and children and post repair of congenital heart lesions [10,11,12]. More recently, RV deformation data using 3D-STE-based software specialized for the RV has been reported and validated against MRI [13, 14]. The potential application of this new modality in evaluating the dimensions and function of the RV in neonates has not been fully explored.
The outcome of patients with persistent pulmonary hypertension was reported by Malowitz et al. [15] to be related to RV systolic indices. Moreover, the function of the tripartite RV systolic function was found by Chikkabyrappa et al. [16] to be a better tool to assess the RV systolic function than the biplane traditional method. Recently Balasubramanian et al. [17] correlated the outcome of patients operated for hypoplastic left heart syndrome, with biplane fractional area change of the RV. Hence establishing a robust, reproducible, and geometrical free modality to evaluate the RV size and systolic function will add to the diagnostic power of echocardiography in the management of those patients.
In this study, we sought to analyze full-volume 3D datasets of the RV obtained from apical window using a recently modified commercially available 3D software and to obtain reference values for 3D volumes, EF, TAPSE, FAC, and LS of the RV free wall in a group of healthy neonates.
Methods
Study Population
Fifty consecutive normal full-term neonates were recruited prospectively to undergo screening 2D and 3D echocardiography for the purpose of measuring ventricular volumes and systolic function. All subjects were born in one institution and were either awaiting the discharge of their mothers or presenting for their first well baby checkup.
The inclusion criteria were (1) parental consent for echocardiographic evaluation, (2) full-term pregnancy (more than 37 weeks gestation), and (3) completely normal results on 2D and Doppler echocardiography, with normal chamber size and systolic function. Exclusion criteria were (1) structural congenital lesions or significant valve regurgitation, (2) abnormal cardiac rhythms, (3) age more than 4 weeks, and (4) hypertension and/or acute illnesses.
Data Analysis
All echocardiography studies were performed by an experienced cardiac sonographer using iE 33 platform scanner (Philips Medical Systems, Andover, MA). Matrix array transducer (S8) was used for the routine M-mode, 2D, and Doppler study, and a dedicated 3D full sampling transducer (X7) was used for the acquisition of the 3D full-volume datasets. Studies were performed with the babies asleep or quietly feeding on a bottle of formula; attention was made to acquire the datasets during the resting phase of the periodic breathing or during shallow breathing to minimize heart translation and breathing artifacts. Several, full-volume datasets were acquired from each: the apical, parasternal, and subcostal transducer position. Only datasets obtained from the 4-chamber view were used in the analysis.
The echocardiograms were transferred to a stand-alone Tomtec server (Tomtec Imaging Systems, Unterschleissheim, Germany) and were analyzed offline. 3D datasets (without stitching artifacts and containing the whole of the RV without significant area of dropout in the free wall) were analyzed for each subject, using the latest 4D RV-FUNCTION-2.0 software, (Tomtec Imaging Systems, Unterschleissheim, Germany). The same experienced observer analyzed and reported the 3D study on each subject.
The 4D RV-FUNCTION 2.0 software is a semi-automated program that requires the operator’s input in the initial steps to identify certain landmarks and later in the process for contour adjustments. Initially, the system will slice the full volume and automatically generate five end-diastolic 2D frames of the ventricles: three are used to identify and mark the LV and two for the RV. Sequentially, a short-axis cut at mid ventricular level is automatically developed (Fig. 1). The operator initially identifies the LV apex and mitral valve in each 4 and 2 chamber views. On the remaining two longitudinal frames, the mid TV and the RV apex are marked. Finally, the operator places 2 landmarks corresponding to the aortic valve hinge points (AV1–2) on the LV apical long-axis view (Fig. 1). When this step is completed a short-axis cut at the mid ventricular level is generated. On this image, the anterior (AJL) and posterior junction (PJL) of the RV free wall with the interventricular septum are identified and marked. Finally, the septum-to-RV free wall distance at mid ventricular level is set. This will conclude the alignment of the 3D image.
Operator identifies the LV apex and mitral valve in the 4 and 2 chamber views of the LV and the TV and the RV apex in the remaining two longitudinal frames of the RV. On the LV apical 3-chamber view, the operator places two landmarks corresponding to the aortic valve hinge points (AV1–2). In the last short-axis view two points are placed on the anterior and posterior junctions between the septum and the free wall of RV. AJ anterior junction, AV aortic valve, Ch chamber, LV left ventricle, PJ posterior junction, RV right ventricle, SAX short axis, TV tricuspid valve
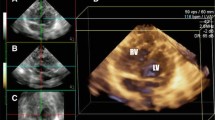
The software, using reflected speckles, automatically traces the boundaries of the RV in the longitudinal 4-chamber view at end diastole and end systole and in three corresponding short-axis cuts at preset levels of the RV. Following automated tracing of the endocardium by the system, additional manual adjustments are feasible. Whenever, the final adjustments are approved by the operator, the RV endocardial surface is automatically traced over the entire cardiac cycle and values of RV diastolic, systolic, and stroke volumes in addition to EF are provided (Fig. 2). The change in RV volumes over time is depicted as a dynamic 3D cast (Fig. 3).
RV endocardial surface is automatically traced over the entire cardiac cycle and is depicted in 3 short and one 4-chamber cuts, both in diastole and systole. Values of RV diastolic, systolic, and stroke volumes and EF are provided. Adjustments in every image and frame are feasible. EDV end-diastolic volume, EF ejection fraction, ESV end-systolic volume, Ch chamber, RV right ventricle, SAX short axis, SV stroke volume
In addition to volumetric data, 2D measurements including tricuspid valve annulus (TVA) diameter, TAPSE, FAC, and free wall LS could be extracted from a non-shortened 4-chamber image derived from the same 3D dataset. Using STE, TAPSE was calculated as the difference in the distance between RV apex and the lateral aspect of the annulus at end diastole and systole. When measuring FAC, the trabeculations were included in the cavity of the RV in diastole. The free wall LS is an automated measurement as well. It was calculated as the difference in length of the line tracing the endocardial surface of the RV free wall from the apex to the lateral TV annulus in diastole (L0) and systole (L1) as per the following formula: LS = (L0 − L1/L0) * 100 (Fig. 4).
Non-foreshortened 2D images of the RV at end diastole and end systole. The free wall LS was calculated as the difference in length of the line tracing the endocardial surface of the RV free wall from the apex to the lateral TV annulus in diastole. LS longitudinal strain, RV right ventricle, TV tricuspid valve
Inter-observer and intra-observer variability of the measurements was assessed in 10 randomly selected subjects. To assess intra-observer variability, the same observer (Z.B.) repeated the dataset analysis 1 month following the initial analysis to avoid recall bias. To assess inter-observer variability, the whole analysis was performed by a second observer (G.S.), who was blinded to the results of the first observer (Z.B.).
Statistical Analysis
Descriptive statistics are expressed as means and standard deviation (± SD) for continuous variables and frequencies and percentages for categorical variables. The correlation between each of the 3D derived parameters: end-diastolic volume (EDV), stroke volume (SV), and EF on one hand and BW and BSA on the other was assessed using scatter plots and Pearson correlation coefficient. The same analysis was applied on the 2D parameters: free wall LS, FAC, and TAPSE. Finally, inter-rater agreement between reader 1 and reader 2 for EDV, ESV, SV, LS, and FAC measurements, as well as the intra-rater agreement between reader 1 and reader 1 repeat was assessed using the intraclass correlation coefficient. P-value < 0.05 was used to indicate statistical significance. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, version 24).
Results
Ten parents refused to consent for this study. Additionally, two babies had more than trivial shunting lesions (one with atrial septal defect and another with a large patent ductus arteriosus); both were excluded from further data analysis.
The final study group consisted of 38 neonates. Previously, we reported on left ventricular volumes and strain using 4-chamber 3D datasets obtained from this group [8].
The mean age was 3.7 ± 4.7 days. Mean weight and body surface area (Du Bois formula) were 3.1 ± 0.5 kg and 0.19 ± 0.02 m2, respectively. Full demographic data are presented in Table 1. Mean time for the completion of the echocardiographic study including the acquisition of the 3D datasets was 42 ± 9 min (range 17–51). Mean heart rate at the time of the acquisition of the dataset that was used in the analysis was 124.7 ± 11.3 beats per minute. The mean frame rate of the analyzed datasets was 50.1 ± 13.3. At least one dataset acquired from the apical window was adequate for analysis in every subject.
The mean end diastolic, systolic, and stroke volumes were measured at 5.6 ± 1.1, 2.5 ± 0.5, and 3.0 ± 0.6 ml, respectively. The resultant calculated 3D EF was 54.6 ± 3.2%.
From a non-foreshortened 4-chamber view of the RV generated from the 3D dataset, several 2D measurements were automatically presented by the software. The mean dimension of the tricuspid annulus was 11.1 ± 0.8 mm and lateral TAPSE measured was 6.8 ± 0.9 mm. In order to standardize the measured TAPSE, the value was divided by the length of the RV. The RV length was defined as the length of the line drawn from the point of intersection of the RV free wall and the interventricular septum to the midpoint of the line joining the TV hinge points at end diastole. The mean of indexed TAPSE (TAPSEi) was calculated at 24.2 ± 2.6%. The mean FAC was 43.9 ± 2.6%.
Right ventricular free wall LS was measured at (− 27.9 ± 2.5%). Conventional 2D echocardiographic parameters are presented in Table 2.
Correlation of three-dimensional EDV, SV, and EF with BW and BSA are presented in Table 3.
In our healthy group of neonates, we found that EDV and SV values correlated well with both BW (r = 0.78, P-value < 0.0001; r = 0.81, P-value < 0.0001, respectively) and BSA (r = 0.83, P-value < 0.0001; r = 0.85, P-value < 0.0001, respectively).
The correlation of 2D variables with BW and BSA are presented in Table 4.
It was found that TVA and TAPSE are significantly correlated with BW and BSA (P value < 0.0001 for either variable). On the other hand, there was no correlation between FAC and either BW or BSA (P value = 0.99 and 0.68, respectively). Similarly, there was no association between RV LS and either the BW or BSA (P value = 0.64 and 0.62), respectively.
Intra-observer and Inter-observer Variability
The intraclass correlation coefficient (ICC) was used to measure the inter-rater agreement between reader 1 and reader 2 for EDV, ESV, SV, LS, and FAC measurements, as well as the intra-rater agreement between reader 1 and reader 1 repeat. There was an excellent strength of agreement for inter- and intra-observer variability as evident by an ICC value > 0.9 and a P value < 0.001 for all variables except FAC (Table 5).
Discussion
Our data show that 3D-STE analysis of the RV is feasible in healthy neonates. As previously published [9], obtaining 3D datasets on a sleeping or feeding neonate could be done without the need for sedation. Acquiring 3D datasets to include the RV anterior free wall is a major impediment in the 3D imaging of the RV in adults [18]. We found that maneuvering the probe from the standard apical window more medially renders imaging of the anterior wall feasible. In addition, we speculate that the presence of an anterior thymus tissue might play a role in shielding the RV anterior wall from the air-filled lung tissue. The preservation of resolution using a 7 MHz 3D probe, as the depth required to image the RV in a small neonate is relatively shallow, produced good quality images at a reasonable frame rate. On the other hand, as voluntary breath holding is not feasible in this age group, translational and stitching artifacts are expected to be common. Neonates, however, often have periodic breathing characterized by a pause following a phase of fast breaths. We attempted to begin the acquisition following the fast breathing phase as there is usually a short pause or a period of shallow breathing, which minimizes breathing artifacts. Despite all the above some datasets were deemed unsuitable for analysis. On average three datasets were obtained from the apical window on each baby; from those at least one set was adequate for analysis.
The recent version of the RV analysis software from Tomtec (4D right ventricle 2) allows for flexible adjustments by the operator, which are crucial when analyzing a small ventricle. We found that frame by frame adjustments of the endocardial tracings in all four planes provided by the software were necessary. Previous versions of this software were suboptimal for the accurate measurement of the RV in this population. Attempts to perform RV volumetric analysis with Version 1.2 of this software were unsuccessful as the automated tracing of the endocardium was suboptimal and manual manipulation of the tracings were limited.
In this study we obtained reference values and ranges for the RV volumes in a healthy group of neonates. 3D obtained volumes were presented indexed to WT and BSA, as we found that both EDV and SV correlated well with either BW or BSA. To the best of our knowledge this is the largest study to report on RV volumes and EF in normal neonates, using Tomtec software.
In an attempt to validate RV volumetric measurements by 3DE, Schindera et al. [19] measured volumes of RV casts of excised human and porcine heart of varying sizes and shapes and concluded that real-time 3D echocardiography, using a novel disc summation method of angled longitudinal and cross-sectional scan planes, can accurately estimate the true volumes. In comparison to MRI, Leibundgut et al. [20] reported on 100 consecutive adult patients with normal or diseased RV and found a good correlation between 3D RV and MRI, with the latter tending to have higher volumes. They attributed this to the difference in the methods used in the analysis. Although MRI has validated 3D echocardiographic measurements in other populations, validation of neonatal measures by CT, MRI, or other methodology should be undertaken as well.
Kutty et al. [21] using an older version of the Tomtec software serially measured RV volumes in patients with hypoplastic left heart syndrome and compared the results to 18 normal infants aged 0–6 months. Of interest, they were able to analyze all 63 studies. In the current study, we could analyze all subjects, only because several datasets were obtained on each subject, out of which one was suitable for analysis. Although, their control group had a wider age range (0–6 months), the EF reported was similar to the EF in our healthy neonates.
Tamborini and his group [22] reported on a large series in normal adults and found that RV volume measurements by Tomtec using 3DE are both feasible and reproducible. RV volumes in their series, as in ours, correlated well with BSA.
Establishing l RV volumes in this age group has significant clinical implications. In newborns suffering from RV inflow or outflow tract obstructive lesions, the “size” of the RV might be crucial in determining which interventional course is to be followed. The feasibility of imaging and measuring RV volumes in 3D and having reference values could be useful in planning palliative or corrective surgery in neonates with congenital heart disease affecting the RV. Moreover, decreased RV systolic function can be valuable predictor of outcome, in sick neonates with a variety of cardiopulmonary disease of newborn. So far, the lack of a clearly defined quantitative parameter for functional assessment of the RV has been a major impediment to early identification of the development of RV dysfunction in newborn infants [23, 24]. Having a robust and reproducible tool to measure RV systolic function will add objectivity to the clinical management of sick neonates.
In addition to 3D volumetric data, the Tomtec RV analysis software automatically generates a non-foreshortened 2D image of the RV. From this image, more traditional RV measurements, like TV annulus, FAC, TAPSE and RV free wall LS, are provided. Using 2D images and including the trabeculations within the cavity of the RV, Levy et al. [25] had reported FAC of 33 ± 5% in full-term newborns. The disagreement with our results might be due to ventricular foreshortening. The 4-chamber view obtained from a 3D dataset, as in our study, is with minimal or no foreshortening leading to larger diastolic volumes and hence larger FAC. Moreover, as shown in the corresponding figure presented in their article, demonstrating how the area was traced, the level of measurement was more posterior at the level of the coronary sinus, as compared to the level at which our measurements were made. Different levels of measurements could potentially produce different values. Prospective studies comparing FAC from 2D images to those derived from the 3D datasets in this age group as well as others would be of interest.
TAPSE is another readily available parameter obtained from the 3D dataset analysis. The advantage of measuring TAPSE from 3D, in addition to abolishing foreshortening, is in its use of angle-independent speckle tacking of the annulus rather than the angle-dependent M-mode traditional method. Our results agree with those obtained by Ghandi et al. [26]. We found that TAPSE correlated very well with BW and BSA. To standardize the value, we index it to the length of the RV, a readily available measurement that could be done at the same time TAPSE is obtained.
Two-dimensional RV Free wall LS could be measured from an apical 2D image obtained from the 3D dataset. Using Tomtec software, LS of the free wall, defined as the wall of the RV between the lateral TV annulus and the RV apex, could be calculated based on the difference in length between the end diastolic and end-systolic frames. Although our 2D images are obtained from a 3D dataset which has a relatively low frame rate, numbers were higher than those reported by Jain et al. (− 22 ± 4%). The difference might be explained by the difference in algorithms used by different venders [27].
Limitations
Optimal image acquisition and quality are important aspect for the analysis of 3DE. Fundamental limitations of ultrasound, such as reverberation and shadowing, and acquisition shortcomings like movement and stitching artifacts, can limit feasibility of 3DE. In newborns, most of these limitations are overcome because of the usual, very good image quality and the shallow depth of field required. Despite that, it was necessary to acquire several datasets to arrive at the level of feasibility attained in our study. This might become a limitation when using this modality in the clinical arena.
The relatively low frame rate of 2D imaging coupled to high heart rates of newborns could potentially lead to underestimating strain values. This become more significant when the 2D image is obtained from a 3D dataset with its inherited low frame rate.
This was a single-center study with a relatively small sample size. Our population was limited to Middle Eastern origin and included neonates with normal hearts. Further larger studies including different ethnic groups and newborns with heart disease are needed.
Finally, the RV volumes obtained by 3DE in this age group need to be validated; moreover, values in this study may not be applicable to other software due to inter-vendor variability.
Conclusion
3DE of the RV is feasible and reproducible in neonates without the need for sedation. Using Tomtec software, reference values of RV 3D volumes and EF were obtained. These data could be potentially helpful in patients where the size or the systolic function of the RV is questionable. In addition, 2D images dissected from the 3D datasets were used to provide normal ranges of 2D systolic function indices, including TAPSE, FAC, and free wall LS. Studies with larger number of subjects are needed to set nomograms for this age group.
Data Availability
Available upon request.
References
Smith A, Purna JR, Castaldo MP et al (2019) Accuracy and reliability of qualitative echocardiographic assessment of right ventricular size and function in neonates. Echocardiography 36:2285–2285. https://doi.org/10.1111/echo.14409
Ho SY, Nihoyannopoulos P (2006) Anatomy, echocardiography, and normal right ventricular dimensions. Heart 92(Suppl_1):I2–I13. https://doi.org/10.1136/hrt.2005.077875
Foale R, Nihoyannopoulos P, Mckenna W et al (1986) Echocardiographic measurement of the normal adult right ventricle. Heart 56(1):33–44. https://doi.org/10.1136/hrt.56.1.33
Lai WW, Geva T, Shirali GS et al (2006) Guidelines and standards for performance of a pediatric echocardiogram: a Report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr 19(12):1413–1430. https://doi.org/10.1016/j.echo.2006.09.001
Sugeng L, Mor-Avi V, Weinert L et al (2006) Quantitative assessment of left ventricular size and function. Circulation 114(7):654–661. https://doi.org/10.1161/circulationaha.106.626143
Jacobs LD, Salgo IS, Goonewardena S et al (2005) Rapid online quantification of left ventricular volume from real-time three-dimensional echocardiographic data. Eur Heart J 27(4):460–468. https://doi.org/10.1093/eurheartj/ehi666
Friedberg MK, Su X, Tworetzky W et al (2010) Validation of 3D echocardiographic assessment of left ventricular volumes, mass, and ejection fraction in neonates and infants with congenital heart disease. Circ Cardiovasc Imaging 3(6):735–742. https://doi.org/10.1161/circimaging.109.928663
Kaku K, Takeuchi M, Tsang W et al (2014) Age-related normal range of left ventricular strain and torsion using three-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr 27(1):55–64. https://doi.org/10.1016/j.echo.2013.10.002
Bulbul Z, Issa Z, Siblini G et al (2015) Normal range of left ventricular strain, dimensions and ejection fraction using three-dimensional speckle-tracking echocardiography in neonates. J Cardiovasc Echogr 25(3):67. https://doi.org/10.4103/2211-4122.166074
Shimada YJ, Shiota M, Siegel RJ, Shiota T (2010) Accuracy of right ventricular volumes and function determined by three-dimensional echocardiography in comparison with magnetic resonance imaging: a meta-analysis study. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 23(9):943–953. https://doi.org/10.1016/j.echo.2010.06.029
Grewal J, Majdalany D, Syed I et al (2010) Three-dimensional echocardiographic assessment of right ventricular volume and function in adult patients with congenital heart disease: comparison with magnetic resonance imaging. J Am Soc Echocardiogr 23(2):127–133. https://doi.org/10.1016/j.echo.2009.11.002
Selly JB, Iriart X, Roubertie F et al (2015) Multivariable assessment of the right ventricle by echocardiography in patients with repaired Tetralogy of Fallot undergoing pulmonary valve replacement: a comparative study with magnetic resonance imaging. Arch Cardiovasc Dis 108(1):5–15. https://doi.org/10.1016/j.acvd.2014.07.054
Atsumi A, Seo Y, Ishizu T et al (2016) Right ventricular deformation analyses using a three-dimensional speckle-tracking echocardiographic system specialized for the right ventricle. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 29(5):402-411.e2. https://doi.org/10.1016/j.echo.2015.12.014
Muraru D, Spadotto V, Cecchetto A et al (2015) New speckle-tracking algorithm for right ventricular volume analysis from three-dimensional echocardiographic data sets: validation with cardiac magnetic resonance and comparison with the previous analysis tool. Eur Heart J Cardiovasc Imaging 17(11):1279–1289. https://doi.org/10.1093/ehjci/jev309
Malowitz JR, Forsha DE, Smith PB et al (2015) Right ventricular echocardiographic indices predict poor outcomes in infants with persistent pulmonary hypertension of the newborn. Eur Heart J Cardiovasc Imaging 16(11):1224–1231. https://doi.org/10.1093/ehjci/jev071
Chikkabyrappa SM, Critser P, Roane J et al (2020) Tripartite assessment of right ventricular systolic function in persistent pulmonary hypertension of the newborn. Pediatr Cardiol 41(6):1206–1211. https://doi.org/10.1007/s00246-020-02376-1
Balasubramanian S, Smith SN, Srinivasan P et al (2021) Longitudinal assessment of right ventricular function in hypoplastic left heart syndrome. Pediatr Cardiol. https://doi.org/10.1007/s00246-021-02624-y.Advanceonlinepublication
Kjaergaard J, Petersen CL, Kjaer A et al (2006) Evaluation of right ventricular volume and function by 2D and 3D echocardiography compared to MRI. Eur J Echocardiogr J Work Group Echocardiogr Eur Soc Cardiol 7(6):430–438. https://doi.org/10.1016/j.euje.2005.10.009
Schindera ST, Mehwald PS, Sahn DJ et al (2002) Accuracy of real-time three-dimensional echocardiography for quantifying right ventricular volume: static and pulsatile flow studies in an anatomic in vitro model. J Ultrasound Med Off J Am Inst Ultrasound Med 21(10):1069–1075. https://doi.org/10.7863/jum.2002.21.10.1069
Leibundgut G, Rohner A, Grize L et al (2010) Dynamic assessment of right ventricular volumes and function by real-time three-dimensional echocardiography: a comparison study with magnetic resonance imaging in 100 adult patients. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 23(2):116–126. https://doi.org/10.1016/j.echo.2009.11.016
Kutty S, Graney BA, Khoo NS et al (2012) Serial assessment of right ventricular volume and function in surgically palliated hypoplastic left heart syndrome using real-time transthoracic three-dimensional echocardiography. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 25(6):682–689. https://doi.org/10.1016/j.echo.2012.02.008
Tamborini G, Marsan NA, Gripari P et al (2010) Reference values for right ventricular volumes and ejection fraction with real-time three-dimensional echocardiography: evaluation in a large series of normal subjects. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 23(2):109–115. https://doi.org/10.1016/j.echo.2009.11.026
Mertens L, Seri I, Marek J, et al, Writing Group of the American Society of Echocardiography, European Association ofEchocardiography, Association for European Pediatric Cardiologists (2011) Targeted Neonatal Echocardiography in the Neonatal Intensive Care Unit: practice guidelines and recommendations for training. Writing Group of the American Society of Echocardiography (ASE) in collaboration with the European Association of Echocardiography (EAE) and the Association for European Pediatric Cardiologists (AEPC). J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 24(10):1057–1078. https://doi.org/10.1016/j.echo.2011.07.014
Levy PT, Holland MR, Sekarski TJ et al (2013) Feasibility and reproducibility of systolic right ventricular strain measurement by speckle-tracking echocardiography in premature infants. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 26(10):1201–1213. https://doi.org/10.1016/j.echo.2013.06.005
Levy PT, Dioneda B, Holland MR et al (2015) Right ventricular function in preterm and term neonates: reference values for right ventricle areas and fractional area of change. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 28(5):559–569. https://doi.org/10.1016/j.echo.2015.01.024
Ghandi Y, Habibi D, Farahani E (2018) Reference values of longitudinal systolic right and left ventricular function measured by M-mode echocardiography in healthy preterm and term neonates. J Cardiovasc Echogr 28(3):177–181. https://doi.org/10.4103/jcecho.jcecho_31_18
Jain A, Mohamed A, El-Khuffash A et al (2014) A comprehensive echocardiographic protocol for assessing neonatal right ventricular dimensions and function in the transitional period: normative data and z scores. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 27(12):1293–1304. https://doi.org/10.1016/j.echo.2014.08.018
Funding
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
ZRB is the principal investigator of the study and he put down the concept, designed the study, analyzed the datasets, wrote the initial draft, and finalized the article. GS helped design the study and in the analysis of the datasets. HT is the leading statistician on the paper and was responsible of data cleaning and analysis. MM assisted in the statistical analysis and interpretation of the data as well as formulation of the study tables. FB had reviewed and critically revised the initial and final drafts of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be interpreted as a potential conflict of interest.
Ethical Approval
The study was performed in accordance with the ethical standards of the Research Ethics Committee and was approved by the Research Advisory Council/Office of Research Affairs at King Faisal Specialist Hospital and Research Center.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bulbul, Z., Siblini, G., Tamim, H. et al. Right Ventricular Volumes, Ejection Fraction, and Systolic Function Indices in Normal Neonates by Three-Dimensional Speckle-Tracking Echocardiography. Pediatr Cardiol 43, 181–190 (2022). https://doi.org/10.1007/s00246-021-02716-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-021-02716-9