Abstract
Increased procalcitonin concentration (PCT) is known to be reliable for the identification of infections even in the presence of the non-specific systemic inflammatory response seen after cardiopulmonary bypass (CPB), whereas increased C-reactive protein concentration (CRP) is not. The present work explored the ability of neonate PCT measured early after cardiac surgery to identify postoperative infections. This was a retrospective case–control study, where PCT was matched between patients with and without infections according to the patient’s age, the CPB length, the use of deep hypothermic circulatory arrest (DHCA), and the postoperative day (POD). The accuracy in the prediction of infections was ascertained and cutoff thresholds were identified. 144 neonates were eligible, and 89 pairs of measurements from 94 patients were analyzed. PCT was a good predictor of infections within POD4, and was a better predictor when compared with CRP at POD1 and POD2. The sum of PCT (pg mL−1) and CRP (mg L−1) > 33 on POD1 or POD2 predicted infections with a 0.68 sensitivity and a 0.82 specificity, and a sum > 49.36 on POD3 or POD4 predicted infections with a 0.82 sensitivity and a 0.93 specificity. In patients with DHCA, PCT was higher than in those without DHCA, and was not predictive of infections. The accuracy of PCT to identify infections after neonatal cardiac surgery is better than that of CRP when measured within 48 h of surgery. The sum of the two markers measured early after surgery is an excellent predictor of postoperative infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blood concentration of procalcitonin (PCT) is considered one of the best markers of bacterial infection [1], and several studies have demonstrated excellent accuracy for the diagnosis of infection in critically ill neonates [1,2,3,4]. Although both PCT and C-reactive protein (CRP) are acute-phase proteins and increase in several non-infectious inflammatory states, PCT is reliable for the identification of infection even in the presence of a non-specific systemic inflammatory response syndrome (SIRS) such as seen after cardiopulmonary bypass (CPB), whereas CRP concentration is not [5]. Recent studies have validated its use for screening of post-cardiac surgery infections in children [5,6,7,8,9]. Neonates usually undergo more complex surgeries when compared with older children; longer bypass and aortic cross-clamp durations, larger transfusions, often require deep hypothermic circulatory arrest (DHCA), and a delay in sternal closure. This combination of factors places them at high risk for both SIRS and sepsis. Both PCT and CRP concentrations are impacted by these specific factors. Nevertheless, it has been suggested that PCT provides increased accuracy for the diagnosis of postoperative infections in neonates when compared with CRP [10].
Previous authors have attempted to identify an accurate PCT threshold for prediction of infections; however, PCT varies with both age [11] and the timing of the measurement relative to surgery and the occurrence of infection [12, 13]. These make it difficult to identify a unique threshold for the entire postoperative period in a wide age-range pediatric population, such as considered previously [5,6,7,8, 14,15,16]. The present work aims to explore the ability of PCT measured within two weeks of cardiac surgery to predict an infection within the next 48 h in neonates.
Materials and Methods
This retrospective cohort study was performed at the Necker-Enfants Malades University Hospital in Paris. All neonates undergoing surgery between November 1, 2014 and November 1, 2017 at the Department of Pediatric cardiac surgery were eligible for inclusion if they had at least 2 PCT measurements within the 15 days following surgery. The institutional ethical committee waived the need for written informed parental consent to perform this retrospective analysis, after de-identification of all patient data.
Anesthesia was induced with sevoflurane, and maintained with alfentanil, midazolam, and atracurium. Patients underwent surgery either with moderate hypothermic CPB, or with DHCA and selective antegrade cerebral perfusion if they required reconstruction of the aortic arch. Antibiotic prophylaxis consisted of cefazolin 50 mg kg−1 at induction of anesthesia, followed by 25 mg kg−1 during CPB, 25 mg kg at sternal closure, and another 25 mg kg−1 every 6 h during 24 h. In the case of a delayed sternal closure, 25 mg kg−1 Cefazolin was administered every 6 h until sternal closure. All neonates received 1 mg kg−1 dexamethasone at induction of anesthesia; if they required DHCA they received 30 mg kg−1 dexamethasone at the beginning of CPB.
Postoperatively, clinical, biological, and radiological examinations were performed daily until discharge from the Cardiac Intensive Care Unit (CICU). Serum samples were drawn daily for CRP measurement, white blood cells and polymorphonuclear leukocytes counts as part of the institutional protocol. PCT was measured at the discretion of the attending intensivist. Besides clinical assessment, screening included chest radiograph, blood cultures, culture of the endotracheal suction (if intubated), urine culture, culture of the wound seepage, and/or intraoperative mediastinal culture in case of reoperation for mediastinitis. Patients were thought to have infections if they had (i) pneumonia—fever, worsening respiratory status, new pulmonary opacity of chest X-ray, and either polymorphonuclear leukocytes or pathogenic organisms on tracheal aspirate; (ii) sepsis—systemic inflammatory response (at least two of the following, one of which must be abnormal temperature or leukocyte count: core temperature > 38.5 or < 36 °C, elevated or depressed leukocyte count, tachycardia, or bradycardia, tachypnea) plus a positive blood culture within 3 days; (iii) urinary tract infection—fever in the setting of increased white blood cells on urinalysis and a positive urine culture; (iv) wound infection—erythema or dehiscence with purulent drainage with or without deep tissue involvement; (v) necrotising enterocolitis not requiring laparotomy—abdominal distension, bilious residuals, melena. Neither PCT nor CRP were shown to be reliable markers of infection in infants with ECMO [17]; therefore, patients with postoperative ECMO were not analyzed here. PCT measurement used the ARCHITECT BRAHMS PCT, a chemiluminescent microparticle immunoassay on the automated ARCHITECT i2000-SR® immunoassay analyzer (Abbott).
PCT concentrations were considered for analysis if measured in neonates older than 3 days, within the 15th postoperative day (POD) and less than 2 days before infection declaring. The day when antibiotics were started was considered to be the day of infection declaring. Each blood sampling was considered an independent patient-day episode; as such, the analysis included one or several measurements per patient, performed on different PODs. First, variables associated with postoperative PCT concentrations were identified in neonates without infections, and further used for matching of patient-day episodes in patients with infections, with patient-day episodes in patients without infections. After matching, associations between PCT, CRP, and white blood cells, polymorphonuclear leukocytes counts and the occurrence of infections were explored using matched tests. The ability of each biomarker to predict infections was explored using the ROC methodology, and their best predictive thresholds were identified using the Youden index [18]. Comparison of the predictive abilities between the biomarkers used the likelihood ratio test for nested models. Analysis used the basic R package, “optmatch” and “verification” packages.
Results
A total of 357 neonates underwent surgery during the study period. Among them, 154 patients were eligible for inclusion. Ten patients required postoperative ECMO, and were not analyzed here, leaving 144 patients under analysis. The characteristics of the study population were age 7.9 ± 5.07 days, weight 3.51 ± 3.81 kg, CPB duration 132.79 ± 61.23 min, aortic cross-clamping duration 71.17 ± 45.68 min. The patients underwent surgery for repair of transposition of the great arteries (35), transposition + ventricular septal defect (VSD) (16), transposition + VSD + coarctation (8), reconstruction of the aortic arch (27), palliation for hypoplastic left heart syndrome (19) and palliation for pulmonary atresia with or without VSD (15), total anomalous pulmonary vein connection (10), Truncus arteriosus (6), and others (8). Overall, 40 patients required DHCA, and in 113, the sternum was left open after surgery, the median delay to sternal closure was 4 days, interquartile range (IQR) [3,4,5,6].
In total, 70 neonates had postoperative infections: 47 had postoperative pneumonia, 12 had sepsis, 5 had mediastinitis, 4 had necrotising enterocolitis, and 2 had urinary tract infections. Among them, 19 neonates had presumed or confirmed infections preoperatively, and received antibiotics prior to surgery. Only the first postoperative infection was considered in patients with several episodes. The median time interval between surgery and the day of infection declaring was 4.5 days, IQR [3–9]. The median time to extubation was 5 days [2–9], the duration of ICU stay was 8 days [5–12], and 5 patients died in-hospital (3.5%).
The variables independently associated with postoperative PCT concentrations in the group of 74 neonates without infections were the POD (coeff estimate − 0.99 ± 0.28, p < 0.001) and the use of DHCA (coeff estimate 6.65 ± 1.83, p < 0.001). Consequently, each patient-day episode in patients with infections was matched with a patient-day episode in patients without infections according to the POD and the use of DHCA, as well as to the patient’s age and the CPB duration (shown to be associated with PCT concentrations in previous work [5, 11, 15]).
Overall, 89 patient-day episodes were matched, including 47 patients without infections and 47 patients with infections. The characteristics of the matched population are shown in Table 1. The kinetics of PCT and CRP concentrations during the first 15 PODs in neonates with or without infection, and with or without DHCA, are shown in Figs. 1 and 2, respectively. In patients without infections, PCT was higher postoperatively if surgery was performed with DHCA when compared with the others, 8.27 ± 11.89 ng mL−1 vs 2.78 ± 4.06 ng mL−1, respectively (p = 0.002), and so was CRP, 32.54 ± 26 ng mL−1 vs 18.55 ± 18.11 ng mL−1, respectively (p = 0.01). White blood cells and polymorphonuclear leukocyte counts were similar in patients with and without infections, whether surgery was performed with DHCA or not.
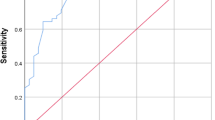
The results of the ROC analysis exploring the predictive ability of the markers, when measured less than 2 days before infection declaring, are shown in Table 2. The ROC curves for both PCT and CRP as well as the sum of the two markers on POD 1 and 2, POD 3 and 4, or later, are shown in Fig. 3. The ensemble shows a better predictive ability of PCT for infections when compared with just CRP on POD 1 and 2. It also shows that the sum of the two markers was a better predictor of infections within 4 days of surgery, when compared with each marker taken alone. In the group of patients undergoing surgery without DHCA, there was a good predictive ability of CRP concentrations for infections, and a good predictive ability of PCT for infections when measured within 4 PODs. The sum of CRP (mg L−1) and PCT (pg mL−1) measured on POD1 or POD2 above 32.87 and above 49.36 when measured at POD3 or POD4 were the best predictors of infections. None of the markers predicted infection in patients undergoing surgery with DHCA (results not shown).
In patients who did not require DHCA, the likelihood ratio test for nested models demonstrated significant added predictive ability of PCT concentrations over a model of postoperative infection using CRP as the unique predictor (1.09, p < 0.001).
Discussion
The present case–control study demonstrates good accuracy of PCT to identify infections occurring within the first week of cardiac surgery in neonates. It shows that the combination of PCT and CRP monitoring to screen neonates early after surgery is a better indicator of infection than CRP alone. In patients undergoing surgery with DHCA, neither PCT nor CRP were predictive of infections within the first two weeks of surgery. To our knowledge, this is the largest study to date to explore the accuracy of PCT as a marker of infection in a neonatal population with cardiac surgery.
PCT was identified as a human precursor of calcitonin in 1989, and was first described as an infectious marker in 1993 [1]. In 1994, Dandona et al. [19] showed that after the injection of endotoxins in normal human volunteers, the PCT concentration became detectable at 4 h, peaked at 6 h, maintained a plateau through 8 to 24 h, and was preceded by an increase in TNF-alpha and IL-6. The short half-life (24–30 h) [19] and the rapid return to undetectable concentrations in uncomplicated patients is a major advantage of PCT over CRP. Therefore, there is a growing body of literature investigating PCT as an alternative to CRP in patients with sepsis, and as a predictor of both complications and poor outcome [20]. In critically ill neonates, PCT has been shown to be an accurate indicator of infection [1,2,3,4], and in older patients with cardiac surgery, PCT has been shown to be a more accurate predictor of postoperative infections when compared with CRP [5,6,7,8, 13,14,15]. These are in accordance with the results in Table 2 and Fig. 3, which indicate that PCT was a better predictor of infections when measured at POD1 or POD2, when compared with CRP.
Uncomplicated cardiac surgery with CPB leads to inflammatory cytokine release, a PCT peak at POD1 and a return to normal levels within the first week [12, 13, 20]. In patients with infections following cardiac surgery, PCT concentrations remain high [10, 12, 13], and proportional to the severity of infection [12]. A further advantage of PCT monitoring in neonates with CPB is the lack of impact of a delayed sternal closure, a common technique in neonates with postoperative hemodynamic instability, whereas CRP concentrations remain high and further increase in patients in whom the sternum is left open [13].
However, PCT concentrations measured after cardiac surgery need to be interpreted with caution. Besides the above-mentioned overlap between the biological features of the post-CPB inflammatory response and an infectious state, the postoperative increase in PCT is also impacted by surgical complexity [20] and CPB duration [15]. Many other situations may trigger PCT synthesis and release in the context of cardiac surgery, including myocardial infarction [21], cardiogenic shock [22], and concomitant organ dysfunction [20]. Surgical trauma per se increases pro-inflammatory cytokines (IL-6), CRP, and PCT after uneventful surgeries in neonates [23, 24]. Also, some peculiarities of PCT concentrations in neonates need to be highlighted. PCT concentrations are high during the first days of life: PCT rises from < 0.08 ng mL−1 soon after birth, to 0.8 ng mL−1 at 24 h after birth, and drops again < 0.08 ng mL−1 afterwards [11]. Lapillone et al. [25] reported high-PCT concentrations in neonates suffering from SIRS with respiratory distress syndrome in the absence of infection. Inclusion of neonates older than 3 days and matching according to age, CPB duration, the requirement for DHCA, and to the POD aimed to minimize all these biases.
The identification of an accurate PCT threshold for the prediction of infection in previous studies has been made difficult by the analysis of populations including neonates along with older infants and adolescents, and by ignoring the expected variation over time of PCT concentrations. As a consequence, in a meta-analysis of PCT studies, Chiesa et al. showed that PCT cutoffs predictive of infection varied between 0.235 and 100 ng mL−1 [20]. Overall, reported concentrations in neonates and infants with cardiac surgery are higher than those reported in adults: cutoffs to identify localized infections or sepsis vary between 2 and 5 ng mL−1 in pediatric studies [6, 9, 10, 14] and between 1 and 1.5 ng mL−1 in adults [12, 26], whereas cutoffs which identify septic shock vary between 15 and 20 ng mL−1 in pediatric studies [4, 7, 16] and 10 ng mL−1 in adult studies [12, 26]. In addition, it is likely that there is no unique PCT cutoff for the whole postoperative period, but a sequence of thresholds according to the time elapsed since surgery. Here we identified a cutoff of 12.97 ng mL−1 on POD1 and POD2 and of 1.33 on POD3 and POD4. With a similar approach in a population of 65 neonates with cardiac surgery, Bobillo et al. [10] identified cutoffs of 4 ng mL−1 at POD1, of 5 ng mL−1 at POD2, and 5.5 ng mL−1 at POD3. However, the kinetics of the cutoff reported here are more in accordance with the PCT kinetics following uncomplicated cardiac surgery with CPB [12, 13, 20].
Both PCT and CRP were higher postoperatively in patients with DHCA when compared with those without DHCA; also, their predictive value was negligible in patients with DHCA. Older studies performed in infants showed that deep hypothermia and rewarming significantly reduce gut mucosal blood flow and increase intestinal oxygen consumption. The mucosal hypoperfusion and hypoxia are thought to be responsible for bacterial translocation from the gut leading to systemic endotoxin release, which acts as a powerful trigger for cytokine and PCT production [27]. It is likely that the intensity of the systemic inflammatory response due to the use of DHCA overwhelmed the inflammatory response due to the infectious state here, and biased the interpretation of PCT and CRP concentrations as indicators of infection.
Limitations
The present study was single-center based, and the results need to be validated on a larger scale. The use of corticosteroids after induction of anesthesia might have had an impact on the rise of both PCT and CRP due to their strong anti-inflammatory effects. Since most of the PCT measurements were performed within 5 days of surgery, the exploration of the ability to predict infection was limited to this period.
Conclusion
The accuracy of PCT to identify infections early after neonatal cardiac surgery is good, and is better than that of CRP within 48 h of surgery. A combination of both biomarkers is the best predictor of infections within 4 days of surgery. Neither PCT nor CRP are predictive of infections after deep hypothermic CPB.
References
Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C (1993) High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 341(8844):515–518
Auriti C, Fiscarelli E, Ronchetti MP, Argentieri M, Marrocco G, Quondamcarlo A, Seganti G, Bagnoli F, Buonocore G, Serra G et al (2012) Procalcitonin in detecting neonatal nosocomial sepsis. Arch Dis Child Fetal Neonatal Ed 97(5):F368–370
Chiesa C, Panero A, Rossi N, Stegagno M, De Giusti M, Osborn JF, Pacifico L (1998) Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. Clin Infect Dis 26(3):664–672
Neunhoeffer F, Plinke S, Renk H, Hofbeck M, Fuchs J, Kumpf M, Zundel S, Seitz G (2016) Serum concentrations of interleukin-6, procalcitonin, and C-reactive protein: discrimination of septical complications and systemic inflammatory response syndrome after pediatric surgery. Eur J Pediatr Surg 26(2):180–185
Celebi S, Koner O, Menda F, Balci H, Hatemi A, Korkut K, Esen F (2006) Procalcitonin kinetics in pediatric patients with systemic inflammatory response after open heart surgery. Intensive Care Med 32(6):881–887
Arkader R, Troster EJ, Lopes MR, Junior RR, Carcillo JA, Leone C, Okay TS (2006) Procalcitonin does discriminate between sepsis and systemic inflammatory response syndrome. Arch Dis Child 91(2):117–120
Michalik DE, Duncan BW, Mee RB, Worley S, Goldfarb J, Danziger-Isakov LA, Davis SJ, Harrison AM, Appachi E, Sabella C (2006) Quantitative analysis of procalcitonin after pediatric cardiothoracic surgery. Cardiol Young 16(1):48–53
Rey C, Los Arcos M, Concha A, Medina A, Prieto S, Martinez P, Prieto B (2007) Procalcitonin and C-reactive protein as markers of systemic inflammatory response syndrome severity in critically ill children. Intensive Care Med 33(3):477–484
Simon L, Saint-Louis P, Amre DK, Lacroix J, Gauvin F (2008) Procalcitonin and C-reactive protein as markers of bacterial infection in critically ill children at onset of systemic inflammatory response syndrome. Pediatr Crit Care Med 9(4):407–413
Bobillo S, Rodriguez-Fanjul J, Sole A, Moreno J, Balaguer M, Esteban E, Cambra FJ, Jordan I (2018) Kinetics of procalcitonin in pediatric patients on extracorporeal membrane oxygenation. Biomark Insights 13:1177271917751900
Chiesa C, Pacifico L, Osborn JF, Bonci E, Hofer N, Resch B (2015) Early-onset neonatal sepsis: still room for improvement in procalcitonin diagnostic accuracy studies. Medicine 94(30):e1230
Aouifi A, Piriou V, Bastien O, Blanc P, Bouvier H, Evans R, Celard M, Vandenesch F, Rousson R, Lehot JJ (2000) Usefulness of procalcitonin for diagnosis of infection in cardiac surgical patients. Crit Care Med 28(9):3171–3176
Davidson J, Tong S, Hauck A, Lawson DS, da Cruz E, Kaufman J (2013) Kinetics of procalcitonin and C-reactive protein and the relationship to postoperative infection in young infants undergoing cardiovascular surgery. Pediatr Res 74(4):413–419
Garcia IJ, Gargallo MB, Torne EE, Lasaosa FJ, Vinas AT, Tolosa CV, Rico AP (2012) Procalcitonin: a useful biomarker to discriminate infection after cardiopulmonary bypass in children. Pediatr Crit Care Med 13(4):441–445
Hammer S, Fuchs AT, Rinker C, Daebritz S, Kozlik-Feldmann R, Netz H (2004) Interleukin-6 and procalcitonin in serum of children undergoing cardiac surgery with cardiopulmonary bypass. Acta Cardiol 59(6):624–629
Hatherill M, Tibby SM, Sykes K, Turner C, Murdoch IA (1999) Diagnostic markers of infection: comparison of procalcitonin with C reactive protein and leucocyte count. Arch Dis Child 81(5):417–421
Rungatscher A, Merlini A, De Rita F, Lucchese G, Barozzi L, Faggian G, Mazzucco A, Luciani GB (2013) Diagnosis of infection in paediatric veno-arterial cardiac extracorporeal membrane oxygenation: role of procalcitonin and C-reactive protein. Eur J Cardio-Thorac Surg 43(5):1043–1049
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3(1):32–35
Dandona P, Nix D, Wilson MF, Aljada A, Love J, Assicot M, Bohuon C (1994) Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab 79(6):1605–1608
Sponholz C, Sakr Y, Reinhart K, Brunkhorst F (2006) Diagnostic value and prognostic implications of serum procalcitonin after cardiac surgery: a systematic review of the literature. Crit Care 10(5):R145
Lecharny JB, Khater D, Bronchard R, Philip I, Durand G, Desmonts JM, Dehoux M (2001) Hyperprocalcitonemia in patients with perioperative myocardial infarction after cardiac surgery. Crit Care Med 29(2):323–325
Picariello C, Lazzeri C, Chiostri M, Gensini G, Valente S (2009) Procalcitonin in patients with acute coronary syndromes and cardiogenic shock submitted to percutaneous coronary intervention. Intern Emerg Med 4(5):403–408
Nguyen-Vermillion A, Juul SE, McPherson RJ, Ledbetter DJ (2011) Time course of C-reactive protein and inflammatory mediators after neonatal surgery. J Pediatr 159(1):121–126
Pavcnik-Arnol M, Bonac B, Groselj-Grenc M, Derganc M (2010) Changes in serum procalcitonin, interleukin 6, interleukin 8 and C-reactive protein in neonates after surgery. Eur J Pediatr Surg 20(4):262–266
Lapillonne A, Basson E, Monneret G, Bienvenu J, Salle BL (1998) Lack of specificity of procalcitonin for sepsis diagnosis in premature infants. Lancet 351(9110):1211–1212
Jebali MA, Hausfater P, Abbes Z, Aouni Z, Riou B, Ferjani M (2007) Assessment of the accuracy of procalcitonin to diagnose postoperative infection after cardiac surgery. Anesthesiology 107(2):232–238
Booker PD, Romer H, Franks R (1996) Gut mucosal perfusion in neonates undergoing cardiopulmonary bypass. Br J Anaesth 77(5):597–602
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical Approval
This study was approved by the institutional ethical committee, who waived the need for written informed parental consent to perform this retrospective analysis, after de-identification of all patient data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aryafar, A., Di Marzio, A., Guillard, O. et al. Procalcitonin Concentration Measured Within the First Days of Cardiac Surgery Is Predictive of Postoperative Infections in Neonates: A Case–Control Study. Pediatr Cardiol 40, 1289–1295 (2019). https://doi.org/10.1007/s00246-019-02150-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-019-02150-y