Abstract
Congenital ventricular outpouchings (CVOs) are rare congenital heart defects with limited data regarding prognosis and outcomes. We aimed to describe the characteristics, outcomes and factors associated with morbidity and mortality of prenatally diagnosed CVOs using our institutional experience and a review of published cases. A total of 86 cases of prenatally diagnosed CVOs were identified, including 3 from our institution and 83 cases identified from a review of the literature. Fetal and postnatal outcomes were analyzed for each case. Pericardial effusions (44%) and ventricular dysfunction (17%) were the most common associated findings. Excluding cases that resulted in pregnancy termination, mortality was 17%, with the majority (11/13) occurring in the prenatal period. Factors associated with mortality included an outpouching located on the left ventricle, a diagnosis of hydrops fetalis, the presence of a pericardial effusion, and an earlier gestational age at diagnosis. Of those that survived to delivery, 57% remained asymptomatic without the need for intervention, and the outpouching regressed or resolved in an additional 15%. Prenatally diagnosed congenital ventricular outpouchings are a dynamic form of congenital heart disease with a high fetal mortality rate. The outcomes associated with the outpouchings appear to be the most variable in the prenatal period and the first year after birth. Serial prenatal and postnatal evaluations should be performed to evaluate for a change in the characteristics of the outpouching.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congenital ventricular outpouchings (CVOs), a term often used to describe both aneurysms and diverticula, are rare congenital anomalies characterized by localized protrusions of the ventricular wall. The estimated incidence is 1 per 200,000 live births, however the actual incidence is difficult to determine as routine obstetrical imaging may not detect all affected individuals, and many remain asymptomatic after birth [1,2,3]. With advances in fetal cardiac imaging, an increasing number are now diagnosed in the prenatal period [4,5,6]. Compared to those diagnosed in the postnatal period, prenatally diagnosed CVOs may represent a unique subgroup with increased risk of morbidity and mortality.
Currently there is a paucity of data on the characteristics and outcomes associated with prenatally diagnosed CVOs and many published reports consist of case reports or relatively small series.
It is our objective to evaluate the clinical course and outcomes associated with prenatally diagnosed CVOs. To accomplish our objective, we aim to report our institutional series in addition to a summary of prenatally diagnosed CVOs published in the literature, thereby supplying a relatively large cohort of patients with this rare congenital anomaly.
Material and Methods
Fetuses with a prenatal diagnosis of CVO were identified from a retrospective review at our institution as well as from a review of previously published reports. Previously published reports were identified using PubMed.gov, Medline and Google Scholar and search terms included a combination of the following terms: aneurysm, diverticulum, ventricular outpouching, and prenatal diagnosis. The publication dates of identified reports ranged from 1990 to 2015. Echocardiographic features, CVO characteristics, prenatal course, outcomes, and postnatal course were reviewed. The Vanderbilt University Institutional Review Board for Research on Human Subjects approved this study.
Prenatal Course
Data collected regarding the prenatal course included: maternal demographic and pregnancy related information, reason for fetal echocardiogram, identified fetal cardiac and extracardiac anomalies, genetic or chromosomal abnormalities, CVO location and size, presence of ventricular dysfunction, and presence of pericardial effusion.
Outcomes
Outcomes were reported as follows: termination of pregnancy, intrauterine fetal demise (IUFD), postnatal death, status post cardiac transplantation, awaiting cardiac transplantation, medically treated for congestive heart failure (CHF), surgical intervention (including resection, plication, patch closure, or primary closure), asymptomatic, CVO regression, or CVO resolution.
Postnatal Course
Data collected regarding the postnatal course among those with live births included: patient demographic information, associated cardiac and extracardiac anomalies, genetic or chromosomal abnormalities, CVO location and size, presence of ventricular dysfunction or pericardial effusion, medical therapies, surgical procedures, and age and status at last follow up.
CVO Classification
CVOs are commonly classified in the literature as either aneurysms or diverticula based on specific histologic and/or morphologic characteristics (Table 1) [2, 5, 7,8,9,10]. The type of CVO classification used by the authors of the published cases reported in the literature was retained. CVOs at our institution were classified according to the morphologic criteria listed in Table 1.
Statistics
Patient characteristics are reported as number (percent) and median (interquartile range). We performed univariate analysis using Pearson Chi-square tests for categorical variables and Mann–Whitney U tests for continuous variables. A two-tailed p-value of < 0.05 was used to determine statistical significance. Data were analyzed using IBM SPSS Statistics Premium 22.
Results
A total of 83 published cases of prenatally diagnosed CVOs were identified from 1990 to 2015. In addition, there were 3 patients prenatally diagnosed with a CVO at our institution from 2008 to 2017. Therefore, a total of 86 patients with prenatally diagnosed CVOs were included in our analysis. Table 2 summarizes the characteristics of all 86 patients.
At our institution, there were 2 left ventricular CVOs (one apical and one free wall) and 1 right ventricular CVO. The details of these 3 cases are described further, along with the details of the 83 published cases, in Supplemental Tables I and II.
CVO Characteristics
The most frequently encountered CVOs were those classified as left ventricular aneurysms, representing 45% of all CVOs. CVOs were more commonly located on the apex as opposed to the free wall (63% vs. 37%). A CVO involving the left ventricle was more often classified as an aneurysm as opposed to a diverticulum (70% vs. 30%), whereas a CVO involving the right ventricle was more often classified as a diverticulum (73% vs. 27%), p < 0.001.
Outcomes
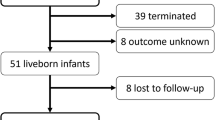
Figure 1 demonstrates the outcomes associated with all 86 identified cases of prenatally diagnosed CVOs, grouped according to the ventricle involved.
Outcome of fetuses diagnosed with congenital ventricular outpouchings (including both aneurysms and diverticula) grouped according to the ventricle involved. CVOs congenital ventricular outpouchings, RV right ventricle, LV left ventricle, IUFD intrauterine fetal demise, TOP termination of pregnancy. *Pending cardiac transplantation or status post transplantation
Mortality
Among the 86 cases of prenatally diagnosed CVOs, there were 21 non-survivors with 19 occurring in the prenatal period. Eight cases were a result of pregnancy termination and 11 were a result of IUFD. The average gestational age at IUFD was 29 3/7 weeks (range 26–37 weeks). The two postnatal deaths both occurred on the first day after birth. Excluding the cases of pregnancy termination, there were 13 total deaths (mortality rate of 17%).
Factors associated with mortality included a diagnosis of hydrops fetalis (p < 0.001), an earlier age at diagnosis (p = 0.006), the presence of a pericardial effusion (p = 0.04), and a CVO involving the left ventricle (p = 0.005). Among the non-survivors (and excluding pregnancy terminations), 12/13 (92%) had a CVO involving the left ventricle. The specific CVO type (aneurysm vs. diverticulum), the CVO location (apex vs. free wall), and the presence of other anomalies, were not associated with mortality.
Survivors
A total of 67 (78%) cases proceeded to delivery and 65 (76%) survived beyond the first day after birth. Of those (with gestational age and mode of delivery documented) 94% were delivered at 37 weeks gestation greater (median gestational age of 39 weeks) and 66% were delivered vaginally.
As shown in Fig. 1, those that survived were classified as “asymptomatic” or “symptomatic” for the purposes of our study. Cases that did not require medical or surgical intervention were classified as “asymptomatic”, while those that required medical or surgical intervention as a result of the CVO were classified as “symptomatic”.
Over a median follow up of 12 months (range 0–120 months), 57% were asymptomatic without complications reported at most recent follow up, 7.5% showed some degree of regression in the size of the CVO and another 7.5% had complete resolution of the CVO. Of those that showed complete resolution, 2 resolved in the prenatal period and all resolved before 1 year.
There was no association between CVO type as classified by the respective authors (aneurysm vs. diverticulum) or ventricle involved (left vs. right ventricle) and whether the child was asymptomatic at last follow up. In both the asymptomatic and symptomatic groups, the most common ventricle involved was the left ventricle (56% and 59% respectively), and the most common location was the apex (65% and 53% respectively).
The CVO type as classified by the respective authors, was similarly not associated with regression/resolution of the CVO, however CVOs involving the right ventricle were more likely to regress/resolve compared to left ventricular CVOs (64% vs. 36%, p = 0.03).
Of note, almost half of the published cases did not report the size of the CVO and the remaining cases used different techniques to describe the size of the CVO (cm2, diameter length, outpouching:ventricular volume or area ratios, or descriptive terms such as “large” “moderate” or “small”). Therefore, there was no reliable method to compare various groups in regard to CVO sizes.
Surgical intervention was performed in 12 (18%). The timing of intervention was not reported in 2 patients. Of those with timing of intervention reported, the majority (90%) were performed before 1 year of age, with just over half (60%) performed before 1 month of age. The surgical interventions reported included resection or plication of the CVO, double pericardial patch closure, mattress suture closure, Dor procedure, and a combined Damus–Kaye–Stansel (DKS)/Dor procedure with later conversion to bidirectional Glenn physiology. There was no significant difference in the specific CVO type or ventricle involved among patients that underwent surgical intervention versus those that did not.
Five (7.5%) were either receiving medications to treat CHF symptoms, awaiting cardiac transplantation or received cardiac transplantation. All 5 CVOs were classified as aneurysms, and all but one involved the left ventricle.
Pericardial Effusions
The most common indication for obtaining a fetal echocardiogram was concern for pericardial effusion, which was diagnosed in 38 (44%) of cases. The presence of a pericardial effusion was associated with an earlier gestational age at diagnosis (20.5 weeks vs. 22.5 weeks, p < 0.001). Pericardial effusions were more commonly associated with CVOs classified as diverticula as opposed to aneurysms (61% vs. 39%, p = 0.04), and when the CVO was located on the apex as opposed to the free wall (76% vs. 24%, p = 0.04).
Of the 38 total cases with documented pericardial effusion, spontaneous resolution of the effusion occurred in 7 (18%) with the majority (5/7) resolving in the prenatal period.
There were 14 non-survivors with 13 of the deaths occurring in the prenatal period. Excluding 5 cases resulting in pregnancy terminations, the mortality rate in this group was 24%. Of the 8 IUFD cases, 7 involved the left ventricle, 1 had a diagnosis of trisomy 18, 3 were following a pericardiocentesis (5 days to 5 weeks post-procedure), and 3 had progressively worsening ventricular dysfunction and/or enlarging pericardial effusion.
Regarding the size of the pericardial effusion, 19 were described as “large” or “massive”, 2 were “moderate”, and 4 were “small” (13 cases did not report the size of the effusion).
In utero pericardiocentesis was performed in 12 (32%). The gestational age at pericardiocentesis ranged from 14 to 25 weeks (average gestational age of 18.5 weeks). All but 1 case undergoing pericardiocentesis was described as having a “large” or “massive” pericardial effusion. All 3 cases with left ventricular CVOs that underwent pericardiocentesis had subsequent intrauterine fetal demise at an average gestational age of 30 weeks (range 26–37 weeks). Conversely, all 9 cases with right ventricular CVOs that underwent pericardiocentesis had documented resolution of the effusion and were asymptomatic at their most recent postnatal follow up.
Of the 19 cases with a large or massive pericardial effusion, 4 pregnancies were terminated and 3 resulted in IUFD (all of which involved the left ventricle). An additional 9 cases underwent in utero pericardiocentesis without recurrence of the effusion (all involved the right ventricle). Three cases with a large/massive pericardial effusion did not undergo a pericardiocentesis and all 3 had spontaneous improvement or resolution of the effusion.
Additional Anomalies and Associated Conditions
Additional congenital anomalies or associated conditions were reported in 40 (47%) cases. Table 2 describes the frequency of the reported anomalies or associated conditions. The most common reported anomalies or associated conditions were ventricular dysfunction in 15 (17%), ventricular septal defects in 12 (14%) and ventricular ectopy in 10 (12%). Of note, ventricular ectopy was most commonly associated with left ventricular CVOs; 9 of the 10 patients with ventricular ectopy had a left ventricular CVO.
When comparing the incidence of associated conditions between those cases that were asymptomatic (required no medical/surgical intervention) and those that were symptomatic, there was no significant difference in the incidence of pericardial effusion (33% vs. 47%, p = 0.3) or ectopy (25% vs. 6%, p = 0.09). Additionally, both groups reported congenital heart defects (most commonly atrial or ventricular septal defects).
Ventricular dysfunction was reported in 15 (17%) cases. The majority (11/15) had ventricular dysfunction beginning in the prenatal period. Of those with prenatal ventricular dysfunction, none had documented improvement in their function prior to birth and 4 (36%) died in the prenatal or immediate postnatal period, all of whom had left ventricular aneurysms. Of the 7 with prenatal ventricular dysfunction that survived to delivery, the function spontaneously improved in 4 (57%), whereas one required cardiac transplantation and two required cardiac surgery with subsequent improvement in function. Four were diagnosed with ventricular dysfunction in the postnatal period. Of those 4, one was awaiting cardiac transplantation, two were receiving medical therapy for the ventricular dysfunction, and one had spontaneous normalization of the ventricular function.
All 15 cases of ventricular dysfunction were classified by the respective authors as aneurysms and 80% involved the left ventricle, with just over half (53%) located at the apex. The size of the CVO was reported in 47%; all of which were reported to be > 10 mm in the prenatal period. Two cases described the CVO as “large”, and another 2 cases described an aneurysm-to-ventricular ratio of > 1 (ratios were 1.4 and 3.3). The remaining four cases with ventricular dysfunction did not have a CVO size reported.
Pulmonary hypoplasia (as diagnosed by the respective authors) was reported in 5 (6%). Four had a left ventricular aneurysm and either resulted in pregnancy termination or death in the immediate newborn period, and all had an associated pericardial effusion. The fifth case had a right ventricular diverticulum with an associated large pericardial effusion and underwent in utero pericardiocentesis with reported improvement in the appearance of the lungs.
Discussion
Our series adds to the current literature by representing a large cohort of prenatally diagnosed congenital ventricular outpouchings involving both the right and left ventricles. Prior series and literature reviews have typically focused on one type of outpouching (aneurysm or diverticulum), one ventricle (right sided or left sided), or those diagnosed both in the prenatal and postnatal periods.
CVO Characteristics
Ideally, the distinction between aneurysms and diverticula would be made using histologic tissue characterization; however, the current literature relies heavily on classification by echocardiographic features of the CVO, including size of the communication with the ventricle and contractility of the CVO (Table 1) [2, 5, 7,8,9,10]. It is possible that these classifications may be useful in understanding the clinical course of these outpouchings and their associated complications and outcomes. For the purposes of this retrospective review, CVO types were classified by the respective publishing authors.
We found that left ventricular aneurysms are the most common type of CVO encountered and CVOs involving the left ventricle were more commonly classified as aneurysms whereas CVOs involving the right ventricle were more commonly classified as diverticula.
Outcomes
Reported outcomes associated with CVOs have ranged from fetal death to complete and spontaneous resolution of the CVO [11,12,13]. In general, CVOs have been thought to have an excellent prognosis and many individuals remain asymptomatic, although significant complications may still occur, including hydrops fetalis, pericardial effusion, ventricular dysfunction, arrhythmias, thromboembolism, CVO rupture, and death [4, 8, 13,14,15,16].
Mortality
Excluding cases resulting in pregnancy termination, our data show a mortality rate of 17% associated with prenatally diagnosed CVOs with the majority (85%) occurring in the prenatal period. Previous studies have identified fetal characteristics that may portend a poor prognosis, including large CVO size, ratio of CVO to ventricular volume > 1, rapid growth, significant atrioventricular valve insufficiency, ventricular dysfunction and an earlier gestational age at diagnosis [5, 10, 17,18,19]. Based on our data, factors associated with mortality include an earlier gestational age at diagnosis, a CVO involving the left ventricle, the presence of a pericardial effusion and a diagnosis of hydrops fetalis.
Furthermore, prior reports have generally considered those with CVOs classified as aneurysms to have a relatively poorer prognosis [5, 12,13,14, 20, 21]. However, our data demonstrate increased mortality with CVOs involving the left ventricle regardless of CVO classification (aneurysm vs. diverticulum).
Survivors
Given the uncertain natural history and prognosis associated with CVOs, the optimal postnatal treatment strategy remains controversial [4, 13]. Some authors have suggested resection of all CVOs to prevent potential complications. Given the potential for spontaneous resolution however, others propose an expectant management strategy, reserving surgical intervention for those with symptoms, those considered at high risk for complications and/or those with other associated cardiac lesions requiring surgical intervention [4, 9, 13].
In our review, of those surviving the prenatal and immediate postnatal period, the majority (72%) remained asymptomatic without medical or surgical intervention or demonstrated regression or resolution of the CVO. Regression or complete resolution of the CVO occurred in 15% and all CVOs that resolved had done so by 1 year of age.
Surgical intervention was performed in 18%. Of those cases where age at surgery was documented, just over half occurred before 1 month, and all but one occurred before 1 year of age. Currently, there are no defined guidelines for surgical management of CVOs, however prior reports have described the following as potential indications for surgical intervention: significant symptoms, significant ventricular dysfunction, arrhythmias unresponsive to medications, substantial CVO size, concern for progressive CVO enlargement, concern for thromboembolic risk, potential for spontaneous CVO rupture, paradoxical motion of the CVO in systole, and CVOs with thin walls [7, 15, 22,23,24,25,26,27,28,29]. The latter two indications are thought to be associated with an increased risk of rupture.
Our findings would suggest that expectant management could be an appropriate strategy in specific cases, particularly in those without clinical symptoms. Close and frequent follow up should be performed, especially during the first year, as this is the time where surgical intervention and/or regression is most likely to occur.
Currently, there are no recommendations in place regarding the use of anticoagulation. Only a small number of previously published reports discussed the use of anticoagulation. At our institution, all three cases were placed on anticoagulation (aspirin or enoxaparin) for thromboprophylaxis. Anticoagulation could be considered, especially in the presence of a large CVO with abnormal CVO wall motion, or with associated ventricular dysfunction.
Pericardial Effusions
Pericardial effusions are a well-documented finding associated with prenatally diagnosed CVOs. A prior study reported a 36% prevalence rate for pericardial effusions associated with prenatally diagnosed left ventricular CVOs [5]. We found a similar rate (44%) associated with all types of CVOs, including both right and left ventricular CVOs.
The etiology and natural history of pericardial effusions remains unclear. Effusions often develop without ventricular dysfunction, arrhythmias, genetic abnormalities or viral infections. Proposed etiologies have included a reactive process related to congestive heart failure or to the initial CVO formation, friction from the CVO against the pericardium, or as a result of spontaneous CVO perforation [8, 11, 14, 22]. Prior reports describe a range of outcomes associated with pericardial effusions, from spontaneous and complete resolution, to the development of hydrops fetalis and fetal demise [12, 14].
In our study, pericardial effusions were more commonly associated with CVOs classified as diverticula, as opposed to aneurysms. While diverticula were more commonly associated with the right ventricle, the ventricle involved (right vs. left) was not associated with the development of the effusion itself. However, those with a pericardial effusion and a CVO involving the left ventricle did have a significantly higher mortality compared to those with an effusion and a CVO involving the right ventricle (89% vs. 11%, p = 0.002).
Spontaneous resolution of the pericardial effusion was noted in 18%. An additional 32% underwent in utero pericardiocentesis. The majority of those that underwent pericardiocentesis had no recurrence of their effusion, however three had subsequent intrauterine fetal demise. At present the indications for pericardiocentesis are unclear as it is difficult to predict which cases will spontaneously resolve and which could result in demise. Prior reports have described the following as possible indications for pericardiocentesis: fetal lung compression/risk of pulmonary hypoplasia, enlarging pericardial effusion, and/or concern for or worsening of hydrops [2, 11,12,13,14, 20, 22, 30, 31]. To avoid potential risks of in utero pericardiocentesis and allow for possible spontaneous resolution, prior recommendations have been expectant management for small to moderate effusions diagnosed early in pregnancy without associated hydrops. Although intervention to prevent pulmonary hypoplasia has been advocated, there are prior reports of fetuses with large pericardial effusions without evidence of postnatal pulmonary compromise [2, 11, 12, 22].
Additional Anomalies and Associated Conditions
Many additional anomalies or associated conditions have been reported with CVOs. In our study, almost half (47%) had an additional congenital anomaly or associated condition, the most common being ventricular dysfunction, ventricular septal defects, ventricular ectopy, and pulmonary hypoplasia. Ventricular dysfunction most commonly started in the prenatal period, was associated with a mortality rate of 36%, and did not tend to resolve or improve until the postnatal period. However, after delivery, the majority (57%) had spontaneous improvement in the ventricular function. The features found to be most associated with ventricular dysfunction include a large CVO involving the left ventricle, and/or a CVO classified as an aneurysm.
Cardiac Imaging
Prenatal and postnatal imaging plays an important role in classifying the type of CVO and associated characteristics (Figs. 2 and 3). It is also important to trend the CVO size over time, however there is no clear consensus for optimal measurement method. Previously reported methods include diameter, area or circumference measurements obtained by 2-dimensional prenatal or postnatal echocardiography, or volume measurements obtained by 3-dimensional echocardiography or cardiac magnetic resonance imaging (Figs. 4 and 5). Regardless of the specific method selected, we suggest consistent serial measurements using the most appropriate and readily available modality at your institution.
Malakan Rad and colleagues proposed a classification scheme to determine left ventricular CVO size (based on circumference, area and volume indices), and morphologic subtypes (based on ventricular distortion, CVO wall thickness and CVO motion) [10]. The authors suggested this classification scheme could have implications for prognosis. For example, type I CVOs were found to be associated with the best prognosis, whereas large Type IIc CVOs were associated with the worst prognosis. Consistent use of these descriptors could be helpful in further delineating the natural history and in comparing data between institutions.
Study Limitations
A significant limitation of our study is related to the classification system used to assign a CVO as either an aneurysm or a diverticulum without histologic data. It is possible that CVOs may have been incorrectly diagnosed as an aneurysm or diverticulum. Additional studies using uniform criteria for classification would be beneficial. Second, the study is limited by its inherent retrospective nature. In many cases the data are incomplete and based on descriptions provided by the writing author(s). Third, the follow up time is limited (median follow up of 12 months). It is possible that if these cases were followed for a longer period, either an increased number may have regressed, or conversely an increased number may have become symptomatic or developed complications. Additionally, cases included in our sample have been drawn solely from the literature, therefore introducing publication bias. Lastly, while prenatal imaging techniques have advanced, not all CVOs are likely to be diagnosed prenatally. It is possible that many asymptomatic cases were missed in our analysis, skewing the results toward more negative outcomes. However, for the purposes of our review, our data reflect what is currently known regarding the spectrum of disease diagnosed in utero and these data will be useful in aiding the practitioners with prenatal counseling.
To better understand the clinical course and outcomes associated with CVOs, a multicenter registry in this population will be required for further investigation.
Conclusion
Prenatally diagnosed CVOs represent a unique subgroup of patients with CVOs, with a high prenatal mortality rate. Because of the variable course of these defects, we recommend serial evaluation with fetal echocardiography. Extensive prenatal counseling is also recommended to discuss factors that may increase the risk for perinatal morbidity and mortality. As with other forms of congenital heart disease, prenatal diagnosis can and should be used to optimize the location and timing of delivery, and possible surgical intervention [32]. Postnatally, all patients should be evaluated by a cardiologist for confirmation of diagnosis. Given the variability in the clinical course during the first year, all should have serial monitoring for changes in CVO size, development of symptoms, and consideration of medical and surgical intervention.
In the setting of limited longitudinal data beyond the first year, multicenter collaboration will be essential to accumulate long term data regarding management and outcomes in this prenatally diagnosed population.
References
Goncalves L, Sims J, Philippe J (1992) Aneurysm, left ventricle. TheFetus.net. https://sonoworld.com/fetus/page.aspx?id=33. Accessed 17 Dec 1992
Williams J, Collardey K, Treadwell M, Owens S (2009) Prenatally diagnosed right ventricular outpouchings: a case series and review of the literature. Pediatr Cardiol 30(6):840–845
Prefumo F, Bhide A, Thilaganathan B, Carvalho J (2005) Fetal congenital cardiac diverticulum with pericardial effusion: two cases with different presentations in the first trimester of pregnancy. Ultrasound Obstet Gynecol. 25(4):405–408
Marijon E, Ou P, Fermont L, Concordet S, Le Bidois J, Sidi D, Bonnet D (2006) Diagnosis and outcome in congenital ventricular diverticulum and aneurysm. J Thorac Cardiovasc Surg 131(2):433–437
Ohlow M, Bruneli M, Lauer B (2014) Characteristics and outcome of primary congenital left ventricular aneurysm and diverticulum: analysis of cases from the literature. Prenat Diagn 34(9):893–899
Awad S, Patel A, Polimenakos A, Braun R, Abdulla R (2009) Left ventricular accessory chamber: a case report and review of the literature. Pediatr Cardiol 30(7):1022–1025
Evans W, Madrid A, Castillo W, Rollins R, Berthoty D, Starnes V, Wiencek R, Ciccolo M, Acherman R (2009) All cardiac right ventricular outpouches are not created equal. Pediatr Cardiol 30(7):954–957
Perlitz Y, Mukary M, Lorber A, Ben-Ami M (2009) Prenatal diagnosis of fetal cardiac right ventricular diverticulum disappearing at three months of age: a case report and literature review. Fetal Diagn Ther 25(1):44–46
Wax J, Moran A, Pinette MG, Reyes A, Cartin A, Blackstone J (2007) Prenatal sonographic diagnosis of fetal right ventricular diverticulum. J Ultrasound Med 26(2):267–270
Malakan Rad E, Awad S, Hijazi Z (2014) Congenital left ventricular outpouchings: a systematic review of 839 cases and introduction of a novel classification after two centuries. Congenit Heart Dis 9(6):498–511
McAuliffe F, Hornberger L, Johnson J, Chitayat D, Ryan G (2005) Cardiac diverticulum with pericardial effusion: report of two new cases treated by in-utero pericardiocentesis and a review of the literature. Ultrasound Obstet Gynecol 25(4):401–404
Abi-Nader K, David A, Yates R, Pandya P (2009) Successful outcome after prenatal treatment of a cardiac diverticulum with massive pericardial effusion. Fetal Diagn Ther. 25(1):148–152
Barberato M, Barberato S, Binotto C, Cavalcanti M, Passos A, Miyague N (2009) Prenatal diagnosis of left ventricular aneurysm and diverticulum. Arg Bras Cardiol 93(2):e36–38
Carrard C, Massardier J, Pangaud N, Champion F (2010) Fetal right ventricular diverticulum with pericardial effusion: report of a new case treated by in utero pericardiocentesis. Pediatr Cardiol 31(6):891–893
Demir F, Ozbarlas N, Gocen U, Buyukkurt S (2015) Prenatal diagnosis of giant left ventricular diverticulum: case report. Echocardiography 32(2):395–397
Nam K, Kwon J, Son G, Cho N, Park Y, Kim Y (2010) Prenatally diagnosed left ventricular diverticulum with thoracoabdominal wall defect: a case and review of the literature. J Perinatol 30(11):760–762
Grethel E, Hornberger L, Farmer D (2007) Prenatal and postnatal management of a patient with pentalogy of Cantrell and left ventricular aneurysm: a case report and literature review. Fetal Diagn Ther 22(4):269–273
Matias A, Fredouille C, Nesmann C, Azancot A (1999) Prenatal diagnosis of left ventricular aneurysm: a report of three cases and a review. Cardiol Young 9(2):175–184
McElhinney D, Silverman N (1999) Left ventricular aneurysm in the fetus: a diagnosis with a mixed prognosis. Cardiol Young 9(2):123–126
Bernasconi A, Delezoide A, Menez F, Vuillard E, Oury J, Azancot A (2004) Prenatal rupture of a left ventricular diverticulum: a case report and review of the literature. Prenat Diagn 24(7):504–507
Oloron P, Ibarra C, Galdeano Miranda J (2011) Right ventricular outpouching associated with a ventricular septal defect: case report. Pediatr Cardiol 32(8):1269–1270
Del Rio M, Martinez J, Bennasar M, Palacio M, Figueras F, Puerto B, Mortera C, Cararach V (2005) Prenatal diagnosis of a right ventricular diverticulum complicated by pericardial effusion in the first trimester. Ultrasound Obstet Gynecol 25(4):409–411
Jacobson R, Perez A, Meyer R, Miodovnik M, Siddigi T (1991) Prenatal diagnosis of fetal left ventricular aneurysm: a case report and review. Obstet Gynecol 78(3 Pt 2):525–528
Sharma J, Oforl-Amando G, Marboe C, Quaegebeur J (2002) Congenital left ventricular aneurysm with pericardial effusion: prenatal diagnosis, surgical management and follow-up. Pediatr Cardiol 23(4):458–461
Seo D, Won H, Ko J, Jhang W (2011) Modified damus-kaye-stansel/dor procedure for a newborn with severe left ventricular aneurysm. Korean Circ J 41(8):494–496
Hirose A, Maeno Y, Suda K, Fusazaki N, Kado H, Matsuishi T (2013) Serial hemodynamic assessment using Doppler echocardiography in a fetus with left ventricular aneurysm presented as fetal hydrops. J Perinatol 33(6):486–489
Davidson A, Witeman V, Gaynor J (2006) Images in cardiovascular medicine fetal cardiac diverticulum. Circulation 113(4):e56
Conway J, Hancock Friesen C, Thompson D, Warren A (2008) Fetal diagnosis of an “extra cardiac chamber”. Pediatr Cardiol 29(1):188–190
Erek E, Odemis E, Tanidir I (2013) Right ventricular diverticulum and associated cyst. Pediatr Cardiol 34(8):2093–2095
El Kady D, Gersovich E, Moon-Grady A, Towner D, McGahan J, Rhee-Morris L, Naderi S (2005) Congenital cardiac left ventricular aneurysm with pericardial effusion: early prenatal diagnosis and intervention. J Ultrasound Med 24(7):1011–1015
Johnson J, Ryan G, Toi A, Smallhorn J (1996) Prenatal diagnosis of a fetal ventricular diverticulum associated with pericardial effusion: successful outcome following pericardiocentesis. Prenat Diagn 16(10):954–957
Killen S, Mouledoux J, Kavanaugh-McHugh A (2014) Pediatric prenatal diagnosis of congenital heart disease. Curr Opin Pediatr 26(5):536–545
Carles D, Maugey-Laulom B, Habboud H, Alberti E, Weichhold W, Leger F (1995) Early prenatal diagnosis of ventricular diverticulum complicated by serous pericardial effusion. Prenat Diagn 15(8):778–780
Cesko I, Hajdu J, Csapo Z, Toth T, Sipos B, Papp Z (1998) Fetal hydropericardium associated with left ventricular diverticulum. Prenat Diagn 18(7):721–724
Sepulveda W, Drysdale K, Kyle P, McNeal A, Moore I (1996) Congenital left ventricular aneurysm causing hydrops fetalis: prenatal diagnosis with color Doppler ultrasonography. J Ultrasound Med 15(4):327–331
Papagiannis J, Van Praagh R, Schwint O, D’Orsogna L, Qureshi F, Reynolds J, Kallfelz C, Nozar J (2001) Congenital left ventricular aneurysm: clinical imaging, pathologic and surgical findings in seven new cases. Am Heart J 141(3):491–499
Cavalle-Garrido T, Cloutier A, Harder J, Boutin C, Smallhorn J (1997) Evolution of fetal ventricular aneurysms and diverticula of the heart: an echocardiographic study. Am J Perinatol 14(7):393–400
Sherman S, Leenhouts K, Utter G, Litaker M, Lawson P (1996) Prenatal diagnosis of left ventricular aneurysm in the late second trimester: a case report. Ultrasound Obstet Gynecol 7(6):456–457
Chaubal N, Dighe M, Shah M, Chaubal J, Raghavan J (2004) Congenital left ventricular aneurysm: prenatal sonographic diagnosis. J Ultrasound Med 23(1):125–128
Jowett V, Miller O (2011) Prenatal diagnosis of left ventricular aneurysm in association with interruption of the aortic arch. Cardiol Young 21(1):113–115
Vernon M, Rutledge J, Kemma M (2014) In utero evolution of an apical left ventricular aneurysm. Circulation 130(22):1982–1983
Patel C, Judge N, Muise K, Levine M (1996) Prenatal myocardial infarction suspected by fetal echocardiography. J Am Soc Echocardiogr 9(5):721–723
Weichert J, Chiriac A, Axt-Fliedner R (2010) Fetal diagnosis of left ventricular aneurysm of the free wall and the interventricular septum: report of two cases and review of the literature. J Matern Fetal Neonatal Med 23(12):1510–1515
Kitchiner D, Leung M, Arnold R (1990) Isolated congenital left ventricular diverticulum: echocardiographic features in a fetus. Am Heart J 119(6):1435–1437
Brachlow A, Sable C, Smith S, Slack M, Martin G (2002) Fetal diagnosis and postnatal follow-up of an asymptomatic congenital left ventricular diverticulum. Pediatr Cardiol 23(6):658–660
Pradhan M, Dalal A, Kapoor A, Kumar S, Manisha A (2008) Fetal left ventricular diverticulum presenting as dysrhythmia: diagnosis and management. Fetal Diagn Ther 23(1):10–14
Paoletti D, Robertson M (2012) Prenatal diagnosis of a left ventricular diverticulum. Am J Ultrasound Med 15(3):112–114
Peters C, Wacker-Gussmann A, Strasburger J, Cuneo B, Gotteiner N, Gulecyuz M, Wakai R (2015) Electrophysiologic features of fetal ventricular aneurysms and diverticula. Prenat Diagn 35(2):129–136
Gembruch U, Steil E, Redel D, Hansmann M (1990) Prenatal diagnosis of a left ventricular aneurysm. Prenat Diagn 10(3):203–209
Hornberger L, Dalvi B, Benacerraf B (1994) Prenatal sonographic detection of cardiac aneurysms and diverticula. J Ultrasound Med 13(12):967–970
Pipitone S, Sperandeo V, Mongiovi M, Roberto G, Centineo G (2002) Prenatal diagnosis of ventricular aneurysm: a report of two cases and a review. Prenat Diagn 22(2):131–136
Chiang Y, Yang C, Shih J, Lee C (2006) Prenatal diagnosis of congenital left ventricular aneurysm by four-dimensional ultrasonography with spatio-temporal image correlation (STIC). Ultrasound Obstet Gynecol 28(3):345–347
Balakumar K (2009) Prenatal diagnosis of left ventricular aneurysm. Indian J Radiol Imaging 19(1):84–86
Fujita Y, Hidaka N, Yumoto Y, Morihana E, Fukushima K, Wake N (2012) Measurement of the fetal isovolumetric contraction time in the fetus with a left ventricular aneurysm. J Obstet Gynaecol Res 38(3):586–588
Gardiner H, Wimalasundera R, Pasquini L, Wawryk S, Ho S (2005) Images in cardiovascular medicine. Pericardiocentesis at 14 weeks: effective treatment of pericardial effusion complicating right ventricular diverticulum. Circulation 112(9):e120
Koshiishi T, Osada H, Hata A, Furugen Y, Murakoshi T, Mitsuhashi N (2007) Prenatal rupture of right ventricular diverticulum: a case report and review of the literature. Prenat Diagn 27(12):1154–1157
John J, Bricker J, Fenrich A, Vick G, El-Said H, Ayres N, Bezold L (2002) Images in cardiovascular medicine. Fetal diagnosis of right ventricular aneurysm associated with supraventricular tachycardia with left bundle-branch block aberrancy. Circulation 106(1):141–142
McCaffrey F (2002) Around PediHeart: aneurysm of the right ventricular apex in a 24-week fetus. Pediatr Cardiol 23(1):8
Funding
This study received no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to report.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shuplock, J.M., Kavanaugh-McHugh, A. & Parra, D. Prenatally Diagnosed Congenital Ventricular Outpouchings: An Institutional Experience and Review of the Literature. Pediatr Cardiol 41, 272–281 (2020). https://doi.org/10.1007/s00246-019-02252-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-019-02252-7