Abstract
We aimed to detect residual cardiac dysfunction—if any—in children with recovered primary dilated cardiomyopathy (DCM) by using the left ventricular (LV) layer-specific myocardial strains. Fifty children with recovered primary DCM both clinically and echocardiographically were included as the patient group. Fifty healthy children of matched age and sex served as the control group. Echocardiographic evaluation was performed for all included children in the form of conventional echocardiography, tissue Doppler imaging (TDI), two-dimensional speckle tracking echocardiography (2D-STE), and LV layer-specific myocardial strain. Both LV systolic and diastolic functions measured by conventional echocardiography were similar in children with recovered DCM and the control group. There was a significant reduction in LV systolic and diastolic functions measured by TDI in the patient group. Moreover, there was a significant reduction of LV global longitudinal systolic strain (GLSS) by 2D-STE in children with recovered DCM. Interestingly, there was a significant reduction of LV layer-specific myocardial strain from endocardium to epicardium in children with recovered DCM compared to the healthy control. There was a significant positive correlation between different layer-specific myocardial strains and LV GLSS, LV ejection fraction, and LV peak systolic velocity. Left ventricular layer-specific myocardial strain can be a promising tool for early identifications of LV dysfunction in children with DCM. Subtle cardiac dysfunction is present in patients with recovered DCM, so long-term follow-up is recommended in these patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Primary dilated cardiomyopathy (DCM) is the presence of left ventricular dilatation with severely reduced systolic function in the absence of a cause. The hallmark of the disease is the left ventricular (LV) dilatation with systolic dysfunction [1]. DCM is the most common cause of cardiomyopathy in children with considerable morbidity and mortality [2]. Nonetheless, several studies reported the recovery of the cardiac function both clinically and echocardiographically to normal in 21% to 37% of the pediatric patients, respectively [3,4,5]. It is unclear if patients who recovered from DCM do or do not have residual cardiac dysfunction.
LV wall is not homogenous but rather a complex multilayered structure. Most imaging techniques analyze the whole myocardial wall thickness for the assessment of myocardial function rather than the individual function of different layers of the myocardium ranging from endocardium to epicardium. Furthermore, different diseases are known to affect the myocardial layers to a different degree, resulting in either global morphological alterations or prominent alterations in a specific layer [6, 7].
Recently, speckle tracking echocardiography (STE) was upgraded to allow the determination of cardiac function in the different three layers of the myocardial wall and known as specific layer myocardial strain [8, 9]. Layer-specific myocardial strain was studied in the normal heart and in different cardiac diseases [9,10,11,12]. Yet, no study reported the use of layer-specific myocardial strain in children with recovered dilated cardiomyopathy.
The aim of this work was to evaluate endocardial, mid-myocardial, and epicardial longitudinal strain using two-dimensional (2D) STE in children with recovered DCM to detect any residual subtle lesion and to correlate these parameters with other echocardiographic data.
Patients and Methods
This was a prospective case-controlled study that was performed in Pediatric cardiolohy unit, Pediatric department, Tanta University Hospital, Egypt during the period from April 2017 to March 2019. Fifty children with recovered primary DCM were included as a patient group. Fifty healthy children of matched age and sex served as a control group. The study was approved by the local ethical committee of our Faculty of Medicine. Written Informed consent was signed by parents of all included children.
Inclusion criteria: children below 18 years with recovered primary DCM both clinically and echocardiographically i.e., normal cardiac size and function by echocardiography (LV EF ≥ 50%) and resolution of all clinical symptoms and signs of heart failure.
Exclusion criteria: children with congenital or acquired heart diseases, children with DCM secondary to systemic diseases, children with chronic diseases, and children with arrhythmias.
All these patients were subjected to the following:
- 1.
Full history taking and complete clinical evaluation
- 2.
Echocardiographic Examination was performed using a commercially available ultrasound transducer and equipment (Vivid 7 and Vivid 9, GE Healthcare, Horten, Norway). Data acquisition was performed with a 3.5-MHz S7 probes. Digital loops were stored on the hard disk of the echocardiography machine, and transferred to a workstation (Echo PAC PC, 113; GE, and Horten, Norway) for offline analysis.
- -
Conventional echocardiography: LV systolic function was assessed by measuring LV ejection fraction (LV EF), where LV EF% = LV end-diastolic dimension (LV EDD)3 − LV end-systolic dimension (LV ESD)3/(LV EDD)3 × 100%. LV diastolic function was evaluated by mitral valve (MV) E/A ratio, where E is the passive LV filling and A-wave is the atrial contraction.
- -
Tissue Doppler imaging (TDI) was performed to assess systolic and diastolic mitral annulus velocities according to the American society of echocardiography (ASE) recommendations [13]. PW-TDI sample volume is placed at the level of the septal mitral annulus. TDI display showed an antegrade systolic wave S’, and two retrograde waves E' and A'. Myocardial Performance Index (MPI) of LV was also calculated (isovolumic contraction time + isovolumic relaxation time/ejection time) which is known as the Tei index. Measurements were repeated three times and the average was recorded.
- -
2 D-STE was also performed to assess 2D-longitudinal strain (2D-LS) using a 3.5-MHz transducer in the standard apical view (4-chamber image). It tracks the characteristic pattern of natural acoustic markers present in the myocardial wall (“speckles”) from frame to frame throughout the cardiac cycle. The myocardial strain is then calculated by the change in position of the speckle pattern with respect to the initial position. Peak systolic LS was calculated by averaging the peak systolic values of 6 segments. For myocardial strain, regional lengthening is expressed as a positive value and shortening as a negative value. Auto LV EF by STE was also performed where the software requires the examiner to define three regions of interest in the LV, the endocardial border is then traced throughout the cardiac cycle and the software automatically locates the end-systolic and end-diastolic frames. End-diastolic and end-systolic volumes are calculated based on the tracings and serve as the basis for the EF calculation.
- -
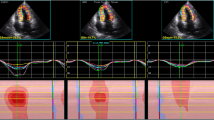
Layer-specific myocardial longitudinal strain: Apical views were analyzed using the new modified speckle tracking software enabling the analysis of strains in three myocardial layers. Layer-specific strain starts similarly to traditional 2D-STE by tracking of the endocardial border in the end-systole at the four apical view then the myocardial wall was automatically defined into three chains of nodes and was divided into 3 separate myocardial wall of similar thickness permitting the examination of the three myocardial layers; endocardial, mid-myocrdial, and epicardial layers. The endocardial, myocardial, and the epicardial borders were traced in the end-systolic frame of the 2D images at the apical view for the analysis of layer-specific strain. Grayscale images were analyzed in a 16 segmental model. Segments that failed to be tracked properly were manually adjusted by the operator. The contours were re-tracked and the quality of segmental tracking was validated by the software. Any segments that subsequently failed to be tracked were excluded.
All images were analyzed 1 week later by the same operator in random order to test intra-observer reliability. Another operator repeated all examinations to test inter-observer reliability without knowing the measurement of the first operator.
The primary outcome of this study was to evaluate the layer-specific myocardial strain in children with recovered DCM to detect any residual subtle dysfunction. The secondary outcomes were to correlate these parameters to other echocardiographic parameters and to compare different layer-specific myocardial strain to detect which layer was the most affected one in such children.
Statistical Analysis
Statistical analysis was performed using SPSS program V.17 (SPSS, Chicago, IL). Continuous variables were presented in the form of mean and standard deviation. Categorical data were presented in the form of number or percentage. Comparison between continuous variables among the two groups was performed using the unpaired Student T test. Comparison between categorical data such as sex among the two groups was performed using Chi-Square test. Correlation between various echocardiographic data was performed using Spearman coefficient analysis. P value below 0.05 was considered significant.
Results
The patient group included 50 children with recovered DCM with a mean age of 4.5 ± 1.8 years. Fifty healthy children with a mean age of 4.6 ± 2 years served as the control group. LV EF and mitral E/A ratio measured by conventional echocardiography were comparable in both groups, but LV S wave and mitral E'/A' ratio measured by TDI were significantly lower in children with recovered DCM compared to the healthy control. Furthermore, LV MPI was significantly increased in the patient group compared to the control group. Additionally, LV EF and LV GLSS measured by 2D-STE showed a significant reduction in the patient group. Interestingly, layer-specific myocardial strain showed marked reduction of LS of all layers of the myocardium in the patient group compared to the healthy control. It was greatest in the endocardium, lower in the mid-myocardial layer, and lowest in the epicardium (Table 1 and Figs. 1, 2, 3).
Table 2 shows that there was a significant positive correlation between endocardial strain and LV GLSS, LV EF, and LV S, while, there was no significant correlation between endocardial strain and both LV MPI and mitral E′/A′. Moreover, there was a significant positive correlation between mid-myocardial strain and LV GLSS, LV EF, LV S, and LV MPI, while, there was no significant correlation between mid-myocardial strain and mitral E′/A′ ratio. In addition, there was a significant positive correlation between epicardial strain and LV GLSS only, while, there was no significant correlation between epicardial strain and LV MPI, LV EF, LV S, or mitral E′/A′ ratio. The intra-observer and inter-observer reliability were excellent for GLSS and layer-specific strain and the results are summarized in Table 3.
Discussion
LV consists of three layers of the myocardium. Different diseases could affect the myocardial layers to a different extent and could result in a predominant dysfunction in a specific layer. Thus, evaluation of the myocardial deformation of the whole ventricular wall thickness is unable to provide comprehensive information about the cardiac function [14]. This is why the layer-specific myocardial strain was developed. To the best of our knowledge, this study is the first to evaluate the layer-specific longitudinal myocardial strain in children with recovered primary DCM.
Our results showed that there was a significant decrease in both systolic and diastolic function in children with recovered DCM compared to healthy control, as detected by TDI. However, conventional echocardiography failed to show any systolic or diastolic LV dysfunction. This was in agreement with other investigators [15, 16].
LV MPI was significantly increased in patients with recovered DCM, indicating that these patients have a residual subtle cardiac dysfunction. Tei index is known to have a close correlation with both systolic and diastolic function. Our results were in accordance with the results of Amorim et al. [15]. Moreover, Dujardin et al. [17] reported that the Tei index and EF were the most significant independent predictors of outcomes in patients with idiopathic DCM.
Patients in this study showed a marked decrease of LV EF measured by auto LVEF compared to the control group. Similar findings were reported by other investigators [18, 19] revealing that auto LVEF is a sensitive tool for the evaluation of LV systolic function.
The assessment of GLS is an important part of the echocardiographic examination as the longitudinal movement of the ventricular wall is the main deformation of the heart. In the current study, we found that 2D-LV GLSS decreased significantly in children with recovered DCM compared to healthy control. It is known that longitudinal cardiac fibers which are located in the sub-endocardium are the first to be affected in myocardial injury. Hence, LV GLSS is an accurate measure of myocardium deformation, allowing angle-independent estimation of myocardial function and is considered better than EF because it does not rely on geometrical assumptions [20, 21]. This result was in accordance with that reported by other investigators [15, 22].
Interestingly, an increased epicardial-to-endocardial gradient of LS was demonstrated in healthy children as well as in children with recovered DCM. LV LS was highest in the endocardium and lowest in the epicardium. LS being lowest in the epicardial layer may be due to technical error as the epicardial layer is the outermost layer, so it is difficult to be tracked well. However, this result needs further research to be explained well. The same results were reported by Shi et al. [23] and Leitman et al. [24], who reported the presence of epicardial-to-endocardial gradient of LS in normal subjects. Previous analysis systems have considered total wall thickness for averaging of deformation parameters for the whole LV. This study used an advanced STE technique which allows the analysis of myocardial deformation separately within each of three myocardial layers. Novel myocardial speckle tracking system demonstrates a prominent difference in the magnitude of myocardial deformation between the endocardial and epicardial layers with a continuous decrease of LS from endocardial to mid-myocardial and epicardial layers. Regardless of the epicardial strain that couldn’t be tracked well in most cases, the mid-myocardial strain was the most affected myocardial layer in DCM and was lower than GLSS as well making it the best choice for following up children with recovered DCM besides other parameters.
Our results revealed the presence of residual subclinical myocardial dysfunction in children with recovered DCM supporting the idea of the absence of a real recovery in DCM as LV mechanics may not return normal even in the presence of normal EF, emphasizing the importance of long-term follow-up in these patients. This was supported by Merlo et al. [25] who revealed that 24% of persistently normalized patients with DCM showed progressive deterioration later on and 5% of them died or underwent heart transplantation even after 3 years of being recovered. Similarly, Everitt et al. [4] revealed that about 20% of children with recovered DCM showed abnormal cardiac function or size on later follow-up. Furthermore, Moon et al. [26] revealed that LV dysfunction recurs in some patients with recovered idiopathic DCM and the recurrence was significantly correlated with the discontinuation of anti-failure treatment. The results raise the question of whether continuous medical therapy may be mandatory in patients who recovered from LV systolic dysfunction. Interestingly, Basuray et al. [27] reported that HF-recovered patients had abnormal BNP and elevated troponin I, indicating that there is persistent neurohormonal activation, increased oxidative stress, cardiomyocyte injury, and stress, despite the apparent recovery of EF. These findings provided a rationale to continue background medical therapy for HF-recovered patients.
Limitations of the study: This study demonstrated a single center study with a relatively small number of patients, so further studies with a larger number is recommended. Follow-up of the children with recovered DCM by specific layer myocardial strain to know at what time the LV function will recover completely (if any) was not performed.
Conclusion
Left ventricular layer-specific myocardial strain can be a promising tool for early identifications of LV dysfunction in children with DCM. Subtle cardiac dysfunction is present in patients with recovered DCM, so long-term follow-up is recommended in these patients.
References
Bakalakos A, Ritsatos K, Anastasakis A (2018) Current perspective on the diagnosis and management of dilated cardiomyopathy beyond heart failure: a cardiomyopathy clinic doctor’s point of view. Hellenic J Cardiol 59(5):254–261
Alvarez JA, Orav EJ, Wilkinson JD, Fleming LE, Lee DJ, Sleeper LA, Rusconi PG, Colan SD, Hsu DT, Canter CE, Webher SA, Cox GF, Jefferies JL, Towbin JA, Lipshultz SE, Pediatric Cardiomyopathy Registry Investigators (2011) Competing risks for death and cardiac transplantation in children with dilated cardiomyopathy: results from the Pediatric Cardiomyopathy Registry. Circulation 124:814–823
Lewis AB (1999) Late recovery of ventricular function in children with dilated cardiomyopathy. Am Heart J 138:334–338
Everitt MD, Sleeper LA, Lu M, Canter CE, Pahl E, Wilkinson JD, Addonizio LJ, Towbin JA, Rossano J, Singh RK, Lamour J, Webber SA, Colan SD, Margossian R, Kantor PF, Jefferies JL, Lipshultz SE (2014) Recovery of echocardiographic function in children with idiopathic dilated cardiomyopathy: results from the pediatric cardiomyopathy registry. J Am Coll Cardiol 63(14):1405–1413
Alexander PM, Daubeney PE, Nugent AW, Lee KJ, Turner C, Colan SD, Robertson T, Davis AM, Ramsay J, Justo R, Sholler GF, King I, Weintraub RG (2013) Long-term outcomes of dilated cardiomyopathy diagnosed during childhood: results from a national population-based study of childhood cardiomyopathy. Circulation 128:2039–2046
Torrent-Guasp F, Ballester M, Buckberg GD, Carreras F, Flotats A, Carrio I, Ferreira A, Samuels LE, Narula J (2001) Spatial orientation of the ventricular muscle band: physiologic contribution and surgical implications. J Thorac Cardiovasc Surg 122(2):389–392
Cong J, Wang Z, Jin H, Wang W, Gong K, Meng Y, Lee Y (2016) Quantitative evaluation of longitudinal strain in layer-specific myocardium during normal pregnancy in China. Cardiovasc Ultrasound 14:45
Adamu U, Schmitz F, Becker M, Kelm M, Hoffmann R (2009) Advanced speckle tracking echocardiography allowing a three myocardial layer-specific analysis of deformation parameters. Eur J Echocardiogr 10:303–308
Zhang Q, Fang F, Liang YJ, Xie JM, Wen YY, Yip GW, Lam YY, Chan JY, Fung JW, Yu CM (2011) A novel multi-layer approach of measuring myocardial strain and torsion by 2D speckle tracking imaging in normal subjects and patients with heart diseases. Int J Cardiol 147:32–37
Ozawa K, Funabashi N, Takaoka H, Kamata T, Kanaeda A, Saito M, Nomura F, Kobayashi Y (2015) Characteristic myocardial strain identified in hypertrophic cardiomyopathy subjects with preserved left ventricular ejection fraction using a novel multi-layer transthoracic echocardiography technique. Int J Cardiol 184:237–243
Bogdanović J, Ašanin M, Krljanac G, Lalić NM, Jotić A, Stanković S, Rajković N, Stošić L, Rasulić I, Milin J, Popović D, Bogdanović L, Lalić K (2019) Impact of acute hyperglycemia on layer-specific left ventricular strain in asymptomatic diabetic patients: an analysis based on two-dimensional speckle tracking echocardiography. Cardiovasc Diabetol 18:68
Eun LY, Lee Y (2018) Myocardial layer-specific strain analysis in children with mitochondrial disease. Yonsei Med J 59(1):128–134
Lai WW, Geva T, Shirali GS, Task Force of the Pediatric Council of the American Society of Echocardiography, Pediatric Council of the American Society of Echocardiography (2006) Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr 19(12):1413–1430
Amorim S, Campelo M, Martins E, Moura B, Sousa A, Pinho T, Silva-Cardoso J, Júlia Maciel M (2016) Prevalence, predictors and prognosis of ventricular reverse remodeling in idiopathic dilated cardiomyopathy. Rev Port Cardiol 35(5):253–260
Amorim S, Rodrigues J, CampeloM MouraB, Martins E, MacedoF S-C, Júlia Maciel M (2017) Left ventricular reverse remodeling in dilated cardiomyopathy maintained subclinical myocardial systolic and diastolic dysfunction. Int J Cardiovasc Imaging 33(5):605–613
Friedberg MK, Roche SL, Mohammed AF, Balasingam M, Atenafu EG, Kantor PF (2008) Left ventricular diastolic mechanical dyssynchrony and associated clinical outcomes in children with dilated cardiomyopathy. Circ Cardiovasc Imaging 1:50–57
Dujardin KS, Tei C, Yeo TC, Hodge DO, Rossi A, Seward JB (1998) Prognostic value of a Doppler index combining systolic and diastolic performance in idiopathic-dilated cardiomyopathy. Am J Cardiol 82(9):1071–1076
van Dalen BM, Kauer F, Vletter WB, Soliman OII, van der Zwaan HB, ten Cate FJ, Geleijnse ML (2010) Influence of cardiac shape on left ventricular twist. J Appl Physiol 108(1):146–151
Nesbitt GC, Mankad S (2009) Strain and strain rate imaging in cardiomyopathy. Echocardiography 26(3):337–344
Cong J, Fan T, Yang X, Squires JW, Cheng G, Zhang L (2015) Structural and functional changes in maternal left ventricle during pregnancy: a three dimensional speckle echocardiography study. Cardiovasc Ultrasound 13:6–13
Marwick TH (2006) Measurement of strain and strain rate by echocardiography: ready for prime time? J Am Coll Cardiol 47(7):1313–1327
Okada M, Tanaka H, Matsumoto K, Ryo K, Kawai H, Hirata K (2012) Subclinical myocardial dysfunction in patients with reverse-remodeled dilated cardiomyopathy. J Am Soc Echocardiogr 25(7):726–732
Shi J, Pan C, Kong D, Cheng L, Shu X (2016) Left ventricular longitudinal and circumferential layer-specific myocardial strains and their determinants in healthy subjects. Echocardiography 33(4):510–518
Leitman M, Lysiansky M, Lysyansky P, Friedman Z, Tyomkin V, Fuchs T, Adam D, Krakover R, Vered Z (2010) Circumferential and longitudinal strain in 3 myocardial layers in normal subjects and in patients with regional left ventricular dysfunction. J Am Soc Echocardiogr 23:64–70
Merlo M, Stolfo D, Anzini M, Negri F, Pinamonti B, Barbati G, Ramani F, Di Lenarda A, Sinagra G (2015) Persistent recovery of normal left ventricular function and dimension in idiopathic dilated cardiomyopathy during long term follow up: does real healing exist? JAHA 4:e001504
Moon J, Ko Y, Chung N, Ha J, Kang S, Choi E, Rim S (2009) Recovery and recurrence of left ventricular systolic dysfunction in patients with idiopathic dilated cardiomyopathy. Can J Cardiol 25(5):e147–e150
Basuray A, French B, Ky B, Vorovich E, Olt C, Sweitzer NK, Cappola TP, Fang GC (2014) Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation 129(23):2380–2387
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no potential conflict of interest to disclose.
Ethical Approval
The study is in accordance with the ethical standards of institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maher, E., Elshehaby, W., El Amrousy, D. et al. Left Ventricular Layer-Specific Myocardial Strains in Children with Recovered Primary Dilated Cardiomyopathy: What Lies Beneath the Iceberg?. Pediatr Cardiol 41, 101–107 (2020). https://doi.org/10.1007/s00246-019-02228-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-019-02228-7